Abstract
Background: The long-term prognosis of patients with stage IV AJCC melanoma is extremely poor. We have previously published short-term clinical outcome and immunological responses to a heat killed Mycobacterium vaccae-based vaccine.
Results: In this study we report on a better than expected long-term survival (3-y DSS 29·6%, 5-y, and 7-y DSS both 23·9%) relative to historical controls in the patients who received the vaccine in these trials, published in 1999 and 2003. Although the complete or partial response was only 10%, it was the remarkable response to other interventions upon relapse, such as surgery and radiotherapy followed by stable disease that was previously unexpected.
Methods: We reviewed the outcome of 72 patients who were treated with M. vaccae for metastatic melanoma between January 1996 and July 2004.
Conclusion: Given this remarkable outcome in stage IV metastatic melanoma and its lack of toxicity we propose that this would make a promising candidate for randomized trials for stage III fully resected melanoma.
Keywords: stage IV melanoma, mycobacterial vaccine, long-term survival
Introduction
Mycobacterium vaccae (M. vaccae) was selected as an alternative to BCG (Bacille Calmette Guérin, a living derivative of a bovine tubercle bacillus Mycobacterium bovis) as a potential candidate for an anti-tuberculosis vaccine. It had become clear that the nature of the response to BCG depended on prior exposure to endogenous mycobacteria, resulting in protection against tuberculosis in some regions and no protection in others. In an attempt to improve on this, a heat killed variant of M. vaccae was selected as, in addition to enhancing the cell mediated (Th-1) immune responses that protect against tuberculosis, it is able to suppress inappropriate and detrimental responses associated with a humoral (Th-2)response.1 BCG has been studied as a potential immunostimulant to treat cancer for several decades, albeit with variable results. It is, however, licensed for intra-vesicular administration for the treatment of bladder cancer, M. vaccae was considered a good substitute for BCG and initially offered to cancer patients with limited options, on an informed consent basis as an intradermal vaccine, prior to the instigation of formal trials., These confirmed that the intradermal administration of M. vaccae on a two weekly, then monthly basis, was capable of reversing the suppression of Th-1-mediated beneficial immune responses, as has been shown to occur in melanoma and prostate cancer.2,3 In the initial report by Maraveyas2 the vaccination schedule resulted in a sustained IL-2 induction as assessed by the intracellular cytokine staining, in 39% of patients. This was associated with an improved outcome, compared with those who did not show an IL-2 response. In order to explore this further, non-responders were given low dose subcutaneous Interleukin-2 (IL-2) (18 units spread over three days, and repeated two weekly). The addition of subcutaneous IL-2 was found to convert non-cytokine responders to responders and an association with an enhanced clinical response was noted (unpublished data, Dalgleish et al.). In a randomized study, those patients who received IL-2 as well as the vaccine had a non-significant trend in clinical response compared with those in the vaccine alone.4 The vaccine has also been on trial under its proprietary name of SRL172 in lung cancer where it was shown to give a trend for increased survival when given with chemotherapy, as opposed to chemotherapy alone.5 A larger multi-center study, however, failed to confirm this but it did demonstrate a significant improvement in quality of life in a patient who received this vaccine in addition to chemotherapy.6 There were considerable issues with the technical conduct of this study7 and a re-analyses excluding patients who received three vaccines, or less, showed a higher significant survival for patients with adenocarcinoma.8 In spite of encouraging results in other tumors, such as renal cancer, SR Pharma, the company which produced the vaccine, decided to abandon further studies.9
Since the publication of the two melanoma studies it has become evident that patients within these studies have survived longer than expected.,2,4 Whereas a few patients had dramatic responses and remained disease free, many more patients developed stable or slowly progressing disease which became manageable with other modalities. Several of these patients have had more than one episode of surgery for recurrences and others have had marked responses, including complete responses, to radiotherapy. Here we present the follow-up of these patients and confirm the enhanced survival suspected but not shown to be significant in the early reports.
Results
Demographic and clinic-pathologic characteristics of the 72 patients included in this analysis are shown in Table 1.
