Abstract
Rotavirus (RV)–specific secretory immunoglobulin (RV-SIg) has been previously detected in serum of naturally RV infected children and shown to reflect the intestinal Ig immune response. Total plasma SIgA and plasma RV-SIg were evaluated by ELISA in children with gastroenteritis due or not due to RV infection and in 50 children vaccinated with the attenuated RIX4414 human RV vaccine and 62 placebo recipients. RV-SIg was only detected in children with evidence of previous RV infection or with acute RV gastroenteritis. Vaccinees had higher RV-SIg titers than placebo recipients and RV-SIg titers increased after the second vaccine dose. RV-SIg measured after the second dose correlated with protection when vaccinees and placebo recipients were analyzed jointly. RV-SIg may serve as a valuable correlate of protection for RV vaccines.
Keywords: rotavirus, vaccine, correlate of protection, secretory immunoglobulin
Introduction
Rotavirus (RV) is the principal cause of severe gastroenteritis (GE) in young children, being responsible, before the introduction of routine immunization, for approximately 453,000 deaths annually worldwide.1 Two RV vaccines are available and recommended for infants worldwide by the WHO2: Rotarix (GlaxoSmithKline Biologicals), an attenuated human RV vaccine, and Rotateq (Merck and Co. Inc.), a bovine-human reassortant vaccine. Both vaccines are less efficacious (39% to 77%) in some low-income countries in Africa and Asia,3 where 85% of worldwide mortality occurs.4 The improvement of these vaccines or the development of new RV vaccines is hindered by the lack of a widely accepted immunological correlate of protection. At present, serum RV-specific IgA (RV-IgA) measured shortly after natural infection or vaccination represents the best practically measured correlate of protection against RV GE.5 However, some vaccinees with serum RV-IgA develop mild RV GE, and protection provided by the vaccines can be higher or lower than the levels predicted by serum RV-IgA detected in vaccinees.6,7
RV preferentially replicates in the intestine, and local mucosal immunity is thought to be key in human RV immunity.7 During an acute RV infection in children, circulating IgD- RV-specific B cells express intestinal-homing receptors (α4β7+, CCR9+), and thus probably reflect mucosal immunity.8 In agreement with this finding, in our previous double blind trial of the attenuated RIX4414 human RV vaccine, correlations between protection from disease and frequencies of RV-memory IgD-, CD27+, α4β7+, CCR9+ circulating B cells measured after dose 1 (D1) and plasma RV-IgA after dose 2 (D2) were found. However, the correlation coefficients for both tests were low, suggesting that other factors are important in explaining protection from disease.9 In this trial, only a minority (32.7%) of vaccinees presented RV-IgA coproconversion, indicating that this is not an optimal parameter to measure vaccine-induced intestinal antibody responses.9
Secretory Ig (SIg) in serum has been proposed as an alternate method for indirectly measuring intestinal Ig.10 Polymeric IgA and IgM are transported across mucosal epithelial cells by the polymeric Ig receptor.11 At the epithelial surface the receptor is cleaved and part of it (the secretory component [SC]) remains attached to the Ig, forming SIg, which may retro-transcytose across epithelial cells and eventually enter the circulation.11 RV-SIg has been detected in serum of children with recent RV infection,10,12 but not in the serum of healthy breast-fed children, even though it was present in the stool and duodenal fluid of some of them and in their mothers’ milk and serum.13,14 Moreover, serum RV-SIg correlated with the amounts detected in duodenal fluid one week after the acute infection.15 These results suggest that serum RV-SIg is frequently observed after RV infection and reflects intestinal Ig.
It is generally accepted that neutralizing antibodies against the RV infecting strain present in the intestine provide protection.16 However, assessment of intestinal fluid after RV vaccination is impractical and measurement of stool antibodies is subject to technical problems, including interference by maternal antibodies.9,17 Hence, circulating RV-SIg could reflect more precisely the intestinal protective immune response induced by the vaccine and be a better correlate of protection than circulating RV-IgA after vaccination.
We here confirm the presence of plasma RV-SIg in children with natural RV infection, and further addressed its occurrence in children vaccinated with the attenuated human RV vaccine RIX4414. We report, for the first time, that vaccinees have higher RV-SIg titers than placebo recipients after each of the two administered doses, and that RV-SIg titers increased after D2. Furthermore, RV-SIg measured after D2 correlated with protection when vaccinees and placebo recipients were analyzed jointly. We propose that plasma RV-SIg may be a valuable correlate of protection for RV vaccines.
Results
Total plasma SIgA, RV-SIg and RV-IgM in children with acute GE
Based on the presence of RV antigen or RNA in stools and RV-IgA in plasma, children with acute GE from prior studies (Table S1)18,19 were classified in 3 groups: group A: children without evidence of previous RV infection (RV-IgA-) and without RV GE (n = 5); group B: children with evidence of previous RV infection (RV-IgA+) but without RV GE (n = 20) and group C: children with acute RV GE with primary infection (RV-IgA-) (n = 7) or secondary infection (RV-IgA+) (n = 4). Umbilical cord blood samples taken from healthy full-term newborn infants, group D (n = 4), were used as controls. Plasma samples from 10 adult healthy volunteers were assessed in parallel.
The mean concentration of total plasma SIgA for adult healthy volunteers was 12.27 μg/ml (6.73 – 25.8 μg/ml), which is comparable to values previously reported,20,21 and there was no significant difference when serum or plasma samples from these volunteers were evaluated (data not shown). Moreover, as previously shown,22,23 umbilical cord blood (group D) had significantly less total SIgA than any of the groups with acute GE (Fig. 1A). Group C (children with RV GE) had significantly less total plasma SIgA than group A (Fig. 1A) and than groups A and B analyzed jointly (children without RV GE, data not shown).
