Abstract
A critical review of cost-effectiveness analyses of HPV vaccination in males was conducted and nine studies were identified in different countries. Due to the heterogeneity among these studies in terms of modeling approach, vaccination strategies, health outcomes considered, assumptions and parameters, limited conclusions can be drawn with regard to the absolute cost-effectiveness. Nevertheless, key drivers were identified. More favorable cost-effectiveness appeared when all HPV-related diseases outcomes were considered, a suboptimal vaccine coverage among girls and/or lower vaccine prices were assumed. There was a general lack of transparency to fully describe the details of the methodological approach of modeling and calibration. Further research should be conducted to generate robust evidence-based data sets (HPV-related diseases epidemiology, costs and quality of life). The best modeling practice for HPV vaccination and how to better capture the true economic value of vaccination beyond cost-effectiveness in a broader policy context need to be investigated.
Keywords: human papillomavirus, vaccine, strategy, males, cost-effectiveness, economic evaluation
Introduction
Background
Human papillomavirus (HPV) is a family of viruses that infect epithelial tissues including skin, cervix, anus, mouth and throat.1,2 The primary path of transmission is sexual contact, although HPV can also be passed on vertically from mother to child.3 HPV infection may lead to anogenital warts, precancerous lesions and cervical cancer, and close to 100% of cervical cancers are caused by HPV infection.4 Worldwide, 493 000 new cases of cervical cancer and 274 000 deaths due to the disease were reported in 2002, making it the second most common cancer in females.5
Two vaccines are currently available in the market. Gardasil® (Sanofi Pasteur MSD), a quadrivalent vaccine that protects against HPV types 6, 11, 16, and 18, holds an indication in individuals (males and females) aged 9 y and older for the prevention of premalignant genital (cervical, vulvar, and vaginal) lesions and cervical cancer and genital warts causally related to these HPV types.6 Another vaccine, Cervarix® (GlaxoSmithKline) protects against HPV types 16 and 18 and is indicated for the prevention of premalignant genital (cervical, vulvar, and vaginal) lesions and cervical cancer causally related to certain oncogenic HPV types in females.7
Routine vaccination programs against HPV in females have been implemented and publicly funded in the majority of Western European countries to protect against the diseases, in addition to cervical screening campaigns.8
Context
The International Agency for Research on Cancer (IARC) has classified HPV as a human carcinogen at different anatomical sites beyond the cervix: penis, anus, oral cavity, oropharynx and tonsils.9 It is estimated that the virus is associated with 80% to 85% of anal cancers, 70% of vaginal cancers, 40% of vulvar cancers, 50% of penile cancers, and 20% to 60% of head and neck cancers.10-12 In Europe, men bear approximately 30% of the overall HPV-related cancers (17 403 incident cases annually), mainly due to head and neck cancers (14 098 incident cases annually).8,13-15 Emerging evidence from Denmark,16 the US,17 and Australia18 highlights a significant increase in the incidence of HPV-related head and neck cancers in males. An increase in the incidence of anal cancer has also been reported in several countries19-21 and HPV infection was found to be the major contributor.21
Genital warts, associated with HPV types 6 and 11 in 90% of cases, are very common and recurrent benign lesions.22,23 Although they do not lead to life-threatening diseases, genital warts affect patients’ quality of life, especially due to emotional and sexual concerns.24,25 It is estimated that between 286 682 and 325 722 new cases of genital warts occur annually among males in Europe as a consequence of HPV types 6 and 11 infection.15
Female-only HPV vaccination protects heterosexual males indirectly via their partners, but these heterosexual men will remain vulnerable to HPV unless vaccinated. To provide direct protection to males, several countries recently recommended vaccinating males against HPV (Austria,26 Saxony in Germany,27 the US,28 Canada,29 and Australia30,31), in addition to female vaccination. The mass vaccination of boys is funded in the US,32 Prince Edward Island in Canada,33 Australia,30 Saxony in Germany,27 and Vorarlberg in Austria.34
Issue and objective
Expanding the vaccination program to males would further control the transmission of HPV and provide direct protection to males. Yet the constraint on healthcare resources requires the program to be financially viable and more specifically, the value for money or cost-effectiveness of such a program needs to be assessed.
Past economic evaluations have concluded that vaccinating females is a cost-effective strategy whatever the country.35,36 This paper aimed to critically review published cost-effectiveness analyses that assessed male HPV vaccination. It intended to (1) identify studies assessing the cost-effectiveness of male vaccination; (2) review the methods used for this assessment, and (3) identify the major drivers of cost-effectiveness of male vaccination and assess the consistency and robustness of the results.
Methods
Cost-effectiveness studies were identified through a pragmatic literature review conducted on PubMed from 1948 to 31st December 2011. The search strategy used was the following: “cost-benefit analysis” AND (“papillomavirus vaccines”[MeSH] OR HPV) AND (boys OR males). As a pragmatic review, we did not run the search on other electronic databases, which might lead to missing some potential studies. It is acknowledged as a limitation of the current study.
Titles and abstracts were screened and publications were excluded if they did not assess the cost-effectiveness of HPV vaccination in males. Selected papers were reviewed and information was extracted using a pre-specified summary table, (Table 1), which included the description of the modeling approach, model structure, main assumptions and parameterization.
Table 1. Modeling aspects reviewed in this study.
| Aspects | Descriptions |
|---|---|
| Modeling approach | Dynamic vs. static; aggregate level vs. individual level; deterministic vs. stochastic. |
| Assumption on natural immunity | Susceptible-infectious-susceptible (SIS), susceptible-infectious-recovered (SIR) and susceptible-infectious-recovered-susceptible (SIRS)1 |
| Model calibration process | Method used for calibration, if any. |
| Diseases modeled | HPV-related diseases considered in the model. |
| HPV transmission patterns modeled | Heterosexual or homosexual. |
| Vaccination and screening strategies assessed | Vaccination strategy (initial vaccination, catch-up vaccination and booster), screening strategy and vaccine coverage rate. |
| Input parameters | Vaccine characteristics (efficacy, against infection and/or disease, waning function), transition probabilities, costs, utilities and discount rate. |
1 An SIR model assumes that, after recovery from infection, the individual will not be re-infected (i.e., natural immunity) whereas an SIS model assumes the individual can be re-infected immediately after recovery from infection (i.e., no natural immunity). An SIRS model assumes that natural immunity only exists for a period of time following recovery from infection.
A factor analysis was conducted in order to identify the major drivers of results as follows. The results of the sensitivity analyses from each study were reviewed and the magnitude and direction of change in the results were assessed where sufficient data were available. Given that both vaccination strategy and vaccine coverage rate strongly impact the level of protection and therefore the epidemiology of the disease, only sufficiently similar vaccination programs (in terms of vaccination coverage, target age, and sex) were used for this analysis.
Finally, the quality of the studies was also compared with the checklist suggested by Garnett et al.37 for mathematical models. The list of items is reproduced in Table 2.
Table 2. Items that should accompany the most rigorous model analyses (reproduced with permission from Garnett et al.37).
| Aspects | Descriptions |
|---|---|
| Diagrams that show model structure | To show how disease natural history is presented, process and determinants of disease acquisition, and how putative intervention could affect the system. |
| Complete list of model parameters | To include clear and precise descriptions of the meaning of each parameter, together with the values or ranges for each, with justification or the primary source cited, and important caveats about the use of these values noted. Where a parameter value comes from another modeling analysis, this caveat should be noted. Parameter values that are fit in this model (not independently measured) should be clearly marked. |
| Assessment of model predictions with data | Illustration of agreement between model (as used in the analyses) and data or observational information. Clear statement about how model was fitted to the data, including goodness-of-fit measure, the numerical algorithm used, which parameter varied, constraints imposed on parameter values, and starting conditions. |
| Presentation of results | Key modeling results to be presented with a scientifically based estimate of uncertainty. Presentation of uncertainty analyses should be accompanied with statement about the sources of uncertainties quantified and not quantified, and these sources can include parameter, data, and model structure. |
| Discussion of model structure | To include the scientific rationale for this choice of model structure and identify points where this choice would influence conclusion drawn. Also to describe the strength of the scientific basis underlying the key model assumptions. |
Review
Overview
Fifty-eight citations were retrieved from the PubMed search. Two cost-effectiveness studies38,39 were excluded as they were very similar to another included study40 and did not focus on male vaccination. A total of nine cost-effectiveness studies that assessed male HPV vaccination were finally included in our review (Fig. 1). Six studies were conducted in the US context and the other three related to the UK, Austria, and Denmark. Quadrivalent vaccines, the only vaccine indicated in both sexes, were considered in all but two studies,41,42 which only looked at HPV types 16 and 18 in the analyses. All studies but one focused on heterosexual relationship. Kim43 specifically assessed the vaccination of men who have sex with men (MSM). Life year was used as the primary effectiveness endpoint in Zechmeister et al.,41 and in other studies health-related quality of life (measured by quality-adjusted life years [QALYs]) was used as the primary endpoint.
