Abstract
Dendritic cells have a central role in HIV infection. On one hand, they are essential to induce strong HIV-specific CD4+ helper T-cell responses that are crucial to achieve a sustained and effective HIV-specific CD8+ cytotoxic T-lymphocyte able to control HIV replication. On the other hand, DCs contribute to virus dissemination and HIV itself could avoid a correct antigen presentation. As the efficacy of immune therapy and therapeutic vaccines against HIV infection has been modest in the best of cases, it has been hypothesized that ex vivo generated DC therapeutic vaccines aimed to induce effective specific HIV immune responses might overcome some of these problems. In fact, DC-based vaccine clinical trials have yielded the best results in this field. However, despite these encouraging results, functional cure has not been reached with this strategy in any patient. In this Commentary, we discuss new approaches to improve the efficacy and feasibility of this type of therapeutic vaccine.
Keywords: therapeutic vaccine, HIV, dendritic cells, functional cure, T cell responses
Introduction
Currently, over 30 million people are infected with HIV worldwide, most of them living in developing countries. Although combined antiretroviral therapy (cART) has proven to be highly effective to prevent clinical progression and death, by itself it is unable to eradicate the infection, thus necessitating therapy throughout life.1,2 Resistance, adverse effects in the medium-long-term, and cost are important limitations for lifelong adherence to this therapy. Although the number of HIV infected patients on treatment in developing countries has increased steadily in the last few years and it has been associated with a decrease of new HIV infections, the treatment demands rise.3 In fact, the number of new infections overcomes the number of new patients on treatment. Economic, social, cultural and political issues impose major obstacles to implement widespread treatment in developing countries. Therefore, for an effective control of the epidemic new cost-effective and viable therapeutic strategies need to be evaluated.
A low proportion of HIV infected patients show a lack of clinical progression associated with strict control of viral replication without any treatment. This “functional cure” has been linked with potent HIV-specific immune responses observed in these patients. Therapeutic vaccination is one of the most promising strategies to restore HIV-specific T-cell responses in HIV infected patients and control viral replication without cART.4 Initially, “classical approaches” such as whole inactivated virus (REMUNE)5 or recombinant protein (gp120)6 were tested as therapeutic vaccines. In general, the capacity of these early vaccines, as well as those based on DNA vectors7 to increase the HIV-specific responses and control viral load were very limited. New approaches have been used in recent years, based on more innovative vectors such as recombinant virus or dendritic cells (DC).8,9 Regretfully, although most viral vector vaccines were able to induce HIV-specific immune responses in clinical trials, they showed very limited efficacy to control viral replication.10 Finally, DC-based vaccines have yielded the best results in this field.
Myeloid-Derived Dendritic Cells (MD-DC) as a Cellular Adjuvant for a Therapeutic Vaccine Against HIV Infection
Immature (im) myeloid DCs patrol mucosal territories to detect invader pathogens. Once they engulf a pathogen, undergo a process of maturation, which involves the antigen processing, and the migration to proximal lymphoid tissues, where they will induce antigen-specific T-cell and B-cell responses.11 Different imDC subsets, positioned in the mucosal surfaces, are among the earliest cells to encounter the HIV-1 transmitted through sexual contact. HIV-1 exploits the antigen-presenting cell functions of DCs to be transferred to CD4+ T cells, through the so-called virological synapse.12 This transfer constitutes a mechanism of HIV-1 spreading and occurs even in the absence of productive infection of DCs, a process called trans-infection.12,13 Nevertheless, DCs are required for induction of T-cell responses to intracellular pathogens,14 and the capacity of DCs loaded with infectious and non-infectious HIV-1 virions to activate naïve HIV-1-specific CD4 and CD8 T cells has been demonstrated in vitro.15 Therefore, the initial contact of HIV-1 with DCs can result in opposite outcomes, either beneficial in inducing strong HIV-specific T-cell responses or deleterious in promoting the spread and dissemination of HIV-1 among HIV-specific and HIV-nonspecific CD4 T cells, leading to the progressive depletion of CD4 T cells that characterize the natural history of chronic HIV-1 infection.
In addition to the contribution of DCs to HIV dissemination, it is likely that antigen presentation could be impaired by HIV infection. Our group has demonstrated in in vitro assays that adenosine deaminase (ADA) enhances the CD3-mediated proliferation of the T cells of HIV-1-infected individuals16 and this effect is directly correlated with the CD4 percentage and inversely with the viral load. We have reported that ADA enhances T helper type 1 (Th1) and pro-inflammatory cytokine secretion in SEA-pulsed dendritic cells with autologous lymphocyte cocultures and CD3-triggered T cells.17,18 and that the HIV envelope glycoprotein gp120 markedly reduces this effect.19 These results could help to explain, at least in part, the progressive impairment of the immune system in HIV-infected patients.
It would be conceivable that DCs can have internal mechanism to control viral infection and improve the antigen presentations. In fact, DCs have APOBEC3G/3F that mediates intrinsic resistance of monocyte-derived dendritic cells to HIV-1 infection.20-22 Moreover, immature (im) MD-DC from healthy individuals produce α-defensins1–323 and α-defensins1–3 are able to modulate the maturation and differentiation process of MD-DC.24 A high production of α-defensins 1–3 by immature DC could act by damaging the virus prior or after its internalization. This would favor a more efficient viral processing and presentation to CD4+ T cells with a minor rate of infectious HIV transmission. In fact, we demonstrate that DC from HIV-infected patients that spontaneously control the infection produced higher levels of α-defensins 1–3, which positively correlated with CD4 T-cell counts and were associated with slower progression25 (Fig. 1). We hypothesize that a high production of α-defensins 1–3 by immature DC could contribute to determine a beneficial outcome of the HIV-1-DC interaction that would involve increased and optimal HIV-1-specific T-cell responses, which in turn would retard the progression of the disease.