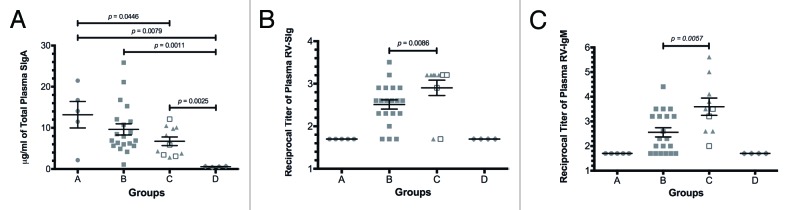
Figure 1. Total plasma SIgA, RV-SIg and RV-IgM in children with acute GE. (A)The concentration of SIgA for each sample was determined based on a standard curve of plasma with a known SIgA concentration (14.6 μg/ml). The limit of detection was 4.8 ng/ml. (B) and (C) The reported values correspond to the log10 of the inverse titer measured by ELISA. The plasma RV-IgM for groups A and D is below the limit of detection. Lines and error bars denote the mean and SEM, respectively. Differences between groups were evaluated with the nonparametric Mann–Whitney test and all p values reported are 1-tailed. Groups of children: Group A: RV-IgA-, RV-GE-; group B: RV-IgA+, RV-GE-; group C: RV-IgA- (triangles) and RV-IgA+ (open squares) RV-GE+ and group D: umbilical cord blood samples taken from healthy full-term newborn infants.
Plasma RV-SIg was detected in children with previous RV infection and with current primary or secondary RV infection (groups B and C, respectively), but not in children without previous RV infection (group A) or in umbilical cord blood samples (group D, Fig. 1B). As expected,12 children with acute RV GE had significantly higher titers of plasma RV-SIg than children with previous RV infection but without an ongoing RV GE (group C vs. group B, Fig. 1B), and none of the 10 adult healthy volunteers had plasma RV-SIg (data not shown).24
The anti-human SC monoclonal antibody (mAb) used as a capture antibody in the ELISAs can recognize both SIgA and SIgM (Fig. 1A and data not shown). The ELISA protocol for total SIgA includes an anti-IgA antibody, and does not identify SIgM (data not shown); in contrast, the RV-SIg ELISA can detect both RV-SIgA and RV-SIgM. Therefore, we next explored RV-IgM responses in all groups of children (Fig. 1C) and potential correlations between RV-SIg and RV-IgA (Fig. 2A) and RV-IgM (Fig. 2B). Plasma RV-IgM was only detected in children with evidence of previous RV infection and those with acute RV GE, and was higher in the latter than the former (Fig. 1C).
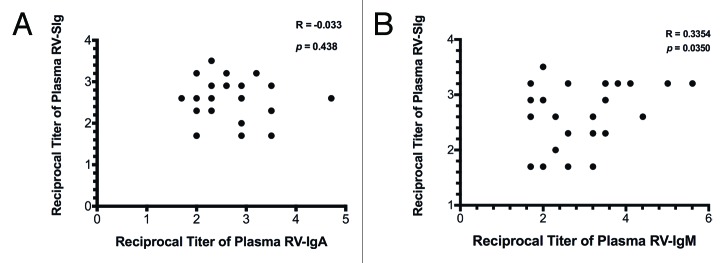
Figure 2. Correlations of RV-SIg with RV-IgA and RV-IgM. (A) Correlation (Spearman one-tailed test) between plasma RV-SIg and plasma RV-IgA titers of children from group B (RV-IgA+, RV-GE-) and children from group C with secondary infection (RV-IgA+, RV-GE+) analyzed jointly. (B) Correlation (Spearman one-tailed test) between plasma RV-SIg and plasma RV-IgM titers of children from groups B and C analyzed jointly.
Correlation tests between RV-SIg and RV-IgA (group B and children from group C with secondary infection) or RV-IgM (groups B and C) were performed either separately or jointly. No correlation between RV-SIg and RV-IgA was observed in any case (Fig. 2A, and data not shown). In contrast, there was a significant correlation between RV-SIg and RV-IgM when groups B and C were analyzed jointly (children with evidence of RV infection) although with a low correlation coefficient (Fig. 2B). Thus, plasma RV-SIg seems to be induced independently of plasma RV-IgA and to a certain degree separately of plasma RV-IgM in children with natural RV infection.
Competitive binding assays with rhSC
To confirm anti-human SC mAb-mediated detection of total SIgA and RV-SIg in ELISAs, competitions with recombinant human secretory component (rhSC) were performed. As shown in Figure 3A, a 50% reduction in binding of colostral SIgA was obtained with 0.38 μg/ml rhSC, which corresponds to the naturally found 1:1 molar ratio occurring between rhSC and SIgA.
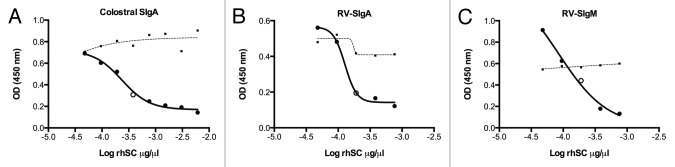
Figure 3. Effect of competing rhSC on measurement of purified colostral SIgA and RV-SIg. Reported values are optical density units (OD, 450 nm) (Y axis) and the log10 of the rhSC concentration in μg/μl (X Axis) used to compete the binding of: (A) purified colostral SIgA (0.076 μg/ml); (B) a plasma sample from a child in which RV-SIg was presumably only RV-SIgA (RV-IgA+, RV-IgM-); and (C) a plasma sample from a child in which RV-SIg was presumably only RV-SIgM (RV-IgA-, RV-IgM+). Open dots in graphics show the concentration of rhSC inducing approximately 50% of inhibition and the dashed lines correspond to the signal observed when samples were competed with albumin at the same concentrations as for rhSC.
As previously stated, the RV-SIg ELISA does not distinguish between RV-SIgA and RV-SIgM. Therefore, a plasma sample with presumably only RV-SIgA (RV-SIg+, RV-IgA+, RV-IgM-) and a plasma sample with presumably only RV-SIgM (RV-SIg+, RV-IgA-, RV-IgM+) were selected to compete with rhSC for the binding to the anti-human SC mAb. As shown in Figure 3B and C, the same concentration of rhSC (0.38 μg/ml) inhibited approximately 50% of the maximum signal observed for both types of samples. Thus, detection of either total SIgA or RV-SIg strictly relies on the binding of SIg to the anti-human SC mAb.
