Abstract
Two novel methods of dengue virus inactivation using iodonaphthyl azide (INA) and aminomethyl trioxsalen (AMT) were compared with traditional virus inactivation by formaldehyde. The AMT inactivated dengue-2 virus retained its binding to a panel of 5 monoclonal antibodies specific for dengue-2 envelope protein, whereas inactivation by formaldehyde and INA led to 30–50% decrease in binding. All three inactivated viruses elicited high level virus neutralizing antibodies in vaccinated mice. However, only mice vaccinated with AMT inactivated virus mounted T cell responses similar to live, uninactivated virus.
Keywords: dengue virus, virus inactivation, vaccine, immunogenicity, mouse
Introduction
Dengue viruses belong to the family Flaviviridae. Four antigenically distinct serotypes of dengue virus have similar clinical presentation, epidemiology, and distribution. An estimated 50 million dengue virus infections occur each year, spread across nearly 100 countries in the tropical and subtropical regions of the world.1,2 Infection with any of the four dengue virus serotypes can cause diseases ranging from mild febrile illness and classic dengue fever to the severe and potentially fatal forms of dengue hemorrhagic fever (DHF) and dengue shock syndrome (DSS).1 Natural infection with any of the dengue virus serotypes provides only long-term homotypic immunity, and available epidemiologic data suggest an increased risk for DHF/DSS during secondary infections with a heterologous serotype.3,4 Global expansion of dengue virus infections in recent decades has made the development of vaccines for dengue viruses a public health priority. Traditional vaccine approaches such as live attenuated viruses (LAV),5,6 inactivated viruses,7 and subunit vaccines,8 as well as novel approaches such as cloned, engineered attenuated viruses9 and chimeric viruses using yellow fever virus (YFV) backbone10 are being pursued. Many of these are at different stages of clinical evaluation; however, a licensed vaccine is not yet available.
To avoid the potential for increased risk of DHF/DSS due to postulated immune enhancement,11 a dengue virus vaccine should elicit protective immunity simultaneously to all four serotypes. Current approaches to a tetravalent dengue vaccine involve mixing four monovalent vaccines. Live, replicating vaccines based on this approach are prone to difficulties stemming from serotype competition and dominance. We are developing dengue vaccine candidates based on non-replicating platforms such as DNA,12-15 adenovirus vectors16,17 and inactivated virus vaccines. Virus neutralizing antibodies are believed to be of paramount importance in dengue immunity, and success of a dengue vaccine in pre-clinical studies is measured by the magnitude and duration of neutralizing antibody response and its ability to protect non-human primates from live virus challenge. Formaldehyde inactivated dengue virus vaccines have been reported earlier.7,18 Although these vaccines were highly immunogenic in animal models, the immune responses declined rapidly and the vaccinated animals had breakthrough viremia after live virus challenge.7 A majority of dengue virus neutralizing antibodies is believed to be against conformational epitopes on the virus envelope protein. Treatment with formaldehyde causes methylene cross links between amino groups on one hand, and amide, guanidyl, indole, phenol or imidazole groups on the other, leading mostly to intermolecular cross linking of proteins.19,20 This implies formation of hetero-oligomers if the protein being treated in not highly purified. Formaldehyde fixation destroyed denaturation transitions of bovine serum albumin, ribonuclease-A and hemoglobin although infrared spectra of native and fixed proteins were essentially identical indicating preservation of secondary structures.20 Nowacek21 has stated that fixation using formaldehyde results in conformational changes in the structure, and changes both chemical and antigenic profiles of proteins. Thus formaldehyde inactivation of dengue viruses could potentially have serious negative impact on its antigenicity/immunogenicity. Inactivation methods in which the surface antigenic proteins are largely unperturbed may yield better, inactivated dengue virus vaccines. Here we report results of two such methods of inactivation; photo-inactivation methods in presence of iodonaphthyl azide (INA) and a psoralen. INA is known to sequester exclusively to lipid bilayers of biological systems. Upon exposure to UV (UV) radiation, the azide moiety is converted to a reactive nitrene radical which interacts with the transmembrane domains of viral proteins.22 Surface antigenic structures are left largely intact. Psoralens are a class of coumarins which in presence of UV radiation, inactivate viruses by interacting with the viral nucleic acid23 and potentially preserving the integrity of surface antigen structures.
Results
Purification and inactivation of dengue-2 virus
Virus purification was followed by western blot analysis of preparations at various stages using dengue-2 envelope specific mouse monoclonal antibody 4G2 (Fig. 1). A typical preparation of partially purified dengue-2 virus yielded 5–6 mg/ml protein and 108-5x108 PFU (plaque forming units)/ml of virus.
