Abstract
We previously demonstrated the immunogenicity and tolerability of the serogroup B meningococcal vaccine, 4CMenB (Bexsero®), in 11−17 y-olds randomized to receive 1, 2, or 3 doses at 1, 2, or 6 mo intervals. Participants in this extension study provided an additional blood sample 18−24 mo after last vaccine dose, to assess persistence of serum bactericidal activity with human complement (hSBA), and to compare with age-matched 4CMenB-naïve controls.
In the original study, one month after one 4CMenB dose, 93% of subjects had seroprotective hSBA titers (≥4) against indicator serogroup B strains for individual vaccine antigens (fHbp, NadA and NZOMV), increasing to ~100% after two or three doses. After 18−24 mo, 62−73% of subjects given one dose had titers ≥4 against the three antigens, significantly lower rates than after two (77−94%) or three (86−97%) doses. Only proportions with titers ≥ 4 against NZOMV were significantly different between the two (77%) and three (90%, p < 0.0001) dose groups. These results confirm that two doses of 4CMenB, administered 1 to 6 mo apart, provide good levels of bactericidal activity against serogroup B meningococci, which were sustained at least 18−24 mo in over 64% of adolescents for all three tested vaccine-related antigens.
Keywords: meningococcal, vaccine, serogroup B, antibodies, adolescents, persistence
Introduction
In spite of advances in treatment and supportive medical therapies, infectious disease due to Neisseria meningitidis, continues to be a major health burden particularly affecting two age groups—infants and adolescents.1 Advances in vaccine development have led to the licensure and widespread use of effective glycoconjugate vaccines against four of the five main disease-causing serogroups—A, C, W-135 and Y—but a suitable candidate for a broadly effective vaccine against serogroup B had remained elusive, until recently.2 Serogroup B meningococcus poses significant specific difficulties for vaccine development: the serogroup B capsular polysaccharide, the basis of the other serogroup vaccines, closely resembles natural human neural tissue so it is poorly immunogenic.3,4 Strain-specific serogroup B vaccines based on outer membrane vesicles (OMV), in which the main antigen is PorA, have been developed in response to local outbreaks, notably in New Zealand, Norway, and Cuba,5 but such vaccines have proven to be highly PorA sub-type specific.6
An innovative approach has been the use of reverse vaccinology to identify and characterize potential protein antigen components for the development of a multicomponent vaccine which could thus be effective against a majority of circulating strains.7 The resulting vaccine contains four protein antigens, 4CMenB (Bexsero®, Novartis Vaccines, and Diagnostics), which has recently received approval for use from 2 mo of age by the European Medicines Agency (EMA).8 This vaccine has been shown to be immunogenic and well tolerated when administered in various dose schedules in all age groups from infants to adults,9,10 including toddlers and our own study in adolescents.11 Inclusion in routine infant vaccination schedules when co-administered with routine vaccines resulted in some increase in reactogenicity, including higher rates of transient fever which usually occurred within six hours of vaccination.9 Our study demonstrated that one month after the last of two doses administered 1, 2, or 6 mo apart, immune responses considered protective against a panel of three meningococcal serogroup B strains, each specifically selected to assess the contribution of one vaccine antigen, were elicited in 99−100% of 11−17 y-olds. The seroresponse to two doses was significantly higher when compared with one dose, and was similar to three doses.
It is important to know how long protective bactericidal antibodies will persist in the circulation. For this reason, we measured antibody persistence in participants of the original study 18−24 mo after their last dose of 4CMenB. We also enrolled 4CMenB vaccine-naive subjects of similar age as a control group.
Materials and Methods
The study was performed at six study centers in Santiago and Valparaíso, Chile, following Good Clinical Practice and the principles outlined in the Declaration of Helsinki. Ethical committee approval was obtained from the relevant local oversight bodies, and informed, written consent was obtained from all participants or their parents/legal guardians, as appropriate.
The original study, registered on clinicaltrials.gov as NCT00661713, was performed from June 2008 until December 2010, and involved 1631 healthy participants, of either sex, aged 11−17 y.11 Of these, 1625 subjects were eligible for enrolment in this extension study (clinicaltrials.gov NCT01148524) performed from August 2010 to January 2012. Recruitment was performed as part of a two-dose hepatitis A vaccination campaign. Eligibility was based on having received at least one dose of 4CMenB and provided a blood sample at the 7 mo time window of the original study, and being in good health based on clinical history, medical examination and the clinical judgment of the investigator. For this extension study an additional cohort of 4CMenB-naive subjects of similar age (13−19 y of age) to the original participant group were recruited at the same study centers. Main exclusion criteria in both groups were any history of household and/or intimate exposure to N. meningitidis disease, any chronic medical condition or known immune impairment, receipt of antibiotics within 6 d of enrolment, or blood or blood products within 90 d, or prior receipt of a serogroup B meningococcal vaccine in the vaccine-naive group.