Total plasma SIgA and RV-SIg in children vaccinated with the attenuated RIX4414 human RV vaccine
In our previous trial of the RIX4414 human RV vaccine, children received the “all-in-one” vaccine formulation, in which the calcium carbonate buffer is lyophilized with the virus and the powder is reconstituted with water before vaccination. This formulation induced lower RV-IgA seroconversion rates25; however, it contains the same vaccine strain virus found in the Rotarix formulation and protected at similar levels against any RV GE and severe RV GE.9
There were no significant differences for total plasma SIgA among study groups (Fig. 4A). As shown in Figure 4B, vaccinees had higher RV-SIg titers than placebo recipients, both after D1 and D2. Titers of RV-SIg in vaccinees were significantly higher after D2 than after D1 (Fig. 4B), suggesting a boost effect of the second dose.

Figure 4. Total plasma SIgA and RV-SIg in vaccinees and placebo recipients. Data for 50 vaccinees and 62 placebo recipients after dose 1 (D1) or dose 2 (D2) are shown. Lines and error bars denote the mean and SEM, respectively. Differences between vaccinees and placebo recipients, as well as between protected and non-protected children, were evaluated with the Mann–Whitney test and between vaccinees after D1 and D2 with the Wilcoxon test. All p values reported are 1-tailed. (A) The reported values correspond to μg/ml interpolated from a plasma pool with a known SIgA concentration (14.6 μg/ml). (B and C) The reported values correspond to the log10 of the inverse titer measured by ELISA.
In an attempt to establish the relationship between RV-IgA and RV-SIg after vaccination, the distribution of RV-SIg was analyzed in children with and without RV-IgA (RV-IgA+ vs. RV-IgA-, Table 1). The frequency of RV-SIg+ children was significantly higher in RV-IgA+ vaccinees than in RV-IgA- vaccinees after D2 (16/25 vs. 8/25, chi-square test p < 0.05, Table 1). Moreover, the frequency of placebo recipients with RV-SIg+ was significantly higher in children RV-IgA+ than in those RV-IgA- after D2 (4/5 vs. 12/57, Fisher exact probability test p < 0.05, Table 1). In addition, a significant correlation was found between RV-IgA and RV-SIg in all study groups, except for vaccinees after D1 (data not shown); however, in all cases the correlation coefficients were low (Spearman test rho < 0.4). The frequency of RV-SIg+ children was significantly higher in RV-IgA- vaccinees than in RV-IgA- placebo recipients after D1 and/or D2 (20/23 vs. 21/54, chi-square test p < 0.05, Table 1). These results suggest that plasma RV-IgA and RV-SIg partially overlap, but depict different antibody responses.
Table 1. Number of vaccinees and placebo recipients with/without (+/−) plasma RV-IgA with plasma RV-Sig.
| Vaccinees n = 50 | Placebo recipients n = 62 | |||
|---|---|---|---|---|
| RV-IgA+ (RV-SIg+) |
RV-IgA- (RV-SIg+) |
RV-IgA+ (RV-SIg+) |
RV-IgA- (RV-SIg+) |
|
| After D1 | 13 (6) | 37 (14) | 4 (2) | 58 (13) |
| After D2 | 25 (16) | 25 (8)a | 5 (4) | 57 (12)b |
| After D1 and/or D2c | 27 (18) | 23 (20) | 8 (6) | 54 (21) |
a, b Statistically significant difference between the subgroups with RV-IgA and without RV-IgA for vaccine and placebo recipients (chi-square test and Fischer exact probability test p < 0.05, respectively). cStatistically significant difference between the subgroups of vaccinees and placebo recipients without RV-IgA (chi-square test p < 0.05).
Next, the relationship between RV-SIg titers and protection was assessed. First, as shown in Table 2, the protection rates for vaccinees, as well as for placebo recipients, increased as a function of RV-SIg titers detected after D2. Second, when vaccinees and placebo recipients were analyzed jointly there was a correlation between protection and RV-SIg titers measured after D2 (Spearman test p < 0.05, rho = 0.22). Third, the frequency of protected children was significantly higher in RV-SIg+ children (titers ≥ 1:100) than in those RV-SIg- (titer < 1:100) (37/40 vs. 55/72, chi-square test p < 0.05) and the presence of RV-SIg conferred an almost four times increase in the probability to be protected against any RV GE (OR: 3.81, CI 95%: 1.04 – 13.93). Finally, protected children had significantly higher RV-SIg titers than non-protected children after D2 (Fig. 4C). In contrast, analysis of samples after D1 did not show any statistically significant correlation or difference between study groups. Altogether, these results suggest that RV-SIg is related to protection both after vaccination and natural RV infection.
Table 2. Correlation between RV-SIg titers after D2 and protection against any RV GE.
| RV-SIg Titers after D2 | Vaccinees* (%) | Placebo recipients* (%) | Vaccinees+placebo recipients* (%) |
|---|---|---|---|
| < 100 | 22/26 (84.61) | 33/46 (71.73)a | 55/72 (76.39) |
| 100 | 10/11 (90.90) | 8/10 (80) | 18/21 (85.71)b |
| ≥ 200 | 13/13 (100) | 6/6 (100) | 19/19 (100)c |
Shown is the number of protected vaccinees, placebo recipients or vaccinees and placebo recipients analyzed jointly / the total number of children in each population for each RV-SIg titer. aThe frequency of protected children was similar in RV-SIg- (titer < 1:100) vaccinees and placebo recipients (chi-square test p > 0.05). bThe frequency of protected children was significantly higher in RV-SIg+ children (titers ≥ 1:100) than in those RV-SIg- (titer < 1:100) when vaccinees and placebo recipients were analyzed jointly (chi-square test p < 0.05). cCorrelation between RV-SIg titers and protection (Spearman test p < 0.05, rho = 0.22) when vaccinees and placebo recipients were analyzed jointly.
Additionally, no correlations were found between any RV-specific B cells subset previously studied, including RV-specific IgD+CD27+α4β7+CCR9+ and IgD-CD27+α4β7+CCR9+, and plasma RV-SIg (data not shown).9
Finally, we addressed the possibility that plasma RV-IgG could correlate with protection after vaccination with RIX4414. Although vaccinees had higher RV-IgG titers than placebo recipients after D2 (Fig. S1A), RV-IgG did not correlate with protection in any case (Table S3, Spearman test p = 0.38, rho = 0.026, when vaccinees and placebo recipients were analyzed jointly). Furthermore, there was no difference in RV-IgG titers between protected and non-protected children (Fig. S1B).