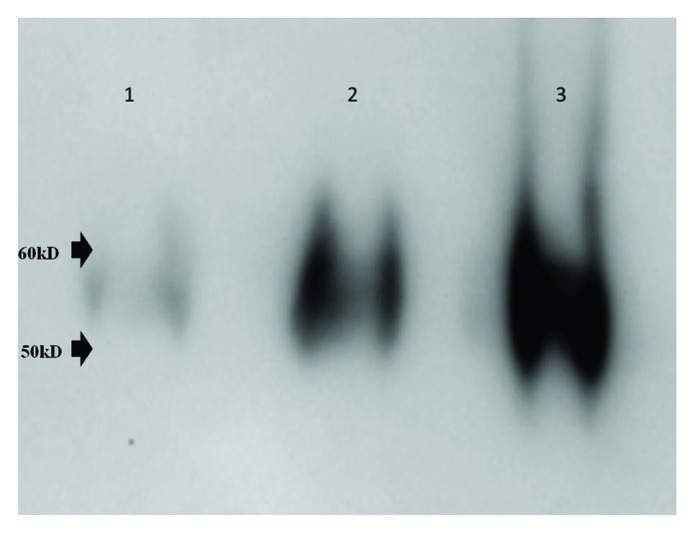
Figure 1. Western Blot analysis. Five ul aliquots each of crude infected cell culture supernatant (lane 1), Minimate tangential flow filtration retentate (lane 2) and fraction from sucrose density gradient (lane 3) were resolved by SDS-PAGE (4–20% gradient gel) and dengue E protein was enumerated by standard western blot analysis using monoclonal antibody 4G2
One hundred and 50 microtiter volumes of partially purified dengue-2 virus (5.4 mg/ml protein; 108 PFU/ml) were treated with formaldehyde, INA or AMT as described under Methods. 2-fold serial dilutions were used to (1) infect vero cell monolayers in 12-well tissue culture plate which were incubated for 7 d and cells examined for presence of viral antigen protein by indirect immunofluorescence assay, (2) determine plaque titer by a direct plaque assay. 10-fold serial dilutions of the un-inactivated control virus were tested similarly. Results (Table 1) showed that no plaques could be detected after inactivation with INA (3 min), AMT (5 min) or formaldehyde (5 d). Similarly, no infected vero cells were detected by immunofluorescence after infecting with INA-, AMT- or formaldehyde-inactivated dengue virus, whereas more than 60% of the cells (++++) were infected even with the highest dilutions of control virus.
Table 1. Inactivation of dengue-2 virus by formaldehyde, INA and AMT.
| Control Untreated Virus | INA (50uM) + UV (5 min.) | AMT (10mg/ml) + UV (3 min.) |
Formaldehyde (5 d) |
|
|---|---|---|---|---|
| Plaque Assay | 1.25 x 107 pfu/ml | No plaques detected | No plaques detected | No plaques detected |
| Immunofluorescence of infected vero cells. | ++++ at highest dilution tested |
Negative | Negative | Negative |
Control and treated virus preparations were tested by direct plaque assay and immunofluorescence (IFA) of vero cells infected by treated virus and incubated for 7 d. IFA slides were scored as ‘+’, ‘++’, ‘+++’ or ‘++++’ to indicate approximately < 10%, 10–30%, 30–60% and > 60% cells infected.
Virus inactivated by AMT retains its ability to bind monoclonal antibodies
To determine if any of the inactivation processes altered the antigenic properties of the virus, we investigated the ability of inactivated viruses to bind to a panel of five monoclonal antibodies specific to dengue-2 E protein. Identical quantities of control untreated and various inactivated viruses were used to coat duplicate wells of a microtiter ELISA plate, and were reacted with a panel of 5 different monoclonal antibodies. Results (Fig. 2) showed that compared with untreated virus, binding to monoclonal antibodies by formaldehyde- and INA-inactivated virus decreased by 30–60%. Such decreased binding was not evident when dengue-2 virus was inactivated by AMT; AMT-inactivated virus and untreated virus bound all 5 antibodies equally well. The experiment was performed two times with similar results.
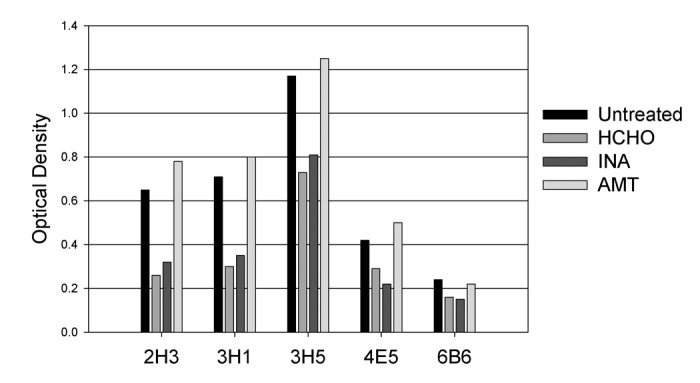
Figure 2. Binding of monoclonal antibodies 2H3, 3H1, 3H5, 4E5 and 6B6 to live dengue-2 virus (untreated) and virus inactivated by HCHO (formaldehyde), INA or AMT. Viruses were coated onto wells of a Microtiter ELISA plate and reacted with antibodies in a standard ELISA assay using an HRP-conjugated anti-mouse IgG. Data shown are representative of two independent determinations.
