Abstract
To characterize the cell mediated immunity (CMI) induced by the investigational CYD tetravalent dengue vaccine (TDV), we developed a whole-blood, intracellular cytokine staining (ICS) assay and a multiplex assay, each requiring 3 mL of blood. We assessed CMI before and 28 d after a first and third injection of CYD-TDV and one year after the third injection in a subset of 80 adolescents and adults enrolled in a phase II trial in Singapore (ClinicalTrial.gov NCT NCT00880893). CD4/IFNγ/TNFα responses specific to dengue NS3 were detected before vaccination. Vaccination induced YF-17D-NS3-specific CD8/IFNγ responses, without significant TNFα, and a CYD-specific Th1/Tc1 cellular response in all participants, which was characterized by predominant IFNγ secretion compared with TNFα, associated with low level IL-13 secretion in multiplex analysis of peripheral blood mononuclear cells (PBMC) supernatants after restimulation with each the CYD vaccine viruses. Responses were directed mainly against CYD-4 after the first vaccination, and were more balanced against all four serotypes after the third vaccination. The same qualitative profile was observed one year after the third vaccination, with approximately 2-fold lower NS3-specific responses, and 3-fold lower serotype-specific cellular responses. These findings confirm previous observations regarding both the nature and specificity of cellular responses induced by CYD-TDV, and for the first time demonstrate the persistence of cellular responses after one year. We also established the feasibility of analyzing CMI with small blood samples, allowing such analysis to be considered for pediatric trials.
Keywords: cell mediated immunity, intracellular cytokine staining, whole blood, cytokines, tetravalent dengue vaccine
Introduction
Dengue virus, a member of the genus Flavivirus, exists as four closely related but antigenically distinct serotypes (DENV1–4). The virus infects humans via blood feeding by infected Aedes mosquitos (Aedes aegypti and Aedes albopictus). Over the past two decades, the number of dengue infections has continued to increase in the endemic areas of South-east Asia, Central and South America and the South Pacific regions.1,2
Immune responses to natural infection are thought to provide lifelong protection against homoserotypic strains, but limited cross-protection between serotypes.3 Secondary infections with a different serotype have been associated with a higher risk of developing severe disease due to inappropriate heterologous humoral and cellular immune responses. Low-affinity heterologous T-cell responses, excessive production of soluble pro-inflammatory mediators and complement activation have been linked to disease severity4,5 while other response profiles are considered indicative of a safe immune response to infection or vaccination.6-8
The investigational, YF-17D-based, recombinant, live, attenuated tetravalent dengue vaccine being developed by Sanofi Pasteur (CYD-TDV),9,10 is one of several dengue vaccines in development11,12 and has demonstrated satisfactory safety and immunogenicity, and protective efficacy for 3 of the 4 serotypes.13-18
We have previously shown that CYD-TDV vaccination in adults induces significant CD8+ T-cell responses against the YF-17D backbone of the CYD vaccine viruses and T helper 1 (Th1) responses to each dengue serotype without inducing changes in serum pro-inflammatory cytokines.19 Here we expand on these investigations and report on cellular responses up to one year after CYD-TDV vaccination of a cohort of adolescents and adults recruited in a dengue low endemic area. One of our objectives was to assess the feasibility of evaluating CMI responses using small volumes of blood.
Two complementary methods were chosen to characterize the cellular immune response. To measure the cellular response against each serotype and the Th1/Th2 balance, freshly isolated PBMCs were stimulated in vitro with each monovalent CYD dengue vaccine, and the cytokines IFNγ, TNFα, IL-13 and IL-5 were measured. It was demonstrated in human recipients of live attenuated dengue vaccine candidate (VDV1 and VDV2) that the cytokine pattern induced upon live or inactivated vaccine in vitro restimulation was very similar, suggesting a CD4 helper response specific for structural proteins.19 By intracellular cytokine staining, the CD4 and CD8 response against the NS3 protein of either wild-type dengue or the YF-17D vaccine virus were analyzed, allowing the differentiation of the memory response against CYD-TDV and wild-type virus in the same subject, since no cross-reactivity between DENV and YF-17D NS3 was previously demonstrated.19
Results
CD4+ T cell responses to DENV NS3
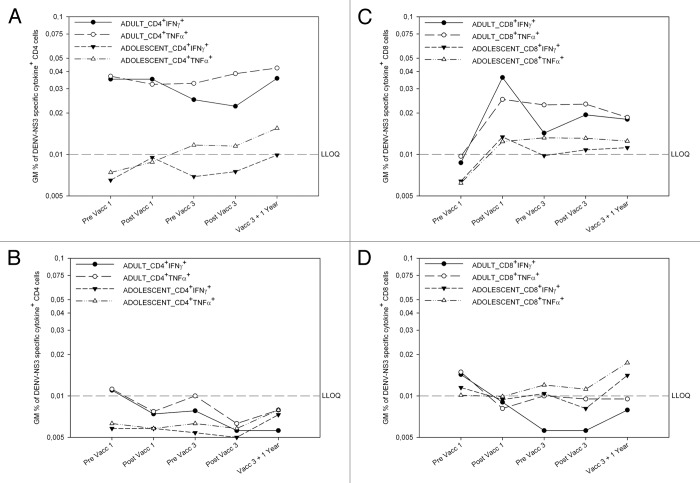
Both before and after vaccination, adult CD4+ T cell populations secreted TNFα and IFNγ (Fig. 1A), but not IL-2 (data not shown), in response to DENV NS3. Levels of TNFα and IFNγ were similar, and most cells co-expressed both cytokines.