Discussion
We confirmed12,15 that RV-SIg can be detected in blood of naturally infected children (Fig. 1B), and showed that children vaccinated with the attenuated RIX4414 human RV vaccine have higher RV-SIg titers than placebo recipients, both after D1 and D2, and in vaccinees higher titers were observed after D2 than after D1 (Fig. 4B). Furthermore, RV-SIg measured after D2 correlated with protection in vaccinees and placebo recipients analyzed jointly (Table 2). The lack of correlation of RV-SIg with protection in vaccinees is probably related to the low number (five) of vaccine failures in these children.9 To our knowledge, this is the first study in which plasma antigen specific SIg has been evaluated as a correlate of protection after vaccination.
Unexpectedly,12 children with acute RV GE (group C) had less total SIgA than children with acute GE of a different etiology (groups A and B analyzed jointly). Considering that plasma SIgA may be short-lived, similar to circulating IgA (4–6 d),26 and that the mean time of blood drawing after onset of diarrhea was 4.2 d, this result suggests that acute RV GE may disrupt the intestine epithelial barrier to a greater extent than other pathogenic conditions, affecting the mechanism by which total SIgA is selectively retro-transcytosed from the intestinal lumen.
RV-SIg has been reported to appear as early as 3–4 d after the onset of RV diarrhea, with the number of individuals positive for serum RV-SIg increasing significantly around day 10, and becoming undetectable approximately a month later.15 The transient nature of RV-SIg is probably one of the reasons why its measurement has not been implemented for evaluating vaccine immunogenicity.27 We used a labeled avidin-biotin ELISA protocol, which is expected to be more sensitive than the one available in previous reports, and detected RV-SIg in 17 out of 20 children with evidence of previous RV infection without an ongoing RV GE. This result challenges the notion that plasma RV-SIg can only be transitorily detected. Nonetheless, RV-SIg was transiently observed in some vaccinated children, since only half of vaccinees with RV-SIg after D1 also had RV-SIg after D2. Of 15 placebo recipients with RV-SIg after D1 only 4 had it after D2. This potential divergence in plasma RV-SIg persistence between naturally infected and vaccinated and placebo recipient children could be explained by differences in the mean age (14.2 vs. 4 mo, respectively), by the number of RV infections in the children, and by differences between the effects of wild type virus and the vaccine virus on the plasma RV-SIg positivity rates. Further studies are necessary to determine the kinetics of RV-SIg in blood after RV vaccination.
Both RV-SIgA and RV-SIgM are probably measured in our RV-SIg ELISA, because RV-SIg can be identified both in children without RV-IgA (children with primary RV infection from group C) and in children with low levels or no RV-IgM (group B). Moreover, plasma RV-SIg is probably produced with a different kinetics than plasma RV-IgA, since there was no significant correlation between RV-SIg and RV-IgA in children with evidence of previous RV infection (Fig. 2A) that were analyzed 2–10 d after the onset of diarrhea. Likewise, RV-SIg is probably produced independently of RV-IgM, because in children with primary RV infection RV-SIg and RV-IgM exhibit no correlation (data not shown). Nonetheless, some of the RV-SIg may include RV-SIgM, due to the fact that there is a significant correlation between RV-SIg and RV-IgM when groups B and C are analyzed jointly, although the correlation coefficient was low (Spearman test rho = 0.33). Thus, RV-SIg induced after natural infection is most probably composed of both RV-SIgA and RV-SIgM, and these Igs are independently produced from RV-IgA and RV-IgM, supporting the hypothesis that plasma RV-SIg is generated in the intestinal compartment.
Correlations were found between RV-SIg and RV-IgA in vaccinees after D2 and placebo recipients after D1 and D2 (data not shown), but not in vaccinees after D1, or in children with evidence of previous RV natural infection (Fig. 2A). This may be explained by differences between these groups: children with evidence of previous RV infection were older (mean age 14.2 mo) than vaccinees (4 mo old) and may have been more exposed to RV infections. Thus, age and history of RV infections seem to be important determinants of RV-SIg responses and will need to be further investigated.
In children that received Rotarix, the main effect of the second dose was to provide a “catch-up’ immunization.7,9,28 Our results for RV-IgA and RV-SIg are in agreement with this finding: RV-IgA was detected in 13 vaccinees after D1 and it was detected in 14 more children after D2. RV-SIg was detected in 20 vaccinees after D1, and it was detected in 14 more children after D2. However, a detectable boost (4-fold increase in preexisting plasma RV-SIg) for RV-SIg was observed in three children whereas for RV-IgA it was detected only in one child. In addition, a significant difference between vaccinees and placebo recipients’ titers was observed for plasma RV-SIg (Fig. 4B) but not for RV-IgA.9
In our trial plasma RV-SIg seems to be a better correlate of protection than plasma RV-IgA, because although RV-IgA also correlated with protection when vaccinees and placebo recipients were analyzed jointly after D2 (Table S2, Spearman test p = 0.05, rho = 0.152),9 in contrast to RV-SIg (Table 2), the frequency of protected children was not significantly higher in RV-IgA+ children (titers ≥ 1:100) than in those RV-IgA- (titer < 1:100) (Table S2, Chi-square test p > 0.05,). Moreover, the frequency of protected children was significantly higher in RV-IgA- (titer < 1:100) vaccinees compared with RV-IgA- placebo recipients (Table S2), which implies that other factors, aside from RV-IgA, are important in explaining protection from disease in vaccinees.
The lack of correlation between RV-SIg detected after vaccination and any RV-memory B cells subsets analyzed may be explained by the fact that RV-SIg detected is probably a mixture of SIgM and SIgA.
Since naturally acquired serum RV-IgG has been correlated with protection against RV infection in children,29 and passively transferred serum RV-IgG mediated mucosal immunity against RV infection in a macaque model,30 we assessed RV-IgG titers in plasma samples of vaccinees and placebo recipients after D2. Although RV-IgG titers were significantly higher in vaccinees than placebo recipients (Fig. S1A), RV-IgG did not correlate with protection (Table S3). This lack of correlation is most probably due to the presence of variable amounts of maternal RV-IgG antibodies, transferred via the placenta, when the second vaccine dose was administered (4 mo old).