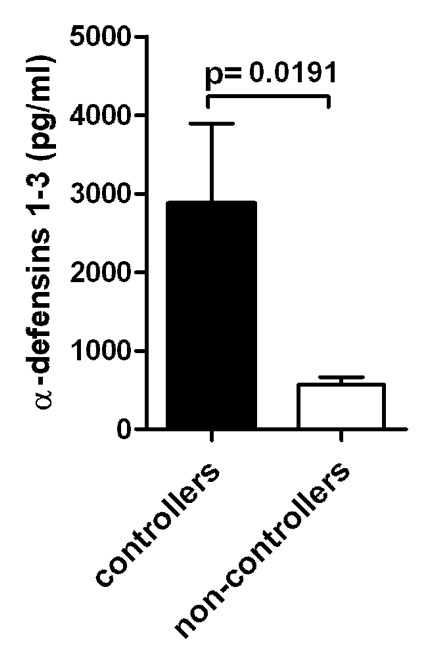
Figure 1. Levels of a-defensins 1–3 produced by imMDDC from HIV-infected individuals. (A) Comparison between the secreted levels of α-defensins 1–3 by imMDDC from HIV-controllers (elite controllers and viremic controllers; n = 13) and HIV non-controllers (viremic non-controllers and patients with HAART; n = 16). Bars indicated the mean of α-defensins 1–3 and error bars represent the SEM from the mean of the replicates.
Therefore, DCs have a central role in HIV infection. On one hand, they are essential to induce strong HIV-specific CD4+ helper T-cell responses that are crucial to achieve a sustained and effective HIV-specific CD8+ cytotoxic T-lymphocyte (CTL) able to control HIV replication.26,27 This concept is entirely consistent with data on chronic viral infections in the murine model.28 On the other hand, DCs contribute to virus dissemination and HIV itself could avoid a correct antigen presentation, surpassing the intrinsic intracellular innate mechanism to control viral infection.
In that setting, it has been hypothesized that ex vivo generated DC therapeutic vaccines might overcome some of these problems. DCs could be the most potent “cellular adjuvant” for a vaccine preparation aimed to induce effective Th1 and CTL responses to intracellular pathogens and tumor antigens29 as it could be HIV without the risk of spreading the infection and improving an adequate antigen presentation. We hypothesize that a therapeutic vaccine using autologous myeloid derived dendritic cells (MD-DC) pulsed with autologous heat-inactivated whole HIV could overcome these problems. It could be argued that HIV has already escaped to the immune system of the patient so boosting with the same virus will not improve the breadth of the immune response and would not be effective. However, although it is true that viral escape to CTL has been extensively demonstrated,30-33 some data suggest that the antigen-presenting functions of dendritic cells are impaired in HIV-1 infected patients and this could contribute to the functional defects of HIV-1-specific helper and CTL responses.34,35 We think that our model could help to know if a correct antigen presentation with this therapeutic vaccine could reverse the functional defect of helper and CTL responses and prevent, at least partially, the viral escape.
Clinical Trials with MD-DC-Based Therapeutic Vaccines
Autologous MD-DCs,36 pulsed ex vivo with a variety of inactivated pathogens and tumor antigens, have been shown to induce a potent protective immunity in experimental murine models of human infections and tumors.11,37,38 Some studies in animal models suggest that animals immunized with DCs loaded with HIV-1 viral lysate, envelope glycoproteins or inactivated virus mount a potent immune response against HIV-1.39-41,42 Although it has been performed at least 13 published clinical trials of DC-based immunotherapy for HIV infection in humans43-50,5152-54,55 (reviewed in ref. 9), most of them were non-controlled, non-randomized studies.
Overall, the safety profile has been excellent with only minor local side effects reported in some clinical trials. No severe side effects or induction of autoimmunity have been reported. The 13 clinical trials published so far suggest that DC immunotherapy in HIV-1 infection can elicit HIV-1 specific immunological responses. However, only five of these studies reported virological responses to immunization,44,45,51,52,55 three have not assessed virological responses47,50,53 and five failed to show any response.43,46,48,49,54
Recently, our group reported the results of a blinded placebo controlled trial of a therapeutic vaccine using autologous MD-DC pulsed with autologous heat-inactivated whole HIV. We immunized 36 patients on cART with high CD4 T-cell counts with 3 doses of MD-DC cells (n = 24) or with non-pulsed MD-DC (n = 12). Vaccination was feasible, safe, well tolerated and clearly shifted the virus/host balance. A peak reduction of 94% of viral load after cART interruption (as compared with viral load before any cART) was observed (Fig. 2). This significant decrease in plasma viral load observed in immunized recipients was associated with a consistent increase in HIV-1-specific T-cell responses. These data suggest that HIV-1 specific immune responses elicited by therapeutic DC vaccines could significantly change pVL set-point after cART interruption in most chronic HIV-1 infected patients treated in early stages. However, despite these encouraging results, functional cure was not reached with this vaccine in any patient. In addition, immunization did not prevent the drop of CD4+ T cells, a third of randomized patients had to reinitiate cART during the follow-up and viral load response to vaccine waned with time. These problems are probably due to persistent viral replication and it is likely that if a therapeutic vaccine would be able to control viral replication to undetectable level these deleterious effects would not be observed.
