Abstract
Dominant negative inhibitor (DNI) is a translocation-deficient homolog of recombinant protective antigen of Bacillus anthracis that is a candidate for a next generation anthrax vaccine. This study demonstrates that the biophysical characteristics of the DNI protein stored in lyophilized form at 4 °C for 8 y were similar to recombinant protective antigen (rPA). To provide information on the accelerated stability of DNI, samples in the lyophilized form were subjected to thermal stress (40 and 70 °C for up to 4 weeks) and thoroughly evaluated using various biophysical and chemical characterization techniques. Results demonstrate preserved structural stability of the DNI protein under extreme conditions, suggesting long-term stability can be achieved for a vaccine that employs DNI, as desired for a biodefense countermeasure. Furthermore, the biological activity of the stressed DNI bound to the adjuvant Alhydrogel® was evaluated in mice and it was found that the immunogenicity DNI was not affected by thermal stress.
Keywords: dominant-negative inhibitor, vaccine, aluminum hydroxide, immunogenicity, accelerated stability, biophysical characterization
Introduction
Spores of the Gram-positive bacterium Bacillus anthracis can initiate lethal anthrax disease in mammalian hosts through cutaneous, inhalation and gastrointestinal exposure.1 The inhaled form of anthrax is the most severe in humans and the mortality in untreated cases is almost 100%.2 Concerns about the potential use of the inhaled form of anthrax as a biological weapon have led to major efforts to develop effective anthrax countermeasures that include programs for mass immunization with vaccines that can be used pre- or post-exposure.3 After germination of spores, vegetative Bacillus anthracis secretes anthrax toxin, a binary AB toxin that includes lethal factor (LF), edema factor (EF) and protective antigen (PA) that is largely responsible for the major pathology of anthrax and contributes to rapid mortality in disseminated disease. Binding of PA to specific receptors in the host results in subsequent activation of PA by furin into two fragments: N-terminal PA20 (20 kDa) and carboxy terminal PA63 (63 kDa), the latter of which assembles into a heptameric or octameric pre-pore structure that binds and translocates edema factor (EF) or lethal factor (LF). Because PA is the sole immunogenic component of the anthrax toxin and can interfere with toxin binding to receptors, recombinant protective antigen (rPA) is the focus of next generation anthrax vaccines currently under development.2,4,5 rPA vaccines differ in several respects from the only currently licensed vaccine in the United States, anthrax vaccine adsorbed (AVA/BioThrax®). Recombinant PA vaccines contain no lethal factor (LF) or edema factor (EF) which are present in small quantities in the culture filtrates used to manufacture AVA. They also contain no other Bacillus anthracis-related contaminating proteins or cross-linking agents which are used to detoxify the toxin in Biothrax®. Such purified recombinant protein vaccines would be expected to have fewer side effects than those associated with use of AVA.2,5 AVA has a limited duration of protection and requires five immunizations and yearly boosts.5 Although AVA has an extended shelf life of at least 4 y, recombinant PA-based vaccines lose potency rapidly in formulations in which rPA is bound to aluminum adjuvants.6 Loss of potency is correlated with chemical changes (e.g., deamination) that can occur in rPA or are associated with other changes resulting in loss of specific epitopes involved in the generation of protective immunity.
Several analogs of rPA have been developed as potential post exposure therapeutic molecules. One such homolog, dominant negative inhibitor (DNI), is a translocation-deficient mutant of rPA with the two mutations K397D and D425K that result in a molecule that does not interfere with heptamer formation or receptor binding.7,8 One molecule of DNI can associate with native cleaved PA63, forms higher order oligomers, and prevents translocation of EF or LF and hence toxin action.7,9 DNI has also been demonstrated to be a potent immunogen that is hyperimmunigenic in comparison to rPA in aluminum adjuvanted vaccine formulations in mice. The use of DNI as a replacement for rPA in next generation anthrax vaccines is therefore possible. DNI has also been shown to be effective against anthrax after spore exposure.9,10
Vaccines to protect against bioweapons such as anthrax require very long-term stability in the event of significant periods of storage before potential use. Consequently, we examined the potential for lyophilized DNI to be used as a vaccine after long-term storage and at extremes of temperature with the intent to overcome potency losses observed in rPA vaccines in aqueous formulations. In the present study, the physical and chemical stability of the DNI protein was evaluated after a storage period of 8 y at 4 °C in the lyophilized form and compared with a recombinant protective antigen (rPA) standard. In addition, DNI samples stressed by storage at 40 and 70 °C for up to 4 weeks in a lyophilized form were characterized and compared with unstressed DNI.
