Abstract
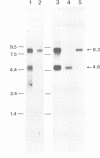
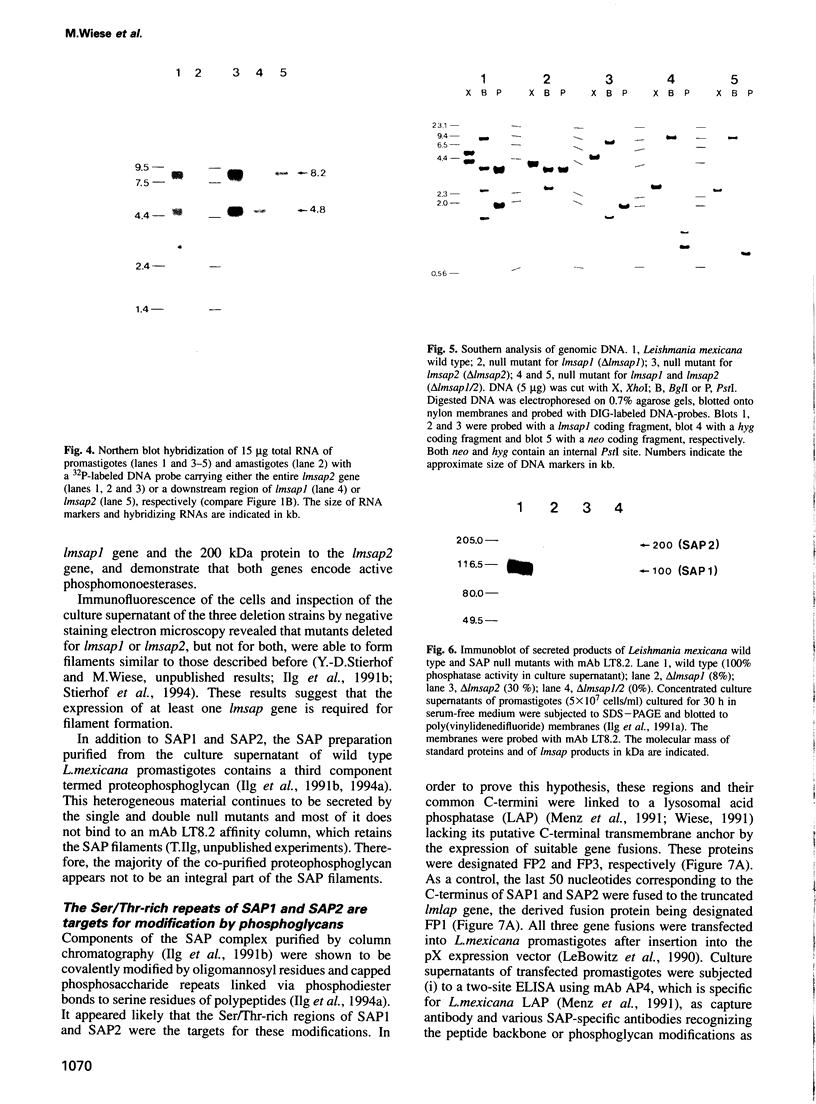
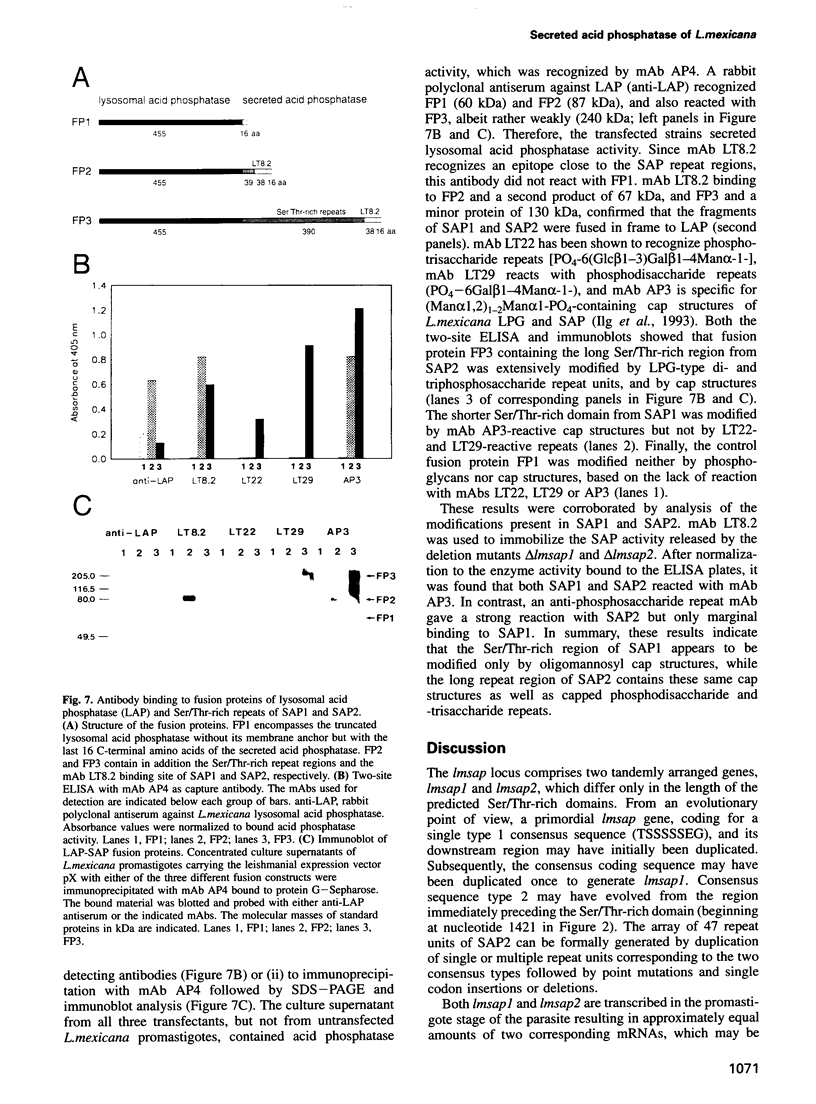
The insect stage of the protozoan parasite Leishmania mexicana secretes a phosphomonoesterase in the form of a filamentous complex. The polypeptide subunits of this polymer are modified by phosphoglycans and/or oligomannosyl residues linked to phosphoserine. Based on peptide sequence data of a predominant 100 kDa protein of the filamentous complex, two tandemly arranged, single copy genes, lmsap1 and lmsap2, were cloned and sequenced. lmsap1 predicts a protein with features characteristic of acid phosphatases and a remarkable serine- and threonine-rich region of 32 amino acids close to the C-terminus. In the otherwise identical lmsap2 product, this region is extended to 383 amino acids and is composed of short Ser/Thr-rich repeats. Deletion analysis demonstrates that lmsap1 encodes the major 100 kDa protein of the complex while a minor 200 kDa component is derived from the lmsap2 gene. Null mutants of either gene retain the ability to secrete acid phosphatase filaments, while a deletion of both genes results in Leishmania defective in enzyme formation. The Ser/Thr-rich domains are the targets for phosphoglycan modifications as shown by the expression of secreted fusion proteins composed of these C-terminal regions and the N-terminal domain of a lysosomal acid phosphatase.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander J., Russell D. G. The interaction of Leishmania species with macrophages. Adv Parasitol. 1992;31:175–254. doi: 10.1016/s0065-308x(08)60022-6. [DOI] [PubMed] [Google Scholar]
- Bahr V., Stierhof Y. D., Ilg T., Demar M., Quinten M., Overath P. Expression of lipophosphoglycan, high-molecular weight phosphoglycan and glycoprotein 63 in promastigotes and amastigotes of Leishmania mexicana. Mol Biochem Parasitol. 1993 Mar;58(1):107–121. doi: 10.1016/0166-6851(93)90095-f. [DOI] [PubMed] [Google Scholar]
- Bates P. A., Dwyer D. M. Biosynthesis and secretion of acid phosphatase by Leishmania donovani promastigotes. Mol Biochem Parasitol. 1987 Dec;26(3):289–296. doi: 10.1016/0166-6851(87)90081-8. [DOI] [PubMed] [Google Scholar]
- Bates P. A., Hermes I., Dwyer D. M. Golgi-mediated post-translational processing of secretory acid phosphatase by Leishmania donovani promastigotes. Mol Biochem Parasitol. 1990 Mar;39(2):247–255. doi: 10.1016/0166-6851(90)90063-r. [DOI] [PubMed] [Google Scholar]
- Berens R. L., Marr J. J. An easily prepared defined medium for cultivation of Leishmania donovani promastigotes. J Parasitol. 1978 Feb;64(1):160–160. [PubMed] [Google Scholar]