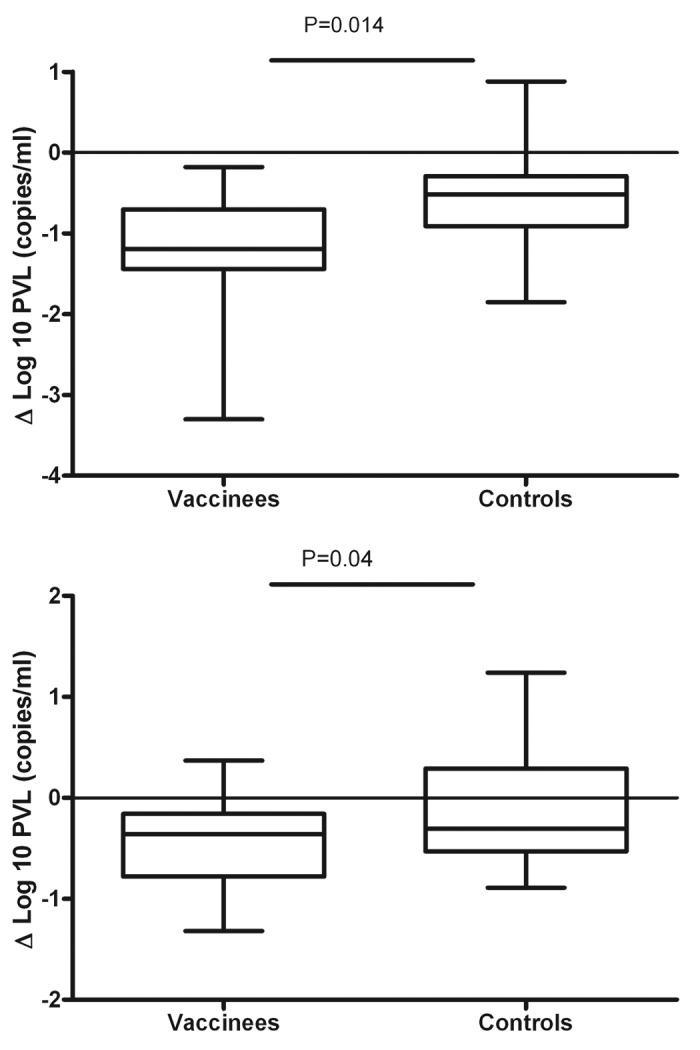
Figure 2. Highest and lowest decrease in pVL set-point in vaccinees and controls (as defined as the highest and lowest difference between pVL set-point at any time point after cART interruption and baseline pVL set-point before any cART). There were significantly different between arms [highest decrease (or peak decrease): mean ∆log10 -1.2 vs -0.56 copies/ml, in vaccinees vs controls, p = 0.014; lowest decrease: mean ∆log10 -0.54 vs -0.06 copies/ml, in vaccinees vs controls, p = 0.04].
These results are proof of concept suggesting that HIV-1 specific immune responses elicited by therapeutic DC vaccines could significantly change pVL set-point after cART interruption. However, new candidates and/or new optimized strategies of vaccination with the final objective of obtaining a functional cure as an alternative to cART for life are needed.
MD-DC Based Therapeutic Vaccines: The Way Ahead
There are a number of potential possibilities to improve the outcomes of MD-DC based therapeutic vaccines (Table 1). First, new designed immunogens to pulse MD-DC should be tested and has to be clarified whether they should be designed using the autologous or heterologous virus. Second, different adjuvants could favor or impair antigen presentation. Third, clinical trial design could be optimized to improve the effectiveness of the vaccine. Finally, the ex vivo pulsing DC has opened the possibility that other in vivo DC targeting immunogens could be tested to improve the capture and antigen presentation avoiding the cumbersome manipulation of DCs.
Table 1. Potential possibilities to improve the outcomes of MD-DC based therapeutic vaccines.
| New immunogens | - autologous vs heterologous immunogens - mRNA - Viral vectors - Dendrimers - Nanoparticles |
|---|---|
| Adjuvants. | - ADA - Anti PD1 and Anti PDL-1 antibodies |
| Clinical trial design. | - booster doses of immunogen after cART interruption - prime-boost immunization with viral vectors and DC–based vaccines |
| in vivo DC targeting immunogens | - to improve the capture and antigen presentation avoiding the cumbersome manipulation of DCs. |
New designed immunogens to pulse MD-DC
It is worth considering whether or not autologous virus is a good immunogen since it could be argued that further boosting of the same antigens could not be effective due to viral escape. One of the strongest arguments favoring autologous virus come from the clinical trials. Those studies performed with heterologous antigens43,46-49 did not find any virological response. Conversely, the five clinical trials that have used the autologus virus as immunogen showed variable levels of virological response.44,45,51,52,55 Lu et al.44 and our group45,51,55 used whole chemically and heat inactivated virus, respectively. Routy et al.56,57 reported the preliminary results of an immunotherapy trial consisting of MD-DCs and RNA encoding autologous HIV-1 antigens (Gag, Nef, Rev, Vpr) administered monthly in 33 subjects in four intradermal doses in combination with cART, followed by two more doses during a 12-week cART interruption (STI). They found that this strategy induced a control of pVL similar to that reported by Lu et al.44 and our group.55 These data suggest that a combination of autologous antigens administered to patients on cART could have a higher virological effect than heterologous immunogens, although it should be tested in a randomized clinical trial.