Results and Discussion
Biophysical characterization of DNI vs. wild type (WT) rPA
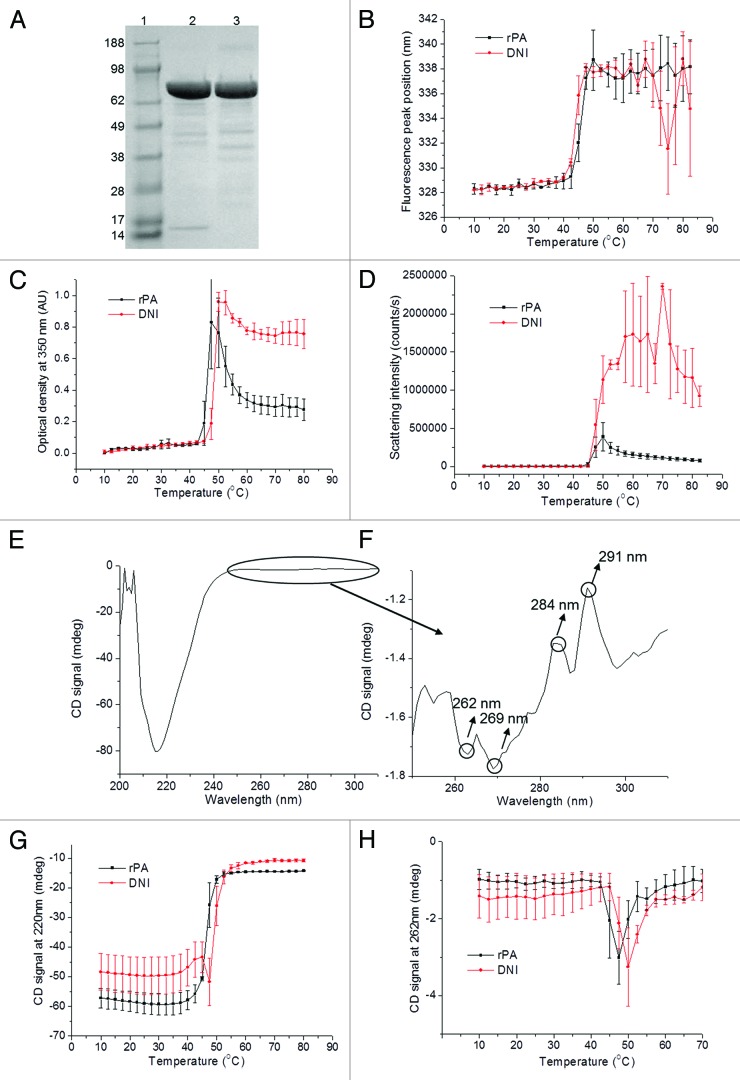
Long-term stability is a highly desirable property for anthrax vaccines due to the chances of extended periods of storage before their potential use. To assess the long-term stability of DNI as well as to further validate its use as an alternative to rPA for a candidate vaccine against anthrax, a comparison between DNI after 8 y of storage at 4 °C in the lyophilized form and a native rPA standard was performed by various methods. As seen by SDS-PAGE, the main species for both proteins appears between the 62 and 98 kDa bands of molecular weight standards and corresponds to the molecular weights of DNI and rPA (83 kDa) (Fig. 1A). Both proteins show minor degradation bands between 38 and 62 kDa, which are in much lower concentration than the main band. An additional minor degradation band at about 15 kDa seen for DNI but not rPA suggests the presence of a degradation product or an impurity. It is, however, in very low concentration compared with the native protein. The tertiary structure of rPA and DNI as a function of temperature was studied using intrinsic fluorescence. The initial peak position is about 328 nm at low temperature and starts shifting at about 40 °C for both proteins (Fig. 1B), suggesting equivalent structural stability. The values are typical of the melting temperatures seen for anthrax PA at similar pH values described in other studies.12,16 Optical density (OD) data at 350 nm was used as a second method to study protein aggregation (Fig. 1C). Here, the Tonset value for DNI was about 2.5 °C lower than for rPA. The large error bars at 45 °C and the light scattering data described above, however, suggest that this difference is not significant. Increases in scattering intensity with similar temperature onsets (Tonset) at about 45 °C implies similar aggregation behavior of the aged DNI and fresh WT rPA (Fig. 1D). The CD in the far-UV region (200–250 nm) of DNI displays a broad peak with a minimum around 218 nm indicating a secondary structure rich in β sheets. In the near-UV region (250–320 nm), the four characteristic peaks at 262, 269, 284, and 291 nm arise from aromatic amino acid residues (Fig. 1E and F). The rPA standard obtained from List Biological Laboratories showed similar near- and far-UV CD spectra (data not shown). Changes in the far-UV CD signal at 220 nm and the near-UV signal at 262 nm were observed with increasing temperature (Fig. 1G and H) and show an overall loss in both secondary and tertiary structures. The other near-UV CD signals at 269, 284, and 291 nm produced equivalent results to the 262 nm signal of DNI and are thus not shown. In the far-UV CD region, the Tonset of thermal transitions occurs at about 40 °C for both rPA and DNI, suggesting equivalent stability of the secondary structures. In the near-UV CD region, the Tonset occurs at about 42.5 °C for rPA, which is 2.5 °C lower than that observed for the DNI protein but again this difference is not significant given the large error bar at 45 °C.
Figure 1. rPA vs DNI characterized by SDS-PAGE, standard in lane 1, DNI in lane 2, rPA in lane 3 (A), intrinsic fluorescence (B), OD-350 nm (C) and static light scattering (D). Far- and near- UV CD spectra of initial DNI at 10 °C (E and F), CD signal at 220 nm (G) and 262 nm (H) vs. temperature.