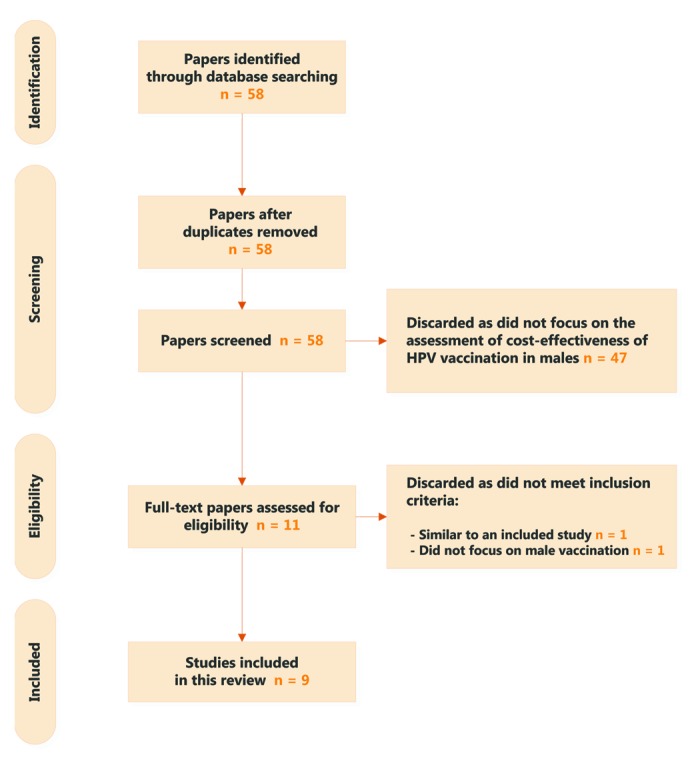
Figure 1. PRISMA diagram.
Only incremental cost-effectiveness ratios (ICERs) associated with the most similar strategies and vaccine coverage rate were reported in Table 3 to facilitate comparison. Yet, males' vaccination is extremely interesting if coverage in females is sub-optimal. These strategies compared male and female to female-only vaccination strategies, except for Kim,43 which focused only on male vaccination. The coverage rate of the female only vaccination strategy was high (approximately 70%). Currencies were also converted to euros using the exchange rate in May 201244 for comparative purposes.
Table 3. Cost-effectiveness studies of vaccinating males.
| Elbasha et al.40 | Elbasha and Dasbach45 | Jit et al.46 | Kim and Goldie47 | Kim43 | Taira et al.42 | Zechmeister et al.41 | Olsen and Jepsen48 | Chesson et al.49 | |
|---|---|---|---|---|---|---|---|---|---|
| Country | US | US | UK | US | US | US | Austria | Denmark | US |
| Modeling approach | Dynamic, aggregate, deterministic | Dynamic, aggregate, deterministic | Dynamic, aggregate, stochastic | Transmission and disease progression were modeled separately. For HPV 16/18 transmission: dynamic, aggregate, stochastic; for disease progression: static, individual-based, stochastic | Static, aggregate, stochastic | Two-part (transmission: dynamic, aggregate, deterministic; disease: static, aggregate, stochastic) | Dynamic, aggregate, deterministic | Agent-based (dynamic, individual, stochastic) | Dynamic, aggregate, deterministic |
| Diseases included | CC, GW | CC, VAG, VUL, PEN, ANA, H&N, JO/AORRP, GW | CC, GW | CC, VAG, VUL, PEN, ANA, H&N (oropharyngeal), JORRP, GW | ANA, GW | CC | CC | CC, GW | CC, VAG, VUL, PEN, ANA, H&N (oropharyngeal), JORRP, GW |
| Natural immunity | SIR | SIRS | SIRS | SIR | n/A | SIS | SIS | SIS | SIR |
| Coverage rate (as for ICER) | 70% | F: 7–80% M: 4–48% |
80% | 75% | 50% | 70% | 65% | 70% | 75% |
| Vaccine and booster price | $360 | $400 | £210 (incl. admin.) | $500 | $500 | $300; $100 (booster) | €360 | €415 | $500 |
| Efficacy | |||||||||
| Against infection | 90% | F: 76–96% M: 41–62% |
100% | F: 100%; M: 85% | 90% | 90% | 90% | 100% | F: 95%; M: 90% |
| Against disease outcome | 100% | F: 98–100% M: 84–91% |
- | F: 100%; M: 90% | 90% | - | - | - | |
| Protection duration (years) | Lifetime | 32 (half life) | 10, 20 or lifetime | Lifetime | Lifetime | 10 after booster | 10 after booster | Lifetime (?) | Lifetime |
| Discount rates (for both costs and benefits) | 3.0% | 3.0% | 3.5% | 3.0% | 3.0% | 3.0% | 5.0% | 3.0% | 3.0% |
| Assessed strategy and ICER | 12FM+CU12–24F vs. 12F+CU12–24F $41 803/QALYg ~€22 442/QALYg |
9–26FM vs. 9–26F $25 664/QALYg ~€20 541/QALYg |
12FM vs. 12F+CU12–25F £520 255/QALYg ~€650 319/QALYg |
12FM vs. 12F $90 870/QALYg ~€72 696/QALYg |
12M vs. NoVac $15 290/QALYg ~€12 232/QALYg |
12FM +B22FM vs. 12F+B22F $422 039/QALYg ~€337 631/QALYg |
12FM+B22FM vs. 12F+B22F €311 000/LYg |
12FM vs. NoVac €18 677/QALYg |
12FM+CU13–26F vs. 12F+CU13–26F $184 270/QALYg ~€147 416/QALYg |
CC, cervical cancer; VAG, vaginal cancer; VUL, vulvar cancer; PEN, penile cancer; ANA, anal cancer; H&N, head and neck cancers; JO, juvenile-onset; AO, adult-onset; RRP, recurrent respiratory papillomatosis; GW, genital warts; F, female; M, male; CU, catch-up; B, booster; NoVac, no vaccination; QALYg, QALY gained; LYg, life year gained; SIS, Susceptible-Infectious-Susceptible; SIR, Susceptible-Infectious-Recovered; SIRS, Susceptible-Infectious-Removed-Susceptible. Exchange rate: €1 = $1.25 = £0.80 (as of May 2012)44
Because vaccination strategies and comparators varied across studies, direct comparison of ICERs was not relevant. Nevertheless, the ICER results varied greatly among studies, ranging from €12 232 to €650 319 per QALY gained, a 53-fold increase. Two studies accounted for the prevention of HPV 16 and 18 related cervical cancers only.41,42 The ICERs were high (approximate €300 000 per QALY or life-year gained) in both studies. In addition to the different comparators considered, such variation was most likely the result of significant differences in the design of the models.
The level of detail reported for each study varied greatly. Since most of the models are very complex, the lack of transparency makes it difficult to compare the different base case analyses.
Modeling methods and calibration approach
Four modeling methods were identified in the nine studies. Kim43 employed a standard static Markov cohort model. Kim and Goldie,47 Taira et al.42 and Chesson et al.49 used hybrid models in which transmission of HPV and progression of HPV-related diseases were modeled separately. An agent-based model was used by Olsen and Jepsen.48 Although seldom used in the field of health economics,50,51 this type of model offers one of the most flexible approaches allowing individuals to act autonomously according to a set of if/then rules. Other studies were based on classical transmission models that utilized a set of differential equations to reflect the probability of infection as a function of the number of infected in the population of interest.