Human antigen-specific SIg has been quantified in secretions,31-33 as well as in serum,34-36 both after natural infection or vaccination with multiple pathogens and antigens. Mucosal priming seems to be required for induction of SIg, but immunization at a given mucosal surface may induce a response at multiple mucosal sites.37 Although SIg in serum may potentially come from colostrum,38 this is unlikely in our study, because the children studied were older than two months of age, and very few (7%) of the placebo recipients had RV-SIg while almost all of them (> 96%) were breast fed.9
The mechanism by which SIgA produced at mucosal surfaces is transported to the circulation has not been clearly established: passive leakage of SIgA or active transport mediated by an unknown receptor could be involved.11,39 The transferrin receptor (CD71) has been proposed as an IgA receptor.40 CD71 is abnormally expressed at the apical pole of enterocytes in patients with active celiac disease and evidence that polymeric/secretory IgA mediates protected transport of pathogenic gliadin peptides through their binding to CD71 has been obtained.41 Whether this is the case for pathogen-specific SIg detected in serum remains to be determined. Although some RV-SIg could be retro-transcytosed bound to the viral antigen during acute infection, it seems that several months after the virus has disappeared RV-SIg continues to be retro-transcytosed (Fig. 1B). Frequent RV infections have been associated with celiac disease in genetically predisposed children.42 Thus, further studies associating RV infection with celiac disease and IgA nephropathy (in which abnormally localized SIg may play a pathogenic role)36,43 are required.
Since present RV vaccines are less efficacious in countries where they are most needed, new vaccine formulations are being tested. For this reason, the RV’s field would greatly benefit of the use of a correlate of protection to accelerate this process. Our results suggest that RV-SIg could be complementary to RV-IgA in evaluating vaccine immunogenicity and could be a valuable correlate of protection for RV vaccines. However, because of our lack of preimmune plasma samples, our study does not demonstrate vaccine induced RV-SIg. In addition, due to the fact that children received an “all-in-one” vaccine formulation, which is less immunogenic than the final RIX4414 formulation, the correlation of RV-SIg with protection may be underestimated. In fact, the Rotarix commercial formulation induces higher RV-IgA seroconversion rates than the RIX4414 formulation used in the present study, and this may also be the case for RV-SIg.28 Hence, further studies are needed to assess plasma RV-SIg kinetics in vaccinees, and to properly determine its value as a RV correlate of protection for existing vaccines.
Materials and Methods
Ethics statement
Written informed consent was obtained from each volunteer or infant’s parents or legal guardian. Studies were approved by the Ethics Committee of the San Ignacio Hospital and Pontificia Universidad Javeriana and conducted in accordance with the guidelines of the Helsinki Declaration.
Subjects and sample processing
We studied plasma samples from 36 children with acute GE from prior published studies.18,19 These children (14 females and 22 males; mean age: 13.5 mo, range: 6–22; 20 breast fed and 16 not breast fed) were admitted with gastroenteritis to the pediatric emergency service or were hospitalized at the moment of sample collection. Demographic and clinical data of the children are presented in Table S1 arranged in the 3 groups described in the results section. The mean time of blood drawing after onset of diarrhea was 5 d (range: 1–12).
Plasma and serum samples were obtained from 10 healthy adult volunteers (7 females and 3 males; mean age: 28 y, range: 25–38), without any gastrointestinal symptoms, during the month previous to the blood drawing. Additionally, 4 umbilical cord blood samples taken from healthy full-term newborn infants were also included.
In our previous double-blind randomized controlled study,9 children received two doses of either placebo (n = 160) or 106.7 focus-forming units of the attenuated RIX4414 human RV vaccine (n = 159). Vaccine and placebo groups were very similar in terms of the median age at the time of the first and second vaccination (60 and 122 d, respectively), gender (M/F 85/74 and 84/76, respectively), and percentage of breast fed at dose 1 and dose 2 (96.9% and 95%, 88.6% and 88.1%, respectively). Details about the vaccine trial and the strategy for clinical evaluation of protection were previously published.9 Briefly, from the moment infants received their second dose of vaccine/placebo, they were contacted every 2 weeks until they were 13 mo old to identify cases of GE. Of the 319 children who received two doses of vaccine/placebo, a subgroup of 119 was randomly selected for immunological assessments (50 vaccinees and 69 placebo recipients). Plasma samples from all these children, except seven placebo recipients (children in whom the informed consent form did not authorize further studies), were included in the present studies. Plasma samples were collected 14–16 d after receiving each dose of RIX4414 or placebo.
All plasma samples from previous studies,9,18,19 which had been stored at -80°C, were thawed, diluted in 50% glycerol and preserved at -20°C for use. All assays, except competitive binding assays, were blinded experiments.
ELISA for measuring plasma RV secretory immunoglobulin
96-well vinyl microtiter ELISA plates (Thermo Electron Corporation, Cat. No. 2401) were coated with an anti-human SC mAb (clone GA-1) (Sigma-Aldrich, Cat. No. I 6635) (70 μl of 1/10,000 dilution) and incubated overnight at 4°C. After blocking with 5% blotto, serial dilutions of plasma samples in 2.5% blotto were deposited in each well. After incubation, samples were discarded and 1/10 dilutions of a supernatant from RF (Bovine RV strain P6[1]G6, 107 focus forming units/ml) virus-infected MA104 cells or the supernatant of mock-infected cells (negative control) were added. Of note, most antibodies detected with this antigen are specific for the major capsid protein VP6, which contains group- and subgroup-specific antigenic determinants and exhibits a high level of sequence conservation.44,45 Then, the following sequence of reagents was added: guinea pig anti-rhesus RV hyperimmune serum (70 μl of 1/4,000 dilution); biotinylated goat anti-guinea pig serum (Vector Laboratories, Cat. No. BA-7000) (70 μl of 1/2,000 dilution); peroxidase-labeled streptavidin (SP) (Kirkegaard and Perry Laboratories [KPL], Cat. No. 14-30-00) (70 μl of 1/1,000 dilution) and tetramethyl benzidine substrate (Sigma-Aldrich, Cat. No. 50-76-00). The reaction was stopped by the addition of 17.5 μl 2 M sulfuric acid. Absorbance was read at a wavelength of 450 nm on an ELISA plate reader (Multiskan EX; Thermo Labsystems). Serial dilutions of a pool of plasmas from children with RV-SIg was used as a positive control and a plasma from a child without evidence of previous RV infection (RV-IgA-) was used as a negative control in each plate. Samples were considered positive if the optical density in the experimental wells was > 0.1 units and 2-fold greater than the optical density in the corresponding negative control wells. To be accepted for analysis, the titer of the positive control plasma could not differ by more than one dilution from plate to plate.