Table 1. Clinic-pathologic characteristics of patients.
| Patients (n = 72) |
||
|---|---|---|
| Sex | Female Male |
37 (51.4%) 35 (48.6%) |
| Age (years) | Median (range) ≤ 52 > 52 |
52 (23–80) 39 (54.2%) 33 (45.8%) |
| Primary melanoma site | Head Limb Trunk Unknown |
10 (13.9%) 40 (55.6%) 17 (23.6%) 5 (6.9%) |
| Metastases timing | Synchronous Metachronous |
9 (12.5%) 63 (87.5%) |
| M-stage at study entry (TNM VII. ed) | 1a 1b 1c |
25 (34.7%) 6 (8.3%) 41 (56.9%) |
| Number of metastases | ≤ 2 > 2 |
18 (25%) 54 (75%) |
| Serum LDH | Normal High NA |
27 (37.5%) 21 (29.2%) 24 (33.3%) |
| Neutrophiles/Lymphocites ratio | ≤ 5 > 5 NA |
45 (62.5%) 7 (9.7%) 20 (27.8%) |
| Combined medical therapy | No Yes |
17 (23.6%) 55 (76.4%) |
| Surgical resection | No Yes |
57 (79.2%) 15 (20.8%) |
| Radiotherapy | No Yes |
57 (79.2%) 15 (20.8%) |
NA, not available data.
Overall, 63 (87·5%) patients were treated with M. vaccae (SRL-172) combined with other treatment modalities, such as chemo- or cytokine medical therapy, radiotherapy or surgery. As regards associated medical therapy, 53 (73·6%) patients received SRL172 in combination with IL-2, and two (2·8%) patients were on thalidomide. Systemic adverse events were minor and involved mild “flu-like” symptoms in seven (9·7%) patients on the day of treatment and moderate urticaria in one patient (1·4%). Local toxicity was restricted to erythema and swelling at the SRL172 injection site. In no case was SRL172 stopped due to adverse events. The mean duration of SRL172 administration was 22 mo (median six mo, range 1–130). The most frequent reason for treatment discontinuation was progressive disease or death. Six patients who achieved a R0 resection during the first six months of therapy were excluded from the clinical response assessment. Clinical response data are shown in Table 2. A complete response was observed in two patients (3·0%) and a partial response in 6 (9·1%). At the time of writing, after a prolonged follow-up, CR was achieved in 7 (10·8%) patients. The median time required to achieve CR was 17 mo (range 2–63). Both patients who obtained a complete response during the first six months of therapy had no other treatment. Among the 5 patients who achieved late complete response, 2 had no other treatment, 2 had complete regression of the residual tumor burden after surgical cytoreduction (lymph node excision in multiple skin and nodal metastases in one patient and lymph node excision in multiple pulmonary and nodal metastases in the other case), and 1 patient achieved complete regression of skin metastases after complete response to radiotherapy of a single lymph node lesion.
Table 2. Clinical response assessment after 6 mo of therapy.
| Patients (n = 66) |
Median (95% CI) disease specific survival (months) |
||
|---|---|---|---|
| Clinical response | CR/PR (Best response) SD PD |
8 (12.1%) 19 (28.8%) 39 (59.1%) |
NR 30.0 (15.8–44.1) 5.0 (3.6–6.3)* |
CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; NR, not reached *, computed on 38 cases because 1 patient lost at follow-up was excluded.
Of the 72 patients enrolled in the study, 14 (19.4%) were alive as of July 2012. One patient, who achieved CR after two months of therapy, died without melanoma from an accidental cause after 131 mo from the start of therapy. Therefore, he was censored for DSS analysis purpose (melanoma unrelated death). All other patients died from melanoma related causes. One patient lost at follow-up after nine months from the beginning of therapy was excluded from DSS analysis.