The study vaccine, 4CMenB (Bexsero®, Novartis Vaccines and Diagnostics, Siena, Italy), contains four main antigens—factor H binding protein (fHbp), Neisserial adhesin A (NadA), Neisseria Heparin Binding Antigen (NHBA), and outer membrane vesicles from New Zealand strain 98/254 (NZOMV). The fHbp and NHBA proteins are presented as fusion proteins with two other protein antigens (GNA2091 and GNA1030, respectively) also identified by reverse vaccinology. The vaccine is supplied in prefilled monodose (0.5 mL) syringes containing 50 µg (each) fHbp-GNA2091, NadA, and NHBA-GNA1030, 25 µg NZ 98/254 OMV and 1.5 mg aluminum hydroxide.
In the original study participants had been randomly allocated to eight different study groups to receive either one, two or three doses of 4CMenB in different schedules as shown in Table 1, all subjects providing a final blood sample at 7 mo, one month after their last placebo injection or dose of 4CMenB. In the present study all subjects provided a 15 mL blood sample at 24 mo, so 18 mo after their last placebo injection or dose of 4CMenB.
Table 1. Demographics of subjects.
| One dose | Two doses | Three doses | Naive | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Schedule (study months when 4CMenB administered) | 0 | 6 | 0,1 | 0,2 | 0,6 | 0,1,2 | 0,1,6 | 0,2,6 | - |
| Enrolled (n = ) | 95 | 51 | 102 | 106 | 49 | 153 | 53 | 57 | 151 |
| Age (years) | 16.0 ± 2.0 | 16.0 ± 1.9 | 16.1 ± 1.9 | 15.9 ± 1.8 | 16.2 ± 1.9 | 15.9 ± 1.9 | 16.2 ± 2.1 | 15.6 ± 1.9 | 15.6 ± 1.7 |
| Male | 42 (44%) | 18 (35%) | 45 (44%) | 45 (42%) | 19 (39%) | 57 (37%) | 20 (38%) | 19 (33%) | 90 (60%) |
| Hispanic | 95 (100%) | 51 (100%) | 102 (100%) | 106 (100%) | 49 (100%) | 151 (99%) | 53 (100%) | 57 (100%) | 150 (99%) |
| Other | 1 Asian 1 Other |
1 Other | |||||||
| Pooled MITT population | 146 | 257 | 263 | 151 | |||||
| Male | 60 (41%) | 109 (42%) | 96 (37%) | 90 (60%) | |||||
| Time since last dose (months) | 24 | 18 | 23 | 22 | 18 | 22 | 18 | 18 | 0 |
| % of pooled group | 65% | 35% | 40% | 41% | 19% | 58% | 20% | 22% | |
Serology
Serum was obtained and stored within 24 h in aliquots at –18°C until transport in appropriate cold-chain conditions to the Novartis Clinical Serology Laboratory (Marburg, Germany). Samples were identified by a 6-digit code originally assigned to each subject in the first study to maintain blinding during serology analyses; newly enrolled vaccine-naive subjects were assigned new 6-digit codes. As described in the report of the original study,11 immune responses to 4CMenB were assessed as serum bactericidal activity using human complement (hSBA) against three N. meningitidis serogroup B strains selected to assess responses to the individual vaccine antigen components, namely 44/76-SL for fHbp variant 1.1, 5/99 for NadA, and NZ98/254 for the PorA P1.4 component of NZOMV. At the time of the study a suitable strain to assess responses against the NHBA antigen had not been identified. Interpolated hSBA titers were based on the reciprocal of the final serum dilution giving >50% killing at 60 min, compared with the number of colony-forming units at time zero, with a detection limit of 2.
Statistics
Geometric mean titers (GMTs) and 95% CIs were computed by exponentiating (base 10) the least square means of the log-transformed titers from a two-way analysis of variance (ANOVA) with factors for vaccine group and study center. Titers below the limit of detection were set at half the limit of detection for these calculations. Percentages of participants with an hSBA titer ≥ 4, which represents the accepted correlate of protection against meningococcal disease,12 were evaluated for each of the three Neisseria meningitidis strains. Data are presented according to the original study groups, and for pooled groups consisting of those who received one, two or three doses of 4CMenB in the primary study. There was no formal statistical hypothesis testing or sample size calculation planned in the protocol; ad hoc pair-wise statistical comparisons between groups (pooled data) were made by Chi-square test.