Immunogenicity of inactivated viruses in Balb/C mice
Sixteen groups of 8 mice each were immunized with 1 µg or 10 µg of untreated live dengue-2 virus, or virus inactivated by formaldehyde, INA or AMT, with or without alum. A control group was injected with PBS. In general, antibody responses (Fig. 3) in mice vaccinated with any of the three inactivated viruses with alum were significantly higher than in mice vaccinated with the same vaccine without alum (t-test; p < 0.05). Only other significant difference (ANOVA [p < 0.05] followed by Holm-Sidack post-hoc analysis) in antibody responses were on day 30, between formaldehyde inactivated virus on one hand and AMT or INA inactivated virus on the other. On day 30, which was four weeks after a single immunization, it was evident that all inactivated virus preparations elicited poor neutralizing antibody responses when injected without alum adjuvant, at both 1 µg and 10 µg dose levels (panels A and C). When administered with alum adjuvant, 1 µg dose of INA- and AMT-inactivated dengue-2 elicited responses similar to that elicited by 1 µg un-inactivated dengue-2 virus (panel B). Formaldehyde inactivated virus did not elicit detectable neutralizing antibody (GMT < 10). However, this significant difference was not apparent at the 10-µg dose level (with alum) where all inactivated viruses elicited responses similar to one another and to the un-inactivated virus control (panel D; GMT 424–563). Alum adjuvanted inactivated virus vaccines, on day 60 produced comparable high PRNT50 titers both at 1 µg and 10 µg dose levels (panel B and D). These high titers were maintained through day 150.
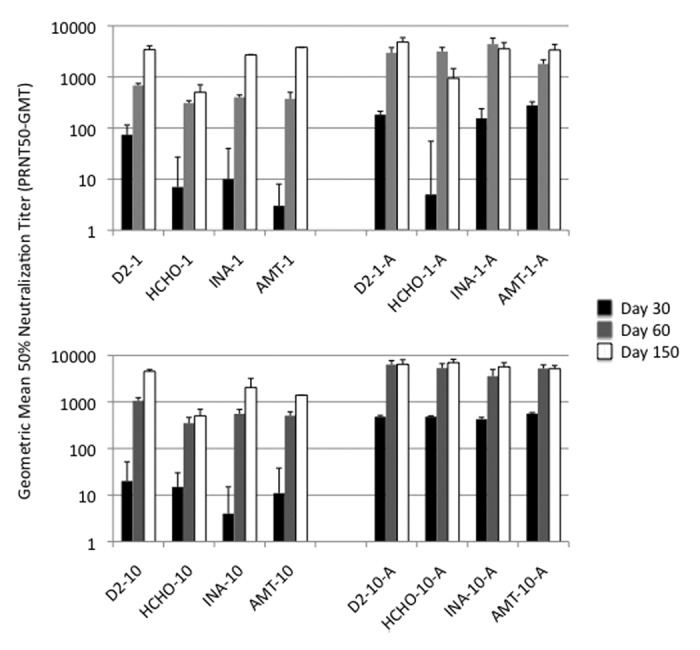
Figure 3. Immunogenicity of inactivated viruses: Neutralizing antibody response. Geometric mean titers (GMT) for groups of mice vaccinated with virus inactivated with formaldehyde (HCHO), INA or AMT, and control un-inactivated virus are shown. Numbers 1 and 10 refer to 1 and 10 ug dose levels and letter ‘A’ denotes alum adjuvant.
T cell responses to vaccination were measured by IFN-γ (interferon gamma) ELISPOT assay using splenocytes of vaccinated mice stimulated in vitro by peptide pools representing capsid (C), envelope (E) and non-structural protein NS-1 of dengue-2 virus. As with antibody response, T cell responses were greater when vaccines were administered with alum adjuvant (not shown). Highest T cell responses against both C and E peptide pools were noted in mice vaccinated with 10-ug un-inactivated control dengue-2 virus (Fig. 4). These responses were matched only by mice vaccinated with 10-ug AMT-inactivated virus. All other inactivated viruses elicited relatively lower responses. As expected, only mice vaccinated with live control dengue-2 virus mounted NS-1 protein specific T cell responses.