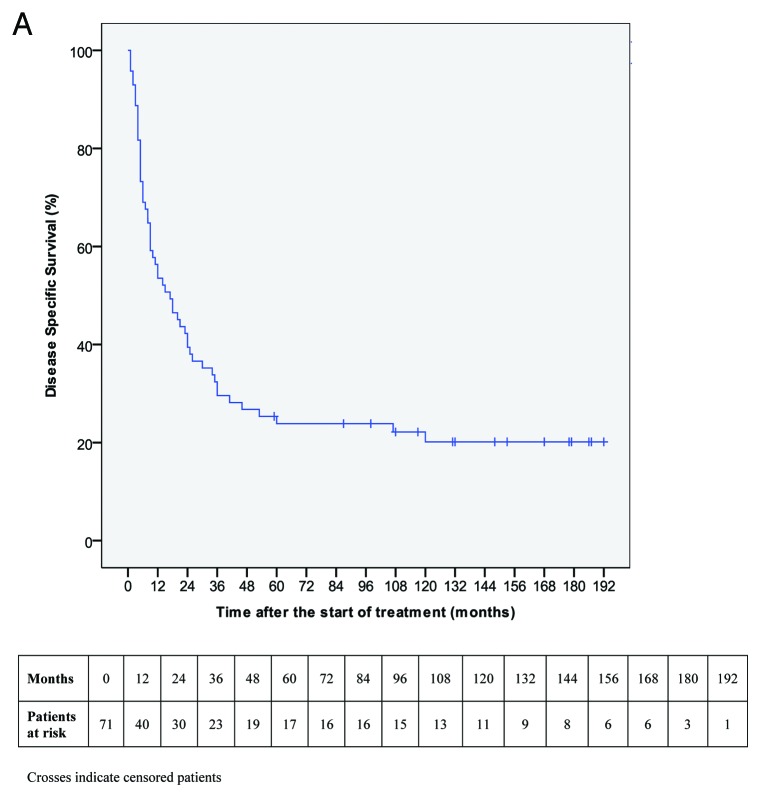
Median follow-up was 148 mo (range 59–192) for living patients and nine months for dead patients (range 1–120). In all patients, median DSS was 17 mo (95% CI 7.8–26·2). DSS rate was 53·5% (95% CI 41·9–65·1) at one year, 29·6% (95% CI 19·0–40·2) at three years, 23·9% (95% CI 13·9–33·9) at both five years and seven years and 20·1% (95% CI 10·5–29.7) at ten years (Fig. 1). Survival rate differences according the main clinico-pathologic variables and clinical response are shown in Table 3. Duration of SRL172, considered as months of administration, was statistically associated with DSS (continuous variable: HR 0·957 95% CI 0·940–0·975, p < 0·001).
Figure 1. Kaplan-Meier estimate for disease specific survival.
Table 3. Kaplan-Meier disease specific survival analysis (71 patients).
| n | 3 y DSS % | 5 y DSS % | p | ||
|---|---|---|---|---|---|
| Sex | Female Male |
37 34 |
43.2 14.7 |
32.2 14.7 |
0.006* |
| Age (years) | ≤ 52 > 52 |
39 32 |
28.2 31.3 |
28.2 18.8 |
0.463 |
| Primary melanoma site | Head Limb Trunk Unknown |
10 40 16 5 |
50.0 27.5 12.5 60.0 |
50.0 20.0 12.5 40.0 |
0.020* |
| Metastases timing | Synchronous Metachronous |
9 62 |
33.3 29.0 |
22.2 24.2 |
0.938 |
| M-stage at study entry (TNM VII. ed) | 1a 1b 1c |
24 6 41 |
50.0 0.0 22.0 |
37.5 0.0 19.5 |
0.002* |
| Number of metastases | ≤ 2 > 2 |
17 54 |
76.5 14.8 |
76.5 6.9 |
< 0.001* |
|
Serum LDH (48 patients) |
Normal High |
27 21 |
51.9 9.5 |
44.4 4.8 |
< 0.001* |
|
Neutrophiles/Lymphocites Ratio (51 patients) |
≤ 5 > 5 |
44 7 |
36.4 28.6 |
29.5 14.3 |
0.068 |
| Combined medical therapy | No Yes |
17 54 |
29.4 29.6 |
29.4 22.1 |
0.620 |
| Surgical resection | No Yes |
56 15 |
17.9 73.3 |
12.5 66.7 |
< 0.001* |
| Radiotherapy | No Yes |
56 15 |
32.1 20.0 |
26.8 13.3 |
0.444 |
|
Clinical Response+ (65 patients) |
CR/PR (best response) SD PD |
8 19 38 |
87.5 31.6 5.3 |
87.5 15.8 2.6 |
< 0.001* |
, statistically significant log-rank test; +, only patient with measurable disease included (6 patients underwent R0 resection were excluded); CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease.