ELISA for measuring total plasma secretory IgA
To detect total SIgA, a sandwich ELISA was developed using a previously described approach.43 Briefly, plates were coated with the anti-human SC mAb or PBS (Gibco, Cat. No. 21600-069) (negative control). After blocking, serial dilutions of plasma samples were applied in each well. The following sequence of reagents was then added: biotin-labeled goat anti-human IgA (KPL, Cat. No. 16-10-01) (70 μl of 1/1,000 dilution), SP, and plates were developed and analyzed as described above. The concentration of total SIgA in the plasma pool, used as a positive control, was interpolated from a standard curve generated with purified SIgA from human colostrum (AbD Serotec, Cat. No. PHP133). The corresponding concentration for each plasma sample tested was in turn interpolated from the plasma pool curve using a four-parameter logistic-log function.46
ELISA for measuring plasma RV-IgM
RV-IgM ELISA was performed as previously described,24 with minor modifications. Briefly, 96-well Immulon 2 microtiter ELISA plates (Dynex Technologies) were coated overnight at 4°C with Goat F(ab’)2 anti-human IgM (Invitrogen, Cat. No. AHI1601) (70 μl of 1/500 dilution). The plates were then blocked and incubated with plasma samples diluted in 5% blotto. The remaining steps of the assay are as described for plasma RV-SIg ELISA. Samples were considered as positive using the same criteria previously described.
ELISA for measuring plasma RV-IgG
Briefly, and as previously described with minor modifications,24 plates were coated with either a supernatant from RF virus-infected MA104 cells or the supernatant of mock-infected cells (negative control) and incubated overnight at 4°C. After blocking, serial dilutions of plasma samples were deposited in each well. After incubation, the following sequence of reagents was added: biotin-labeled goat anti-human IgG (KPL, Cat. No. 16-10-06) (70 μl of 1/1,000 dilution), SP, and plates were developed and analyzed as described. Serial dilutions of a pool of plasmas from children with RV-IgG was used as a positive control and a plasma from a child without evidence of previous RV infection (RV-IgA-) and without RV-IgG from placental transfer of maternal IgG antibodies was used as a negative control.
Recombinant human SC and competitive binding assays
Recombinant human secretory component (rhSC) was obtained as previously described,47 and was used to ensure the activity of the capture antibody in the SIg ELISAS. Bovine serum albumin (Merck, 1120180100) (used as a negative control) or rhSC was added in 1/2 serial dilutions (starting from 6.1 μg/ml onwards) after the blocking step, and incubated for 10 min at 37°C. Next, purified SIgA was added at a concentration of 0.076 μg/ml (the concentration of SIgA present in a 1/200 dilution of the positive control plasma pool), plates were incubated for 2 h and the assay continued as described above. A similar strategy was used for RV-SIg, using a dilution of plasma (1/200) that gave a sub-saturating signal in the ELISA.
Statistical analyses
Analysis was performed with SPSS software version 20.0 (IBM Inc.) and with GraphPad Prism version 6 for Mac. Differences between groups were evaluated with nonparametric Wilcoxon, Mann–Whitney, Fisher exact probability or chi-square tests, as required. Correlations were evaluated using Spearman's test. Significance was established if p ≤ 0.05, in 1 tailed tests.
Supplementary Material
Acknowledgments
This publication was financed by the Pontificia Universidad Javeriana (ID4215). We thank the subjects who participated in the previous studies for their generosity, the personnel of the Pediatrics Department of the San Ignacio Hospital for their help in identifying children with diarrhea, Luz-Stella Rodríguez and Olga L. Rojas for their support, Harry B. Greenberg and Peter Burrows for reading the manuscript and Marisol Machetá for help in administrating the research projects.
Glossary
Abbreviations:
- RV
rotavirus
- SIg
secretory immunoglobulin
- SIgA
secretory immunoglobulin A
- SC
secretory component
- GE
gastroenteritis
- D1
dose 1
- D2
dose 2
- rhSC
recombinant human secretory component
Disclosure of Potential Conflicts of Interest
JA and MF were co-principal investigators for a trial of the RIX4414 rotavirus vaccine (the precursor to the Rotarix vaccine) between October 2002 and October 2003, which was partially funded by GlaxoSmithKline.
Footnotes
Previously published online: www.landesbioscience.com/journals/vaccines/article/25610
References
- 1.Tate JE, Burton AH, Boschi-Pinto C, Steele AD, Duque J, Parashar UD, WHO-coordinated Global Rotavirus Surveillance Network 2008 estimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:136–41. doi: 10.1016/S1473-3099(11)70253-5. [DOI] [PubMed] [Google Scholar]
- 2.WHO Meeting of the Strategic Advisory Group of Experts on immunization, October 2009--Conclusions and recommendations. Biologicals. 2010;38:170–7. doi: 10.1016/j.biologicals.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 3.Patel MM, Steele D, Gentsch JR, Wecker J, Glass RI, Parashar UD. Real-world impact of rotavirus vaccination. Pediatr Infect Dis J. 2011;30(Suppl):S1–5. doi: 10.1097/INF.0b013e3181fefa1f. [DOI] [PubMed] [Google Scholar]
- 4.Patel M, Shane AL, Parashar UD, Jiang B, Gentsch JR, Glass RI. Oral rotavirus vaccines: how well will they work where they are needed most? J Infect Dis. 2009;200(Suppl 1):S39–48. doi: 10.1086/605035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Angel J, Franco MA, Greenberg HB. Rotavirus immune responses and correlates of protection. Curr Opin Virol. 2012;2:419–25. doi: 10.1016/j.coviro.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Franco MA, Angel J, Greenberg HB. Immunity and correlates of protection for rotavirus vaccines. Vaccine. 2006;24:2718–31. doi: 10.1016/j.vaccine.2005.12.048. [DOI] [PubMed] [Google Scholar]