Figure 1. DEN NS3-specific CD4 responses pre-exist in adults, and DENV NS3-specific CD8 responses can be recalled by vaccination Dengue virus NS3-specific CD4+ (left) and CD8+ (right) T cell responses in CYD-TDV vaccinees (top) and placebo controls (bottom). Geometric mean (GM) interferon-gamma (IFNγ) and tumor-necrosis-factor-α (TNFα) production in CD4 (CD3+CD8-) (left) and CD8 T cells (right) following ex vivo stimulation of whole blood with DENV NS3 peptides in dengue vaccine group (top panel) and placebo group (bottom) before and 28 d after the first, before and 28 d after the third vaccination, and 1 y after the last vaccination as measured by ICS. For each subject, first the background cytokine levels in the negative control (medium+DMSOs) were subtracted from DENV NS3 peptide pool A and B stimulated cytokine levels. Second, the responses from pool A and pool B were combined by taking the higher percentage of the two pools for each subject. Responses shown are the geometric mean (GM) cytokine percentages of these combined results. (A) Percentages of IFNγ+ or TNFα+ CD4 cells in adult and adolescent vaccinees (B). Percentages of IFNγ+ or TNFα+ CD4 cells in adult and adolescent placebo recipients (C) Percentages of IFNγ+ and TNFα+ CD8 cells in adult and adolescent vaccinees. (D) Percentages of IFNγ+ and TNFα+ CD8 cells in adult and adolescent placebo recipients
Results of the PRNT50 assay at baseline for all four serotypes and results of CMI are available for 29 adults in our cohort of 39. Of these 29, 10 had DENV-NS3 specific CD4+TNFα+ responses, 50% of whom had no detectable antibodies. Similarly, of the 12 of these 29 subjects with neutralizing antibodies against at least one serotype, 7 had no detectable DENV-NS3 specific CD4+TNFα+ response (data not shown).
In the adult control group no or very low cytokine secretion was detected before or after vaccination (Fig. 1B). No adolescent in either group, displayed cytokine responses to dengue NS3 before or after vaccination (Fig. 1A and B).
CD8+ T cell responses to DENV NS3
Before vaccination, no IFNγ, TNFα, or IL-2 was secreted by CD8+ T cells in response to DENV NS3. After vaccination, adult and to a lesser extent adolescent CD8+ T cells secreted IFNγ and TNFα at comparable levels at each timepoint (Fig. 1C).
CD4+ T cell responses to YF-17D NS3
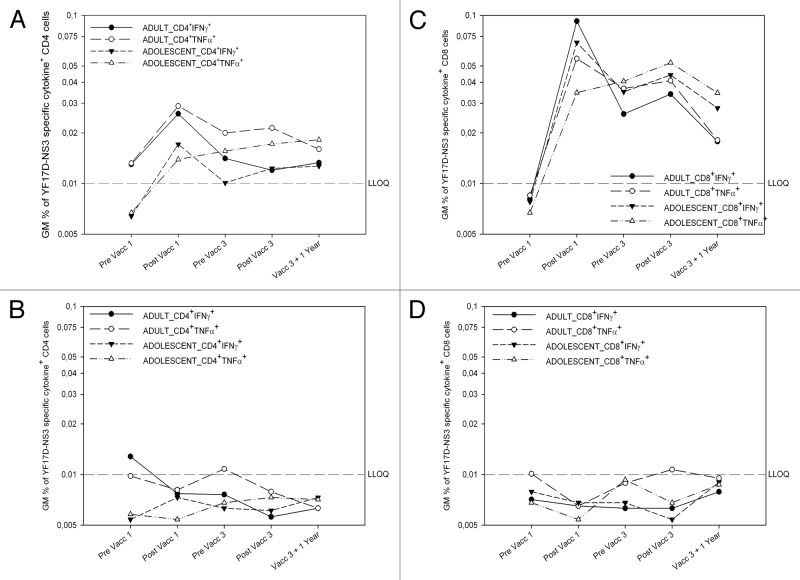
Before vaccination, no IFNγ, TNFα, or IL-2 was secreted by adult and adolescent CD4+ T cell populations in response to YF-17D NS3. Low levels of IFNγ and TNFα were initially expressed after the first vaccination, but were not boosted by third vaccination and stayed stable over time (Fig. 2A).
Figure 2. Tetravalent CYD vaccination induces mostly CD8+ YF-17D NS3-specific responses YF-17D NS3-specific CD4+ (left) and CD8+ (right) T cell responses in CYD-TDV vaccinees (top) and placebo controls (bottom). Geometric mean (GM) interferon-gamma (IFNγ) and tumor-necrosis-factor-α (TNFα) production in CD4 (CD3+CD8-) (left) and CD8 T cells (right) following ex vivo stimulation of whole blood with YF-17D NS3 peptides in dengue vaccine group (top) and placebo group (bottom panel) before and 28 d after the first, before and 28 d after the third vaccination, and 1 y after the last vaccination (V07) as measured by ICS. For each subject, first the background cytokine levels in the negative control (medium+DMSOs) were subtracted from YF-17D NS3 peptide pool A and B stimulated cytokine levels. Second, the responses from pool A and pool B were combined by taking the higher percentage of the two pools for each subject. Responses shown are the geometric mean cytokine percentages of these combined results.(A) Percentages of IFNγ+ or TNFα+ CD4 cells in adult and adolescent vaccinees (B). Percentages of IFNγ+ or TNFα+ CD4 cells in adult and adolescent placebo recipients (C) Percentages of IFNγ+ and TNFα+ CD8 cells in adult and adolescent vaccinees. (D) Percentages of IFNγ+ and TNFα+ CD8 cells in adult and adolescent placebo recipients
CD8+ T cell responses to YF-17D NS3
In adults and adolescents, no IFNγ, TNFα, or IL-2 was secreted by CD8+ T cells before vaccination in response to YF-17D NS3, however significant levels of IFNγ were detected in samples from both age groups in response to YF-17D NS3 (Fig. 2C) after the first vaccination and, to a lesser extent, before and after the third. TNFα was also detected after vaccination, but at lower levels than IFNγ, with most cells co-expressing both cytokines. One year later, the CD8+ T cell IFNγ/TNFα response decreased about 2-fold.