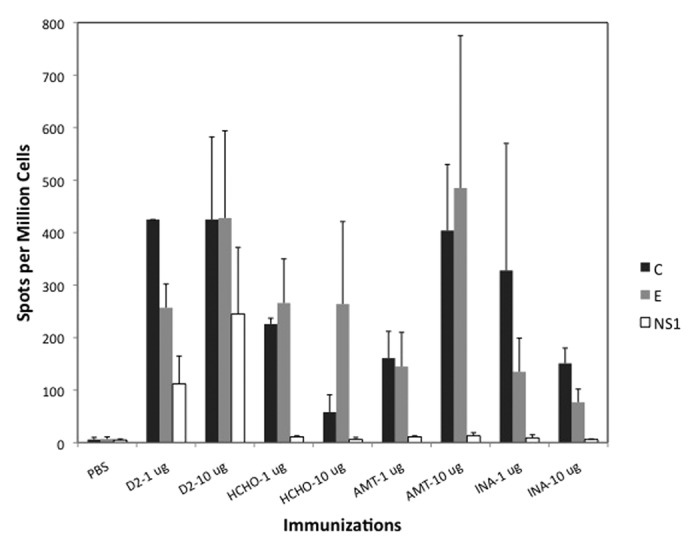
Figure 4. T cell responses measured by IFN-γ ELISPOT assay. Average Spots per Million Cells (3 mice per group) for each vaccine group (with alum) is shown. Cells were stimulated in vitro by peptide pools representing Capsid (C), pre-membrane (prM), Envelope (E) or non-structural protein 1 (NS1). prM-specific T cell response was not detected and is not shown.
Discussion
Development of a vaccine to prevent dengue infections has posed many a challenge. Requirement that the vaccine provide protection against all four dengue serotypes has meant developing four monovalent vaccines and mixing them to formulate a tetravalent vaccine. There have also been some efforts at creating a single shuffled recombinant DNA vaccine that expresses antigen, containing epitopes from all four serotypes.13 Current leading vaccine candidate, the CYD vaccine of Sanofi Pasteur comprised of four replicating virus chimeras has produced only modest immunogenicity and efficacy in phase 2 clinical trials.24 It is important to continue to develop and test simpler dengue vaccine candidates. The least complicated platform for viral vaccines is perhaps the use of inactivated whole viruses. Since the early development of the influenza vaccine in 1933 and polio virus vaccine in1955 by Jonas Salk by inactivating viruses using formaldehyde, this method of virus inactivation has been successfully used in developing hepatitis A virus vaccine,25 and vaccines for flaviviruses related to dengue such as tick-borne encephalitis virus26 and Japanese encephalitis virus.27 A purified formaldehyde-inactivated dengue virus vaccine has also been described in experimental animal models.7,18 Although formaldehyde has been historically used as the choice agent for virus inactivation, its applications have limitations. As noted earlier, formaldehyde treatment can lead to alterations in the molecular architecture of antigenic proteins, which can have serious negative impact for viruses such as dengue where many virus neutralizing epitopes are believed to be conformational. Inactivation of viruses by formaldehyde appear to be nonlinear, requiring extended inactivation periods at the cost of reduced antigenicity of the product.28 Methods for inactivation of dengue viruses require formaldehyde treatment for up to 5 d.29 Incomplete inactivation by formaldehyde of a polio virus vaccine, led to the “Cutter incident” in which several hundred children became infected with polio.30 A vaccine trial of formaldehyde inactivated respiratory syncytial virus (RSV), actually increased disease severity in vaccinated children.31 This is especially relevant to dengue vaccine, where antibody dependent enhancement of infection is a potential risk. In reviewing the “accidents” of inactivated virus vaccines, Brown et al.30 have opined that ‘it is remarkable that formaldehyde is still being used for the preparation of inactivated vaccines, particularly since it is known that the procedure also affects immunogenic epitopes of viruses.’
Alternate methods of virus inactivation that preserve the antigenic structure are being sought. In light of recent concerns regarding safety of attenuated yellow fever virus vaccine (strain 17DD), Gaspar et al.32 have attempted inactivating 17DD using high pressure. Virus inactivation was achieved by treatment at 310 MPa for 3 h. Although the inactivated virus protected mice as well as the live 17DD strain, it elicited significantly lower antibody response and the long-term efficacy of this inactivated vaccine is not known. Photo-inactivation in presence of certain chemical agents offers the potential to inactivate viruses without affecting their antigenicity. Photochemical inactivation of both DNA virus (herpes simplex) and RNA virus (western equine encephalitis) using psoralen derivatives was reported more than two decades ago.23 More recently, Maves et al.33,34 have reported immunogenicity of a psoralen inactivated denegue-1 virus in mice and non-human primates. Iodonaphthyl azide (INA) has also been used as an agent for photochemical inactivation of viruses.22,35,36 While psoralen derivatives target pyrimidines in the viral nucleic acid as the site of action, INA specifically targets the membrane portion of viral integral membrane proteins. This study compares immunogenicity of dengue-2 virus photo-inactivated using INA or AMT with virus chemically inactivated using formaldehyde. Virus inactivation was achieved using all three methods. However, inactivation with formaldehyde or INA resulted in reduced binding to monoclonal antibodies, suggesting that antigenicity may have been compromised. Many dengue virus neutralizing antibody epitopes are believed to target conformational epitopes and formation of methylene cross links during formaldehyde treatment can potentially destroy such structures. Exposure of INA to UV radiation results in production of free radicals which can potentially damage surface antigenic structures. It may still be possible to optimize conditions for dengue virus inactivation by INA by using proper free radical scavengers in the reaction. Lack of information on target epitopes of the monoclonal antibodies used, makes it difficult to speculate what regions of the envelope protein may be vulnerable to changes by treatment with formaldehyde and INA. This decreased binding to monoclonal antibodies is in contrast to HIV inactivation by INA, where