Biophysical characterization of stressed DNI
The efficacy of a vaccine can be affected by various forms of protein degradation during long-term storage. In an effort to determine the effects of storage on a potential DNI vaccine in a lyophilized form, accelerated studies were performed on the 8-y old DNI samples by employing thermal stress at 40 and 70 °C for up to 4 weeks and compared with unstressed vaccine. SDS-PAGE shows no significant difference when DNI was exposed to 40 °C for up to 4 weeks (Fig. 2A, lanes 1 to 4). In all DNI samples stressed at 70 °C, however, a high molecular weight species between the 188 and 98 kDa bands is detected, suggesting the formation of a covalently linked dimer, which increases slightly when stressed for longer periods of time (Fig. 2A, lanes 5 to 7). Two peaks were observed by SEC-HPLC at retention times of 10.4 and 11.7 min (Fig. 2B). The larger peak (peak 2) corresponds to the monomeric form of the protein while the smaller peak (peak 1) to a dimer based on comparisons to a series of protein standards. There seems to be no differences between the unstressed samples and the results seen after four weeks at 40 °C. For the sample stressed for four weeks at 70 °C, the dimer peak area increases from 4.9% to 9.1% while the monomer peak area decreases from 95.1% to 90.9% (values shown in Table 1), confirming the formation of dimers in DNI at high temperature. Nevertheless, the majority of the sample (90.9%) maintained its monomeric state.
Figure 2. DNI under various stressed vs non-stressed conditions characterized by SDS-PAGE (A): Lane 1: unstressed DNI; lanes 2, 3, and 4: DNI protein stressed at 40 °C for 1, 2, and 4 weeks respectively; lanes 5, 6, and 7, DNI protein stressed at 70 °C for 1, 2, and 4 weeks respectively, by SEC-HPLC (B): black curve: unstressed DNI, red curve: DNI at 40 °C for 4 weeks, blue curve: DNI at 70 °C for 4 weeks. Duplicate measurements showed similar patterns.
Table 1. Quantification of the SEC peak 1 and peak 2 areas for DNI samples stressed at 40 and 70 °C from one to four weeks.
| 40 °C | Time 0 | Time 1 week 40 °C | Time 2 weeks 40 °C | Time 4 weeks 40 °C |
|---|---|---|---|---|
| Peak 1 | 4.9 ± 0.13% | 5.0 ± 0.03% | 5.2 ± 0.06% | 5.4 ± 0.05% |
| Peak 2 | 95.1 ± 0.13% | 95.0 ± 0.03% | 94.8 ± 0.06% | 94.6 ± 0.05% |
| 70 °C | Time 0 | Time 1 week 70 °C | Time 2 weeks 70 °C | Time 4 weeks 70 °C |
| Peak 1 | 4.9 ± 0.13% | 6.8 ± 0.06% | 7.5 ± 0.03% | 9.1 ± 0.04% |
| Peak 2 | 95.1 ± 0.13% | 93.2 ± 0.06% | 92.5 ± 0.03% | 90.9 ± 0.04% |
Triplicate measurements were made
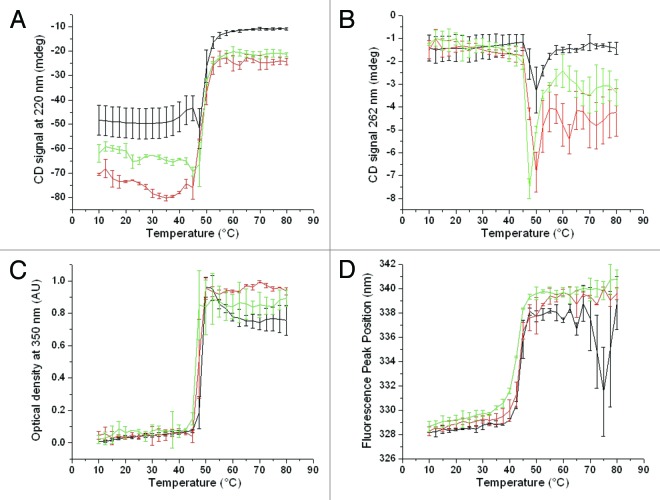
The secondary and tertiary structure of stressed DNI samples was compared by near- and far-UV CD. In the far-UV CD region (signals at 220 nm), the thermal transitions occur at about 40 °C for unstressed DNI (Fig. 3A). After 4 weeks at 40 and 70 °C, the transitions occurred slightly earlier at about 37.5 and 32.5 °C, respectively, suggesting some structural alteration in terms of secondary structure under stressed conditions. In the near-UV region, a drop in the CD signal at 262 nm is observed for all samples at the similar temperature of about 45°C (Fig. 3B), showing no major difference between unstressed and stressed samples. The formation of insoluble aggregates was evaluated by the optical density at 350 nm and showed the initiation of aggregation at about 45 °C for all DNI samples (Fig. 3C), indicating equivalent aggregation behavior. Similar trends were observed by static light scattering (data not shown). Changes in the tertiary structure of DNI were also monitored by intrinsic fluorescence spectroscopy. A slight decrease in Tonset of about 2.5 °C is observed after 4 weeks for the stressed samples (Fig. 3D).
Figure 3. Changes in near- and far-UV CD signals as a function of temperature at 220 nm (A) and 262 nm (B), optical density at 350 nm (C) and intrinsic fluorescence peak position shifts (D) for unstressed DNI samples (black curves) and DNI samples stressed for 4 weeks at 40 °C (red curve) and 70 °C (green curve). Error bars reflect standard deviations from two separate runs (three runs for CD measurements).