In addition, new designed immunogens to pulse MD-DC should be tested. MD-DCs pulsed with recombinant virus has been tested in vitro58 and in clinical trials.48 We have reported that MVA-B (MVA expressing the HIV-1 genes Env-Gag-Pol-Nef of clade B) infected MDDC co-cultured with autologous T lymphocytes induced a highly functional HIV-specific CD8+ T-cell response including proliferation, secretion of IFN-gamma, IL-2, TNF-α, MIP-1beta, MIP-1alpha, RANTES, and IL-6, and strong cytotoxic activity against autologous HIV-1-infected CD4+ T lymphocytes. Ghandi et al.48 conducted a phase I/II clinical trial to evaluate whether adding MD-DCs to a Canarypox-HIV vaccine improved virologic control as compared with Canarypox-HIV vaccine alone during analytic treatment interruption in HIV-1-infected subjects. They found that higher percentage of subjects in the DC group had a VL setpoint < 5000 c/mL during cART interruption, but virologic control was transient. Although lymphoproliferative responses were higher in patients receiving DC-Canarypox-HIV vaccine, ELISPOT responses to HIV-1 antigens did not differ by treatment arm. They conclude that new methods to enhance the immunogenicity and antiviral efficacy of DC-based vaccines for HIV-1 infection are needed.
In the last few years, synthetic molecules have been proved to be highly efficient to transduce various types of cells in less invasive and potentially more harmless approaches. High numbers of studies have been focused on dendrimers59 and other nanoparticles60,61,62 as carriers of drugs and biomolecules. In vitro and animal models have shown that dendritic cells loaded with HIV-1 p24 proteins adsorbed on surfactant-free anionic polylactic acid colloidal biodegradable nanoparticles induced enhanced cellular immune responses against HIV.61 Clinical trials should be performed with some of these new immunogens to confirm the results obtained in animal models and test the safety of this approach.
Adjuvants to favor or impair antigen presentation
The second issue to take in consideration to improve the effectiveness of MD-DCs based vaccine is the role of adjuvants. During Ag presentation by DCs, T-cell activation is subjected to regulation by several intercellular interactions mediated by surface and soluble molecules that stimulate their receptors expressed on T cells. These regulatory interactions might modulate the activation and differentiation of T cells and could favor or impair antigen presentation. An example of a molecule that favors antigen presentation is adenosine deaminase (ADA). Extracellular ADA mediates extracellular adenosine degradation and acts as a costimulatory molecule in T-cell activation processes.17 By acting as a bridge between A2B adenosine receptors on dendritic cells (DCs) surface and CD26 on T-cells surface, ADA acts as a costimulatory molecule in cocultures of SEA-presenting DCs and autologous T cells, not only enhancing T-cell proliferation, Th-1/pro-inflammatory cytokine secretion,17 and naïve T-CD4+ cell activation, memory, and FOXP3+ generation,18 but also increasing DCs immunogenicity in both healthy and HIV-infected subjects.63 Although HIV gp120 envelope protein disrupts ADA-CD26 interaction,64 possibly contributing to the HIV-promoted immunodeficiency,19 ADA is still able to enhance autologous T-cell proliferation against inactivated-HIV presentation by DC in individuals under cART (Fig. 3),16 suggesting a beneficial role for ADA on improving HIV-specific T-cell responses in those individuals. All these observations, together with the availability of ADA in clinical grade indicate that ADA could be a potential adjuvant capable of robustly and safely boosting cellular responses against HIV. Conversely, programmed death-1(PD-1) and PD-1 ligand PD-L1 mediates immunosuppression. There are data showing that the blockade of PD-1 and PDL-1 induces durable tumor regression and prolonged stabilization of disease in cancer patients65,66 and enhances SIV-specific immunity in animal model.67 All these data suggest that modulation of antigen presentation could help to improve the antigen presentation effectively. However, defining the optimal protocol for in vivo DC maturation, without abrogating the uptake/translation of immunogen has proven to be challenging. Some authors hypothesized that simultaneous delivery of classical activation stimuli might result in impairment of the induction of an immune response.68 On the other hand, an intense immune response could lead to a cytokines storm that could be dangerous for the patient.65,66,69
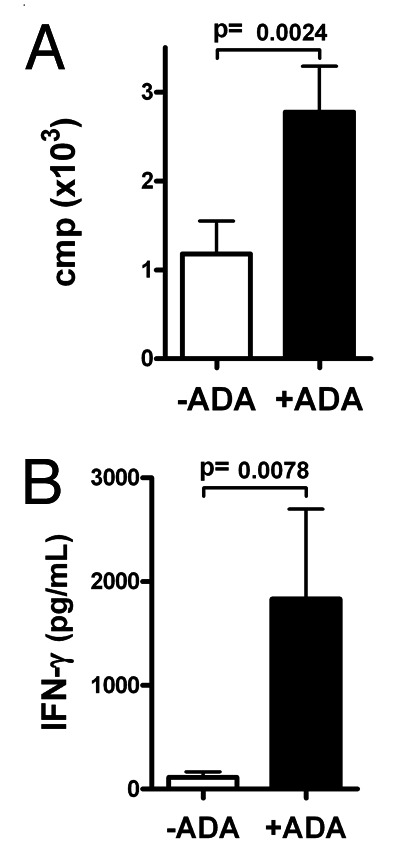
Figure 3. ADA increases proliferation and IFN-γ secretion in co-cultures of HIV-inactivated-pulsed DC and autologous T cells of HIV-1-infected individuals. (A) T-cell proliferation in a 7 d assay, measured as c.p.m. of [3H]-thymidine incorporation during last 18 h of co-culture in absence (white columns) or presence (black columns) of ADA (B) IFN-γ secretion in absence (white), or presence (black) of ADA at day 2 of co-culture of T cells with DC pulsed with inactivated HIV-1 BaL (30 min heat inactivated). Bars indicated the mean of proliferation and IFN-γ secretion, respectively, and error bars represent the SEM from the mean of the replicates (n = 8).