CYD-serotype specific responses measured by Luminex assays
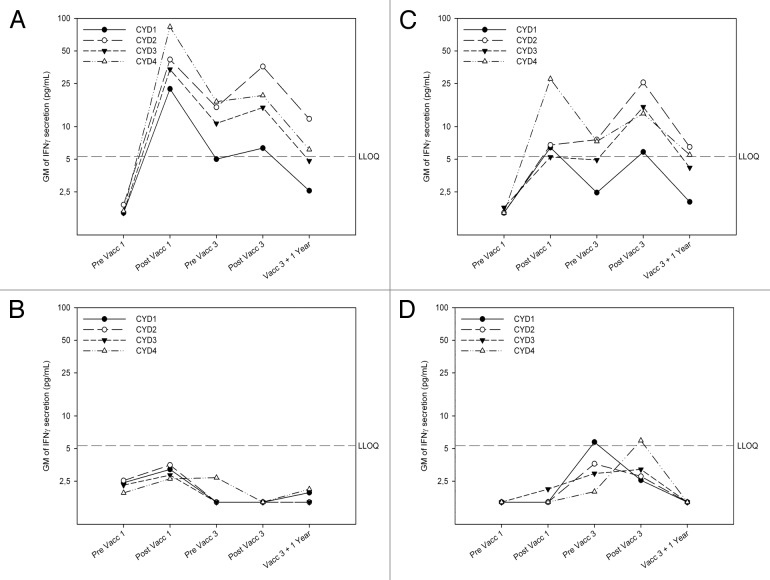
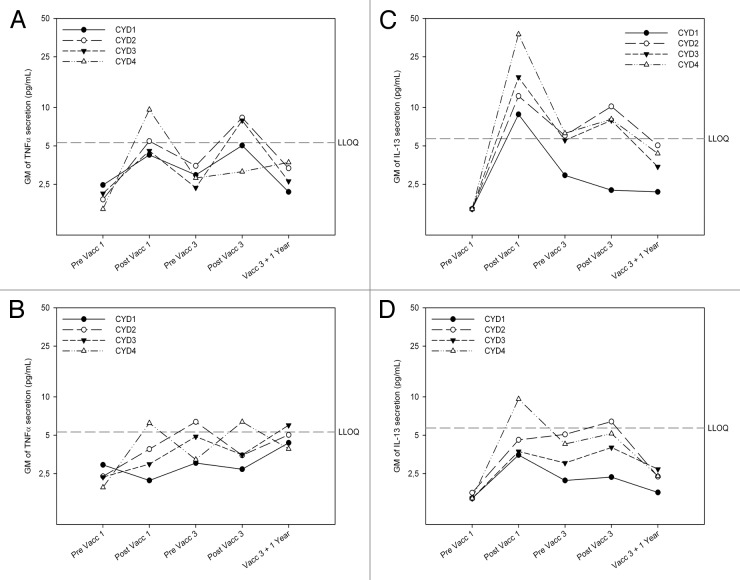
Before vaccination, there was no detectable cytokine secretion in response to in vitro stimulation with the CYD viruses (Figs. 3 and 4). One month later, an overall increase in IFNγ secretion was observed in the vaccinated group (Fig. 3A) but not in controls (Fig. 3B), with higher concentrations detected among adults than adolescents (Fig. 3A and 3C). TNFα levels increased in each group, but only slightly (Fig. 4A). More IFNγ than TNFα was produced in response to CYD re-stimulation in both age groups of vaccinees, but not among controls among who the ratio was often in favor for TNFα (Table S1), although the sample size for this calculation was small. IL-13 levels increased in both age groups of vaccines (Fig. 4C and D) but not in controls (data not shown). Before the third vaccination, cytokine responses were generally lower than after the first vaccination. They increased after vaccination, but remained lower than the levels seen after the first. However, while responses after the first vaccination were predominantly against serotype 4, the balance between serotypes was different after the third dose, with a weaker response against serotype 1 and some dominance of serotype 2 (Fig. 3).