Results
Of the 1625 eligible original participants, 666 were enrolled in the extension study, together with 151 newly recruited vaccine-naive controls. Participants were distributed across the eight original study groups (Table 1). The mean age at the time of enrolment in the extension study was 16 y, with more female than male participants, 60% vs. 40% of the overall population, with similar proportions across the groups. The 151 vaccine-naive participants were of similar age (15.6 y), with proportionately more males (60%) than females (40%) (P < 0.0001).
Seroresponse rates observed 1–6 and 18–24 mo after the last dose according to one, two or three dose vaccine schedules
The proportions of children achieving protective antibody titers for the three different N. meningitidis strains before, 1 mo after, and 18−24 mo after vaccination according to the different vaccine schedules are displayed in Table 2. Before any vaccination in the original study, 38.9%, 29.6% and 28.3% of participants of this persistence study (all groups combined) had titers ≥ 4 against the strains selective for fHbp, NadA, and NZOMV, respectively. The vaccine-naive group recruited for the persistence study, although older (mean age ± S.D., 15.6 ± 1.7 y) than the children in the original vaccine groups (13.8 ± 1.9 y), had similar rates to this earlier group, with protective hSBA levels present in 50, 25, and 40%, respectively (Table 2).
Table 2. Percentages (with 95% CI in parentheses) of participants with hSBA titers ≥ 4 against the three test strains in the individual study groups and as totals in those given 1, 2, or 3 doses at baseline (before dose 1), and 1 mo (from previous study11) and 18–24 mo after the last dose of 4CMenB, in the indicated schedules, and in vaccine-naïve subjects.
| One dose | Two doses | Three doses | Naive | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Schedule (mo)a | 0 | 6 | Total | 0,1 | 0,2 | 0,6 | Total | 0,1,2 | 0,1,6 | 0,2,6 | Total | - | |
| N | 95 | 51 | 146 | 102 | 106 | 49 | 257 | 153 | 53 | 57 | 263 | 151 | |
| Strain 44/76SL (fHbp) | |||||||||||||
| Baseline | 40% (30–51) |
47% (33–62) |
42% (34–51) |
32% (23–42) |
41% (31–51) |
31% (18–45) |
35% (30–42) |
44% (36–52) |
40% (26–54) |
32% (20–45) |
40% (34–47) |
||
| 1 Month after last dose | 93% (85–97) |
94% (84–99) |
93% (88–97) |
100% (96–100) |
100% (97–100) |
100% (93–100) |
100% (99–100) |
100% (98–100) |
100% (93–100) |
100% (94–100) |
100% (99–100) |
||
| 18–24 Months after last dose | 73% (63–81) |
73% (58–84) |
73% (65–80) |
82% (74–89) |
81% (72–88) |
84% (70–93) |
82% (77–87) |
83% (76–89) |
92% (82–98) |
86% (74–94) |
86% (81–90) |
50% (42–59) |
|
| Strain 5/99 (NadA) | |||||||||||||
| Baseline | 33% (23–43) |
33% (21–48) |
33% (25–41) |
26% (18–36) |
30% (22–40) |
22% (18–45) |
27% (22–33) |
34% (27–42) |
28% (17–42) |
21% (11–34) |
30% (25–36) |
||
| 1 Month after last dose | 96% (90–99) |
88% (76–96) |
93% (88–97) |
100% (96–100) |
100% (97–100) |
98% (89–100) |
100% (98–100) |
100% (98–100) |
100% (93–100) |
100% (94–100) |
100% (99–100) |
||
| 18–24 Months after last dose | 65% (55–75) |
73% (58–84) |
68% (60–75) |
93% (86–97) |
95% (89–98) |
94% (83–99) |
94% (91–97) |
96% (92–89) |
98% (90–100) |
100% (94–100) |
97% (95–99) |
25% (18–33) |
|
| Strain NZ98/254 (NZOMV) | |||||||||||||
| Baseline | 31% (21–41) |
29% (17–44) |
30% (23–38) |
24% (16–33) |
30% (22–40) |
29% (17–43) |
27% (22–33) |
30%* (22–58) |
32% (20–46) |
21% (11–34) |
28%# (23–34) |
||
| 1 Month after last dose | 94% (87–98) |
92% (81–98) |
93% (88–97) |
100% (96–100) |
100% (97–100) |
100% (93–100) |
100% (99–100) |
99%* (96–100) |
100% (93–100) |
98% (91–100) |
99%# (97–100) |
||
| 18–24 Months after last dose | 62% (52–72) |
61% (46–74) |
62% (53–70) |
75% (65–83) |
75% (66–83) |
86% (73–94) |
77% (71–82) |
86% (79–91) |
98% (90–100) |
96% (88–100) |
90% (86–94) |
40% (32–48) |
|
a Schedule shows study months when 4CMenB administered.*n = 152; # n = 262.