Among the six patients who underwent R0 resection within the first six months of SRL172, one patient developed recurrence after 51 mo (median PFS not reached). Four of eight patients who obtained best response, and 18 of 19 patients with stable disease developed disease progression, with a median PFS of 36 mo (last event at 36 mo, 95% CI not computed) and 13 mo (95% CI 5·9–20·1), respectively.
In three patients, who previously achieved a CR or PR, a complete cure of recurrent disease was obtained through surgery (complete surgical resection in one patient with multiple cutaneous and lymphatic metastases and in one patient with multiple visceral metastases) and radiotherapy (complete response in one patient with brain metastases). These patients were still alive at last follow-up (respectively 45, 77 and 80 mo after recurrence).
At multivariate analysis, including the entire series, the independent prognostic factors associated with DSS were sex, number of metastases, and duration of SRL172 administration. In the subgroups of 65 patients with measurable disease after six months of therapy, the independent prognostic factors were sex, number of metastases, and clinical response grade. Clinical response grade was not inserted in the first regression because it was not estimable for all 71 patients. Serum LDH and neutrophils/lymphocytes ratio were not included in either Cox’s proportional hazards models because of missing data for many patients. The duration of SRL172 administration was not included in the second model to avoid multi-colinearity phenomenon with clinical response grades. The results of multivariate analysis, considering only the variables that after forward selection reached a statistical significance, are shown in Table 4 and Table 5.
Table 4. Cox’s multivariate disease specific survival analysis (71 patients).
| HR (95% CI) | p | ||
|---|---|---|---|
| Sex | Female Male |
1 2.667 (1.490–4.775) |
0.001 |
| Number of metastases | ≤ 2 > 2 |
1 2.763 (1.137–6.714) |
0.025 |
| SRL172 duration (months) | continuous variable | 0.960 (0.941–0.980) | < 0.001 |
Goodness of the model: -2 Log likelihood 350.677; χ2 47.536; p < 0.001. Included variables: sex, primary melanoma site, M-stage at study entry, number of metastases, SRL172 duration, surgical resection.
Table 5. Cox’s multivariate disease specific survival analysis (65 patients).
| HR (95%CI) | p | ||
|---|---|---|---|
| Sex | Female Male |
1 3.345 (1.767–6.332) |
< 0.001 |
| M number | ≤ 2 > 2 |
1 3.012 (1.104–8.219) |
0.031 |
| Clinical Response | CR/PR (best response) SD PD |
1 7.399 (0.911–60.083) 32.420 (4.024–261.181) |
< 0.001 0.061 0.001 |
Goodness of the model: -2 Log likelihood 329.158; χ2 51.039; p < 0.001. Included variables: sex, primary melanoma site, M-stage at study entry, number of metastases, surgical resection, clinical response grade.
Discussion
In this study we evaluated the long-term outcome of all patients who received M. vaccae for stage IV melanoma at our institution. Most patients were also treated with other cancer therapy modalities. We report a survival rate of 29.6% at three years and 24% at five years, a median survival of 17 mo and a best overall response rate (CR/PR) of 12·1%. Considering that a stable disease over a period of six months might be a significant finding in this patient group, a favorable response including SD was achieved in about 40% of cases. Moreover, this longer than expected survival, based on historical controls and published databases was in unselected patients usually referred to us with very advanced metastatic disease. More than half of our patients (56.9%) had M1c stage and two-thirds had three or more metastatic lesions, 8.5% were M1b and the remainder were M1a stage disease. Patients with M1a disease have a similar survival to our cohort. However, with most patients not being M1a but b and c then the survival would be expected to be half that reported here from other studies.10
Importantly, M. vaccae was demonstrated to be safe and well tolerated given that toxicity was minimal and no III or IV grade adverse events were observed in marked contrast to other immune based therapies, such as α-interferon.