- Cruz A., Coburn C. M., Beverley S. M. Double targeted gene replacement for creating null mutants. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7170–7174. doi: 10.1073/pnas.88.16.7170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckerskorn C., Mewes W., Goretzki H., Lottspeich F. A new siliconized-glass fiber as support for protein-chemical analysis of electroblotted proteins. Eur J Biochem. 1988 Oct 1;176(3):509–519. doi: 10.1111/j.1432-1033.1988.tb14308.x. [DOI] [PubMed] [Google Scholar]
- Gavel Y., von Heijne G. Sequence differences between glycosylated and non-glycosylated Asn-X-Thr/Ser acceptor sites: implications for protein engineering. Protein Eng. 1990 Apr;3(5):433–442. doi: 10.1093/protein/3.5.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilg T., Harbecke D., Wiese M., Overath P. Monoclonal antibodies directed against Leishmania secreted acid phosphatase and lipophosphoglycan. Partial characterization of private and public epitopes. Eur J Biochem. 1993 Oct 15;217(2):603–615. doi: 10.1111/j.1432-1033.1993.tb18283.x. [DOI] [PubMed] [Google Scholar]
- Ilg T., Menz B., Winter G., Russell D. G., Etges R., Schell D., Overath P. Monoclonal antibodies to Leishmania mexicana promastigote antigens. I. Secreted acid phosphatase and other proteins share epitopes with lipophosphoglycan. J Cell Sci. 1991 May;99(Pt 1):175–180. doi: 10.1242/jcs.99.1.175. [DOI] [PubMed] [Google Scholar]
- Ilg T., Overath P., Ferguson M. A., Rutherford T., Campbell D. G., McConville M. J. O- and N-glycosylation of the Leishmania mexicana-secreted acid phosphatase. Characterization of a new class of phosphoserine-linked glycans. J Biol Chem. 1994 Sep 30;269(39):24073–24081. [PubMed] [Google Scholar]
- Ilg T., Stierhof Y. D., Etges R., Adrian M., Harbecke D., Overath P. Secreted acid phosphatase of Leishmania mexicana: a filamentous phosphoglycoprotein polymer. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8774–8778. doi: 10.1073/pnas.88.19.8774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilg T., Stierhof Y. D., McConville M. J., Overath P. Purification, partial characterization and immunolocalization of a proteophosphoglycan secreted by Leishmania mexicana amastigotes. Eur J Cell Biol. 1995 Feb;66(2):205–215. [PubMed] [Google Scholar]
- Jaffe C. L., Perez L., Schnur L. F. Lipophosphoglycan and secreted acid phosphatase of Leishmania tropica share species-specific epitopes. Mol Biochem Parasitol. 1990 Jun;41(2):233–240. doi: 10.1016/0166-6851(90)90186-p. [DOI] [PubMed] [Google Scholar]
- LAINSON R., STRANGWAYS-DIXON J. LEISHMANI MEXICANA: THE EPIDEMIOLOGY OF DERMAL LEISHMANIASIS IN BRITISH HONDURAS. Trans R Soc Trop Med Hyg. 1963 Jul;57:242–265. doi: 10.1016/0035-9203(63)90182-2. [DOI] [PubMed] [Google Scholar]