As previously reported for the full cohort of the original study,11 the immunogenicity of a single dose of 4CMenB was such that one month later 88−96% of participants across both study groups who received one dose had titers ≥4 against the three test strains (Table 2). Although 18−24 mo after their only dose of 4CMenB these proportions had waned to 62−73%, these proportions were all significantly higher than those in the vaccine-naive age-matched subjects (P ≤ 0.0002).
In contrast, one month after the administration of the last of two or three doses of 4CMenB, one, two or six months apart, all participants displayed titers ≥4 against all three strains, except against NadA in one subject in the 0, 6 mo group (Table 2). When these subjects were assessed 18−24 mo after their last vaccination, 42, 64, and 75% had titers ≥4 against all three antigens after one, two or three doses of 4CMenB, respectively, compared with 15% of the 4CMenB-naïve group. Proportions with titers ≥ 4 against at least two antigens were 60, 85, and 92% after one, two or three doses of 4CMenB, respectively, compared with 32% of the 4CMenB-naïve group. There were no differences between the percentages with titers ≥4 of those given two or three doses when assessed against fHbp (82% and 86%, P = 0.285) or NadA (94% and 97%, P = 0.072). However, the proportion of participants with titers ≥4 against NZOMV was lower after two doses (77%) than after three doses (90%, P < 0.0001) at this time-point.
Geometric mean titers observed 1–6 and 18–24 mo after the last dose according to one, two or three dose vaccine schedules
Participants had low hSBA GMTs before 4CMenB vaccination, consistent across groups, a situation also reflected in the GMTs observed in the vaccine-naive controls enrolled for this persistence study (Table 3). The hSBA GMTs assessed one month after the last vaccination with 4CMenB increased proportionally with the numbers of doses received, such that GMTs for all three strains in both two and three dose groups (pooled data) were significantly higher (P < 0.0001) than in the one dose group (Fig. 1). In contrast to the percentages with titers ≥4, the third dose produced an incremental increase in titers when measured as GMTs such that there were significant differences observed after three doses vs. after two doses for all tested strains (P = 0.0275, 0.0150 and 0.0033 for fHbp, NadA and NZOMV, respectively [Table 3]).
Table 3. hSBA GMTs against the three test strains in the individual study groups and as totals in those given 1, 2, or 3 doses at baseline (before dose 1), and 1 mo (from previous study11) and 18–24 mo after the last dose of 4CMenB, in the indicated schedules, and in vaccine-naïve subjects.
| One dose | Two doses | Three doses | Naive | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Schedule (mon)a | 0 | 6 | Total | 0,1 | 0,2 | 0,6 | Total | 0,1,2 | 0,1,6 | 0,2,6 | Total | - | |
| N | 95 | 51 | 146 | 102 | 106 | 49 | 257 | 153 | 53 | 57 | 263 | 151 | |
| Strain 44/76SL (fHbp) | |||||||||||||
| Baseline | 3.8 (2.8–5.1) |
4.3 (2.9–6.6) |
4.0 (3.1–5.1) |
2.7 (2.0–3.7) |
3.2 (2.4–4.3) |
2.8 (1.9–4.3) |
3.0 (2.5–3.6) |
3.9 (3.0–4.9) |
3.6 (2.4–5.3) |