- 7.Angel J, Franco MA, Greenberg HB. Rotavirus vaccines: recent developments and future considerations. Nat Rev Microbiol. 2007;5:529–39. doi: 10.1038/nrmicro1692. [DOI] [PubMed] [Google Scholar]
- 8.Jaimes MC, Rojas OL, Kunkel EJ, Lazarus NH, Soler D, Butcher EC, et al. Maturation and trafficking markers on rotavirus-specific B cells during acute infection and convalescence in children. J Virol. 2004;78:10967–76. doi: 10.1128/JVI.78.20.10967-10976.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rojas OL, Caicedo L, Guzmán C, Rodríguez LS, Castañeda J, Uribe L, et al. Evaluation of circulating intestinally committed memory B cells in children vaccinated with attenuated human rotavirus vaccine. Viral Immunol. 2007;20:300–11. doi: 10.1089/vim.2006.0105. [DOI] [PubMed] [Google Scholar]
- 10.Grauballe PC, Hjelt K, Krasilnikoff PA, Schiøtz PO. ELISA for rotavirus-specific secretory IgA in human sera. Lancet. 1981;2:588–9. doi: 10.1016/S0140-6736(81)90981-8. [DOI] [PubMed] [Google Scholar]
- 11.Mantis NJ, Rol N, Corthésy B. Secretory IgA’s complex roles in immunity and mucosal homeostasis in the gut. Mucosal Immunol. 2011;4:603–11. doi: 10.1038/mi.2011.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hjelt K, Grauballe PC, Schiøtz PO, Andersen L, Krasilnikoff PA. Intestinal and serum immune response to a naturally acquired rotavirus gastroenteritis in children. J Pediatr Gastroenterol Nutr. 1985;4:60–6. doi: 10.1097/00005176-198502000-00012. [DOI] [PubMed] [Google Scholar]
- 13.Hjelt K, Grauballe PC, Nielsen OH, Schiøtz PO, Krasilnikoff PA. Rotavirus antibodies in the mother and her breast-fed infant. J Pediatr Gastroenterol Nutr. 1985;4:414–20. doi: 10.1097/00005176-198506000-00016. [DOI] [PubMed] [Google Scholar]
- 14.Rahman MM, Yamauchi M, Hanada N, Nishikawa K, Morishima T. Local production of rotavirus specific IgA in breast tissue and transfer to neonates. Arch Dis Child. 1987;62:401–5. doi: 10.1136/adc.62.4.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hjelt K, Grauballe PC, Andersen L, Schiøtz PO, Howitz P, Krasilnikoff PA. Antibody response in serum and intestine in children up to six months after a naturally acquired rotavirus gastroenteritis. J Pediatr Gastroenterol Nutr. 1986;5:74–80. doi: 10.1097/00005176-198601000-00014. [DOI] [PubMed] [Google Scholar]
- 16.Ward RL, Clark HF, Offit PA. Influence of potential protective mechanisms on the development of live rotavirus vaccines. J Infect Dis. 2010;202(Suppl):S72–9. doi: 10.1086/653549. [DOI] [PubMed] [Google Scholar]
- 17.Wood D, WHO Informal Consultative Group WHO informal consultation on quality, safety and efficacy specifications for live attenuated rotavirus vaccines Mexico City, Mexico, 8-9 February 2005. Vaccine. 2005;23:5478–87. doi: 10.1016/j.vaccine.2005.07.035. [DOI] [PubMed] [Google Scholar]
- 18.Mesa MC, Rodríguez LS, Franco MA, Angel J. Interaction of rotavirus with human peripheral blood mononuclear cells: plasmacytoid dendritic cells play a role in stimulating memory rotavirus specific T cells in vitro. Virology. 2007;366:174–84. doi: 10.1016/j.virol.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 19.Mesa MC, Gutiérrez L, Duarte-Rey C, Angel J, Franco MA. A TGF-beta mediated regulatory mechanism modulates the T cell immune response to rotavirus in adults but not in children. Virology. 2010;399:77–86. doi: 10.1016/j.virol.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 20.Kvale D, Brandtzaeg P. An enzyme-linked immunosorbent assay for differential quantitation of secretory immunoglobulins of the A and M isotypes in human serum. J Immunol Methods. 1986;86:107–14. doi: 10.1016/0022-1759(86)90272-3. [DOI] [PubMed] [Google Scholar]
- 21.Zhang JJ, Xu LX, Liu G, Zhao MH, Wang HY. The level of serum secretory IgA of patients with IgA nephropathy is elevated and associated with pathological phenotypes. Nephrol Dial Transplant. 2008;23:207–12. doi: 10.1093/ndt/gfm492. [DOI] [PubMed] [Google Scholar]
- 22.van Furth R, Schuit HR, Hijmans W. The immunological development of the human fetus. J Exp Med. 1965;122:1173–88. doi: 10.1084/jem.122.6.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cleveland MG, Bakos MA, Pyron DL, Rajaraman S, Goldblum RM. Characterization of secretory component in amniotic fluid. Identification of new forms of secretory IgA. J Immunol. 1991;147:181–8. [PubMed] [Google Scholar]
- 24.Rojas OL, Narváez CF, Greenberg HB, Angel J, Franco MA. Characterization of rotavirus specific B cells and their relation with serological memory. Virology. 2008;380:234–42. doi: 10.1016/j.virol.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zaman KSD, Yunus MD, Arifeen SE, Azim T. Pod- der G, Faruque ASG, Karim S, Luby S, and Breiman RF. Rotavirus vaccine trials in Bangladesh: past, present, and future. VIIIth Commonwealth Association of Paediatric Gastroenterology and Nutrition [CAPGAN] Congress on Diarrhea and Malnutrition, 2006. [Google Scholar]
- 26.Morell A, Skvaril F, Noseda G, Barandun S. Metabolic properties of human IgA subclasses. Clin Exp Immunol. 1973;13:521–8. [PMC free article] [PubMed] [Google Scholar]
- 27.Grimwood K, Lund JC, Coulson BS, Hudson IL, Bishop RF, Barnes GL. Comparison of serum and mucosal antibody responses following severe acute rotavirus gastroenteritis in young children. J Clin Microbiol. 1988;26:732–8. doi: 10.1128/jcm.26.4.732-738.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salinas B, Pérez Schael I, Linhares AC, Ruiz Palacios GM, Guerrero ML, Yarzábal JP, et al. Evaluation of safety, immunogenicity and efficacy of an attenuated rotavirus vaccine, RIX4414: A randomized, placebo-controlled trial in Latin American infants. Pediatr Infect Dis J. 2005;24:807–16. doi: 10.1097/01.inf.0000178294.13954.a1. [DOI] [PubMed] [Google Scholar]