the inactivated HIV reacted as well as the live virus with conformation sensitive monoclonal antibodies.22 It is not clear why INA-inactivated dengue-2 virus bound monoclonal antibodies less well compared with live virus. Intra-nasal immunization of mice with influenza virus similarly inactivated using INA induced antibodies and protected mice from live virus challenge. However, the antibodies induced were generally 3-fold lower compared with that induced by live virus.35 In contrast, we have shown that dengue-2 virus inactivated by AMT retained the ability to bind to all tested monoclonal antibodies. Although dengue virus type 1 inactivation and immunogenicity was reported previously33,34 a comparison with other methods of inactivation, including formaldehyde inactivation has not been previously made. Photo-inactivation of dengue-2 virus with AMT was more efficient compared with other methods; complete inactivation (below limits of detection by the methods employed) was achieved in only 3 min of UV exposure. This is in agreement with the observation that amotosalen (AMO, another psoralen) was significantly more effective compared with INA in the photoinactivation of vaccinia and pixuna viruses and resulted in only minimal impairment of subsequent ELISA testing.37 No significant sustained differences were noticed in antibody responses of mice vaccinated with alum adjuvanted live or inactivated virus. All vaccines elicited high titer neutralizing antibodies. Because only AMT inactivated dengue-2 retained its ability to bind monoclonal antibodies, it is possible that there is a difference in the quality of antibodies elicited by these vaccines, which is not apparent in the vero cell based virus neutralization assays. We also hypothesize that antibody responses are already at peak levels even at the lower dose used. On the other hand, only AMT inactivated virus (10 µg dose) was able to elicit T cell responses that matched those of live virus. We believe that this unusual observation has to do with how these different inactivated viruses are internalized and processed by the cells, and needs further investigation. Thus, because of the ability of AMT inactivated dengue-2 to trigger superior T cell responses, and no loss in its ability to bind monoclonal antibodies, AMT inactivation of dengue viruses may offer a better platform for production of inactivated dengue virus vaccines compared with current methods using formaldehyde. Preliminary studies have indicated that photo-inactivation by AMT is applicable to a variety of viruses such as yellow fever virus, Venezuelan equine encephalitis virus and influenza virus, and may be an effective platform for producing inactivated viral vaccines in general.
Materials and Methods
Cells, viruses and antibodies
Dengue virus type 2, strain OBS8041 was grown by infecting monolayers of vero cells. The culture supernatant containing virus was concentrated by passing through a tangential flow cell (Minimate, Pall Corp.). The concentrated virus was pelleted through a sucrose cushion (1.5 ml, 20%) by centrifugation at 221,000 x g for 2 h (Beckman SW41 rotor). Virus pellet was resuspended in 1 ml of PBS and further purified by centrifugation through a linear sucrose gradient (20–70%) for 90 min at 175,000 x g (Beckman SW41 rotor). The virus band was collected, aliquoted and stored at -80°C. A typical preparation yielded 5–6 mg/ml protein and 108-109 PFU (plaque forming units)/ml of virus. The following mouse monoclonal antibodies were used in the study: 4G2 (dengue E group specific), 2H2 (dengue prM group specific), and a panel of five antibodies specific for dengue serotype 2 E protein (2H3, 3H1, 3H5, 4E5 and 6B6).
Virus inactivation and binding to monoclonal antibodies
Partially purified dengue-2 virus was inactivated by formaldehyde according to Putnak et al.18 Briefly, virus stock was diluted 10-fold and incubated with 0.02% formaldehyde (pH 7.4) for 120 h at 22°C. Unreacted formaldehyde was neutralized with NaHCO3 (0.04% final concentration). Virus inactivation using INA (Biotium Inc.) was performed by exposing 150 µl volumes of virus preparation containing 50 µM INA and 2 mM glutathione to UV radiation (~365 nm, 5 min, ~145 mW/cm2). Inactivation by psoralen was achieved by exposing 50 µl volumes of virus preparation containing 10 µg/ml (35µM) aminomethyl-trioxsalen (Sigma-Aldrich) (AMT) to UV radiation (~365 nm, 2 min, ~145 mW/cm2). Inactivation of virus was confirmed by (1) incubating vero cells with inactivated virus for 7 d followed by immunofluorescence of cells for dengue antigens using antibodies specific to dengue E and/or prM protein (mouse monoclonal antibodies 4G2 and/or 2H2), (2) direct plaque assay of inactivated virus preparations. Monoclonal antibody binding to inactivated and control live virus was determined by standard enzyme linked immunosorbant assay (ELISA). Briefly, 2- fold serial dilutions of each virus preparation were used to coat wells of a microtiter plate to determine the quantity of virus that is needed to achieve a final absorbance of 0.25 to 1.5 when reacted with each of the monoclonal antibodies (1:100 dilution). ELISAs were then run in which duplicate wells of a microtiter plate coated with the pre-determined quantity of virus preparation was reacted with 1:100 dilution of each of the five monoclonal antibodies. Bound monoclonal antibodies were detected with peroxidase labeled anti-mouse IgG.