2.8 (1.9–4.1) |
3.5 (2.9–4.3) |
||
| 1 Month after last dose | 47 (36–60) |
62 (44–86) |
52 (42–63) |
189 (149–241) |
227 (179–287) |
227 (161–320) |
211 (181–247) |
253 (208–309) |
316 (227–439) |
265 (192–365) |
268 (230–312) |
||
| 18–24 Months after last dose | 16 (11–23) |
19 (12–32) |
17 (12–23) |
29 (20–42) |
34 (24–49) |
27 (16–45) |
31 (24–39) |
42 (31–56) |
50 (30–83) |
44 (27–73) |
44 (35–55) |
4.5 (3.5–5.8) |
|
| Strain 5/99 (NadA) | |||||||||||||
| Baseline | 2.7 (2.0–3.4) |
2.7 (1.9–3.8) |
2.7 (2.2–3.3) |
2.2 (1.7–2.9) |
2.4 (1.8–3.0) |
2.3 (1.6–3.3) |
2.3 (2.0–2.7) |
2.6 (2.1–3.2) |
2.6 (1.8–3.6) |
1.7 (1.2–2.4) |
2.4 (2.0–2.8) |
||
| 1 Month after last dose | 63 (49–81) |
68 (49–94) |
65 (53–79) |
445 (352–562) |
727 (577–916) |
802 (574–1121) |
610 (523–710) |
605 (499–735) |
1181 (856–1630) |
1105 (808–1510) |
790 (679–920) |
||
| 18–24 Months after last dose | 7.1 (5.2–9.6) |
9.9 (6.6–15) |
8.0 (6.2–10) |
40 (30–54) |
43 (33–58) |
65 (43–98) |
46 (38–55) |
73 (57–92) |
121 (82–180) |
100 (68–146) |
86 (72–104) |
2.1 (1.7–2.6) |
|
| Strain NZ98/254 (NZOMV) | |||||||||||||
| Baseline | 2.7 (2.0–3.5) |
2.7 (1.8–3.9) |
2.7 (2.1–3.4) |
2.2 (1.6–2.8) |
2.6 (2.0–3.4) |
2.7 (1.8–3.9) |
2.4 (2.0–2.9) |
2.8* (2.2–3.4) |
3.7 (2.5–5.4) |
2.2 (1.6–2.8) |
2.8# (2.3–3.3) |
||
| 1 Month after last dose | 33 (25–43) |
43 (30–62) |
36 (29–45) |
78 (60–100) |
115 (89–148) |
154 (107–221) |
104 (88–123) |
129* (105–160) |
174 (123–248) |
170 (121–238) |
146# (124–172) |
||
| 18–24 Months after last dose | 8.7 (6.1–12) |
9.2 (5.7–15) |
8.9 (6.7–12) |
17 (12–24) |
19 (14–27) |
27 (17–43) |
20 (16–24) |
23 (18–31) |
42 (26–66) |
41 (26–64) |
30 (24–37) |
3.2 (2.5–4.1) |
|
a Schedule shows study months when 4CMenB administered. *n = 152; # n = 262.
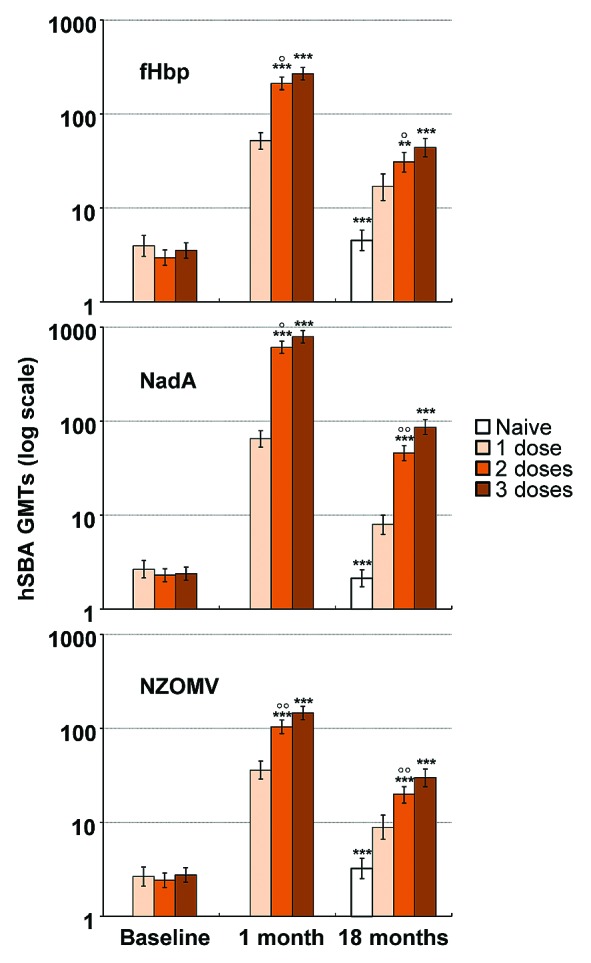
Figure 1. Pooled GMTs in groups having received 0, 1, 2, or 3 doses of 4CMenB, measured at baseline, 1 mo after the last dose of the original study, and 18 mo after completion of that study. * and ◦ indicate statistically significant differences from the 1-dose and 3-dose groups, respectively, at p < 0.05 (*,◦),P < 0.01 (**,◦◦) and P < 0.001 (***,◦◦◦) levels.
Following the pattern observed for protective antibody levels achieved, GMTs against all three test strains waned to some extent when assessed 18−24 mo after the last dose of 4CMenB, but GMTs for all vaccine groups were still statistically significantly higher than the equivalent prevaccination levels and those observed in the age-matched vaccine-naive controls (Table 3). GMTs of persisting antibodies were also dose-dependent, reflecting the initially higher GMTs at 7 mo, and were generally consistent across groups who received the same number of doses, irrespective of the immunisation schedule (see Table 2). There was some indication of higher titers against NadA and NZOMV when assessed at 18 mo after the last dose in the 2-dose (0,6 schedule) and 3-dose groups (schedules 0,1,6 and 0,2,6) than when this interval was 22/23 mo (0,1 and 0,2 schedule for 2-doses, 0,1,2 schedule for 3-doses), but this was not apparent for fHbp. No differences were evident when one dose had been given 18 or 24 mo earlier.