New clinical trial designs to improve the effectiveness of the vaccine
Despite clinical trials using autologous whole inactivated virus or mRNA have shown virological and immunological efficacy, no patient has been cured with these strategies. It is possible that optimized clinical trial design could improve the outcomes of these studies. Some of the studies have reported that virological response waned with time. These problems are probably due to persistent viral replication or viral rebound after a good initial response. A new strategy could be including booster doses after cART interruption that could potentially avoid this decline of the vaccine effect. This is suggested by preliminary data reported by Routy et al.52 It is possible that the results of an ongoing clinical trial using 4 immunization while on cART plus 2 additional booster doses after cART interruption could help to answer this question.9
Although some preventive vaccines have demonstrated low immunogenicity used alone, they are shown to be effective as a prime-boost strategy.70,71 Similarly, it has been shown in animal models that heterologous prime-boost immunization with viral vectors (Modified Vaccinia Ankara: MVA) and DC-targeting protein-based vaccines is a promising vaccination approach to optimize humoral and cellular immunity for therapeutic applications against AIDS.72 These prime-boost strategies deserve to be tested in the therapeutic vaccine setting to test whether they improve both HIV specific immune responses and virological responses as compared with one immungen alone.
In vivo DC targeting immunogens
Despite the advances in DC-based therapeutic vaccines for the treatment of HIV infection, the logistics of developing a tailored vaccine by ex vivo manipulation of autologous DCs for each individual patient may prove to be prohibitive, as significant expertise and specialized facilities are required, limiting its application to a small number of centers. Therefore, direct administration of immunogen targeting DCs has been suggested as an alternative. Flynn et al.73 compared in nonhuman primates (NHPs) immune responses to HIV Gag p24 within 3G9 antibody to DEC205, an uptake receptor on dendritic cells, to nontargeted protein, with or without poly ICLC as adjuvant. They showed qualitative differences in antibody and T-cell responses to DEC-HIV Gag p24 and Gag p24 protein and show that prime boost with protein and adjuvant followed by NYVAC elicits potent cellular immunity. It has been confirmed by other groups.72
At least 4 clinical trials with mRNA electroporated DC-based vaccines have been performed.50,52-54 The results of these clinical trials have been promising, but transfection of mRNA into DCs for adoptive transfer is cumbersome. Additional data in mouse tumor models obtained by Van Lint et al.68 suggested that intranodal immunization with mRNA based therapeutic vaccine encoding a tumor associated antigen plus a mixture of antigen presenting cell activation molecules, including CD40L, a constitutively active variant of Toll-like receptor (TLR) 4 and CD70 has the potential to efficiently augment the induction of tumor-specific immune responses compared with a mRNA electroporated DC-based vaccine. These data suggest that in vivo DC-targeting immunogens are good alternatives to ex vivo pulsed immunogens to be tested in humans in HIV infection.
Conclusion
DC-based vaccines have yielded the best results in this field. In spite of these encouraging results, functional cure has not been reached with this vaccine in any patient. New candidates and/or new optimized strategies of vaccination with the final objective of obtaining a functional cure as an alternative to cART for life are needed. We propose a number of potential strategies to improve the outcomes of MD-DC based therapeutic vaccines. New immunogens to pulse MD-DC should be tested and has to be clarified whether they should be designed using an autologous or heterologous virus. In addition, different adjuvants could be used combined with the immunogen to favor or impair antigen presentation. Different clinical trial designs (as a prime-boost strategy) could optimize the effectiveness of the vaccine. Finally, the ex vivo pulsing DC is a cumbersone strategy, but has opened the possibility that other in vivo DC targeting immunogens could be tested to improve the capture and antigen presentation avoiding the cumbersome manipulation of DCs.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This study was partially supported by grants: FP7-HEALTH-2013-INNOVATION-1 Proposal No: 602570-2, SAF 2012-39075, EC10-153, PI10/02984, TRA-094, FIS PS09/01297, SAF2008-04395, RIS*, HIVACAT**, ORVACS***. *Red Temática Cooperativa de Grupos de Investigación en Sida del Fondo de Investigación Sanitaria (FIS). **HIVACAT: HIV development program in Catalonia. ***ORVACS: Objectif recherche vaccin sida.