- Laird P. W. Trans splicing in trypanosomes--archaism or adaptation? Trends Genet. 1989 Jul;5(7):204–208. doi: 10.1016/0168-9525(89)90082-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBowitz J. H., Coburn C. M., Beverley S. M. Simultaneous transient expression assays of the trypanosomatid parasite Leishmania using beta-galactosidase and beta-glucuronidase as reporter enzymes. Gene. 1991 Jul 15;103(1):119–123. doi: 10.1016/0378-1119(91)90402-w. [DOI] [PubMed] [Google Scholar]
- LeBowitz J. H., Coburn C. M., McMahon-Pratt D., Beverley S. M. Development of a stable Leishmania expression vector and application to the study of parasite surface antigen genes. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9736–9740. doi: 10.1073/pnas.87.24.9736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovelace J. K., Gottlieb M. Comparison of extracellular acid phosphatases from various isolates of Leishmania. Am J Trop Med Hyg. 1986 Nov;35(6):1121–1128. doi: 10.4269/ajtmh.1986.35.1121. [DOI] [PubMed] [Google Scholar]
- McConville M. J., Ferguson M. A. The structure, biosynthesis and function of glycosylated phosphatidylinositols in the parasitic protozoa and higher eukaryotes. Biochem J. 1993 Sep 1;294(Pt 2):305–324. doi: 10.1042/bj2940305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menz B., Winter G., Ilg T., Lottspeich F., Overath P. Purification and characterization of a membrane-bound acid phosphatase of Leishmania mexicana. Mol Biochem Parasitol. 1991 Jul;47(1):101–108. doi: 10.1016/0166-6851(91)90152-v. [DOI] [PubMed] [Google Scholar]
- Ostanin K., Harms E. H., Stevis P. E., Kuciel R., Zhou M. M., Van Etten R. L. Overexpression, site-directed mutagenesis, and mechanism of Escherichia coli acid phosphatase. J Biol Chem. 1992 Nov 15;267(32):22830–22836. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider G., Lindqvist Y., Vihko P. Three-dimensional structure of rat acid phosphatase. EMBO J. 1993 Jul;12(7):2609–2615. doi: 10.1002/j.1460-2075.1993.tb05921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schägger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987 Nov 1;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Stierhof Y. D., Ilg T., Russell D. G., Hohenberg H., Overath P. Characterization of polymer release from the flagellar pocket of Leishmania mexicana promastigotes. J Cell Biol. 1994 Apr;125(2):321–331. doi: 10.1083/jcb.125.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turco S. J., Descoteaux A. The lipophosphoglycan of Leishmania parasites. Annu Rev Microbiol. 1992;46:65–94. doi: 10.1146/annurev.mi.46.100192.000433. [DOI] [PubMed] [Google Scholar]
- Ziegelbauer K., Multhaup G., Overath P. Molecular characterization of two invariant surface glycoproteins specific for the bloodstream stage of Trypanosoma brucei. J Biol Chem. 1992 May 25;267(15):10797–10803. [PubMed] [Google Scholar]