When data were pooled according to total number of doses received, there were significant differences between GMTs for the one, two and three dose groups for all three strains (Fig. 1). The one dose group had significantly higher GMTs than the vaccine-naive group (P < 0.001 in all cases), but these levels were significantly lower than either 2- or 3-dose groups (Fig. 1). When breaking down the data into those adolescents with apparent previous exposure to vaccine antigens, reflected in having hSBA titers ≥ 4 before vaccination, there was significantly better persistence against all three antigens after one dose in these exposed subjects (90–100%) than in those with no apparent antibodies (45–57%, P < 0.001). Persisting hSBA ≥ 5 rates were also significantly greater in initially seropositive than seronegative subjects after two doses against fHbp (97% vs. 74%, P < 0.001) and NZOMV (97% vs. 70%, P < 0.001) and three doses (98% vs. 77%, P < 0.001 for fHbp; 99% vs. 87%, P < 0.005 for NZOMV), but not for NadA where the range was 93–100% across the groups.
Discussion
We have previously reported on the immunogenicity and acceptable tolerability of the novel meningococcal serogroup B vaccine, 4CMenB, when administered as one, two or three doses to healthy adolescents.11 That study indicated that the best schedule in terms of immune response and tolerability was two doses administered one to six months apart, which elicited hSBA titers ≥4, a level generally considered to be protective,12 in almost all subjects. A third vaccine dose did not provide significant advantage when assessed one month post-vaccination.
In this follow-up evaluation of the persistence of circulating antibodies performed 18 to 24 mo after the last vaccination with 4CMenB, we have confirmed that the two dose schedule continues to afford protection against the serogroup B test strains in the majority of participants, 85% of individuals still having protective titers against two of the three target strains. Although not planned or powered to show differences in persistence between the different schedules with the same number of doses, pooling of the groups who received 1, 2, or 3 doses did allow ad hoc evaluations of differences between these pooled groups. It will be interesting to see future evaluations of persistence over a longer period when the vaccine is in widespread use. Levels of hSBA against all three test strains in subjects who had only received one dose of 4CMenB, while higher than vaccine-naive controls, were significantly lower than in those who had received either two or three doses, expressed either as GMTs or as proportions achieving titers ≥ 4. Although GMTs were higher 18–24 mo after three doses than after two doses, the proportions of adolescents who displayed the protective threshold were not significantly different for two of the three antigens (fHbp and NadA).
Among subjects lacking bactericidal antibodies against a given strain at study entry before any vaccination with 4CMenB, the percentages of subjects with hSBA titers ≥4 for the three test strains in this persistence assessment ranged from 45–57% for fHbp, 70–93% for NadA and 77–96% for NZOMV after 1, 2, or 3 doses of 4CMenB, respectively. In those who displayed titers ≥4 before vaccination, implying some pre-exposure to circulating meningococci, the respective rates were 90–100%, 97–99%, and 98–100%, and were statistically significantly higher in most cases, suggesting that vaccination with 4CMenB had boosted a naturally-acquired immunity.
The clinical consequences of this difference in response to one antigen in providing protection against meningococcal serogroup B remain to be determined. Other antigens present in the vaccine, such as NHBA, the fusion proteins GNA2091 and GNA1030, and other proteins contained in the OMV, and synergies between potential antibody responses to these and those described above may also play roles in the induced protective response.13 A recent study of over 1000 clinical isolates from several European countries, applying pooled sera from 4CMenB-vaccinated infants in the meningococcal antigen typing system (MATS), estimated coverage by the vaccine of 78% (95% CI: 63−90) with half of all strains being covered by two or more antigens.14 Additional strain testing of representative non-homologous strains (and NHBA) will also be important to further predict longer term protection. Until we can understand fully the role of the interactions outlined above, the full strain coverage and protective effects of 4CMenB can only be estimated. Ultimately, these estimates based on immune surrogates will need to be replaced by field effectiveness assessments after use of the vaccine, ideally in a setting where implementation incorporates catch-up vaccination of adolescents and herd impact may also be assessed.