Footnotes
Previously published online: www.landesbioscience.com/journals/vaccines/article/25876
References
- 1.Palella FJJ, Jr., Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, et al. HIV Outpatient Study Investigators Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N Engl J Med. 1998;338:853–60. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 2.Siliciano JD, Kajdas J, Finzi D, Quinn TC, Chadwick K, Margolick JB, et al. Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4+ T cells. Nat Med. 2003;9:727–8. doi: 10.1038/nm880. [DOI] [PubMed] [Google Scholar]
- 3.Sidibé M. Newsmaker interview: Michel Sidibé. New HIV infections drop, but treatment demands rise. Interview by Jon Cohen. Science. 2010;330:1301. doi: 10.1126/science.330.6009.1301. [DOI] [PubMed] [Google Scholar]
- 4.Autran B, Carcelain G, Combadiere B, Debre P. Therapeutic vaccines for chronic infections. Science. 2004;305:205–8. doi: 10.1126/science.1100600. [DOI] [PubMed] [Google Scholar]
- 5.Kahn JO, Cherng DW, Mayer K, Murray H, Lagakos S. Evaluation of HIV-1 immunogen, an immunologic modifier, administered to patients infected with HIV having 300 to 549 x 10(6)/L CD4 cell counts: A randomized controlled trial. JAMA. 2000;284:2193–202. doi: 10.1001/jama.284.17.2193. [DOI] [PubMed] [Google Scholar]
- 6.Gorse GJ, Simionescu RE, Patel GB. Cellular immune responses in asymptomatic human immunodeficiency virus type 1 (HIV-1) infection and effects of vaccination with recombinant envelope glycoprotein of HIV-1. Clin Vaccine Immunol. 2006;13:26–32. doi: 10.1128/CVI.13.1.26-32.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dorrell L, Yang H, Iversen AK, Conlon C, Suttill A, Lancaster M, et al. Therapeutic immunization of highly active antiretroviral therapy-treated HIV-1-infected patients: safety and immunogenicity of an HIV-1 gag/poly-epitope DNA vaccine. AIDS. 2005;19:1321–3. doi: 10.1097/01.aids.0000180104.65640.16. [DOI] [PubMed] [Google Scholar]
- 8.García F, León A, Gatell JM, Plana M, Gallart T. Therapeutic vaccines against HIV infection. Hum Vaccin Immunother. 2012;8:569–81. doi: 10.4161/hv.19555. [DOI] [PubMed] [Google Scholar]
- 9.García F, Routy JP. Challenges in dendritic cells-based therapeutic vaccination in HIV-1 infection Workshop in dendritic cell-based vaccine clinical trials in HIV-1. Vaccine. 2011;29:6454–63. doi: 10.1016/j.vaccine.2011.07.043. [DOI] [PubMed] [Google Scholar]
- 10.Papagno L, Alter G, Assoumou L, Murphy RL, Garcia F, Clotet B, et al. ORVACS Study Group Comprehensive analysis of virus-specific T-cells provides clues for the failure of therapeutic immunization with ALVAC-HIV vaccine. AIDS. 2011;25:27–36. doi: 10.1097/QAD.0b013e328340fe55. [DOI] [PubMed] [Google Scholar]
- 11.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature 1998; 1998 Mar 19;392: 245-252. [DOI] [PubMed]
- 12.Wu L, KewalRamani VN. Dendritic-cell interactions with HIV: infection and viral dissemination. Nat Rev Immunol. 2006;6:859–68. doi: 10.1038/nri1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Izquierdo-Useros N, Naranjo-Gómez M, Archer J, Hatch SC, Erkizia I, Blanco J, et al. Capture and transfer of HIV-1 particles by mature dendritic cells converges with the exosome-dissemination pathway. Blood. 2009;113:2732–41. doi: 10.1182/blood-2008-05-158642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jung S, Unutmaz D, Wong P, Sano G, De los Santos K, Sparwasser T, et al. In vivo depletion of CD11c+ dendritic cells abrogates priming of CD8+ T cells by exogenous cell-associated antigens. Immunity. 2002;17:211–20. doi: 10.1016/S1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lubong Sabado R, Kavanagh DG, Kaufmann DE, Fru K, Babcock E, Rosenberg E, et al. In vitro priming recapitulates in vivo HIV-1 specific T cell responses, revealing rapid loss of virus reactive CD4 T cells in acute HIV-1 infection. PLoS One. 2009;4:e4256. doi: 10.1371/journal.pone.0004256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Climent N, Martinez-Navio JM, Gil C, Garcia F, Rovira C, Hurtado C, et al. Adenosine deaminase enhances T-cell response elicited by dendritic cells loaded with inactivated HIV. Immunol Cell Biol. 2009;87:634–9. doi: 10.1038/icb.2009.53. [DOI] [PubMed] [Google Scholar]
- 17.Pacheco R, Martinez-Navio JM, Lejeune M, Climent N, Oliva H, Gatell JM, et al. CD26, adenosine deaminase, and adenosine receptors mediate costimulatory signals in the immunological synapse. Proc Natl Acad Sci U S A. 2005;102:9583–8. doi: 10.1073/pnas.0501050102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martinez-Navio JM, Casanova V, Pacheco R, Naval-Macabuhay I, Climent N, Garcia F, et al. Adenosine deaminase potentiates the generation of effector, memory, and regulatory CD4+ T cells. J Leukoc Biol. 2011;89:127–36. doi: 10.1189/jlb.1009696. [DOI] [PubMed] [Google Scholar]
- 19.Martinez-Navio JM, Climent N, Pacheco R, Garcia F, Plana M, Nomdedeu M, et al. Immunological dysfunction in HIV-1-infected individuals caused by impairment of adenosine deaminase-induced costimulation of T-cell activation. Immunology. 2009;128:393–404. doi: 10.1111/j.1365-2567.2009.03121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mangeat B, Turelli P, Caron G, Friedli M, Perrin L, Trono D. Broad antiretroviral defence by human APOBEC3G through lethal editing of nascent reverse transcripts. Nature. 2003;424:99–103. doi: 10.1038/nature01709. [DOI] [PubMed] [Google Scholar]