Due to the relatively low number of studies, it is difficult to investigate the structural uncertainty (i.e., impact of the choice of modeling method on the results).
Only two studies (Elbasha et al.40 and Chesson et al.49) derived parameters directly from existing literature whereas other studies estimated some parameters through a fitting procedure (i.e., calibration). A detailed description of the method of calibration was not reported in any of the studies. In some studies, such as Kim and Goldie,47 calibration targets were based on epidemiological and clinical data from other countries, which could raise questions of data transferability.
HPV-related diseases considered
As reported in Table 1, only three studies (Elbasha and Dasbach,45 Kim and Goldie,47 and Chesson et al.49) considered the full spectrum of HPV-related diseases, i.e., anogenital diseases, head and neck cancers, recurrent respiratory papillomatosis (RRP) and genital warts.
Considering more diseases directly resulted in a decrease of the ICER as it accounted for the prevention of further HPV-related diseases (Fig. 2). Compared with cervical disease only (i.e., dynamic models only considered a reduction in the incidence of cervical cancer through indirect protection), considering the full spectrum of diseases reduced the ICER by 75.1% (from $741 280 to $184 270 per QALY gained) in Chesson et al.,49 by 68.7% (from $290 290 to $90 870 per QALY gained) in Kim and Goldie,47 and by 86.9% (from $195 322 to $25 664 per QALY gained) in Elbasha and Dasbach.45 Genital warts alone accounted for more than half of the ICER reduction, as it is much more prevalent than other HPV-related diseases. When looking at the vaccination of MSM, Kim et al.43 reported that inclusion of genital warts in additional to anal cancer would reduce the ICER by approximately 20% (from $19 070 to $15 290 per QALY gained).
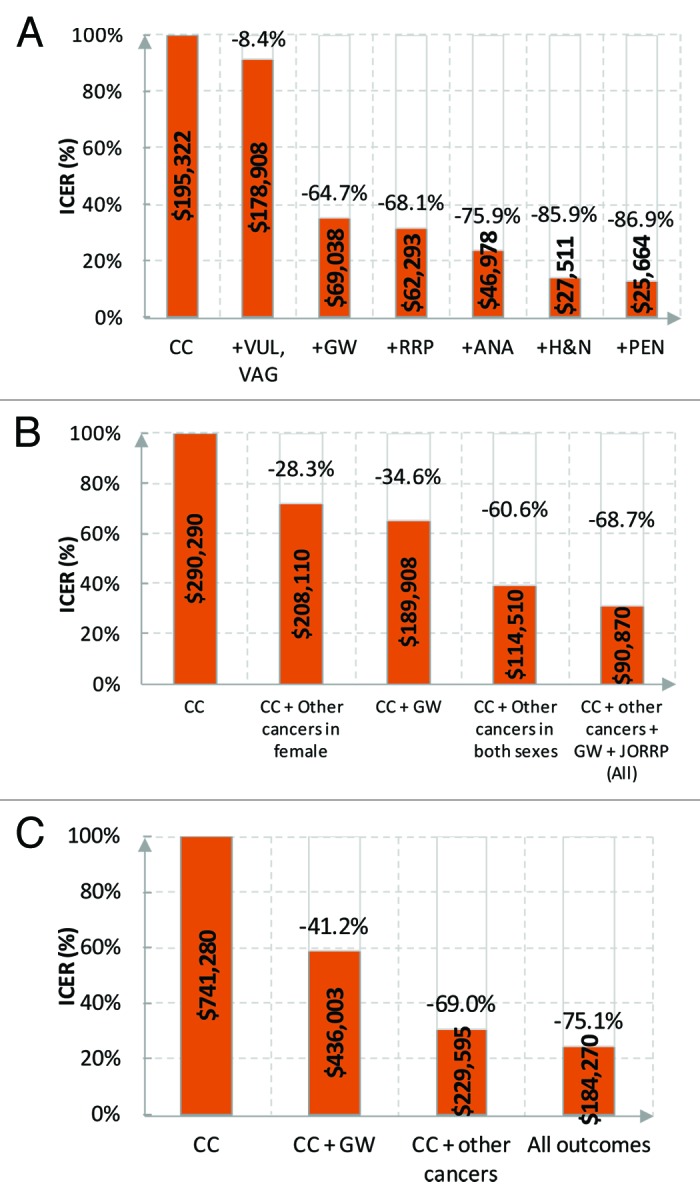
Figure 2. HPV-related diseases and ICERs. (A) 9–26 FM vs. 9–26F. Reprinted with permission of Elbasha and Dasbach.45 (B) 12FM vs. 12F. Reprinted with permission of Kim and Goldie.47 For cervical cancer plus genital warts, estimated from the graph in the publication. (C) 12FM+CU13–26F vs. 12F+CU13–26F. Reprinted with permission of Chesson et al.49 CC, cervical cancer; VAG, vaginal cancer; VUL, vulvar cancer; PEN, penile cancer; ANA, anal cancer; H&N, head and neck cancers; JORRP, juvenile-onset RRP, recurrent respiratory papillomatosis; GW, genital warts; F, female; M, male; CU, catch-up.
Conversely, none of the analyses explored the impact of changing epidemiological estimates of some HPV-related diseases and a constant incidence was used. As noted earlier, a significant increase was witnessed in the incidence of head and neck cancers in males and of anal cancer in both sexes.16-21 Disregarding such trend may have led to underestimating the benefits of vaccinating males.
Assumptions around natural immunity
In the studies, the assumption regarding natural immunity ranged from no immunity to full and lifelong immunity after the clearance of an episode of HPV infection. With no immunity, individuals become susceptible again immediately after the clearance of the infection, leading to an SIS (susceptible-infectious-susceptible) model structure. Similarly, full and lifelong immunity links to an SIR (susceptible-infectious-recovered) model and if immunity was assumed to wane and disappear an SIRS (susceptible-infectious-recovered-susceptible) model was used.
Theoretically, the assumption around natural immunity will affect transmission parameters when they are calibrated. In most of the models, calibration targets included observed incidence of diseases associated with HPV and prevalence of HPV infection. An SIR model will require higher transmission rates than an SIS model to match the observed epidemiology, as a proportion of the population becomes naturally immune after infection and is therefore not contributing to the transmission of the disease. Hence, a higher natural immunity level mechanically predicts a lower vaccine effect (i.e., higher ICER). This was confirmed in Elbasha et al.,40 which reported a decrease of 74.3% in the ICER (from $45 056 to $11 567 per QALY gained; primary vaccination at 12 y with a catch-up campaign at the age of 12 to 24 y, in both sexes vs. in females) in ICER when the duration of natural immunity was reduced from lifelong to 10 y.
Vaccination strategies and coverage rate
In most of the studies, the primary vaccination was given to those aged 12 y for both females and males, except in Elbasha et al.,40 where individuals of 9 to 26 y old were vaccinated. In the MSM study, Kim43 assessed vaccination strategies at the age of 12, 20, or 26 y old. Catch-up campaigns were explored in Elbasha et al.,40 Jit et al.46 and Olsen and Jepsen.48 The effect of booster vaccination was tested in Taira et al.42 and Zechmeister et al.41
Given the different ages (or age range) for the primary vaccination, catch-up vaccination or booster, there were limited scenarios that were comparable between studies.
The effect of the vaccine coverage rate on ICERs was tested in five studies (Elbasha et al.,40 Elbasha and Dasbach,45 Kim and Goldie,47 Taira et al.,42 and Chesson et al.49). As expected, the ICER of extending the vaccination to males increases with higher coverage rate of the existing vaccination program as a result of herd protection: the marginal benefit decreases when one more individual is vaccinated but the additional cost does not decrease. The impact of the coverage rate was found to be substantial. For instance, Chesson et al.49 reported that the ICER would increase from $23 649 to $41 379 per QALY gained when the coverage rate of the existing vaccination program increased from 20% to 30%, with all diseases considered. If the coverage rate further increased to 75%, the ICER would quickly increase to $184 270 per QALY gained (Fig. 3).