These results suggest the administration of this heat killed vaccine, in combination with other cancer therapies, as an approach to metastatic melanoma treatment may be of benefit.
Although initially referred to as a vaccine it is evident that M. vaccae is more of a non-specific immune modulator acting on the innate immune system11 with downregulation of Th-2 responses and the restoration of Th-1 mediated immunity, which results in effective immune responses against the tumor.12
Downregulation of Th-1 associated cytokines is seen in many tumor types and reverts when the tumor is completely excised.13,14
M. vaccae is likewise able to restore Th-1 responses in patients whose disease is not resectable, and thus modulates the immune response in patients with unresectable disease in a similar manner to that seen following complete resection.13,14 Thus, the ability of M. vaccae to restore Th-1 immune function may enable other treatment modalities, such as radiotherapy, to be more effective.
A specific noteworthy issue is the role of surgery in patients with metastatic melanoma. There is a strong rationale and increasing evidence that justify the use of surgery in the management of these patients. Several studies have demonstrated that metastasectomy can significantly improve survival in selected patients, achieving a five-year survival rate of 11–49% for M1a stage, 5–31% for M1b and 7–28% for M1c.15-19 Different theories have been advanced as explanation of these findings. One of them is that a reduction of tumor burden leads to a more efficiently function of the host’s immune system. A phase III randomized trial comparing Canvaxin (an allogeneic irradiated whole tumor cell vaccine – CancerVax, Carlsbad, CA) plus BCG vs. placebo with BCG in completely resected stage IV melanoma failed to demonstrate an overall survival benefit, but reported a five-year survival of 42·3% in the combined control and treatment arms indicating that BCG along may confer a survival benefit.16 This high five-year survival rate has never been achieved in any randomized controlled trial for stage IV melanoma patients. The fact that the outcome in both arms was better than predicted may suggest that BCG had similar activity to M. vaccae in a disease free setting. In our series, the small subgroup of patients who were treated with surgery and M. vaccae achieved a three-year and five-year DSS of 73·3% and 66·7%, respectively. This would further support the role of SRL172 as immunomodulator combined with surgery in patients affected by metastatic melanoma. Prospective randomized trials are necessary to clarify the potential benefits of combination of surgical resection with immune modulators.
It would appear that M. vaccae is more effective in controlling low burden tumors, regardless the site of metastases, which may reflect the overall immunosuppressive effect of the increasing tumor bulk and a threshold above which immune modulation cannot overcome tumor escape. A limitation of the present study is that, although most patients were on clinical trials they were treated and followed up outside a trial setting and that there is no direct control group, only historical databases. Nevertheless, the extended follow-up of this series provides definitive data about DSS and has led to a reassessment of this approach and its possible use to increase the outcome to other modalities.
The maintained improved survival seen in these patients has led to renewed interest in this vaccine approach. M. obuense (IMM-101), a strain closely related taxonomically to M. vaccae, has been selected for further clinical development. IMM-101 has now undergone a phase I/II study in a very similar group of patients to those reported here with stage IV melanoma (ClinicalTrials.gov Identifier: NCT01308762). Similar clinical observations with responses in cutaneous and lung lesions have been reported, as well as a trend to increased survival, which is currently the focus of a long-term follow up study (ClinicalTrials.gov Identifier NCT01559818).20 IMM-101 is now in phase II trials for pancreatic cancer (ClinicalTrials.gov. Identifier: NCT01303172), and metastatic colon cancer (ClinicalTrials.gov. Identifier: NCT01539824).
The data reported here suggest that this class of immune modulators represents a promising candidate for randomized phase III trials in stage III resected melanoma.