As another independent measure of the chemical stability of the DNI, it was incubated with the von-Willebrand factor A domain of the host cellular receptor capillary morphogenesis 2 (CMG2). This protein is known to bind tightly to PA (KD ~200 pM)17 and gel-shift analysis using Native PAGE indicates that a 1:1 stoichiometry leads to a complete shift in the band that corresponds to PA to a higher MW.13 Binding is in part due to the presence of an aspartic acid in PA (D683) in domain 4 which helps coordinate a magnesium ion on the surface of CMG2.18 Also required for high affinity binding is a loop that is present in domain 2, the 2β3–2β4 loop, that sits in a groove on the surface of CMG2.19 Therefore, the ability to bind CMG2 is in large part dictated by correct native the structure of PA. Dry, lyophilized DNI was incubated for up to 3 weeks at 4, 40, and 70 °C, and after each week the ability to bind CMG2 was assessed by Native PAGE. Figure 4 shows that even after three weeks at 70 °C (panel B), and despite the presence of minor degradation, the majority of the protein present is able to bind to CMG2.
Figure 4. Native PAGE analysis of DNI thermal stability, as measured by binding to CMG2 (1:1 stoichiometry). Analysis after 1 week of incubation of dried material (A) and after 3 weeks (B) is shown. Despite some decomposition of the dried material at 70 °C at three weeks, notably the presence of smaller bands, the majority of the protein maintains the ability to form a stable complex with CMG2.
Various studies have revealed the occurrence of significant chemical alteration by deamidation in protective antigen and its mutants.20-22 The chemical stability of DNI was evaluated using capillary isoelectric focusing (cIEF). The presence of urea (6 M) was necessary to obtain peaks with acceptable resolution. For the unstressed DNI sample, a total of 5 peaks were identified: the major peak at a pI value of 6.19 and four other smaller peaks at 6.00, 6.05, 6.12, and 6.27 (Fig. 5). This measured pI of DNI is slightly higher than the pI calculated from its sequence (5.64) and the presence of smaller peaks suggests that the starting material is a heterogeneous mixture of isoforms. This was also observed previously in different rPA starting materials,20 due to the susceptibility of the protein to deamidation during purification and storage and the presence of 68 asparagine residues in its sequence. The cIEF profile of DNI stressed at 40 °C for 4 weeks was not notably different than that of the unstressed sample, indicating little deamidation under these conditions. After 4 weeks at 70 °C, however, the largest peak (pI = 6.19) decreased while the 4 other peaks increased, providing clear evidence of deamidation. Using quantification of the peak areas (Table 2), the major isoform decreased from 59.6% to 40.2% while the 4 other isoforms increase their area percentages for the samples stressed at 70 °C.
Figure 5. DNI under various stressed vs non-stressed conditions characterized by cIEF; electropherograms of unstressed DNI (blue line), DNI stressed at 40 °C for 4 weeks (green line) and DNI at 70 °C for 4 weeks (brown line). Duplicate measurements showed similar patterns.
Table 2. Quantification by cIEF of the isoform peak areas of the unstressed and stressed samples at 40 and 70 °C.
| DNI Stressed at 40 °C | ||||
|---|---|---|---|---|
| Time 0 | 1 week | 2 weeks | 4 weeks | |
| Peak 1 | 1.42 ± 0.16% | 1.48 ± 0.13% | 1.55 ± 0.32% | 1.85 ± 0.15% |
| Peak 2 | 7.22 ± 0.16% | 7.46 ± 0.10% | 7.79 ± 0.20% | 8.24 ± 0.27% |
| Peak 3 | 27.10 ± 0.21% | 27.83 ± 0.38% | 28.38 ± 0.05% | 29.15 ± 0.29% |
| Peak 4 | 59.62 ± 0.18% | 58.62 ± 0.73% | 57.35 ± 0.52% | 55.93 ± 0.60% |
| Peak 5 | 4.64 ± 0.03% | 4.61 ± 0.11% | 4.93 ± 0.05% | 4.81 ± 0.11% |
| DNI Stressed at 70 °C | ||||
|---|---|---|---|---|
| Time 0 | 1 week | 2 weeks | 4 weeks | |
| Peak 1 | 1.42 ± 0.16% | 2.69 ± 0.01% | 3.47 ± 0.15% | 4.15 ± 0.31% |
| Peak 2 | 7.22 ± 0.16% | 10.75 ± 0.21% | 12.63 ± 0.02% | 14.79 ± 0.32% |
| Peak 3 | 27.10 ± 0.21% | 31.00 ± 0.29% | 32.13 ± 0.30% | 32.67 ± 0.18% |
| Peak 4 | 59.62 ± 0.18% | 48.90 ± 0.37% | 44.33 ± 0.42% | 40.24 ± 0.58% |
| Peak 5 | 4.64 ± 0.03% | 6.66 ± 0.14% | 7.43 ± 0.04% | 8.15 ± 0.58% |
Even though only slight conformational changes were observed by CD and fluorescence spectroscopy, chemical analysis revealed increased deamidation of DNI stressed at 70 °C while SDS-PAGE and SEC-HPLC revealed the susceptibility of DNI to form higher molecular weight species at high temperatures. It is important to point out the excellent stability of lyophilized DNI at 40 °C for up to 4 weeks, with only minor changes in structural behavior.
Immunogenicity of DNI following thermal stress
In an effort to determine the effects of long-term storage on the biological activity of DNI, accelerated decay studies were performed in which DNI samples were subjected to incubation at 40 or 70 °C for either 2 or 4 weeks. Control (DNI kept at 4 °C) and heat-stressed DNI preparations were adsorbed to Alhydrogel® (0.85 mg/ml)23 and administered to groups of BALB/c mice by subcutaneous (SC) immunization.