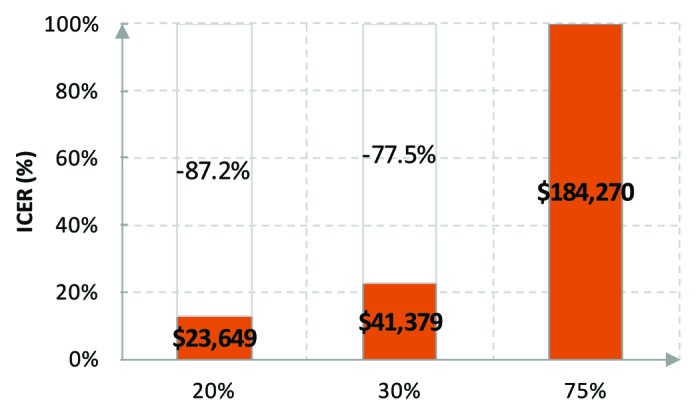
Figure 3. Vaccine coverage rate and ICERs. 12FM+CU13–26F vs. 12F+CU13–26F. Reprinted with permission of Chesson et al.49
With regard to the vaccine coverage rate, most of the studies assumed a rate of around 70%. However, only Elbasha and Dasbach45 clearly stated that this was based on the observed data in the country of interest. Coverage assumptions in other studies might lead to inconsistency with the real world data and incorrect conclusion.
Vaccine price
Vaccine price varied between approximately €250 and €450 for a 3-dose course (including or excluding administration and wastage). Given the bulk procurement of HPV vaccines in many countries, the actual price would be expected to be lower than the list price due to negotiation or a tendering process. None of the studies accounted for this. In practice, the unit price of vaccines often decrease with time, especially given the timeframes of the analyses was relatively long (e.g., 100 y). Nevertheless this was not accounted for in any of the studies.
Four studies have tested the effect and sensitivity of the price in the context of overall cost-effectiveness (Elbasha et al.,40 Kim and Goldie,47 Kim,43 and Chesson et al.49). A linear relationship was seen between the proportional change in vaccine price and the proportional change in ICER.
Vaccine efficacy and protection duration
The base case assumptions on vaccine efficacy against infection came from clinical trial data and were numerically similar across studies. Only Elbasha et al.,40 Elbasha and Dasbach,45 Kim and Goldie,47 and Kim43 considered efficacy against disease outcomes in addition to efficacy against HPV infection.
Most studies (except Elbasha et al.40 and Elbasha and Dasbach45) assumed that those protected by the vaccine could not transmit infection. Nevertheless, it may be possible that infections occurred in the vaccinated (without leading to disease) and whether these infections can be transmitted is unknown.52
The effect of vaccine efficacy on cost-effectiveness was not consistent in the four studies in which sensitivity analyses were conducted for this endpoint.40,42,47,49 This might be due to the effect of herd protection masking the benefit of higher efficacy.
Partial efficacy due to lack of compliance and duration of protection conferred by the vaccine were also studied in several publications. Their impact differed between studies.
Transition rates, disease costs and health-related quality of life
Comparison of transition rates between studies was difficult because of different assumptions on HPV transmission and disease progression or regression. This is further complicated by the fact that some rates were estimated in the calibration process, which was not well described and also heterogeneous between studies.
Disease costs were generally similar across studies, except for genital warts, which were consistently lower in European studies (Table 4). Costs were often limited to direct medical costs and did not take into account certain costs such as those associated with functional and psychological sequelae of treatment and psychological impact of HPV-related morbidities.53-61
Table 4. Costs.
| Elbasha et al.40 | Elbasha and Dasbach45 | Jit et al.46 | Kim and Goldie47 | Kim43 | Taira et al.42 | Zechmeister et al.41 | Olsen and Jepsen48 | Chesson et al.49 | |
|---|---|---|---|---|---|---|---|---|---|
| Perspective | TPP (?) | n/a | TPP | Societal | n/a | n/a | TPP/Societal | TPP | Societal |
| Currency | USD | USD | GBP | USD | USD | USD | EUR | EUR | USD |
| Year | 2005 | 2008 | 2006–7 | 2006 | 2006 | 2001 | 2007 | 2007 | 2008 |
| Vaccine* | 360 | 400 | 180–241.5 | 500 | 500 | 300 + 100(B) | 330 | 415 | 500 |
| Administration* | ~13.11 | 30 | |||||||
| Screening | Cytology: 99 Colpos.: 165 Colpos. +Biopsy: 318 |
Cytology: 112 Colpos.: 187 |
Cytology: 55 Colpos.: 216 |
Cytology: 32 HPV DNA:49 Colpos.: 364 Biopsy: 53 |
- | 81 | 27.51 | n/a | n/a |
| CIN 1 | 1554 | 1764 | 332 | - | n/a | 630 (LSIL) | 2778 | 33 | 1959 |
| CIN 2 | 3483 | 3955 | 3438 | 1218 (HSIL) | 2676 | 3642 | |||
| CIN 3 | 4135 | ||||||||
| Cervical cancer | Loc.: 26 470 Reg.: 28 330 Dist.: 45 376 |
Loc.: 30 059 Reg.: 32 171 Dist.: 51 527 |
S I: 13 339 S II: 22 386 S III: 21 269 S IV: 22 927 |
Loc.: 26 540 Reg.: 28 430 Dist.: 45 540 |
- | S I: 14 979 S II: 21 811 S III: 21 811 S IV: 24 004 |
S I: 16 536 S II: 26 724 S III: 27 611 S IV: 28 192 |
24 689 | 35 693 |
| Other cancers | - | Ranging from 12 380 (loc. vulvar) to 40 463 (head and neck) | - | Ranging from 17 110 (penile) to 37 370 (oral) | 31 300 (anal) | - | - | - | Ranging from 18 528 (penile) to 40 463 (oral) |
| Genital warts | 489 | ♀: 515 ♂: 607 |
134 | 430 | 430 | - | - | 239 | 568 |
| RRP | - | 214 952 | - | 62 010 | - | - | - | - | 137 308 |
TTP, third-party payer; B, booster; Colpos., colposcopy; CIN, cervical intraepithelial neoplasia; CIS, carcinoma in situ; L/HSIL, low-/high-grade squamous intraepithelial leson; Loc., localized; Reg., regional; Dist., distant; S, stage; mo, months; RRP, recurrent respiratory papillomatosis; JO, juvenile-onset. * For a 3-dose course.
For health-related quality of life, additive (i.e., using utility decrements) or multiplicative (i.e., using utility multiplier) methods were used in different studies (Table 5). The health-state utility values or weights were largely consistent across studies as the primary studies on this topic were limited. The major difference was seen in the assumption of the duration of the disutility associated with genital warts: 3 mo in Kim and Goldie,47 8.5 mo in Elbasha and Dasbach,45 and 6 mo in Chesson et al.49 Given the prevalence of genital warts, such an assumption could have a significant impact and help explain the different results between studies. Difference was also seen in the treatment of quality of life in cured cancer patients. As long-term consequences have detrimental effects in these patients, many studies considered a lower utility value for them, although methods differed.
Table 5. Utility.