Figure 3. Tetravalent CYD vaccination induces serotype-specific responses dominated by IFNγ. IFNγ was produced following ex vivo stimulation of PBMCs with CYD1–4 monovalent vaccine with a predominant response against serotype 4 after primary CYD-TDV vaccination and a different balance after booster vaccination. Geometric mean (GM) interferon-gamma (IFNγ) production following ex vivo stimulation of freshly purified PBMCs with CYD-1, CYD-2, CYD-3, and CYD-4 monovalent vaccine in dengue vaccine group (top) and placebo group (bottom) in adults (left) and adolescents (right) before and 28 d after the first, before and 28 d after the third vaccination and 1 y after the last vaccination as measured by Luminex. Responses shown are geometric mean IFNγ levels in pg/mL in the CYD-TDV group and placebo group in adults and adolescents from CYD1–4 stimulated PBMCs with the background IFNγ level in the negative control (CY110 stimulated PBMCs) subtracted. (A) IFNγ secretion in adult vaccinees (B) IFNγ secretion in adult placebo recipients (C) IFNγ secretion in adolescent vaccines (D) IFNγ secretion in adolescent placebo recipients
Figure 4. Tetravalent CYD vaccination induces serotype-specific TNFα and IL-13 responses lower than for IFNγ, but with a good pattern correlation in both age groups. Geometric mean TNFα and IL-13 production following ex vivo stimulation of freshly purified PBMCs with CYD-1, CYD-2, CYD-3, and CYD-4 monovalent vaccine in adults (top) and adolescents (bottom) before and 28 d after the first, before and 28 d after the third vaccination, and 1 y after the last vaccination as measured by Luminex. Responses shown are geometric mean TNFα (left) and IL-13 levels (right) in pg/mL in the dengue vaccine (CYD-TDV) group in adults and adolescents from CYD1–4 stimulated PBMCs with the background cytokine level in the negative control (CY110 stimulated PBMCs) subtracted. (A) TNFα secretion in adult vaccinees (B) TNFα secretion in adolescent vaccinees (C) IL-13 secretion in adult vaccinees (D) IL-13 secretion in adolescent vaccines.
One year after the third vaccination response were still directed against all four serotypes, albeit at lower levels than 11 mo earlier, with the strongest responses detected against CYD-2 and the weakest against CYD-1 (Fig. 2).
Discussion
Our CMI study, conducted as part of a wider Phase II study of the immunogenicity and safety of the CYD-TDV dengue vaccine candidate in an area where dengue is semi-endemic20 confirms the importance of three vaccinations CYD-TDV for a broad Th1 response against the 4 serotypes and shows that cellular immune responses persist with an unchanged qualitative profile until at least one year after the third vaccination, although at lower levels. As memory T cells generated in response to vaccination persist, a benefit of this vaccine would be a more rapid immune response to dengue infection in vaccinees, compared with naïve T cells.
Opportunities to assess CMI have been limited by the need for large volumes of blood, typically of the order of 30 to 50 mL, which is particularly problematic in pediatric studies. As understanding CMI in children is of particular interest in the case of dengue,19 we adapted two complementary assays to using smaller sample volumes by using whole blood instead of purified PBMCs in the antigenic stimulation step of the ICS assay, and using freshly purified, rather than thawed PBMCs in the multiplex assay. This required that a laboratory close to the clinical site perform the stimulation of whole blood samples and freshly purified PBMCs, which in turn required that samples be collected in the morning to allow fresh blood to be simulated for 5 h. Due to school, morning study visits proved unfeasible for adolescents and instead adolescent participants were recruited in the afternoon and samples were processed the next day. While this limits the validity of comparing results between age groups as it may have contributed to the lower responses observed in adolescents compared with adults,21,22 it did not prevent us from demonstrating the feasibility of performing these assays with just 6 mL of blood.
This study was performed in an area where dengue viruses circulate. Despite this, baseline samples, including those displaying a positive anti-dengue PRNT50 antibody response, did not detectably respond to stimulation in multiplex assays with any of the four CYD vaccine viruses. In contrast, CD4+ T-cell responses, characterized by IFNγ and TNFα expression, were detected at baseline in ICS assays but only among adults, consistent with a greater risk of exposure to dengue infection.20 Interestingly, among participants with positive DENV NS3-specific responses at baseline, some were seronegative in the PRNT50 assay, possibly due to a greater persistence of memory T cells than antibodies (data not shown). Thus neither antibody nor cellular assays can be used to demonstrate with certainty that an individual is dengue-naïve. The different outcome of ICS and multiplex assays might be to a higher sensitivity of the ICS assay, or to a greater persistence of anti-NS3 memory responses than anti-envelope responses.
After vaccination, we observed increased IFNγ, TNFα and IL-13 secretion in both age groups of vaccinees upon separate restimulation with each CYD virus. The fact that the cellular response was strongest against serotype 4 after the first vaccination was in accordance with data from PRNT50 assay as described in Leo et al.23 In addition, the only serotype which consistently caused viremia after vaccination is serotype 4, as shown in Morrison et al.16 The IFNγ/TNFα ratio in vaccinees (but not in controls), was in favor of IFNγ, which is supportive of a safe vaccine profile as it indicates homologous responses, rather than cross-reactive heterologous responses.5,8,24 The absence of the Th2 cytokine, IL-5, is also favorable since a Th2 response has been suggested to be linked to more severe outcomes after natural infection. IL-13 has been described as an IL-4-like type 2 cytokine,25 however some of their properties differ due to the differing expression of the cytokine themselves and their receptors on human T cells, B cells, and monocytes.26,27 IL-13 does not regulate T cell differentiation due to a lack of IL-13 receptors on T lymphocytes, and IL-4 expression is restricted to Th2 cells. In HIV-1 infection it was demonstrated that IL-13 and IFNγ secretion by activated T cells was associated with viral suppression and a lack of disease progression.28 The co-expression of IL-13 and IFNγ could therefore be seen as a positive feature to control viremia after injection of the dengue vaccine or after natural infection. In previous clinical trials we sought to detect IL-4, and detected none.19
IFNγ and TNFα secretion by CD8+ T cells in response to YF-17D NS3, increased after vaccination in both age groups, as seen previously,19 and IFNγ dominated, again in support of vaccine safety. These responses persisted over time, but were not boosted by the second or third vaccinations. In contrast to previous results in samples from naïve individuals,19 DENV NS3-specific IFNγ and TNFα expression by CD8+ T cells was detected after vaccination in adults but not at baseline, and not in adolescents. Rather than a vaccine-induced primary cross-reactive response between dengue and YF-17D NS3, it is likely that this CD8+ memory response was already present at baseline at undetectable levels and was boosted by vaccination. Similarly, DENV NS3-specific IFNγ and TNFα secretion by CD4+ T cells was observed both before and after vaccination, but exclusively in adults and was most likely due to previous natural infection. CD4+ cells responded only weakly to YF-17D NS3, as seen in previous studies.19 Natural infection therefore seems to induce CD4+ T cell responses with secretion of TNFα and IFNγ, whereas vaccination induced CD8+ T cell responses, with predominant secretion of IFNγ.