- 29.Velázquez FR, Matson DO, Guerrero ML, Shults J, Calva JJ, Morrow AL, et al. Serum antibody as a marker of protection against natural rotavirus infection and disease. J Infect Dis. 2000;182:1602–9. doi: 10.1086/317619. [DOI] [PubMed] [Google Scholar]
- 30.Westerman LE, McClure HM, Jiang B, Almond JW, Glass RI. Serum IgG mediates mucosal immunity against rotavirus infection. Proc Natl Acad Sci U S A. 2005;102:7268–73. doi: 10.1073/pnas.0502437102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fox PD, Khaw PT, McBride BW, McGill JI, Ward KA. Tear and serum antibody levels in ocular herpetic infection: diagnostic precision of secretory IgA. Br J Ophthalmol. 1986;70:584–8. doi: 10.1136/bjo.70.8.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rocha-Zavaleta L, Pereira-Suarez AL, Yescas G, Cruz-Mimiaga RM, Garcia-Carranca A, Cruz-Talonia F. Mucosal IgG and IgA responses to human papillomavirus type 16 capsid proteins in HPV16-infected women without visible pathology. Viral Immunol. 2003;16:159–68. doi: 10.1089/088282403322017893. [DOI] [PubMed] [Google Scholar]
- 33.Kozlowski PA, Williams SB, Lynch RM, Flanigan TP, Patterson RR, Cu-Uvin S, et al. Differential induction of mucosal and systemic antibody responses in women after nasal, rectal, or vaginal immunization: influence of the menstrual cycle. J Immunol. 2002;169:566–74. doi: 10.4049/jimmunol.169.1.566. [DOI] [PubMed] [Google Scholar]
- 34.Herremans MM, van Loon AM, Reimerink JH, Rümke HC, van der Avoort HG, Kimman TG, et al. Poliovirus-specific immunoglobulin A in persons vaccinated with inactivated poliovirus vaccine in The Netherlands. Clin Diagn Lab Immunol. 1997;4:499–503. doi: 10.1128/cdli.4.5.499-503.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Herremans TM, Reimerink JH, Buisman AM, Kimman TG, Koopmans MP. Induction of mucosal immunity by inactivated poliovirus vaccine is dependent on previous mucosal contact with live virus. J Immunol. 1999;162:5011–8. [PubMed] [Google Scholar]
- 36.Eijgenraam JW, Oortwijn BD, Kamerling SWA, de Fijter JW, van den Wall Bake AWL, Daha MR, et al. Secretory immunoglobulin A (IgA) responses in IgA nephropathy patients after mucosal immunization, as part of a polymeric IgA response. Clin Exp Immunol. 2008;152:227–32. doi: 10.1111/j.1365-2249.2008.03616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Belyakov IM, Ahlers JD. What role does the route of immunization play in the generation of protective immunity against mucosal pathogens? J Immunol. 2009;183:6883–92. doi: 10.4049/jimmunol.0901466. [DOI] [PubMed] [Google Scholar]
- 38.Ogra SS, Weintraub D, Ogra PL. Immunologic aspects of human colostrum and milk. III. Fate and absorption of cellular and soluble components in the gastrointestinal tract of the newborn. J Immunol. 1977;119:245–8. [PubMed] [Google Scholar]
- 39.Corthésy B. Roundtrip ticket for secretory IgA: role in mucosal homeostasis? J Immunol. 2007;178:27–32. doi: 10.4049/jimmunol.178.1.27. [DOI] [PubMed] [Google Scholar]
- 40.Moura IC, Centelles MN, Arcos-Fajardo M, Malheiros DM, Collawn JF, Cooper MD, et al. Identification of the transferrin receptor as a novel immunoglobulin (Ig)A1 receptor and its enhanced expression on mesangial cells in IgA nephropathy. J Exp Med. 2001;194:417–25. doi: 10.1084/jem.194.4.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matysiak-Budnik T, Moura IC, Arcos-Fajardo M, Lebreton C, Ménard S, Candalh C, et al. Secretory IgA mediates retrotranscytosis of intact gliadin peptides via the transferrin receptor in celiac disease. J Exp Med. 2008;205:143–54. doi: 10.1084/jem.20071204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stene LC, Honeyman MC, Hoffenberg EJ, Haas JE, Sokol RJ, Emery L, et al. Rotavirus infection frequency and risk of celiac disease autoimmunity in early childhood: a longitudinal study. Am J Gastroenterol. 2006;101:2333–40. doi: 10.1111/j.1572-0241.2006.00741.x. [DOI] [PubMed] [Google Scholar]
- 43.Oortwijn BD, van der Boog PJM, Roos A, van der Geest RN, de Fijter JW, Daha MR, et al. A pathogenic role for secretory IgA in IgA nephropathy. Kidney Int. 2006;69:1131–8. doi: 10.1038/sj.ki.5000074. [DOI] [PubMed] [Google Scholar]
- 44.Youngman KR, Franco MA, Kuklin NA, Rott LS, Butcher EC, Greenberg HB. Correlation of tissue distribution, developmental phenotype, and intestinal homing receptor expression of antigen-specific B cells during the murine anti-rotavirus immune response. J Immunol. 2002;168:2173–81. doi: 10.4049/jimmunol.168.5.2173. [DOI] [PubMed] [Google Scholar]
- 45.Tang B, Gilbert JM, Matsui SM, Greenberg HB. Comparison of the rotavirus gene 6 from different species by sequence analysis and localization of subgroup-specific epitopes using site-directed mutagenesis. Virology. 1997;237:89–96. doi: 10.1006/viro.1997.8762. [DOI] [PubMed] [Google Scholar]
- 46.Plikaytis BD, Turner SH, Gheesling LL, Carlone GM. Comparisons of standard curve-fitting methods to quantitate Neisseria meningitidis group A polysaccharide antibody levels by enzyme-linked immunosorbent assay. J Clin Microbiol. 1991;29:1439–46. doi: 10.1128/jcm.29.7.1439-1446.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Phalipon A, Cardona A, Kraehenbuhl JP, Edelman L, Sansonetti PJ, Corthésy B. Secretory component: a new role in secretory IgA-mediated immune exclusion in vivo. Immunity. 2002;17:107–15. doi: 10.1016/S1074-7613(02)00341-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