- 21.Chiu YL, Soros VB, Kreisberg JF, Stopak K, Yonemoto W, Greene WC. Cellular APOBEC3G restricts HIV-1 infection in resting CD4+ T cells. Nature. 2005;435:108–14. doi: 10.1038/nature03493. [DOI] [PubMed] [Google Scholar]
- 22.Pion M, Granelli-Piperno A, Mangeat B, Stalder R, Correa R, Steinman RM, et al. APOBEC3G/3F mediates intrinsic resistance of monocyte-derived dendritic cells to HIV-1 infection. J Exp Med. 2006;203:2887–93. doi: 10.1084/jem.20061519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rodríguez-García M, Oliva H, Climent N, García F, Gatell JM, Gallart T. Human immature monocyte-derived dendritic cells produce and secrete alpha-defensins 1-3. J Leukoc Biol. 2007;82:1143–6. doi: 10.1189/jlb.0507295. [DOI] [PubMed] [Google Scholar]
- 24.Rodríguez-García M, Oliva H, Climent N, Escribese MM, García F, Moran TM, et al. Impact of alpha-defensins1-3 on the maturation and differentiation of human monocyte-derived DCs. Concentration-dependent opposite dual effects. Clin Immunol. 2009;131:374–84. doi: 10.1016/j.clim.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 25.Rodríguez-García M, Climent N, Oliva H, Casanova V, Franco R, Leon A, et al. Increased alpha-defensins 1-3 production by dendritic cells in HIV-infected individuals is associated with slower disease progression. PLoS One. 2010;5:e9436. doi: 10.1371/journal.pone.0009436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Letvin NL, Walker BD. Immunopathogenesis and immunotherapy in AIDS virus infections. Nat Med. 2003;9:861–6. doi: 10.1038/nm0703-861. [DOI] [PubMed] [Google Scholar]
- 27.Day CL, Walker BD. Progress in defining CD4 helper cell responses in chronic viral infections. J Exp Med. 2003;198:1773–7. doi: 10.1084/jem.20031947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaech SM, Ahmed R. Immunology. CD8 T cells remember with a little help. Science. 2003;300:263–5. doi: 10.1126/science.1084511. [DOI] [PubMed] [Google Scholar]
- 29.Steinman RM, Pope M. Exploiting dendritic cells to improve vaccine efficacy. J Clin Invest. 2002;109:1519–26. doi: 10.1172/JCI15962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goulder PJ, Brander C, Tang Y, Tremblay C, Colbert RA, Addo MM, et al. Evolution and transmission of stable CTL escape mutations in HIV infection. Nature. 2001;412:334–8. doi: 10.1038/35085576. [DOI] [PubMed] [Google Scholar]
- 31.Allen TM, Altfeld M, Geer SC, Kalife ET, Moore C, O’sullivan KM, et al. Selective escape from CD8+ T-cell responses represents a major driving force of human immunodeficiency virus type 1 (HIV-1) sequence diversity and reveals constraints on HIV-1 evolution. J Virol. 2005;79:13239–49. doi: 10.1128/JVI.79.21.13239-13249.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Allen TM, Yu XG, Kalife ET, Reyor LL, Lichterfeld M, John M, et al. De novo generation of escape variant-specific CD8+ T-cell responses following cytotoxic T-lymphocyte escape in chronic human immunodeficiency virus type 1 infection. J Virol. 2005;79:12952–60. doi: 10.1128/JVI.79.20.12952-12960.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feeney ME, Tang Y, Pfafferott K, Roosevelt KA, Draenert R, Trocha A, et al. HIV-1 viral escape in infancy followed by emergence of a variant-specific CTL response. J Immunol. 2005;174:7524–30. doi: 10.4049/jimmunol.174.12.7524. [DOI] [PubMed] [Google Scholar]
- 34.Donaghy H, Gazzard B, Gotch F, Patterson S. Dysfunction and infection of freshly isolated blood myeloid and plasmacytoid dendritic cells in patients infected with HIV-1. Blood. 2003;101:4505–11. doi: 10.1182/blood-2002-10-3189. [DOI] [PubMed] [Google Scholar]
- 35.Chehimi J, Campbell DE, Azzoni L, Bacheller D, Papasavvas E, Jerandi G, et al. Persistent decreases in blood plasmacytoid dendritic cell number and function despite effective highly active antiretroviral therapy and increased blood myeloid dendritic cells in HIV-infected individuals. J Immunol. 2002;168:4796–801. doi: 10.4049/jimmunol.168.9.4796. [DOI] [PubMed] [Google Scholar]
- 36.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med. 1994;179:1109–18. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 38.Larsson M, Fonteneau JF, Bhardwaj N. Cross-presentation of cell-associated antigens by dendritic cells. Curr Top Microbiol Immunol. 2003;276:261–75. doi: 10.1007/978-3-662-06508-2_12. [DOI] [PubMed] [Google Scholar]
- 39.Lapenta C, Santini SM, Logozzi M, Spada M, Andreotti M, Di Pucchio T, et al. Potent immune response against HIV-1 and protection from virus challenge in hu-PBL-SCID mice immunized with inactivated virus-pulsed dendritic cells generated in the presence of IFN-alpha. J Exp Med. 2003;198:361–7. doi: 10.1084/jem.20021924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoshida A, Tanaka R, Murakami T, Takahashi Y, Koyanagi Y, Nakamura M, et al. Induction of protective immune responses against R5 human immunodeficiency virus type 1 (HIV-1) infection in hu-PBL-SCID mice by intrasplenic immunization with HIV-1-pulsed dendritic cells: possible involvement of a novel factor of human CD4(+) T-cell origin. J Virol. 2003;77:8719–28. doi: 10.1128/JVI.77.16.8719-8728.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aline F, Brand D, Bout D, Pierre J, Fouquenet D, Verrier B, et al. Generation of specific Th1 and CD8+ T-cell responses by immunization with mouse CD8+ dendritic cells loaded with HIV-1 viral lysate or envelope glycoproteins. Microbes Infect. 2007;9:536–43. doi: 10.1016/j.micinf.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 42.Lu W, Wu X, Lu Y, Guo W, Andrieu JM. Therapeutic dendritic-cell vaccine for simian AIDS. Nat Med. 2003;9:27–32. doi: 10.1038/nm806. [DOI] [PubMed] [Google Scholar]