Lower cytokine secretion levels in adolescents, including in response to the positive control stimuli, may have also been due to age, but may also have been due to the longer duration between sampling and processing.29 To improve conditions, samples collected from adolescents from the booster vaccination onwards were placed under constant agitation during overnight storage, and increased expression of all cytokines in response to positive control stimulation was observed. While absolute levels differed, qualitative cytokine profiles in response to CYD restimulation were comparable between age groups, while the NS3-specific qualitative response profile after vaccination of adolescents was similar to that previously seen in naïve adult vaccinees.
In conclusion, we demonstrated the feasibility of assessing CMI on blood sample volumes compatible with their potential use in pediatric trials. The CMI profile in both adults and adolescents support the safety of the CYD-TDV dengue vaccine candidate, consistent with the clinical data reported from this and other clinical trials. Finally, this study shows for the first time the ability of CYD-TDV vaccination to elicit memory responses for at least one year after vaccination, but whether this translates into protection against subsequent infection or plays a role in decreasing clinical symptoms upon infection in the field remains to be demonstrated in clinical efficacy trials.
The limitations of the study were first the low number of volunteers analyzed, which allowed only a descriptive analysis of the data, and second, the fact that the adolescent blood was processed differently than the adult blood, which limited the comparison between the two age groups. A major strength of the study was the ability to compare the adaptive immune response elicited by vaccination or by natural infection in the same subject by stimulating the cells in vitro with YF-17D NS3 or DENV NS3 peptides, respectively. Furthermore, it is the first time that we show the persistence of the cellular response 1 y after completion of the 3-dose vaccination regimen. Finally these analyses were performed using only a limited amount of blood, allowing the analysis of cellular immunity in future pediatric trials.
Material and Methods
This analysis was undertaken as an observational objective as part of a multicenter, randomized, controlled Phase II trial in Singapore, designed primarily to assess the safety and immunogenicity of the CYD-TDV vaccine candidate in 1200 subjects aged 2–45 y, as reported elsewhere.23 Three vaccinations of CYD-TDV or placebo control vaccine were given in a 3:1 ratio at study months 0, 6 and 12. The trial was conducted in accordance with the Declaration of Helsinki, Good Clinical Practices, applicable national and local requirements, and was registered with ClinicalTrials.gov (NCT00880893). Written informed consent was obtained for all participants before enrollment.
This analysis was performed on the first 39 adults (40 planned) aged 18–45 y, and 40 adolescents aged 12–17 y, enrolled at one participating hospital, in samples collected before and 28 d after the first and third vaccinations, and one year after the third vaccination. Main baseline demographic characteristics are presented in Table 1. There were fewer males enrolled in the CYD-TDV group compared with the control group in both age groups and more participants among controls had detectable anti-dengue antibody responses at baseline than in the CYD-TDV group. More adults than adolescents had anti-dengue antibodies.
Table 1. Baseline demographic characteristics of participants who were randomized to the CMI group, by age and vaccine group.
| All participants | Age group | |||||
|---|---|---|---|---|---|---|
| 12–17 y | 18–45 y | |||||
| CYD-TDV | Control | CYD-TDV | Control | CYD-TDV | Control | |
| (n = 59) | (n = 20) | (n = 30) | (n = 10) | (n = 29) | (n = 10) | |
| Male n (%) | 26 (44.1) | 14 (70) | 15 (50.0) | 7 (70.0) | 11 (37.9) | 7 (70.0) |
| Age, mean (SD), years | 22.5 (9.3) | 22.3 (9.6) | 14.8 (1.7) | 14.3 (1.4) | 30.4 (6.8) | 30.2 (7.3) |
| Height, mean [SD], cm | 161.7 (8.3) | 163.6 (9.1) | 160.4 (8.6) | 159.8 (10.4) | 163.1 (7.9) | 167.3 (5.9) |
| Weight, mean [SD], kg | 60.0 (14.9) | 62.6 (18.5) | 56.3 (14.7) | 54.5 (14.9) | 63.8 (14.2) | 70.7 (18.8) |
| Body mass index, mean [SD], kg/m2 | 22.7 (4.6) | 23.2 (5.6) | 21.7 (4.6) | 21.1 (4.1) | 23.8 (4.4) | 25.2 (6.3) |
| Number of subjects with known baseline immune status | 53 | 19 | 30 | 10 | 23 | 9 |
| Dengue Immune subjects* n (%) | 9 (17) | 10 (52.6) | 2 (6.7) | 3 (30) | 7 (30.4) | 7 (77.8) |
Dengue Immune subjects at baseline are defined as those subjects with PRNT50 titers ≥ 10 1/dil against at least one dengue serotype at baseline (V01)
We selected two methods—intracellular cytokine staining (ICS) in whole blood and multiplex analyses in cell culture supernatants—requiring a total sampling volume of 6 mL (3 mL per assay).