Immunogenicity of inactivated virus
The experiments reported herein were conducted in compliance with the Animal Welfare Act and in accordance with the principles set forth in Guide for the Care and Use of Laboratory Animals, Institute of Laboratory Animal Resources, National Research Council, National Academy Press, 1996. Various vaccine formulations were tested for immunogenicity in Balb/C mice (Charles River), 6–8 weeks of age. Groups of mice (n = 8) were vaccinated with PBS, live dengue-2 virus, dengue-2 virus inactivated by treatment with formaldehyde, INA or AMT. One µg or 10 µg (protein) doses with or without alum adjuvant were used for immunization. Animals were vaccinated by intra-dermal inoculation of 50-ul vaccine near the base of the tail on day 1 and day 30. Animals were bled on days 30, 60 and 150, and the serum preparations were tested for dengue-2 virus neutralizing antibody by plaque reduction neutralization test (PRNT) as described.38 Fifty percent virus neutralization titers (PRNT50) were determined by probit analysis (Minitab) and reported as geometric mean titers (GMT). On day 45, three mice from each group were euthanized and their spleens were harvested. The splenocytes were stimulated in vitro using overlapping peptide pools from dengue-2 Capsid, prM, E or NS1 protein, and used in a standard interferon gamma (IFN-γ) ELISPOT assay.39 The peptides were obtained from BEI-Resources (Biodefense and Emerging Infections Research Resource Repository); they comprised of 14, 21, 67 and 47 peptides (for C, prM, E and NS-1 proteins respectively) of 15–20 residues with a 10 amino acid residue overlap.
Acknowledgments
This work was funded by a research grant from the Office of Naval Research, NMRC work unit # A0311. This work was supported in whole or in part by the Frederick National Laboratory for Cancer Research, and the Intramural Research Program of the National Institutes of Health (contract HHSN26120080001E). We thank Dan Ewing, Karla Block, Liang Zhaodong and Susana Widjaja for excellent technical assistance. Authors Raviprakash, Martin and Kochel are employees of the United States government or military service members. This work was prepared as part of their official duties. Title 17 U.S.C. section 101 defines a US. Government work as a work prepared by a military service member or employee of the US. Government as part of that person’s official duties. The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, the Department of Health and Human Services, nor the United States Government.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/vaccines/article/25602
References
- 1.Halstead SB. Dengue. Lancet. 2007;370:1644–52. doi: 10.1016/S0140-6736(07)61687-0. [DOI] [PubMed] [Google Scholar]
- 2.Simmons CP, Farrar JJ, Nguyen V, Wills B. Dengue. N Engl J Med. 2012;366:1423–32. doi: 10.1056/NEJMra1110265. [DOI] [PubMed] [Google Scholar]
- 3.Kliks SC, Nimmanitya S, Nisalak A, Burke DS. Evidence that maternal dengue antibodies are important in the development of dengue hemorrhagic fever in infants. Am J Trop Med Hyg. 1988;38:411–9. doi: 10.4269/ajtmh.1988.38.411. [DOI] [PubMed] [Google Scholar]
- 4.Kliks SC, Nisalak A, Brandt WE, Wahl L, Burke DS. Antibody-dependent enhancement of dengue virus growth in human monocytes as a risk factor for dengue hemorrhagic fever. Am J Trop Med Hyg. 1989;40:444–51. doi: 10.4269/ajtmh.1989.40.444. [DOI] [PubMed] [Google Scholar]
- 5.Sun W, Cunningham D, Wasserman SS, Perry J, Putnak JR, Eckels KH, et al. Phase 2 clinical trial of three formulations of tetravalent live-attenuated dengue vaccine in flavivirus-naïve adults. Hum Vaccin. 2009;5:33–40. doi: 10.4161/hv.5.1.6348. [DOI] [PubMed] [Google Scholar]
- 6.Edelman R, Wasserman SS, Bodison SA, Putnak RJ, Eckels KH, Tang D, et al. Phase I trial of 16 formulations of a tetravalent live-attenuated dengue vaccine. Am J Trop Med Hyg. 2003;69(Suppl):48–60. doi: 10.4269/ajtmh.2003.69.48. [DOI] [PubMed] [Google Scholar]