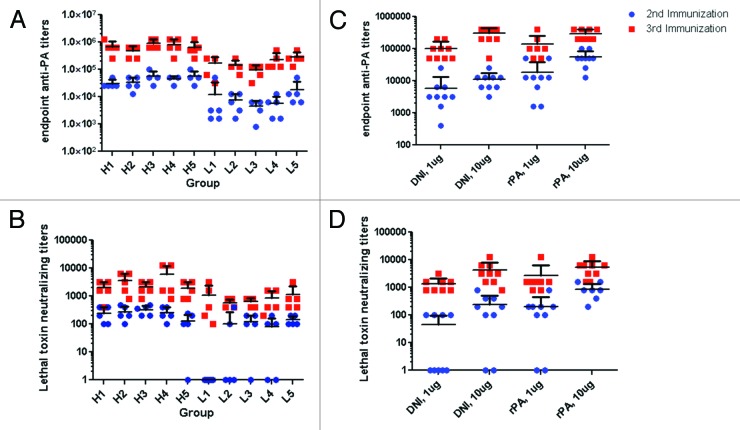
Groups of mice that received the high dose DNI, irrespective of whether or not it had been subjected to thermal stress, showed consistently high PA-specific serum IgG antibody responses after the second and third immunizations (Table 3A; Fig. 6A). There was a profound booster effect after the third immunization, as evidenced by the fact that PA-specific serum IgG endpoint titers on day 38 were on average 20 times greater than on day 24. These values are virtually identical to those obtained with rPA immunizations (Fig. 6C). Groups of mice that received the low dose DNI vaccine preparations also demonstrated uniform seroconversion, although PA-specific serum IgG antibody titers in these animals were 5–10 times less than those observed in the high dose groups. There were, however, no significant differences in PA-specific serum antibody titers among the five groups of animals, underscoring the fact that prolonged incubation of DNI at 40 or 70 °C did not appreciably affect the ability of DNI to elicit PA-specific antibodies in a mouse model.
Table 3. PA-Specific Endpoint Titers and LF Neutralizing Activity.
| A | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 2nd immunization | 3rd immunization | ||||||||
| Sample | Dose (μg) | Endpoint* | Neutralizing† | Endpoint | Neutralizing | ||||
| DNI T 0 |
10 | 29 407 | 200 | 612 066 | 1600 | ||||
| 1 | 4222 | 1 | 128 000 | 528 | |||||
| DNI 2 w, 40 °C |
10 | 29 407 | 230 | 443 613 | 2786 | ||||
| 1 | 5572 | 8 | 128 000 | 528 | |||||
| DNI 2 w, 70 °C |
10 | 51 200 | 264 | 844 485 | 1838 | ||||
| 1 | 3676 | 53 | 84 449 | 606 | |||||
| DNI 4 w, 40 °C |
10 | 44 572 | 230 | 703 079 | 3200 | ||||
| 1 | 4222 | 18 | 194 012 | 606 | |||||
| DNI 4 w, 70 °C |
10 | 51 200 | 53 | 559 801 | 1600 | ||||
| 1 | 12 800 | 132 | 256 000 | 800 | |||||
| B | |||||||||
| 2nd immunization | 3rd immunization | ||||||||
| Sample | Dose (μg) | Endpoint* | Neutralizing† | Endpoint | Neutralizing | ||||
| DNI control | 10 | 9406 | 72 | 258 032 | 3200 | ||||
| 1 | 3456 | 8 | 87 782 | 1176 | |||||
| rPA control | 10 | 47 405 | 741 | 278 690 | 4355 | ||||
| 1 | 10 159 | 62 | 110 598 | 1728 | |||||
values are the geometric mean of five samples. †Lowest dilution tested, 1:100. Samples with no detectable neutralizing titers were set to 1 for the sake of calculating geometric means.
Figure 6. Immunogenicity in mice of control and heat-stressed DNI. (A) rPA-specific endpoint titers and (B) toxin-neutralizing titers in the sera of mice immunized with either a high (H, 10 µg) or low (L, 1 µg) dose of DNI that had been stored at 4 °C (H1 and L1), 40 °C for 2 weeks (H2 and L2) or 4 weeks (H4 and L4), or 70 °C for 2 weeks (H3 and L3) or 4 weeks (H5 and L5) were bled after the second (blue circles) and third (red squares) immunization. (C) PA-specific endpoint titers and (D) neutralizing titers in the sera of mice immunized with either DNI or rPA at doses of 1 µg and 10 µg.
Sera from the DNI-immunized mice was assessed for toxin-neutralizing antibodies (TNA) using an in vitro cytotoxicity assay in which the murine macrophage cell line J774A.1 was subjected to LF. Sera collected from mice 10 d after the third high or low dose DNI-immunization all had measureable TNA (Fig. 6B). The TNA from high dose DNI-immunized mice ranged from 1600–3200 with no statistical difference between groups. Similarly, the TNA in the low dose DNI-immunized mice ranged from 528–800, but again the TNA values in the control DNI immunized mice were not statistically different from those observed in the mice that received DNI that had been subjected to thermal stress. These values are also very similar to those obtained upon immunization with rPA (Table 3B and Fig. 6D). Based on the immunological data, it appears that DNI is immunogenic even after 8 y of storage at 4 °C, and upon thermal stress at 40 and 70 °C for up to 4 weeks.