Cell sampling and processing
Blood was collected in sodium-heparin tubes (BD Vacutainer, ref. 367876). Samples from adults were collected in the morning and processed within 6 h of sampling. For adolescents, due to organizational constraints at the study site related to school scheduling, samples were collected in the afternoon and processed the following day: samples collected before and after the first vaccination were stored overnight without agitation; subsequent samples were gently agitated overnight with the aim reducing the number of contaminated granulocytes and improving T cell responses.21
Responses to NS3 assessed in intracellular cytokine staining
To differentiate between responses to natural dengue infection and CYD-TDV vaccination, we stimulated samples with peptides from the NS3 protein of either wild-type dengue or the YF-17D vaccine virus, the backbone for the CYD vaccine viruses. NS3 peptide pools consist of overlapping 15-mer peptides (11 amino acid overlap) (NeoMPS) spanning the entire NS3 sequence of DENV2/3 NS3 or YF-17D, as described previously.19 Each peptide was resuspended in DMSO at a concentration of 30 mg/mL. The peptides for DENV were grouped into two pools each containing 74–77 peptides (NS3 DENV pool A [aa 1–313] and B [aa 303–619]) with a concentration of 0.35 mg/mL for each peptide. Peptides pools for YF-17D were prepared in the same way (NS3 YF-17D pool A [aa1–316] and B [aa 306–623]). Peptides pools were divided into 20 µL aliquots and stored at ≤ -70°C.
Three milliliters of whole blood was distributed into six 15 mL tubes and stimulated with one of the four NS3 peptide pools at a final concentration of 2µg/mL, or with medium with DMSO as negative control, and CytoStim (Miltenyi Biotec, ref. 130-092-173) as positive control. Brefeldin A (Sigma, Ref. B7671) was immediately added at 10 µg/mL. Tubes were incubated for 5 h at 37 °C, 5% CO2. After incubation, 5 mL of BD FACS Lysing buffer (BD Biosciences, ref. 349202) was added to lyse the red blood cells and to fix the stimulated cells. Samples were frozen at ≤ -70 °C and shipped on dry ice to Sanofi Pasteur France for analysis. Frozen samples were rapidly thawed in a water bath at 37 °C and homogenized by inverting the tubes several times. After two consecutive washings, 2 mL of BD FACS Permeabilizing Solution 2 (BD Biosciences, ref. 340973) was added and samples were incubated at room temperature for 10 min followed by the addition of 10 mL FACS buffer (PBS + 0.1% BSA). Cells were centrifuged at 600 g for 10 min at room temperature. Supernatants were discarded and cell pellets were resuspended in FACS buffer and transferred to 96-well plate. Cells were then stained with a customized lyophilized antibody cocktail from BD Biosciences containing anti-CD3-APC-H7 (SK7), anti-CD8-PerCPCy5 (SK1), anti-IFNγ-FITC (25723.11), anti-TNFα-PE (6404.1111), and anti-IL-2-APC (5344.111). Stimulated, permeabilized and lyophilized PBMCs (BD Biosciences) served as positive control throughout the study. Samples were acquired on a FACS Canto II. Data were analyzed using FACS DIVA 6.0 software (BD Biosciences).
Cell debris and small particles were excluded by gating out events with low forward scatter. A freehand gate was used to gate loosely around the lymphocytes (R1) in the FSC vs. SSC dot plot (according to size and granularity). A gate on CD4+ (CD8-) and CD8+ cells using a dot plot of CD8-PerCPCy5 vs. CD3-APC-H7 was drawn. CD8+ (CD3+CD8+) and CD4+ (CD3+CD8-) cells were gated on IFNγ, TNFα, and IL-2 positive cells using a dot blot of SSC vs. IFNγ-FITC, TNFα-PE, and IL-2-APC respectively. Boolean gating was applied to calculate the percentages of double-positive cytokine and triple positive cytokine CD3+CD8+ or CD3+CD8- (CD4+) secreting cells. For each in vitro re-stimulation (6 per sample: negative control, DENV NS3 pool A and B, YF-17D NS3 pool A and B, CytoStim), 14 results were obtained (7 different cytokine combinations in CD4+ and CD8+ lymphocytes). Values obtained under unstimulated conditions (medium + DMSO) were subtracted from each result. For analysis, the higher of the two responses against peptide pool A or B was retained for each sample, and results below the lower limit of quantification (LLOQ) of 0.01% were replaced with one-half the LLOQ.
Cytokine quantification in multiplex analysis
A Luminex kit (Millipore, Ref. MPXHCYTO-60K) was used to monitor the secretion of IFNγ, TNFα, IL-13, and IL-5 by peripheral blood mononuclear cells (PBMCs), in response to in-vitro (re)stimulation by each vaccine serotype. These cytokines are most likely secreted by serotype-specific CD4+ cells.19
PBMCs were isolated from 3 mL of whole blood diluted 1:2 with medium (RPMI 1640 + 2% Glutamine + antibiotics) and layered above 3 mL of LymphoprepTM (Axis-Shield, ref. 1114547) using 12 mL LeucosepTM tubes (Greiner bio-one, ref. 163290). After gradient centrifugation (800 g for 15 min without break) cells were harvested, washed twice, counted by acetic blue, and diluted to the appropriate concentration. PBMCs were added to 96-well plates at a concentration of 1 × 106 cells/mL and were incubated separately with each of the four serotypes of CYD vaccine virus at a multiplicity of infection (MOI) of 0.5 for 4 d at 37 °C. Phytohemagglutinin (PHA) (Remel, Ref. HA16/30852801) in combination with phorbol-myristate-13-acetate (PMA) (Sigma, Ref. P8139) was used as positive control at a final concentration of 1 µg/mL and 10 ng/mL. Medium with CY110 vaccine stabilizer was used as negative control. Supernatants were harvested and kept at ≤ -70 °C and were shipped to Sanofi Pasteur France for analysis.