- 7.Robert Putnak J, Coller BA, Voss G, Vaughn DW, Clements D, Peters I, et al. An evaluation of dengue type-2 inactivated, recombinant subunit, and live-attenuated vaccine candidates in the rhesus macaque model. Vaccine. 2005;23:4442–52. doi: 10.1016/j.vaccine.2005.03.042. [DOI] [PubMed] [Google Scholar]
- 8.Coller BA, Clements DE, Bett AJ, Sagar SL, Ter Meulen JH. The development of recombinant subunit envelope-based vaccines to protect against dengue virus induced disease. Vaccine. 2011;29:7267–75. doi: 10.1016/j.vaccine.2011.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Durbin AP, Whitehead SS. Next-generation dengue vaccines: novel strategies currently under development. Viruses. 2011;3:1800–14. doi: 10.3390/v3101800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guy B, Barrere B, Malinowski C, Saville M, Teyssou R, Lang J. From research to phase III: preclinical, industrial and clinical development of the Sanofi Pasteur tetravalent dengue vaccine. Vaccine. 2011;29:7229–41. doi: 10.1016/j.vaccine.2011.06.094. [DOI] [PubMed] [Google Scholar]
- 11.Kliks S. Antibody-enhanced infection of monocytes as the pathogenetic mechanism for severe dengue illness. AIDS Res Hum Retroviruses. 1990;6:993–8. doi: 10.1089/aid.1990.6.993. [DOI] [PubMed] [Google Scholar]
- 12.Beckett CG, Tjaden J, Burgess T, Danko JR, Tamminga C, Simmons M, et al. Evaluation of a prototype dengue-1 DNA vaccine in a Phase 1 clinical trial. Vaccine. 2011;29:960–8. doi: 10.1016/j.vaccine.2010.11.050. [DOI] [PubMed] [Google Scholar]
- 13.Raviprakash K, Apt D, Brinkman A, Skinner C, Yang S, Dawes G, et al. A chimeric tetravalent dengue DNA vaccine elicits neutralizing antibody to all four virus serotypes in rhesus macaques. Virology. 2006;353:166–73. doi: 10.1016/j.virol.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 14.Raviprakash K, Kochel TJ, Ewing D, Simmons M, Phillips I, Hayes CG, et al. Immunogenicity of dengue virus type 1 DNA vaccines expressing truncated and full length envelope protein. Vaccine. 2000;18:2426–34. doi: 10.1016/S0264-410X(99)00570-8. [DOI] [PubMed] [Google Scholar]
- 15.Raviprakash K, Luke T, Doukas J, Danko J, Porter K, Burgess T, et al. A dengue DNA vaccine formulated with Vaxfectin (®) is well tolerated, and elicits strong neutralizing antibody responses to all four dengue serotypes in New Zealand white rabbits. Hum Vaccin Immunother. 2012;8:1764–8. doi: 10.4161/hv.21806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holman DH, Wang D, Raviprakash K, Raja NU, Luo M, Zhang J, et al. Two complex, adenovirus-based vaccines that together induce immune responses to all four dengue virus serotypes. Clin Vaccine Immunol. 2007;14:182–9. doi: 10.1128/CVI.00330-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raviprakash K, Wang D, Ewing D, Holman DH, Block K, Woraratanadharm J, et al. A tetravalent dengue vaccine based on a complex adenovirus vector provides significant protection in rhesus monkeys against all four serotypes of dengue virus. J Virol. 2008;82:6927–34. doi: 10.1128/JVI.02724-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Putnak R, Barvir DA, Burrous JM, Dubois DR, D’Andrea VM, Hoke CH, et al. Development of a purified, inactivated, dengue-2 virus vaccine prototype in Vero cells: immunogenicity and protection in mice and rhesus monkeys. J Infect Dis. 1996;174:1176–84. doi: 10.1093/infdis/174.6.1176. [DOI] [PubMed] [Google Scholar]
- 19.Fraenkel-Conrat H, Mecham DK. The reaction of formaldehyde with proteins; demonstration of intermolecular cross-linking by means of osmotic pressure measurements. J Biol Chem. 1949;177:477–86. [PubMed] [Google Scholar]
- 20.Mason JT, O’Leary TJ. Effects of formaldehyde fixation on protein secondary structure: a calorimetric and infrared spectroscopic investigation. J Histochem Cytochem. 1991;39:225–9. doi: 10.1177/39.2.1987266. [DOI] [PubMed] [Google Scholar]
- 21.Nowace, JM. Fixation and Tissue Processing. 2010;141-152