Conclusion
In this study, we have shown that the double mutation in rPA to produce DNI did not significantly affect its conformational stability and thermal behavior. It was also very stable during a storage time of 8 y at 4 °C in lyophilized form. By using a variety of techniques, stability studies of DNI under stressed conditions showed DNI was stable at temperatures as high as 40 °C after 4 weeks with some minor degradation and structural changes when stressed at 70 °C. These changes, however, did not have measurable effects on the immune response of DNI in mice, including the capacity to generate toxin neutralizing antibodies which are the most important determinant of protective immunity. The potency of DNI was therefore not affected by the stress conditions at elevated temperatures for up to 4 weeks. Furthermore, stressed DNI was able to bind to its receptor in vitro, indicating preservation of functionality, indicative of its native structure in the dried states (prior to reconstitution for receptor binding assessment) at extreme temperature. These results indicate that it is possible to circumvent chemical and physical degradation of a key vaccine component by lyophilization. The data obtained demonstrate the long-term stability of DNI during storage in a lyophilized form and its promising use as a vaccine against anthrax, with further evidence that a DNI (or rPA)-based vaccine could be the basis of a vaccine product that avoids the cold chain requirements for vaccines that are to be stockpiled for emergency.
Materials and Methods
Lyophilized dominant negative inhibitor (DNI) was produced by Baxter Pharmaceutical Solutions LLC in vials containing 25 mg of DNI, 113 mg of mannitol, 33 mg of sucrose, and 2.4 mg of dibasic sodium phosphate and was stored for 8 y at 4 °C (date of manufacture: 12/15/2003, batch number: 803918). Vials of DNI were stored at 40 and 70 °C and withdrawn after 1, 2, and 4 weeks. The lyophilized formulations were reconstituted with 3.3 ml of water for injection and then diluted to 0.5 mg/ml with 20 mM citrate phosphate buffer containing NaCl (I = 0.15) at pH 7 (except for cIEF and SEC-HPLC studies as described below). Recombinant protective antigen (rPA) of recent production from List Biological Laboratories Inc. was used as a control for these studies.
Biophysical characterization and chemical stability of DNI
SDS-PAGE was performed with 50 mM of the reducing agent Dithiothreitol (DTT), 4–12% NuPAGE gels and MOPS running buffer. All samples were heated at 80 °C for 5 min before loading onto the gels and 20 µg of protein was loaded into each well.
Size-exclusion liquid chromatography was performed with a Shimadzu HPLC system at a protein concentration of 1.0 mg/ml in 10 mM histidine, 150 mM NaCl, pH 7. A TSKgel G2000SWxL analytical column (7.8 mm × 30 cm, 5 µm) was used at 30 °C with 50 mM sodium phosphate, 200 mM NaCl pH 6.8 as the mobile phase. An amount of 50 µl was injected in triplicate with a flow rate of 0.65 ml/min. The data was analyzed using LC Solution software (Shimadzu Corporation).
Circular dichroism was performed with a Chirascan-plus Circular Dichroism Spectrometer (Applied Photophysics). Far- and near-UV spectra were collected during a single measurement in the range of 200–360 nm as described previously.11,12 A 0.2 cm path length cuvette sealed with a Teflon stopper was used and filters were placed between the cuvettes and the incident light during data collection. A sampling time per point of 3 s and a bandwidth of 1 nm were used. Measurements were made in triplicate over the temperature range of 10 to 80 °C at 2.5 °C intervals with a 1 min equilibration at each temperature and a heating rate of 1 °C/min. The optical density at 350 nm of DNI solutions was collected simultaneously during the CD experiments.
The intrinsic fluorescence of the DNI samples was measured using a QuantaMaster spectrofluorometer (Photon Technology International [PTI] Inc.). Fluorescence emission spectra were recorded from 10 to 82.5 °C at 2.5 °C intervals with a 3 min equilibration time at each temperature using 2 × 10 mm quartz cuvettes. Samples were measured in duplicate using an excitation wavelength of 295 nm. Emission spectra were collected from 300 to 400 nm with a 1 nm step size and a 1 s integration time. The excitation and emission slits were set at 2 and 3 nm, respectively. Data analysis was performed using FelixTM software (Photon Technology International, Inc.). The peak position of the emission wavelength maximum was determined using a polynomial derivative fitting method executed with Origin 7.0 software. Static light scattering was determined simultaneously during the intrinsic fluorescence experiments by a second photomultiplier located at 180° from the fluorescence detector.
Chemical stability studies using capillary isoelectric focusing (cIEF) were performed with an iCE280 instrument from Convergent Biosciences. Experiments were performed in duplicate at 10 °C using a temperature controlled auto-sampler. Samples in 10 mM histidine buffer at pH 7 were mixed with urea, methyl cellulose (Convergent Bioscience), Pharmalyte 3–10 (GE Healthcare) and two PI markers with pH values of 4.65 and 8.18. The final solution protein concentration was 0.4 mg/ml and contained 6 M urea. Data analysis was performed using Chrom Perfect® software.