Antigen-specific vaccines have been disappointing in this setting, presumably due to the ability of the tumors to downregulate HLA-1 epitopes, as well as specific antigens. The long-term outcome with M. vaccae and its effect on the innate immune system suggest that the observed effects may not be antigen-specific, although restoration of the innate and cell-mediated responses will consequently enhance suppressed antigen-specific CD8 T-cell responses.
Currently stage III melanoma patients have been enrolled in studies looking at Avastin or Ipilimumab, and results are not yet available. Both of these agents are extremely expensive for the adjuvant setting and not without significant toxicity. The immunomodulatory agents reported here has minimal toxicity and, unlike Avastin and Ipilimumab, does not require IV infusions, just intradermal inoculation.
With regards to the management of stage IV melanoma two new agents have recently been approved. Ipilimumab, an antibody against the cytotoxic T-lymphocyte-associated-4 molecule (CTLA-4) and Vemurafenib, a serine-threonine protein kinase B-RAF (BRAF) inhibitor, were approved for treatment of metastatic melanoma after encouraging results in phase 3 trials. Ipilimumab has shown a significant improvement in overall survival with a median overall survival of 10.1 mo and 11.2 mo and response rate of 10.9% and 15.2% in previously treated and untreated patients, respectively.21,22 Vemurafenib has been associated with a response rate of 48% and a six-months overall survival of 84% (compared with a response rate of 5% and a six months survival of 64% in the dacarbazine group)23 The response rate to Ipilimumab is, however, low and there is significant autoimmune toxicity and the median duration of response to Vemurafenib is relatively short. The data reported here would suggest that both agents may benefit from combination strategies with IMM-101. As previously discussed, IMM-101 would make a promising candidate for a randomized Phase III trial in stage III melanoma but for stage IV melanoma it could be given with or without standard therapies, including DTIC, Vemurafenib and Ipilimumab.
It is increasingly becoming clear that combinations of treatment modalities are more likely to lead to a better clinical outcome that single agents alone. This has already been shown for ipilimimab where it is more effective with DTIC than DTIC alone. Further studies with IMM 101 and chemotherapy may lead to a reduced side effect profile, as seen in the previous lung studies, it may also reduce the side effect profile of ipilimumab due to the regulatory properties of IMM 101.
Materials and Methods
Patients
All 72 patients treated with SRL172 (heat-killed Mycobacterium vaccae) for malignant melanoma at our institution were reviewed. All had histologically confirmed metastatic melanoma of a non-ocular primary site, and were included in this analysis. The first patient received the first dose of SRL172 on January 1996 and the last patient on July 2004. Among these patients, 41 received SRL172 during a phase II randomized trial of SRL172 ± low-dose IL-2 that was closed because no responses were seen in the first 16 patients receiving SRL172 alone.4 Six patients were treated in a second phase II trial with the same study design that was stopped because no more SRL172 was available at that time. A further 19 patients received SRL172 as compassionate use, when other options were exhausted. All patients had measurable metastatic disease, a WHO performance status of 0–2 and adequate hepatic, renal and hematological indices.
Procedures
SRL172 (SR Pharma) was supplied in borate-buffered saline at 1 mg (109 bacilli) per 0·1 ml dose. SRL172 was administered in doses of 0·05 or 0·1 ml by intradermal injection over the deltoid muscle. Patients received 0·5 mg of SRL172 on day 0, followed by 1 mg on days 14 and 28. Treatment was recommenced on day 56 and continued monthly unless symptomatic disease developed or progression occurred. All adverse events and toxicity were graded according to NCI common toxicity criteria (ctep.cancer.gov/reporting/ctc.html), if the SRL172 injection site was infected (a very rare occurrence) or ulcerated, the due dose of SRL172 was withheld and the next dose was given on schedule but at half dose. Clinical response was assessed by clinical examination, measurements of cutaneous disease and CT re-staging. Clinical response was defined according to WHO criteria in complete response (CR), partial response (PR), stable disease (SD) at six months and progressive disease (PD).