- 22.Raviv Y, Viard M, Bess JW, Jr., Chertova E, Blumenthal R. Inactivation of retroviruses with preservation of structural integrity by targeting the hydrophobic domain of the viral envelope. J Virol. 2005;79:12394–400. doi: 10.1128/JVI.79.19.12394-12400.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanson CV, Riggs JL, Lennette EH. Photochemical inactivation of DNA and RNA viruses by psoralen derivatives. J Gen Virol. 1978;40:345–58. doi: 10.1099/0022-1317-40-2-345. [DOI] [PubMed] [Google Scholar]
- 24.Sabchareon A, Wallace D, Sirivichayakul C, Limkittikul K, Chanthavanich P, Suvannadabba S, et al. Protective efficacy of the recombinant, live-attenuated, CYD tetravalent dengue vaccine in Thai schoolchildren: a randomised, controlled phase 2b trial. Lancet. 2012;380:1559–67. doi: 10.1016/S0140-6736(12)61428-7. [DOI] [PubMed] [Google Scholar]
- 25.Hilleman MR. Hepatitis and hepatitis A vaccine: a glimpse of history. J Hepatol. 1993;18(Suppl 2):S5–10. doi: 10.1016/S0168-8278(05)80370-8. [DOI] [PubMed] [Google Scholar]
- 26.Barrett PN, Dorner F, Plotkin SA. Tick-Borne Encephalitis Vaccine. Vaccines, 3rd Ed. Editors Plotkin SA and Orenstein WA, 767-780 (1999). [Google Scholar]
- 27.Tsai TF. C. G. a. Y. Y. Japanese encephalitis vaccines. Vaccines, 3rd Ed. Editors Plotkin SA and Orenstein WA, 672-710 (1999). [Google Scholar]
- 28.Murdin AD, Inactivated Virus Vaccines RBSS. New Vaccine Technologies, Ed: Ellis RW., 133-150 (2001). [Google Scholar]
- 29.Putnak R, Cassidy K, Conforti N, Lee R, Sollazzo D, Truong T, et al. Immunogenic and protective response in mice immunized with a purified, inactivated, Dengue-2 virus vaccine prototype made in fetal rhesus lung cells. Am J Trop Med Hyg. 1996;55:504–10. doi: 10.4269/ajtmh.1996.55.504. [DOI] [PubMed] [Google Scholar]
- 30.Brown F. Review of accidents caused by incomplete inactivation of viruses. Dev Biol Stand. 1993;81:103–7. [PubMed] [Google Scholar]
- 31.Kim HW, Canchola JG, Brandt CD, Pyles G, Chanock RM, Jensen K, et al. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol. 1969;89:422–34. doi: 10.1093/oxfordjournals.aje.a120955. [DOI] [PubMed] [Google Scholar]
- 32.Gaspar LP, Mendes YS, Yamamura AM, Almeida LF, Caride E, Gonçalves RB, et al. Pressure-inactivated yellow fever 17DD virus: implications for vaccine development. J Virol Methods. 2008;150:57–62. doi: 10.1016/j.jviromet.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 33.Maves RC, Castillo Oré RM, Porter KR, Kochel TJ. Immunogenicity of a psoralen-inactivated dengue virus type 1 vaccine candidate in mice. Clin Vaccine Immunol. 2010;17:304–6. doi: 10.1128/CVI.00353-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maves RC, Oré RM, Porter KR, Kochel TJ. Immunogenicity and protective efficacy of a psoralen-inactivated dengue-1 virus vaccine candidate in Aotus nancymaae monkeys. Vaccine. 2011;29:2691–6. doi: 10.1016/j.vaccine.2011.01.077. [DOI] [PubMed] [Google Scholar]
- 35.Raviv Y, Blumenthal R, Tompkins SM, Humberd J, Hogan RJ, Viard M. Hydrophobic inactivation of influenza viruses confers preservation of viral structure with enhanced immunogenicity. J Virol. 2008;82:4612–9. doi: 10.1128/JVI.02233-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sharma A, Gupta P, Glass PJ, Parker MD, Maheshwari RK. Safety and protective efficacy of INA-inactivated Venezuelan equine encephalitis virus: implication in vaccine development. Vaccine. 2011;29:953–9. doi: 10.1016/j.vaccine.2010.11.033. [DOI] [PubMed] [Google Scholar]
- 37.Sagripanti JL, Marschall HJ, Voss L, Hülseweh B. Photochemical inactivation of alpha- and poxviruses. Photochem Photobiol. 2011;87:1369–78. doi: 10.1111/j.1751-1097.2011.00998.x. [DOI] [PubMed] [Google Scholar]
- 38.Raviprakash K, Porter KR, Kochel TJ, Ewing D, Simmons M, Phillips I, et al. Dengue virus type 1 DNA vaccine induces protective immune responses in rhesus macaques. J Gen Virol. 2000;81:1659–67. doi: 10.1099/0022-1317-81-7-1659. [DOI] [PubMed] [Google Scholar]
- 39.Chen L, Ewing D, Subramanian H, Block K, Rayner J, Alterson KD, et al. A heterologous DNA prime-Venezuelan equine encephalitis virus replicon particle boost dengue vaccine regimen affords complete protection from virus challenge in cynomolgus macaques. J Virol. 2007;81:11634–9. doi: 10.1128/JVI.00996-07. [DOI] [PMC free article] [PubMed] [Google Scholar]