Native PAGE analysis of binding to CMG2 as a function of temperature
A vial of lypophilized DNI was resuspended in 3.1 ml of water and aliquots (200 μL) were removed and lyophilized in separate glass vials. Vials were subsequently incubated at 4, 40, and 70 °C. After one week, samples were removed and resuspended in 200 μL of water at room temperature. Six and a half μL of DNI (7.7 μM) was added to 6 μL of 8.3 μM CMG2, purified as described previously in PBS.13 This gave a final concentration of 2.5 μM DNI, 2.5 μM CMG2 (1:1 stoichiometry). Next, 1.3 μL of 80% glycerol (final conc. ~5%) was added, and the volume adjusted to 20 μL using 20 mM TRIS-HCl pH 8. This was allowed to incubate for 1 h at room temperature. For the WT PA, PA was purified as described previously14 treated as above but the sample was not lyophilized, and was instead was purified in 20 mM TRIS-HCl, pH 8.0, 150 mM NaCl. This liquid sample was incubated at 4 °C with or without CMG2 for up to three weeks. Samples were loaded onto a 4–20% native PAGE gel and run at 40 V for 17 h in a cold room (4 °C).
Adsorption of DNI to Alhydrogel
Control or thermal stressed DNI samples (0.2 mg/ml) were mixed with Alhydrogel® (0.85 mg/ml) and incubated at 4 °C for 24 h. The amount of DNI adsorbed to the adjuvant surface was determined by subtracting the protein concentration in the supernatant after centrifugation from the initial protein content and was determined to >95% for all formulations examined.
Mouse Immunizations
BALB/c mice ages 8–10 weeks were purchased from Taconic. Mice were divided into groups of five and housed in the Wadsworth Center’s animal facility for at least a week prior to the initiation of DNI immunization studies. All animal experiments were approved by and conducted in compliance with the Wadsworth Center’s Institutional Animal Care and Use Committee (IACUC). Mice were immunized by the SC route on days 0, 14, and 28 with either 1 µg (low dose, L) or 10 µg (high dose, H) DNI-adsorbed. Blood was collected on days 24 and 38 by tail vein bleed from mice under isoflurane anesthesia.
For enzyme-linked immunosorbant assays (ELISA), Nunc-Immunoplates (Thermo Scientific) were coated overnight at 4 °C with 1 µg/mL of DNI, blocked for 2 h with 2% goat serum in 0.05% PBS-Tween (PBST) and then incubated with 2× serial dilutions of mouse serum for 1 h. The plates were then washed with PBST and incubated for 1 h with HRP-conjugated anti-mouse IgG secondary antibody (1:2000). The plates were then washed with PBST and developed with SureBlue Peroxidase Substrate (KPL). The reaction was quenched with 1M phosphoric acid and the absorbance was read at 450 nm.
Lethal toxin (LT) cytotoxicity assays using the murine macrophage cell line J774A.1 (ATCC TIB-67) were performed as previously described,15 with the exception that cell viability was determined using CellTiter-Glo® (Promega). Briefly, serial dilutions of mouse sera were mixed with LT (300 ng/mL) and then applied to white 96 well mirotiter plates containing J774A.1 cells (5 × 103 per well). Viability of the J774A.1 cells was determined 24 h later using CellTiter-Glo and a Spectramax L Luminometer (Molecular Devices). Toxin-neutralizing activity is reported as the inverse of the titer of serum that protected at least 50% of the cells from LT. Toxin-neutralizing activity (TNA) was defined as the reciprocal endpoint titer of serum required to protect 50% of the J774A.1 cells from the effects of LT, a value commonly referred to as the TCEC50.
Acknowledgments
Funding from NIH/NIAID U01AI082210 (to RNB). We would like to thank Dr Karen Chave of the Northeast Biodefense Center’s (NBC) Protein Expression Core at the Wadsworth Center (supported by award 5 U54-AI057158-10 from the NIH) for providing us with lethal factor and recombinant PA.
Glossary
Abbreviations:
- DNI
dominant negative inhibitor
- rPA
recombinant protective antigen
- CD
circular dichroism
- cIEF
capillary isoelectric focusing
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/vaccines/article/25852
REFERENCES
- 1.Dixon TC, Meselson M, Guillemin J, Hanna PC. Anthrax. N Engl J Med. 1999;341:815–26. doi: 10.1056/NEJM199909093411107. [DOI] [PubMed] [Google Scholar]
- 2.Donegan S, Bellamy R, Gamble CL. Vaccines for preventing anthrax. Cochrane Database Syst Rev. 2009:CD006403. doi: 10.1002/14651858.CD006403.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Inglesby TV, O’Toole T, Henderson DA, Bartlett JG, Ascher MS, Eitzen E, et al. Working Group on Civilian Biodefense Anthrax as a biological weapon, 2002: updated recommendations for management. JAMA. 2002;287:2236–52. doi: 10.1001/jama.287.17.2236. [DOI] [PubMed] [Google Scholar]
- 4.Peachman KK, Li Q, Matyas GR, Shivachandra SB, Lovchik J, Lyons RC, et al. Anthrax vaccine antigen-adjuvant formulations completely protect New Zealand white rabbits against challenge with Bacillus anthracis Ames strain spores. Clin Vaccine Immunol. 2012;19:11–6. doi: 10.1128/CVI.05376-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wright JG, Quinn CP, Shadomy S, Messonnier N, Centers for Disease Control and Prevention (CDC) Use of anthrax vaccine in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR Recomm Rep. 2010;59(RR-6):1–30. [PubMed] [Google Scholar]