| Elbasha et al.40 | Elbasha and Dasbach45 | Jit et al.46 | Kim and Goldie47 | Kim43 | Taira et al.42 | Zechmeister et al.41 | Olsen and Jepsen48 | Chesson et al.49 | |
|---|---|---|---|---|---|---|---|---|---|
| Method | Multiplic. | Additive (?) | Multiplic. | n/a | n/a | Not used – life-years gained only | Multiplic. (?) | QALY loss per case | |
| Baseline utility | Based on data from the US National Health Interview Survey and the Healthy People 2000 study | n/a | US Beaver Dam Study | n/a | US Beaver Dam Study | Unpublished Danish data (method not reported) | Not reported | ||
| CIN | CIN 1: 0.91 CIN 2/3: 0.87 CIS: 0.87 |
CIN 1: 0.012* CIN 2: 0.065* CIN 3: 0.054* |
n/a | n/a | SIL: 0.97** | CIN 1: 0.9333 CIN 2/3: 0.8658 |
CIN 1/2: 0.03/0.07 for 18 mos CIN 3: 0.2 for 4 mos and 0.03 for 2 y |
||
| Cervical cancer | Loc.: 0.76 Reg.: 0.67 Dist.: 0.48 |
S I: 0.35* S II: 0.44* S III: 0.44* S IV: 0.52* |
Loc.: 0.76 (over 5 y) Reg.: 0.67 (over 5 y) Dist.: 0.48 (lifetime) |
- | S I: 0.79** S II-IV: 0.62** |
S I: 0.7598 S II-IV: 0.6693 |
Loc., reg. and dist. = 0.36, 0.41 and 0.45 for 3 y, followed by death | ||
|
Cervical cancer survivor |
0.76 | S I: 0.10* S II: 0.15* S III: 0.15* S IV: 0.38* |
- | S I: 0.90** S II-IV: 0.62** Well: 1.00** |
n/a | Loc.: 0.27 for 4 mos and then a further 0.07 Reg. and dist.: 0.37/0.45 for 3 y and then a further 0.1/0.24 |
|||
| Other cancers | - | Same as cervical cancer | - | 0.68 (lifetime) | 0.68 (Anal) | - | - | The same as cervical | |
| Genital warts | 0.91 (8.5 mo in average in Elbasha 2010) | 0.039* | 0.91 (3 mo) | 0.91 | - | 0.9142 | Sex-specific, combination of 3 decrements (0.05–0.15) and 2 duration (3 mos and 6 mos) | ||
| RRP | - | 0.80 | - | 0.69 (JO, over 4 y) | - | - | - | 1.05 QALYs per case | |
Multiplic, multiplicative; HALex, Health and Activities Limitation Index; QWB, Quality of Well-Being; TTO, time trade-off; CIN, cervical intraepithelial neoplasia; CIS, carcinoma in situ; SIL, squamous intraepithelial lesion; Loc., localized; Reg., regional; Dist., distant; S, stage; mo, months; RRP, recurrent respiratory papillomatosis; JO, juvenile-onset. *Utility decrement. ** Not clear if it is a decrement or a utility weight.
Rigor of the studies
The assessment of the quality of each study is reported in Table 6, using the checklist suggested by Garnett et al.37 Most of the studies did not discuss the model structure, the implications of using the selected model structure or the possible results if using an alternative one. Although calibration has been conducted in numerous studies, there was a general lack of transparency regarding how it was performed, what targets and variables were used and the comparison between the model prediction and the observation.
Table 6. Rigor of the studies.
| Studies | Diagram | Complete list of model parameters (including natural history parameters) |
Assessment of model predictions with data (calibration details) |
Presentation of results (including sensitivity analyses)* | Discussion of model structure | Quality regarding number of items |
|---|---|---|---|---|---|---|
| Elbasha et al. 40 | Yes | Yes (appendix) | No calibration | Yes One-way SA |
No | 3/5 |
| Elbasha and Dasbach 45 | Yes | Yes (appendix) | Calibrated but method not reported | Yes PSA | Yes (appendix) |
4/5 |
| Jit et al. 46 | No | No | Yes | Yes One-way SA |
No | 2/5 |
| Kim and Goldie 47 | No | Yes (incomplete) | Yes (in another paper) | Yes One-way SA |
No | 3/5 |
| Kim 43 | No | No | Not calibrated | No sensitivity analyses | No | 0/5 |
| Taira et al. 42 | Yes | No | Calibrated but method not reported | Yes One-way SA |
No | 2/5 |
| Zechmeister et al. 41 | Yes | Yes | Calibrated but method not reported | Yes | No | 3/5 |
| Olsen and Jepsen 48 | Yes (online appendix) | Yes | Calibrated but method not reported | One-way SA but results not shown | No | 2/5 |
| Chesson et al. 49 | Yes | Yes (appendix) | No calibration Based on published literatures | Yes | No | 3/5 |
Sensitivity analyses are conducted for vaccine parameters, cost and utilities. Natural history parameters such as the probability of transmission are not included in sensitivity analyses. Cross-protection has been included in some sensitivity analyses. SA, sensitivity analyses; PSA, probabilistic sensitivity analyses.
Discussion
A pragmatic critical literature review of published cost-effectiveness analyses that assessed male HPV vaccination was performed and nine studies were reviewed. Six were conducted in the US context, and all except one focused on heterosexual relationships.
When considering extending the female-only vaccination strategy to males in an environment where female vaccination coverage of around 70%, the ICER ranged from €12 232 to €650 319 per QALY gained. Nevertheless direct comparison of ICERs was not appropriate due to vast heterogeneity among studies, in terms of modeling approach, assumptions around natural immunity, vaccination strategy, vaccine coverage rate, whether most HPV-related diseases were accounted for, and other inputs.
The factor analysis was not able to investigate the absolute levels of ICER associated with male vaccination because of the fundamental difference among models. One of the main drivers was which of the HPV-related diseases considered. The ICER decreased with more diseases considered; this type of analysis more accurately captures all benefits of vaccination. Genital warts were found to have the largest impact on the assessment of the quadrivalent vaccine. A shortened duration of natural immunity contributed to an improved ICER but this was only assessed in one of the cost-effectiveness studies. Vaccination strategy and coverage rate also played an important role. With more females vaccinated through catch-up campaigns or with a higher coverage rate, the ICER spiked as the marginal benefit decreased. Lastly, the ICER was almost directly proportionally to vaccine price.
The reporting of the analyses was not very detailed across studies and it led to a lack of transparency. The main issues related to the lack of justification for the selected modeling approach and the lack of transparency regarding the calibration process. More scenarios and sensitivity analyses could contribute to a better understanding of the impact of each parameter on the results. Recently, the International Society for Pharmacoeconomics and Outcomes Research and the Society for Medical Decision Making published recommendations on the best practice for transparency and validity. It includes the report of ‘nontechnical’ description (e.g., type of the model, inputs, outputs and other parameters, data selection), technical documentation that allows audience with necessary expertise to evaluation and potentially reproduce the analysis, and information on the face, internal, cross, external and predictive validity. These recommendations could be considered and followed by future studies.62
In 2012, the European Centre for Disease Prevention and Control (ECDC) published a report on HPV vaccination, with a special focus on male vaccination.63 Although the ECDC recognized the high burden related to HPV in males, the report noted that the analyses were not fully evidence-based and concluded that vaccination of males was unlikely to be cost-effective in the current economic conditions. The current study took a different angle. We tried to identify the major drivers of difference in results seen across studies, but as for the absolute cost-effectiveness, very limited conclusions could be drawn due to vast heterogeneity of the approach.
Following the review, several methodological concerns and suggestions for future studies were raised.
First and foremost, an optimal modeling approach could not be recommended based on the review nor from the literature. However, a key methodological question concerns the degree of complexity a model should include. Most models incorporated an advanced design, program, and parameterization. But with even the most detailed aspects considered, would there be enough data to populate the model? As the review shows, many studies utilized sources from other countries or even other continents, and yet the calibration process and the extent that the estimated parameters fit the observations were either unreported or under-reported. Interestingly, in the official Australian assessment of male HPV vaccination, a positive conclusion was reached with a simple model, even though few details were made available.64 An alternative to using complex models may be to focus more on factors that substantially affect the conclusion. Few studies discussed structural uncertainty in detail and many assumptions were made in the absence of empirical evidence. Methods to tackle this type of uncertainty were discussed but universal agreement has not been reached.65-67
Second, transparency should be improved in future studies. It is recognized that it would be difficult to clearly report all aspects of a complex model in a short publication. Nevertheless, underpinning assumptions should be described in full detail and uncertainties should be tested whenever possible (e.g., reported in appendices). For instance, the assumption around natural immunity was only tested in Elbasha et al.,40 and it is unclear whether the results were coincidental. Fortunately, evidence is available from two other epidemiological studies. Baussano et al.68 assessed the effect of vaccinating 12-y old girls in an unscreened environment. The authors reported that the SIS model predicted a reduction in lifetime incidence of cervical cancer of 16% and 21% higher than the SIR model in the unvaccinated and vaccinated cohorts. This difference could be increased up to 30% with alternative clinical assumptions and vaccination strategy. Similar findings were also reported by Van de Velde et al. (2010).69 The alternative assumption on this, nevertheless, was not tested in other cost-effectiveness studies. Its impact therefore remains uncertain.
The third point relates to the use of real life data. Only Elbasha and Dasbach45 utilized real life vaccine coverage data. Regarding price of vaccination, as it is common to procure vaccines in bulk for national programs, price negotiation or tendering processes are very likely to reduce the actual price of the vaccine.70,71 With many factors affecting the cost-effectiveness profile based on assumptions, the validity of the results is subject to question. Additionally, not accounting for observed epidemiological trends (e.g., of anal cancer and head and neck cancers16-21) and evolving sexual behavior may have further biased the results.