This the first study of its kind to determine the persistence of the immune responses to the novel meningococcal serogroup B vaccine, 4CMenB, in adolescents. Despite having a large study population, of necessity it was limited to those who participated in the original study and were willing to enroll in this one, so there were no formal sample size calculations or statistical analyses planned. The actual extent of protection against circulating strains of meningococcal serogroup B remains to be determined, but preliminary estimates from MATS indicate that a majority of strains will be covered.13,14
Serogroup B meningococcal disease remains a major health concern for parents of very young children, who represent the main target group for the vaccine, but also for adolescents and young adults who are at risk in a second peak of disease incidence.1 Furthermore, the importance of a serogroup B vaccine for adolescents may come both from direct protection afforded to the individual vaccinee, and also herd effects of mass immunization affecting not only vaccinees but also other age groups. Herd protection was observed following mass immunization campaigns with meningococcal serogroup C conjugate vaccines, which resulted in decreased carriage of serogroup C.15 It remains to be seen whether widespread use of 4CMenB will affect carriage of serogroup B. The rapidity of onset of meningococcal disease, with potential fatal consequences within hours of infection, suggests that circulating antibodies are the key to protection. The persistence of the post-vaccination response will therefore be crucial in maintaining protection. The rate of decline of antibodies to the OMV component of the vaccine are similar to those reported for the Norwegian OMV in 13–14 y-olds, in which efficacy corresponded to the GMT of hSBA.16 However, the inclusion of the additional protein antigens in 4CMenB, and particularly the high persistence of antibodies to fHbp and NadA suggest that efficacy will be maintained for longer than with the OMV-only vaccines. Furthermore, some role for priming cannot be discounted as in many instances the interval between carriage acquisition relative and infective invasion may still afford adequate opportunity for the development of relevant bactericidal antibodies.
In conclusion, two doses of 4CMenB provide a good level of seroprotective activity against serogroup B, sustained over at least 18−24 mo in 64% of adolescents for all vaccine-related antigens. Although adolescents given three doses have higher GMTs than those given two doses, this only increased the proportion with protective titers against the NZOMV antigen.
Acknowledgments
We are deeply indebted to the Municipal authorities of Lo Barnechea, Peñalolén and Providencia, Santiago, Chile for their assistance and support in contacting the participating schools and to the directors, teachers, and parents, but most importantly for the generosity and perseverance of the students of the participating schools of the Metropolitan Region of Chile within the Municipality, namely Colegio Parroquial Santa Rosa, Complejo Educacional Eduardo Cuevas Valdés, Colegio San José and Colegio Diego Aracena, from Lo Barnechea, Colegio Antonio Hermida Fabres and Centro Educacional Eduardo de la Barra, from Peñalolén, Liceo José Victorino Lastarria and Liceo Carmela Carvajal de Prat, from Providencia. Two sites were out-patient clinics, one located in the Municipality of Independencia (Centro Para Vacunas en Desarrollo, Hospital de Niños Roberto del Rio,) and one in the city of Valparaiso (Facultad de Medicina, Universidad de Valparaíso), and we are also indebted to the adolescents and parents at these sites. We thank María Lucía Villata and Lorenzo Bertelli (both Novartis Vaccines) for management of the study, and we are grateful to Keith Veitch of Novartis Vaccines for advice and guidance on manuscript development.
The V72P10 Meningococcal B Adolescent Vaccine Study group includes the following researchers actively involved in subject enrollment, vaccination, and follow-up: C Arriagada, I Avendaño, M Bastías, B Benavente, C Bravo, C Bustamante, G Bustos, N Campos, X Cerda, M. Espinoza, C Fuentes, D. Fuentes, MT Henriques, C Ibañez, G Izquierdo, J. Krauss, M Manriquez, J Martinez, E Muñoz, MA O'Ryan-Soriano, C. Osorio, M Rabello, M Reyes, M Salvatierra, S Schiaccaluga, D Silva, D Simian, P Ulloa, K Vera, A Vergara, R Villena, S Vorphal.