- 6.Castelán-Vega J, Corvette L, Sirota L, Arciniega J. Reduction of immunogenicity of anthrax vaccines subjected to thermal stress, as measured by a toxin neutralization assay. Clin Vaccine Immunol. 2011;18:349–51. doi: 10.1128/CVI.00267-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sellman BR, Mourez M, Collier RJ. Dominant-negative mutants of a toxin subunit: an approach to therapy of anthrax. Science. 2001;292:695–7. doi: 10.1126/science.109563. [DOI] [PubMed] [Google Scholar]
- 8.Yan M, Collier RJ. Characterization of dominant-negative forms of anthrax protective antigen. Mol Med. 2003;9:46–51. [PMC free article] [PubMed] [Google Scholar]
- 9.Aulinger BA, Roehrl MH, Mekalanos JJ, Collier RJ, Wang JY. Combining anthrax vaccine and therapy: a dominant-negative inhibitor of anthrax toxin is also a potent and safe immunogen for vaccines. Infect Immun. 2005;73:3408–14. doi: 10.1128/IAI.73.6.3408-3414.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sellman BR, Nassi S, Collier RJ. Point mutations in anthrax protective antigen that block translocation. J Biol Chem. 2001;276:8371–6. doi: 10.1074/jbc.M008309200. [DOI] [PubMed] [Google Scholar]
- 11.Hu L, Olsen C, Maddux N, Joshi SB, Volkin DB, Middaugh CR. Investigation of protein conformational stability employing a multimodal spectrometer. Anal Chem. 2011;83:9399–405. doi: 10.1021/ac201995c. [DOI] [PubMed] [Google Scholar]
- 12.Hu L, Joshi SB, Andra KK, Thakkar SV, Volkin DB, Bann JG, et al. Comparison of the structural stability and dynamic properties of recombinant anthrax protective antigen and its 2-fluorohistidine-labeled analogue. J Pharm Sci. 2012;101:4118–28. doi: 10.1002/jps.23294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rajapaksha M, Eichler JF, Hajduch J, Anderson DE, Kirk KL, Bann JG. Monitoring anthrax toxin receptor dissociation from the protective antigen by NMR. Protein Sci. 2009;18:17–23. doi: 10.1002/pro.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wimalasena DS, Cramer JC, Janowiak BE, Juris SJ, Melnyk RA, Anderson DE, et al. Effect of 2-fluorohistidine labeling of the anthrax protective antigen on stability, pore formation, and translocation. Biochemistry. 2007;46:14928–36. doi: 10.1021/bi701763z. [DOI] [PubMed] [Google Scholar]
- 15.Kelly-Cirino CD, Mantis NJ. Neutralizing monoclonal antibodies directed against defined linear epitopes on domain 4 of anthrax protective antigen. Infect Immun. 2009;77:4859–67. doi: 10.1128/IAI.00117-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang G, Joshi SB, Peek LJ, Brandau DT, Huang J, Ferriter MS, et al. Anthrax vaccine powder formulations for nasal mucosal delivery. J Pharm Sci. 2006;95:80–96. doi: 10.1002/jps.20484. [DOI] [PubMed] [Google Scholar]
- 17.Wigelsworth DJ, Krantz BA, Christensen KA, Lacy DB, Juris SJ, Collier RJ. Binding stoichiometry and kinetics of the interaction of a human anthrax toxin receptor, CMG2, with protective antigen. J Biol Chem. 2004;279:23349–56. doi: 10.1074/jbc.M401292200. [DOI] [PubMed] [Google Scholar]
- 18.Rainey GJ, Wigelsworth DJ, Ryan PL, Scobie HM, Collier RJ, Young JA. Receptor-specific requirements for anthrax toxin delivery into cells. Proc Natl Acad Sci U S A. 2005;102:13278–83. doi: 10.1073/pnas.0505865102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Santelli E, Bankston LA, Leppla SH, Liddington RC. Crystal structure of a complex between anthrax toxin and its host cell receptor. Nature. 2004;430:905–8. doi: 10.1038/nature02763. [DOI] [PubMed] [Google Scholar]
- 20.Powell BS, Enama JT, Ribot WJ, Webster W, Little S, Hoover T, et al. Multiple asparagine deamidation of Bacillus anthracis protective antigen causes charge isoforms whose complexity correlates with reduced biological activity. Proteins. Structure, Function, and Bioinformatics. 2007;68:458–79. doi: 10.1002/prot.21432. [DOI] [PubMed] [Google Scholar]
- 21.Verma A, McNichol B, Domínguez-Castillo RI, Amador-Molina JC, Arciniega JL, Reiter K, et al. Use of site-directed mutagenesis to model the effects of spontaneous deamidation on the immunogenicity of Bacillus anthracis protective antigen. Infect Immun. 2013;81:278–84. doi: 10.1128/IAI.00863-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.D’Souza AJM, Mar KD, Huang J, Majumdar S, Ford BM, Dyas B, et al. Rapid deamidation of recombinant protective antigen when adsorbed on aluminum hydroxide gel correlates with reduced potency of vaccine. J Pharm Sci. 2013;102:454–61. doi: 10.1002/jps.23422. [DOI] [PubMed] [Google Scholar]
- 23.Berthold I, Pombo M-L, Wagner L, Arciniega JL. Immunogenicity in mice of anthrax recombinant protective antigen in the presence of aluminum adjuvants. Vaccine. 2005;23:1993–9. doi: 10.1016/j.vaccine.2004.10.014. [DOI] [PubMed] [Google Scholar]