In patients with stable disease or complete or partial response after the initial six months treatment, continuation of treatment was at the discretion of the consultant (AGD), as well as after further periodic clinical and radiological follow-up.
Patient demographics, clinical and pathological characteristics, duration of SRL172 administration, combined medical therapy, concomitant radiotherapy, and/or surgical resection, and clinical outcome data were registered on a trial database. When surgery was performed, a complete removal of all metastatic lesions was defined as R0 resection. Follow-up information, updated at July 2012, was collected through direct interview with patients and/or their general practitioner.
Statistical analysis
The primary end point of the analysis was disease-specific survival (DSS). Secondary end points were overall response rate (ORR), and progression-free survival (PFS). DSS was calculated from the date of the administration of the first dose of SRL172 to the date of melanoma-related death, the censoring patients who were alive, or the death of patients due to causes unrelated to melanoma. PFS was computed from the start of therapy until the observation of progressive or recurrent disease. Best overall response rate was defined as the proportion of patients with complete or partial responses after six months of therapy. Survival analysis was performed according to the Kaplan-Meier method and the log-rank test was used to assess the statistical difference between groups. Continuous variables were analyzed by means of Cox’s univariate regression.
A multivariate analysis was conducted in order to adjust for possible confounders using Cox’s proportional hazards model with forward likelihood-ratio method, including covariates with a p value of < 0·1 at univariate analysis. The significance level was set at p < 0·05 (two-sided) and the proportional hazard assumption was controlled by means of goodness of fit tests. The statistical analysis was performed with SPSS (19th edition) software for WindowsTM.
Role of the funding source
SRL172 was supplied by SR Pharma. This study was supported by Cancer Vaccine Institute, formerly the Cancer Vaccine Campaign
Acknowledgments
This study and previous trials would not have been possible without funding from the Cancer Vaccine Institute, formerly the Cancer Vaccine Campaign.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/vaccines/article/25618
References
- 1.Grange JM. Effective vaccination against tuberculosis-a new ray of hope. Clin Exp Immunol. 2000;120:232–4. doi: 10.1046/j.1365-2249.2000.01145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maraveyas A, Baban B, Kennard D, Rook GA, Westby M, Grange JM, et al. Possible improved survival of patients with stage IV AJCC melanoma receiving SRL 172 immunotherapy: correlation with induction of increased levels of intracellular interleukin-2 in peripheral blood lymphocytes. Ann Oncol. 1999;10:817–24. doi: 10.1023/A:1008307821189. [DOI] [PubMed] [Google Scholar]
- 3.Hrouda D, Baban B, Dunsmuir WD, Kirby RS, Dalgleish AG. Immunotherapy of advanced prostate cancer: a phase I/II trial using Mycobacterium vaccae (SRL172) Br J Urol. 1998;82:568–73. doi: 10.1046/j.1464-410X.1998.00803.x. [DOI] [PubMed] [Google Scholar]
- 4.Nicholson SGK, Guile K, John J, Clarke IA, Diffley J, Donnellan P, et al. A randomized phase II trial of SRL172 (Mycobacterium vaccae) +/- low-dose interleukin-2 in the treatment of metastatic malignant melanoma. Melanoma Res. 2003;13:389–93. doi: 10.1097/00008390-200308000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Assersohn L, Souberbielle BE, O’Brien ME, Archer CD, Mendes R, Bass R, et al. A randomized pilot study of SRL172 (Mycobacterium vaccae) in patients with small cell lung cancer (SCLC) treated with chemotherapy. Clin Oncol (R Coll Radiol) 2002;14:23–7. doi: 10.1053/clon.2001.0030. [DOI] [PubMed] [Google Scholar]