Fourthly, the lack of data to support parameterization of economic analyses has been witnessed throughout the review. For example, while genital warts contribute to a great proportion of HPV-related disease burden, the utility multiplier of genital warts in most of the studies came from a single conference abstract.72 Without further scrutiny of the method and compatibility of settings and measurement, the use of such a source may introduce additional uncertainties to the analyses. Additional studies on the transmission of the infection, the disease, costs and health-related quality of life are needed to enhance the robustness of future economic assessments.
Lastly, HPV vaccination is a public health intervention and its benefits will only be realized years or decades after the implementation of the program. An equity concern is therefore raised: should we discount the benefit of future generations? There is not yet a consensus on this topic despite extended debates.73 Further to this point, aspects such as the incorporation of outcome- and behavior-related productivity benefits,74 the utility in anticipation (i.e., the increase in utility associated with the knowledge of being protected from the time of vaccination)75 have been raised in the context of assessing public health intervention. Nevertheless no consensus has yet been reached.
Conclusion
A total of nine cost-effectiveness studies of male HPV vaccination have been reviewed. Due to the heterogeneity among the studies, limited conclusions could be drawn with regard to the absolute cost-effectiveness of male vaccination.
However it was found that incorporation of all HPV-related diseases and a suboptimal vaccine coverage rate among girls could improve the cost-effectiveness profile of male vaccination.
Most of the studies did not provide sufficient transparency on the assumptions and calibration process (where conducted), yet fewer studies tested the alternative assumption, such as the natural history of HPV infection and protection duration of the vaccine. Some key factors such as vaccine coverage rate and vaccine price were largely based on assumptions, potentially leading to biased results. None of the studies considered the epidemiological trend of HPV-related diseases, such as the observed increase in the incidence of anal and head and neck cancers.
Future research should be conducted to better understand the disease, as well as to provide costs and health-related quality of life data for economic analysis. Methodologically, the selection of modeling approach and the aspects to be considered for the assessment of public health policy, especially how the true economic value of vaccination is captured beyond cost-effectiveness results, need further discussion and consensus.
Acknowledgments
The authors would like to thank Marjorie Adam (an employee of Sanofi Pasteur MSD), Marine Clementz and Carl Selya-Hammer (employees of Amaris) for critically reviewing and providing inputs for the manuscript.
Glossary
Abbreviations:
- ANA
anal cancer
- AO
adult-onset
- B
booster
- CC
cervical cancer
- CIN
cervical intraepithelial neoplasia
- CIS
carcinoma in situ
- Colpos.
colposcopy
- CU
catch-up
- Dist.
distant
- ECDC
European Centre for Disease Prevention and Control
- F
female
- GW
genital warts
- H&N
head and neck cancers
- HALex
Health and Activities Limitation Index
- HPV
human papillomavirus
- IARC
International Agency for Research on Cancer
- ICER
incremental cost-effectiveness ratio
- JO
juvenile-onset
- L/HSIL
low-/high-grade squamous intraepithelial lesion
- Loc.
localized
- LYg
life year gained
- M
male
- mo
month
- MSM
men who have sex with men
- Multiplic.
multiplicative
- NoVac
no vaccination
- PEN
penile cancer
- PSA
probabilistic sensitivity analyses
- QALY
quality-adjusted life year
- QALYg
QALY gained
- QWB
Quality of Well-Being
- Reg.
regional
- RRP
recurrent respiratory papillomatosis
- S
stage
- SA
sensitivity analyses
- SIR
susceptible-infectious-recovered
- SIRS
susceptible-infectious-recovered-susceptible
- SIS
susceptible-infectious-susceptible
- TTO
time trade-off
- TTP
third-party payer
- VAG
vaginal cancer
- VUL
vulvar cancer
Disclosure of potential conflicts of interest
This study was conducted by Amaris and funded by Sanofi Pasteur MSD. Yiling Jiang and Aline Gauthier are employees of Amaris, which received consulting fees from Sanofi Pasteur MSD and GlaxoSmithKline. Maarten J. Postma has received grants from Sanofi Pasteur MSD and GlaxoSmithKline. Laureen Ribassin-Majed has received consulting fees from Sanofi Pasteur MSD. Nathalie Largeron and Xavier Bresse are employees of Sanofi Pasteur MSD. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/vaccines/article/25754
References
- 1.Schiller JT, Day PM, Kines RC. Current understanding of the mechanism of HPV infection. Gynecol Oncol. 2010;118(Suppl):S12–7. doi: 10.1016/j.ygyno.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Longworth MS, Laimins LA. Pathogenesis of human papillomaviruses in differentiating epithelia. Microbiol Mol Biol Rev. 2004;68:362–72. doi: 10.1128/MMBR.68.2.362-372.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonnez W, Reichman RC. Papillomaviruses. In: Mandell GL, Bennett JE, Dolin R, eds. Principles and practice of infectious diseases. Philadelphia, PA: Churchill Livingstone Elsevier, 2010:2035-2049. [Google Scholar]
- 4.Saslow D, Castle PE, Cox JT, Davey DD, Einstein MH, Ferris DG, et al. Gynecologic Cancer Advisory Group American Cancer Society Guideline for human papillomavirus (HPV) vaccine use to prevent cervical cancer and its precursors. CA Cancer J Clin. 2007;57:7–28. doi: 10.3322/canjclin.57.1.7. [DOI] [PubMed] [Google Scholar]
- 5.Parkin DM, Bray F. Chapter 2: The burden of HPV-related cancers. Vaccine 2006; 24 Suppl 3:S3-11-S3/25. [DOI] [PubMed] [Google Scholar]
- 6.European Medicines Agency. Gardasil: EPAR - product information. 31 July 2012. Available at http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000703/WC500021142.pdf, accessed 14 June 2013.
- 7.European Medicines Agency. Cervarix: summary of opinion (post-authorisation). 21 February 2013. Available at http://www.ema.europa.eu/docs/en_GB/document_library/Summary_of_opinion/human/000721/WC500139202.pdf, accessed 14 June 2013.