Secreted cytokines were measured by Luminex xMAP technology (Millipore, Ref. MPXHCYTO-60K) according to the manufacturer's instructions. Supernatants were tested undiluted for virus stimulation and at a 1/10 dilution for PHA/PMA stimulation. Data were acquired with Bio-Plex Suspension Array System Luminex 100 and analyzed with Bio-Plex Manager 4.0 Security Edition software. Values obtained for unstimulated condition (medium + CY110) were subtracted from values obtained upon stimulation for each subject and visit. Values below the lower limit of quantification (LLOQ) (IFNγ: 4.3 pg/mL; TNFα: 5.3 pg/mL; IL-13: 5.7 pg/mL) were replaced by half of the lower limit of detection (LLOD) (IFNγ,TNFα and IL-13: 3.2 pg/mL).
Neutralizing antibodies
Plaque reduction neutralization test (PRNT50) was used to determine dengue serotype-specific antibody titers, as described elsewhere.23
Supplementary Material
Acknowledgments
The authors would like to thank all the volunteers who participated in the trial, and the study-site personnel in the pediatric and medical department at the National University Hospital in Singapore, and at Sanofi Pasteur Mandy Khoo for her valuable contributions to the study, Huiling Xong and Bill Shi for helping in the statistical analysis and preparation of the graphs for this manuscript, Patricia Londono-Hayes for comments and suggestions on the draft of this manuscript, Grenville Marsh for critical review and help in the preparation of this manuscript.
Potential Conflict of Interest
AH, SB, AM, SGF, TAW, AB, JL, D., CC, BG are employees of Sanofi Pasteur. AWS, SA, and LS were the study principal investigators. They received no direct payment from the study sponsor for their contributions.
Funding
This work was supported by Sanofi Pasteur
Footnotes
Previously published online: www.landesbioscience.com/journals/vaccines/article/25562
Reference List
- 1.Guzman MG, Halstead SB, Artsob H, Buchy P, Farrar J, Gubler DJ, et al. Dengue: a continuing global threat. Nat Rev Microbiol. 2010;8(Suppl):S7–16. doi: 10.1038/nrmicro2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilder-Smith A, Ooi EE, Vasudevan SG, Gubler DJ. Update on dengue: epidemiology, virus evolution, antiviral drugs, and vaccine development. Curr Infect Dis Rep. 2010;12:157–64. doi: 10.1007/s11908-010-0102-7. [DOI] [PubMed] [Google Scholar]
- 3.Adams B, Holmes EC, Zhang C, Mammen MP, Jr., Nimmannitya S, Kalayanarooj S, et al. Cross-protective immunity can account for the alternating epidemic pattern of dengue virus serotypes circulating in Bangkok. Proc Natl Acad Sci U S A. 2006;103:14234–9. doi: 10.1073/pnas.0602768103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rothman AL. Dengue: defining protective versus pathologic immunity. J Clin Invest. 2004;113:946–51. doi: 10.1172/JCI21512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Green S, Rothman A. Immunopathological mechanisms in dengue and dengue hemorrhagic fever. Curr Opin Infect Dis. 2006;19:429–36. doi: 10.1097/01.qco.0000244047.31135.fa. [DOI] [PubMed] [Google Scholar]
- 6.Guy B, Almond JW. Towards a dengue vaccine: progress to date and remaining challenges. Comp Immunol Microbiol Infect Dis. 2008;31:239–52. doi: 10.1016/j.cimid.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 7.Mathew A, Rothman AL. Understanding the contribution of cellular immunity to dengue disease pathogenesis. Immunol Rev. 2008;225:300–13. doi: 10.1111/j.1600-065X.2008.00678.x. [DOI] [PubMed] [Google Scholar]
- 8.Mangada MM, Rothman AL. Altered cytokine responses of dengue-specific CD4+ T cells to heterologous serotypes. J Immunol. 2005;175:2676–83. doi: 10.4049/jimmunol.175.4.2676. [DOI] [PubMed] [Google Scholar]
- 9.Blaney JE, Jr., Durbin AP, Murphy BR, Whitehead SS. Development of a live attenuated dengue virus vaccine using reverse genetics. Viral Immunol. 2006;19:10–32. doi: 10.1089/vim.2006.19.10. [DOI] [PubMed] [Google Scholar]
- 10.Guirakhoo F, Arroyo J, Pugachev KV, Miller C, Zhang ZX, Weltzin R, et al. Construction, safety, and immunogenicity in nonhuman primates of a chimeric yellow fever-dengue virus tetravalent vaccine. J Virol. 2001;75:7290–304. doi: 10.1128/JVI.75.16.7290-7304.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Durbin AP, Whitehead SS. Dengue vaccine candidates in development. Curr Top Microbiol Immunol. 2010;338:129–43. doi: 10.1007/978-3-642-02215-9_10. [DOI] [PubMed] [Google Scholar]
- 12.Whitehead SS, Blaney JE, Durbin AP, Murphy BR. Prospects for a dengue virus vaccine. Nat Rev Microbiol. 2007;5:518–28. doi: 10.1038/nrmicro1690. [DOI] [PubMed] [Google Scholar]
- 13.Capeding RZ, Luna IA, Bomasang E, Lupisan S, Lang J, Forrat R, et al. Live-attenuated, tetravalent dengue vaccine in children, adolescents and adults in a dengue endemic country: randomized controlled phase I trial in the Philippines. Vaccine. 2011;29:3863–72. doi: 10.1016/j.vaccine.2011.03.057. [DOI] [PubMed] [Google Scholar]