Glossary
Abbreviations:
- 4CMenB
multicomponent investigational meningococcal serogroup B vaccine
- hSBA
serum bactericidal activity with human complement
- fHbp
factor H binding protein
- NadA
Neisserial adhesin A
- NHBA
Neisseria Heparin Binding Antigen
- NZOMV
New Zealand strain 98/254 outer membrane vesicles
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/vaccines/article/25505
References
- 1.Harrison LH, Trotter CL, Ramsay ME. Global epidemiology of meningococcal disease. Vaccine. 2009;27(Suppl 2):B51–63. doi: 10.1016/j.vaccine.2009.04.063. [DOI] [PubMed] [Google Scholar]
- 2.Granoff DM. Review of meningococcal group B vaccines. Clin Infect Dis. 2010;50(Suppl 2):S54–65. doi: 10.1086/648966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jennings HJ, Lugowski C. Immunochemistry of groups A, B, and C meningococcal polysaccharide-tetanus toxoid conjugates. J Immunol. 1981;127:1011–8. [PubMed] [Google Scholar]
- 4.Jennings HJ, Gamian A, Ashton FE. N-propionylated group B meningococcal polysaccharide mimics a unique epitope on group B Neisseria meningitidis. J Exp Med. 1987;165:1207–11. doi: 10.1084/jem.165.4.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zollinger WD, Poolman JT, Maiden MCJ. Meningococcal serogroup B vaccines: will they live up to expectations? Expert Rev Vaccines. 2011;10:559–61. doi: 10.1586/erv.11.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tappero JW, Lagos R, Ballesteros AM, Plikaytis B, Williams D, Dykes J, et al. Immunogenicity of 2 serogroup B outer-membrane protein meningococcal vaccines: a randomized controlled trial in Chile. JAMA. 1999;281:1520–7. doi: 10.1001/jama.281.16.1520. [DOI] [PubMed] [Google Scholar]
- 7.Pizza M, Scarlato V, Masignani V, Giuliani MM, Aricò B, Comanducci M, et al. Identification of vaccine candidates against serogroup B meningococcus by whole-genome sequencing. Science. 2000;287:1816–20. doi: 10.1126/science.287.5459.1816. [DOI] [PubMed] [Google Scholar]
- 8.http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/002333/smops/Positive/human_smop_000447.jsp&mid=WC0b01ac058001d127 Accessed 18 Feb 2013.
- 9.Vesikari T, Esposito S, Prymula R, Ypma E, Kohl I, Toneatto D, et al. EU Meningococcal B Infant Vaccine Study group Immunogenicity and safety of an investigational multicomponent, recombinant, meningococcal serogroup B vaccine (4CMenB) administered concomitantly with routine infant and child vaccinations: results of two randomised trials. Lancet. 2013;381:825–35. doi: 10.1016/S0140-6736(12)61961-8. [DOI] [PubMed] [Google Scholar]
- 10.Toneatto D, Oster P, deBoer AC, Emerson A, Santos GF, Ypma E, et al. Early clinical experience with a candidate meningococcal B recombinant vaccine (rMenB) in healthy adults. Hum Vaccin. 2011;7:781–91. doi: 10.4161/hv.7.7.15997. [DOI] [PubMed] [Google Scholar]
- 11.Santolaya ME, O’Ryan ML, Valenzuela MT, Prado V, Vergara R, Muñoz A, et al. V72P10 Meningococcal B Adolescent Vaccine Study group Immunogenicity and tolerability of a multicomponent meningococcal serogroup B (4CMenB) vaccine in healthy adolescents in Chile: a phase 2b/3 randomised, observer-blind, placebo-controlled study. Lancet. 2012;379:617–24. doi: 10.1016/S0140-6736(11)61713-3. [DOI] [PubMed] [Google Scholar]
- 12.Frasch CE, Borrow R, Donnelly J. Bactericidal antibody is the immunologic surrogate of protection against meningococcal disease. Vaccine. 2009;27(Suppl 2):B112–6. doi: 10.1016/j.vaccine.2009.04.065. [DOI] [PubMed] [Google Scholar]
- 13.Donnelly J, Medini D, Boccadifuoco G, Biolchi A, Ward J, Frasch C, et al. Qualitative and quantitative assessment of meningococcal antigens to evaluate the potential strain coverage of protein-based vaccines. Proc Natl Acad Sci U S A. 2010;107:19490–5. doi: 10.1073/pnas.1013758107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vogel U, Taha M-K, Vazquez JA, Findlow J, Claus H, Stefanelli P, et al. Predicted strain coverage of a meningococcal multicomponent vaccine (4CMenB) in Europe: a qualitative and quantitative assessment. Lancet Infect Dis. 2013; February 13, 2013 10.1016/ S1473-3099(13)70006-9. [DOI] [PubMed]
- 15.Maiden MC, Ibarz-Pavón AB, Urwin R, Gray SJ, Andrews NJ, Clarke SC, et al. Impact of meningococcal serogroup C conjugate vaccines on carriage and herd immunity. J Infect Dis. 2008;197:737–43. doi: 10.1086/527401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holst J, Feiring B, Fuglesang JE, Høiby EA, Nøkleby H, Aaberge IS, et al. Serum bactericidal activity correlates with the vaccine efficacy of outer membrane vesicle vaccines against Neisseria meningitidis serogroup B disease. Vaccine. 2003;21:734–7. doi: 10.1016/S0264-410X(02)00591-1. [DOI] [PubMed] [Google Scholar]