- 8.Bonanni P, Levi M, Latham NB, Bechini A, Tiscione E, Lai P, et al. An overview on the implementation of HPV vaccination in Europe. Hum Vaccin. 2011;7(Suppl):128–35. doi: 10.4161/hv.7.0.14575. [DOI] [PubMed] [Google Scholar]
- 9.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans Human papillomaviruses. IARC Monogr Eval Carcinog Risks Hum. 2007;90:1–636. [PMC free article] [PubMed] [Google Scholar]
- 10.De Vuyst H, Clifford GM, Nascimento MC, Madeleine MM, Franceschi S. Prevalence and type distribution of human papillomavirus in carcinoma and intraepithelial neoplasia of the vulva, vagina and anus: a meta-analysis. Int J Cancer. 2009;124:1626–36. doi: 10.1002/ijc.24116. [DOI] [PubMed] [Google Scholar]
- 11.Giuliano AR, Tortolero-Luna G, Ferrer E, Burchell AN, de Sanjose S, Kjaer SK, et al. Epidemiology of human papillomavirus infection in men, cancers other than cervical and benign conditions. Vaccine. 2008;26(Suppl 10):K17–28. doi: 10.1016/j.vaccine.2008.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gillison M. HPV and its effect on head and neck cancer prognosis. Clin Adv Hematol Oncol. 2010;8:680–2. [PubMed] [Google Scholar]
- 13.Syrjänen S, Lodi G, von Bültzingslöwen I, Aliko A, Arduino P, Campisi G, et al. Human papillomaviruses in oral carcinoma and oral potentially malignant disorders: a systematic review. Oral Dis. 2011;17(Suppl 1):58–72. doi: 10.1111/j.1601-0825.2011.01792.x. [DOI] [PubMed] [Google Scholar]
- 14.Goon PK, Stanley MA, Ebmeyer J, Steinsträsser L, Upile T, Jerjes W, et al. HPV & head and neck cancer: a descriptive update. Head Neck Oncol. 2009;1:36. doi: 10.1186/1758-3284-1-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hartwig S, Syrjänen S, Dominiak-Felden G, Brotons M, Castellsagué X. Estimation of the epidemiological burden of human papillomavirus-related cancers and non-malignant diseases in men in Europe: a review. BMC Cancer. 2012;12:30. doi: 10.1186/1471-2407-12-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blomberg M, Nielsen A, Munk C, Kjaer SK. Trends in head and neck cancer incidence in Denmark, 1978-2007: focus on human papillomavirus associated sites. Int J Cancer. 2011;129:733–41. doi: 10.1002/ijc.25699. [DOI] [PubMed] [Google Scholar]
- 17.Marur S, D’Souza G, Westra WH, Forastiere AA. HPV-associated head and neck cancer: a virus-related cancer epidemic. Lancet Oncol. 2010;11:781–9. doi: 10.1016/S1470-2045(10)70017-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hocking JS, Stein A, Conway EL, Regan D, Grulich A, Law M, et al. Head and neck cancer in Australia between 1982 and 2005 show increasing incidence of potentially HPV-associated oropharyngeal cancers. Br J Cancer. 2011;104:886–91. doi: 10.1038/sj.bjc.6606091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brewster DH, Bhatti LA. Increasing incidence of squamous cell carcinoma of the anus in Scotland, 1975-2002. Br J Cancer. 2006;95:87–90. doi: 10.1038/sj.bjc.6603175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jin F, Stein AN, Conway EL, Regan DG, Law M, Brotherton JM, et al. Trends in anal cancer in Australia, 1982-2005. Vaccine. 2011;29:2322–7. doi: 10.1016/j.vaccine.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 21.Nielsen A, Munk C, Kjaer SK. Trends in incidence of anal cancer and high-grade anal intraepithelial neoplasia in Denmark, 1978-2008. Int J Cancer. 2012;130:1168–73. doi: 10.1002/ijc.26115. [DOI] [PubMed] [Google Scholar]
- 22.von Krogh G, von KG Management of anogenital warts (condylomata acuminata) Eur J Dermatol. 2001;11:598–603, quiz 604. [PubMed] [Google Scholar]
- 23.Lacey CJ, Lowndes CM, Shah KV. Chapter 4: Burden and management of non-cancerous HPV-related conditions: HPV-6/11 disease. Vaccine 2006; 24 Suppl 3:S3-35-S3/41. [DOI] [PubMed] [Google Scholar]
- 24.Maw RD, Reitano M, Roy M. An international survey of patients with genital warts: perceptions regarding treatment and impact on lifestyle. Int J STD AIDS. 1998;9:571–8. doi: 10.1258/0956462981921143. [DOI] [PubMed] [Google Scholar]
- 25.Woodhall S, Ramsey T, Cai C, Crouch S, Jit M, Birks Y, et al. Estimation of the impact of genital warts on health-related quality of life. Sex Transm Infect. 2008;84:161–6. doi: 10.1136/sti.2007.029512. [DOI] [PubMed] [Google Scholar]
- 26.Bundesministerium für Gesundheit. Impfplan Österreich 2013. Evidenz-basierte Empfehlungen des Nationalen Impfgremiums. 2013. Available at http://bmg.gv.at/cms/home/attachments/3/3/6/CH1100/CMS1327680589121/impfplan2013.pdf, accessed 14 June 2013.
- 27.Landesärztekammer S. Empfehlungen der Sächsischen Impfkommission zur Durchführung von Schutzimpfungen im Freistaat Sachsen. 1 January 2013. Available at http://www.slaek.de/de/03/36impfen/pdf/E1_2013_Druck.pdf, accessed 14 June 2013.
- 28.Centers for Disease Control and Prevention (CDC) Recommendations on the use of quadrivalent human papillomavirus vaccine in males--Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep. 2011;60:1705–8. [PubMed] [Google Scholar]
- 29.National Advisory Committee on Immunization Update on human papillomavirus (HPV) vaccines. Can Commun Dis Rep. 2012;38:1–62. doi: 10.14745/ccdr.v38i00a01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Department of Health and Ageing. HPV vaccine extended to boys. 2012. Available at http://www.health.gov.au/internet/ministers/publishing.nsf/Content/59C397F61CACF02FCA257A390000C65A/$File/TP059.pdf, accessed 14 June 2013.
- 31.Department of Health and Ageing. Fact sheet. National Immunisation Program - HPV vaccination for boys. 2012. Available at http://www.health.gov.au/internet/immunise/publishing.nsf/Content/1958E18142193688CA2575BD001C80CA/$File/HPV-vaccination-for-boys-factsheet.pdf, accessed 14 June 2013.
- 32.Centers for Disease Control and Prevention. The VFC program: at a glance. 31 August 2012. Available at http://www.cdc.gov/vaccines/programs/vfc/about/index.html, accessed 14 June 2013.
- 33.Prince Edward Island Department of Health and Wellness. HPV vaccine program expands to include boys. 18 April 2013. Available at http://www.gov.pe.ca/health/index.php3?number=news&dept=&newsnumber=8937&lang=Econtent, accessed 14 June 2013.
- 34.Vorarlberg L. Verbesserung der Gesundheitsvorsorge für Jugendliche. HPV-Impfung wird deutlich billiger. 24 January 2013. Available at http://www.vorarlberg.at/doc/hpv_pku.doc, accessed 14 June 2013.
- 35.Marra F, Cloutier K, Oteng B, Marra C, Ogilvie G. Effectiveness and cost effectiveness of human papillomavirus vaccine: a systematic review. Pharmacoeconomics. 2009;27:127–47. doi: 10.2165/00019053-200927020-00004. [DOI] [PubMed] [Google Scholar]
- 36.Seto K, Marra F, Raymakers A, Marra CA. The cost effectiveness of human papillomavirus vaccines: a systematic review. Drugs. 2012;72:715–43. doi: 10.2165/11599470-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 37.Garnett GP, Cousens S, Hallett TB, Steketee R, Walker N. Mathematical models in the evaluation of health programmes. Lancet. 2011;378:515–25. doi: 10.1016/S0140-6736(10)61505-X. [DOI] [PubMed] [Google Scholar]
- 38.Insinga RP, Dasbach EJ, Elbasha EH, Puig A, Reynales-Shigematsu LM. Cost-effectiveness of quadrivalent human papillomavirus (HPV) vaccination in Mexico: a transmission dynamic model-based evaluation. Vaccine. 2007;26:128–39. doi: 10.1016/j.vaccine.2007.10.056. [DOI] [PubMed] [Google Scholar]
- 39.Kulasingam S, Connelly L, Conway E, Hocking JS, Myers E, Regan DG, et al. A cost-effectiveness analysis of adding a human papillomavirus vaccine to the Australian National Cervical Cancer Screening Program. Sex Health. 2007;4:165–75. doi: 10.1071/SH07043. [DOI] [PubMed] [Google Scholar]
- 40.Elbasha EH, Dasbach EJ, Insinga RP. Model for assessing human papillomavirus vaccination strategies. Emerg Infect Dis. 2007;13:28–41. doi: 10.3201/eid1301.060438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zechmeister I, Blasio BF, Garnett G, Neilson AR, Siebert U. Cost-effectiveness analysis of human papillomavirus-vaccination programs to prevent cervical cancer in Austria. Vaccine. 2009;27:5133–41. doi: 10.1016/j.vaccine.2009.06.039. [DOI] [PubMed] [Google Scholar]
- 42.Taira AV, Neukermans CP, Sanders GD. Evaluating human papillomavirus vaccination programs. Emerg Infect Dis. 2004;10:1915–23. doi: 10.3201/eid1011.040222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim JJ. Targeted human papillomavirus vaccination of men who have sex with men in the USA: a cost-effectiveness modelling analysis. Lancet Infect Dis. 2010;10:845–52. doi: 10.1016/S1473-3099(10)70219-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.XE.com. Universal currency converter. 19 September 2012. Available at http://www.xe.com, accessed 19 September 2012.