- 6.O’Brien ME, Saini A, Smith IE, Webb A, Gregory K, Mendes R, et al. A randomized phase II study of SRL172 (Mycobacterium vaccae) combined with chemotherapy in patients with advanced inoperable non-small-cell lung cancer and mesothelioma. Br J Cancer. 2000;83:853–7. doi: 10.1054/bjoc.2000.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dalgleish AG. Therapeutic cancer vaccines: why so few randomised phase III studies reflect the initial optimism of phase II studies. Vaccine. 2011;29:8501–5. doi: 10.1016/j.vaccine.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 8.Stanford JL, Stanford CA, O’Brien ME, Grange JM. Successful immunotherapy with Mycobacterium vaccae in the treatment of adenocarcinoma of the lung. Eur J Cancer. 2008;44:224–7. doi: 10.1016/j.ejca.2007.08.021. [DOI] [PubMed] [Google Scholar]
- 9.Patel PM, Sim S, O’Donnell DO, Protheroe A, Beirne D, Stanley A, et al. An evaluation of a preparation of Mycobacterium vaccae (SRL172) as an immunotherapeutic agent in renal cancer. Eur J Cancer. 2008;44:216–23. doi: 10.1016/j.ejca.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 10.Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Atkins MB, Byrd DR, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199–206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fowler D, Liu WM, Smith P, Dalgleish AG. Supernatant from tumour cells treated with chemotherapy stimulate professional antigen presenting cells in vitro. Ejc Suppl. 2009;7:115–6. [Google Scholar]
- 12.Lahey T, Arbeit RD, Bakari M, Horsburgh CR, Matee M, Waddell R, et al. Immunogenicity of a protective whole cell mycobacterial vaccine in HIV-infected adults: a phase III study in Tanzania. Vaccine. 2010;28:7652–8. doi: 10.1016/j.vaccine.2010.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heriot AG, Marriott JB, Cookson S, Kumar D, Dalgleish AG. Reduction in cytokine production in colorectal cancer patients: association with stage and reversal by resection. Br J Cancer. 2000;82:1009–12. doi: 10.1054/bjoc.1999.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evans C, Morrison I, Heriot AG, Bartlett JB, Finlayson C, Dalgleish AG, et al. The correlation between colorectal cancer rates of proliferation and apoptosis and systemic cytokine levels; plus their influence upon survival. Br J Cancer. 2006;94:1412–9. doi: 10.1038/sj.bjc.6603104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allen PJ, Coit DG. The surgical management of metastatic melanoma. Ann Surg Oncol. 2002;9:762–70. doi: 10.1007/BF02574498. [DOI] [PubMed] [Google Scholar]
- 16.Morton DLMN, Thompson JF, Kelley MC, Faries M, Wagner J, Schneebaum S, et al. MMAIT Clinical Trials Group An International, ranodmized, phase III trial of bacillus Calmette-Guerin (BCG) plus allogeneic melanoma vaccine (MCV) or placebo after complete resection of melanoma metastatic to regional or distant sites. J Clin Oncol. 2007;25:8508. [Google Scholar]
- 17.Sanki A, Scolyer RA, Thompson JF. Surgery for melanoma metastases of the gastrointestinal tract: indications and results. Eur J Surg Oncol. 2009;35:313–9. doi: 10.1016/j.ejso.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 18.Wasif N, Bagaria SP, Ray P, Morton DL. Does metastasectomy improve survival in patients with Stage IV melanoma? A cancer registry analysis of outcomes. J Surg Oncol. 2011;104:111–5. doi: 10.1002/jso.21903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ollila DW, Gleisner AL, Hsueh EC. Rationale for complete metastasectomy in patients with stage IV metastatic melanoma. J Surg Oncol. 2011;104:420–4. doi: 10.1002/jso.21961. [DOI] [PubMed] [Google Scholar]
- 20.Stebbing J, Dalgleish A, Gifford-Moore A, Martin A, Gleeson C, Wilson G, et al. An intra-patient placebo-controlled phase I trial to evaluate the safety and tolerability of intradermal IMM-101 in melanoma. Ann Oncol. 2012;23:1314–9. doi: 10.1093/annonc/mdr363. [DOI] [PubMed] [Google Scholar]
- 21.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robert C, Thomas L, Bondarenko I, O’Day S, M D JW, Garbe C, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–26. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 23.Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, et al. BRIM-3 Study Group Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–16. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]