- 14.Poo J, Galan F, Forrat R, Zambrano B, Lang J, Dayan GH. Live-attenuated Tetravalent Dengue Vaccine in Dengue-naïve Children, Adolescents, and Adults in Mexico City: Randomized Controlled Phase 1 Trial of Safety and Immunogenicity. Pediatr Infect Dis J. 2010 doi: 10.1097/INF.0b013e3181fe05af. In Press. [DOI] [PubMed] [Google Scholar]
- 15.Qiao M, Shaw D, Forrat R, Wartel-Tram A, Lang J. Priming effect of dengue and yellow fever vaccination on the immunogenicity, infectivity, and safety of a tetravalent dengue vaccine in humans. Am J Trop Med Hyg. 2011;85:724–31. doi: 10.4269/ajtmh.2011.10-0436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morrison D, Legg TJ, Billings CW, Forrat R, Yoksan S, Lang J. A novel tetravalent dengue vaccine is well tolerated and immunogenic against all 4 serotypes in flavivirus-naive adults. J Infect Dis. 2010;201:370–7. doi: 10.1086/649916. [DOI] [PubMed] [Google Scholar]
- 17.Guy B, Barrere B, Malinowski C, Saville M, Teyssou R, Lang J. From research to phase III: preclinical, industrial and clinical development of the Sanofi Pasteur tetravalent dengue vaccine. Vaccine. 2011;29:7229–41. doi: 10.1016/j.vaccine.2011.06.094. [DOI] [PubMed] [Google Scholar]
- 18.Sabchareon A, Wallace D, Sirivichayakul C, Limkittikul K, Chanthavanich P, Suvannadabba S, et al. Protective efficacy of the recombinant, live-attenuated, CYD tetravalent dengue vaccine in Thai schoolchildren: a randomised, controlled phase 2b trial. Lancet. 2012;380:1559–67. doi: 10.1016/S0140-6736(12)61428-7. [DOI] [PubMed] [Google Scholar]
- 19.Guy B, Nougarede N, Begue S, Sanchez V, Souag N, Carre M, et al. Cell-mediated immunity induced by chimeric tetravalent dengue vaccine in naive or flavivirus-primed subjects. Vaccine. 2008;26:5712–21. doi: 10.1016/j.vaccine.2008.08.019. [DOI] [PubMed] [Google Scholar]
- 20.Ler TS, Ang LW, Yap GSL, Ng LC, Tai JC, James L, et al. Epidemiological characteristics of the 2005 and 2007 dengue epidemics in Singapore – similarities and distinctions. Western Pacific Surveillance and Response Journal. 2011;2:1–6. doi: 10.5365/wpsar.2010.1.1.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Afonso G, Scotto M, Renand A, Arvastsson J, Vassilieff D, Cilio CM, et al. Critical parameters in blood processing for T-cell assays: validation on ELISpot and tetramer platforms. J Immunol Methods. 2010;359:28–36. doi: 10.1016/j.jim.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 22.McKenna KC, Beatty KM, Vicetti Miguel R, Bilonick RA. Delayed processing of blood increases the frequency of activated CD11b+ CD15+ granulocytes which inhibit T cell function. J Immunol Methods. 2009;341:68–75. doi: 10.1016/j.jim.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 23.Leo YS, Wilder-Smith A, Archuleta S, Shek LP, Chong CY, Leong HN, et al. Immunogenicity and safety of recombinant tetravalent dengue vaccine (CYD-TDV) in individuals aged 2-45 y: Phase II randomized controlled trial in Singapore. Hum Vaccin Immunother. 2012;8:1259–71. doi: 10.4161/hv.21224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mangada MM, Endy TP, Nisalak A, Chunsuttiwat S, Vaughn DW, Libraty DH, et al. Dengue-specific T cell responses in peripheral blood mononuclear cells obtained prior to secondary dengue virus infections in Thai schoolchildren. J Infect Dis. 2002;185:1697–703. doi: 10.1086/340822. [DOI] [PubMed] [Google Scholar]
- 25.Zurawski G, de Vries JE. Interleukin 13, an interleukin 4-like cytokine that acts on monocytes and B cells, but not on T cells. Immunol Today. 1994;15:19–26. doi: 10.1016/0167-5699(94)90021-3. [DOI] [PubMed] [Google Scholar]
- 26.Minty A, Asselin S, Bensussan A, Shire D, Vita N, Vyakarnam A, et al. The related cytokines interleukin-13 and interleukin-4 are distinguished by differential production and differential effects on T lymphocytes. Eur Cytokine Netw. 1997;8:203–13. [PubMed] [Google Scholar]
- 27.Chomarat P, Banchereau J. Interleukin-4 and interleukin-13: their similarities and discrepancies. Int Rev Immunol. 1998;17:1–52. doi: 10.3109/08830189809084486. [DOI] [PubMed] [Google Scholar]
- 28.Bailer RT, Holloway A, Sun J, Margolick JB, Martin M, Kostman J, et al. IL-13 and IFN-gamma secretion by activated T cells in HIV-1 infection associated with viral suppression and a lack of disease progression. J Immunol. 1999;162:7534–42. [PubMed] [Google Scholar]
- 29.Hoffmann F, Albert MH, Arenz S, Bidlingmaier C, Berkowicz N, Sedlaczek S, et al. Intracellular T-cell cytokine levels are age-dependent in healthy children and adults. Eur Cytokine Netw. 2005;16:283–8. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.