- 45.Elbasha EH, Dasbach EJ. Impact of vaccinating boys and men against HPV in the United States. Vaccine. 2010;28:6858–67. doi: 10.1016/j.vaccine.2010.08.030. [DOI] [PubMed] [Google Scholar]
- 46.Jit M, Choi YH, Edmunds WJ. Economic evaluation of human papillomavirus vaccination in the United Kingdom. BMJ. 2008;337:a769. doi: 10.1136/bmj.a769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim JJ, Goldie SJ. Cost effectiveness analysis of including boys in a human papillomavirus vaccination programme in the United States. BMJ. 2009;339:b3884. doi: 10.1136/bmj.b3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Olsen J, Jepsen MR. Human papillomavirus transmission and cost-effectiveness of introducing quadrivalent HPV vaccination in Denmark. Int J Technol Assess Health Care. 2010;26:183–91. doi: 10.1017/S0266462310000085. [DOI] [PubMed] [Google Scholar]
- 49.Chesson HW, Ekwueme DU, Saraiya M, Dunne EF, Markowitz LE. The cost-effectiveness of male HPV vaccination in the United States. Vaccine. 2011;29:8443–50. doi: 10.1016/j.vaccine.2011.07.096. [DOI] [PubMed] [Google Scholar]
- 50.Bonabeau E. Agent-based modeling: methods and techniques for simulating human systems. Proc Natl Acad Sci U S A. 2002;99(Suppl 3):7280–7. doi: 10.1073/pnas.082080899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim SY, Goldie SJ. Cost-effectiveness analyses of vaccination programmes : a focused review of modelling approaches. Pharmacoeconomics. 2008;26:191–215. doi: 10.2165/00019053-200826030-00004. [DOI] [PubMed] [Google Scholar]
- 52.Regan DG, Philp DJ, Waters EK. Unresolved questions concerning human papillomavirus infection and transmission: a modelling perspective. Sex Health. 2010;7:368–75. doi: 10.1071/SH10006. [DOI] [PubMed] [Google Scholar]
- 53.Allal AS, Sprangers MA, Laurencet F, Reymond MA, Kurtz JM. Assessment of long-term quality of life in patients with anal carcinomas treated by radiotherapy with or without chemotherapy. Br J Cancer. 1999;80:1588–94. doi: 10.1038/sj.bjc.6690567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.de Graeff A, de Leeuw JR, Ros WJ, Hordijk GJ, Blijham GH, Winnubst JA. Long-term quality of life of patients with head and neck cancer. Laryngoscope. 2000;110:98–106. doi: 10.1097/00005537-200001000-00018. [DOI] [PubMed] [Google Scholar]
- 55.Jeffreys M, Rachet B, McDowell S, Habib AG, Lepage C, Coleman MP. Survival from rectal and anal cancers in England and Wales, 1986-2001. Eur J Cancer. 2006;42:1434–40. doi: 10.1016/j.ejca.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 56.Maddineni SB, Lau MM, Sangar VK. Identifying the needs of penile cancer sufferers: a systematic review of the quality of life, psychosexual and psychosocial literature in penile cancer. BMC Urol. 2009;9:8. doi: 10.1186/1471-2490-9-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pirotta M, Ung L, Stein A, Conway EL, Mast TC, Fairley CK, et al. The psychosocial burden of human papillomavirus related disease and screening interventions. Sex Transm Infect. 2009;85:508–13. doi: 10.1136/sti.2009.037028. [DOI] [PubMed] [Google Scholar]
- 58.Bray F, Klint A, Gislum M, Hakulinen T, Engholm G, Tryggvadóttir L, et al. Trends in survival of patients diagnosed with male genital cancers in the Nordic countries 1964-2003 followed up until the end of 2006. Acta Oncol. 2010;49:644–54. doi: 10.3109/02841860903575315. [DOI] [PubMed] [Google Scholar]
- 59.Marur S, D’Souza G, Westra WH, Forastiere AA. HPV-associated head and neck cancer: a virus-related cancer epidemic. Lancet Oncol. 2010;11:781–9. doi: 10.1016/S1470-2045(10)70017-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Arbyn M, Castellsagué X, de Sanjosé S, Bruni L, Saraiya M, Bray F, Ferlay J. Worldwide burden of cervical cancer in 2008. Ann Oncol. 2011;22:2675–86. doi: 10.1093/annonc/mdr015. [DOI] [PubMed] [Google Scholar]
- 61.Mortensen GL, Paaske PB. Patients perceive tonsil cancer as a strike at psycho-socially “vital organs”. Dan Med J. 2012;59:A4504. [PubMed] [Google Scholar]
- 62.Eddy DM, Hollingworth W, Caro JJ, Tsevat J, McDonald KM, Wong JB, ISPOR-SMDM Modeling Good Research Practices Task Force Model transparency and validation: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force-7. Med Decis Making. 2012;32:733–43. doi: 10.1177/0272989X12454579. [DOI] [PubMed] [Google Scholar]
- 63.European Centre for Disease Prevention and Control. Introduction of HPV vaccines in European Union countries – an update. September 2012. Available at http://ecdc.europa.eu/en/publications/publications/20120905_gui_hpv_vaccine_update.pdf, accessed 14 June 2013.
- 64.Department of Health and Ageing. Quadrivalent human papillomavirus (Types 6, 11, 16, 18) recombinant vaccine, solution for injection, 0.5 mL, solution for injection pre-filled syringe single dose, Gardasil®. November 2011. Available at http://www.health.gov.au/internet/main/publishing.nsf/Content/pbac-psd-quadrivalent-nov11, accessed 14 June 2013.
- 65.Kim LG, Thompson SG. Uncertainty and validation of health economic decision models. Health Econ. 2010;19:43–55. doi: 10.1002/hec.1444. [DOI] [PubMed] [Google Scholar]
- 66.Jackson CH, Bojke L, Thompson SG, Claxton K, Sharples LD. A framework for addressing structural uncertainty in decision models. Med Decis Making. 2011;31:662–74. doi: 10.1177/0272989X11406986. [DOI] [PubMed] [Google Scholar]
- 67.Strong M, Oakley JE, Chicott J. Managing structural uncertainty in health economic decision models: a discrepancy approach. J R Stat Soc Ser C Appl Stat. 2012;61:25–45. doi: 10.1111/j.1467-9876.2011.01014.x. [DOI] [Google Scholar]
- 68.Baussano I, Garnett G, Segnan N, Ronco G, Vineis P. Modelling patterns of clearance of HPV-16 infection and vaccination efficacy. Vaccine. 2011;29:1270–7. doi: 10.1016/j.vaccine.2010.11.082. [DOI] [PubMed] [Google Scholar]
- 69.Van de Velde N, Brisson M, Boily MC. Understanding differences in predictions of HPV vaccine effectiveness: A comparative model-based analysis. Vaccine. 2010;28:5473–84. doi: 10.1016/j.vaccine.2010.05.056. [DOI] [PubMed] [Google Scholar]
- 70.Garattini L, van de Vooren K, Curto A. Pricing human papillomavirus vaccines: lessons from Italy. Pharmacoeconomics. 2012;30:213–7. doi: 10.2165/11596560-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 71.Wallace LA, Young D, Brown A, Cameron JC, Ahmed S, Duff R, et al. Costs of running a universal adolescent hepatitis B vaccination programme. Vaccine. 2005;23:5624–31. doi: 10.1016/j.vaccine.2005.06.034. [DOI] [PubMed] [Google Scholar]
- 72.Myers ER, Green S, Lipkus I. 542 Patient preferences for health states related to HPV infection: visual analog scales vs time trade-off elicitation. Proceedings of 21st International Papillomavirus Conference. [Google Scholar]
- 73.Weatherly H, Drummond M, Claxton K, Cookson R, Ferguson B, Godfrey C, et al. Methods for assessing the cost-effectiveness of public health interventions: key challenges and recommendations. Health Policy. 2009;93:85–92. doi: 10.1016/j.healthpol.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 74.Bärnighausen T, Bloom DE, Cafiero ET, O’Brien JC. Economic evaluation of vaccination: capturing the full benefits, with an application to human papillomavirus. Clin Microbiol Infect. 2012;18(Suppl 5):70–6. doi: 10.1111/j.1469-0691.2012.03977.x. [DOI] [PubMed] [Google Scholar]
- 75.Drummond M, Chevat C, Lothgren M. Do we fully understand the economic value of vaccines? Vaccine. 2007;25:5945–57. doi: 10.1016/j.vaccine.2007.04.070. [DOI] [PubMed] [Google Scholar]