- 43.Kundu SK, Engleman E, Benike C, Shapero MH, Dupuis M, van Schooten WC, et al. A pilot clinical trial of HIV antigen-pulsed allogeneic and autologous dendritic cell therapy in HIV-infected patients. AIDS Res Hum Retroviruses. 1998;14:551–60. doi: 10.1089/aid.1998.14.551. [DOI] [PubMed] [Google Scholar]
- 44.Lu W, Arraes LC, Ferreira WT, Andrieu JM. Therapeutic dendritic-cell vaccine for chronic HIV-1 infection. Nat Med. 2004;10:1359–65. doi: 10.1038/nm1147. [DOI] [PubMed] [Google Scholar]
- 45.García F, Lejeune M, Climent N, Gil C, Alcami J, Morente V, et al. Therapeutic immunization with dendritic cells loaded with inactivated autologous HIV-1 in chronic HIV-1 infected patients. J Infect Dis. 2005;191:1680–5. doi: 10.1086/429340. [DOI] [PubMed] [Google Scholar]
- 46.Ide F, Nakamura T, Tomizawa M, Kawana-Tachikawa A, Odawara T, Hosoya N, et al. Peptide-loaded dendritic-cell vaccination followed by treatment interruption for chronic HIV-1 infection: a phase 1 trial. J Med Virol. 2006;78:711–8. doi: 10.1002/jmv.20612. [DOI] [PubMed] [Google Scholar]
- 47.Connolly NC, Whiteside TL, Wilson C, Kondragunta V, Rinaldo CR, Riddler SA. Therapeutic immunization with human immunodeficiency virus type 1 (HIV-1) peptide-loaded dendritic cells is safe and induces immunogenicity in HIV-1-infected individuals. Clin Vaccine Immunol. 2008;15:284–92. doi: 10.1128/CVI.00221-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gandhi RT, O’Neill D, Bosch RJ, Chan ES, Bucy RP, Shopis J, et al. AIDS Clinical Trials Group A5130 team A randomized therapeutic vaccine trial of canarypox-HIV-pulsed dendritic cells vs. canarypox-HIV alone in HIV-1-infected patients on antiretroviral therapy. Vaccine. 2009;27:6088–94. doi: 10.1016/j.vaccine.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kloverpris H, Karlsson I, Bonde J, Thorn M, Vinner L, Pedersen AE, et al. Induction of novel CD8+ T-cell responses during chronic untreated HIV-1 infection by immunization with subdominant cytotoxic T-lymphocyte epitopes. AIDS. 2009;23:1329–40. doi: 10.1097/QAD.0b013e32832d9b00. [DOI] [PubMed] [Google Scholar]
- 50.Routy JP, Boulassel MR, Yassine-Diab B, Nicolette C, Healey D, Jain R, et al. Immunologic activity and safety of autologous HIV RNA-electroporated dendritic cells in HIV-1 infected patients receiving antiretroviral therapy. Clin Immunol. 2010;134:140–7. doi: 10.1016/j.clim.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.García F, Climent N, Assoumou L, Gil C, González N, Alcamí J, et al. DCV2/MANON07- AIDS Vaccine Research Objective Study Group A therapeutic dendritic cell-based vaccine for HIV-1 infection. J Infect Dis. 2011;203:473–8. doi: 10.1093/infdis/jiq077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Routy JP, Angel J, Vezina S, Tremblay C, Loutfy M, Gill J, et al. Final Analysis of a Phase 2 Study of an Autologous DC Immunotherapy (AGS-004) Showed Positive Outcomes in Primary Endpoint of Viral Load Control, and Favorable Safety and Immunogenicity Profile, in Subjects Undergoing Structured Treatment Interruption of ART (Abstract 385). 18th Conference on Retroviruses and Opportunistic Infections Boston February 27-March 2 2011. [Google Scholar]
- 53.Van Gulck E, Vlieghe E, Vekemans M, Van Tendeloo VF, Van De Velde A, Smits E, et al. mRNA-based dendritic cell vaccination induces potent antiviral T-cell responses in HIV-1-infected patients. AIDS. 2012;26:F1–12. doi: 10.1097/QAD.0b013e32834f33e8. [DOI] [PubMed] [Google Scholar]
- 54.Allard SD, De Keersmaecker B, de Goede AL, Verschuren EJ, Koetsveld J, Reedijk ML, et al. A phase I/IIa immunotherapy trial of HIV-1-infected patients with Tat, Rev and Nef expressing dendritic cells followed by treatment interruption. Clin Immunol. 2012;142:252–68. doi: 10.1016/j.clim.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 55.Garcia F, Climent N, Guardo AC, Gil C, Leon A, Autran B, et al. A Dendritic Cell-Based Vaccine Elicits T Cell Responses Associated with Control of HIV-1 Replication. Sci Transl Med 2013; 5: 166ra2. [DOI] [PubMed]
- 56.Routy JP, Boulassel MR, Yassine-Diab B, Nicolette C, Healey D, Jain R, et al. Immunologic activity and safety of autologous HIV RNA-electroporated dendritic cells in HIV-1 infected patients receiving antiretroviral therapy. Clin Immunol. 2010;134:140–7. doi: 10.1016/j.clim.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Routy JP, Boulassel MR, Mona L, Sylvie V, Cecile T, Jonathan A, et al. Safety and viral load changes in HIV-1 infected subjects treated with autologous dendritic immune therapy following ART discontinuation (CTN#239) Abstract OA04-05. AIDS Vaccine 2009 Conference Paris, France from 19 - 22 October 2009.
- 58.Climent N, Guerra S, García F, Rovira C, Miralles L, Gómez CE, et al. Dendritic cells exposed to MVA-based HIV-1 vaccine induce highly functional HIV-1-specific CD8(+) T cell responses in HIV-1-infected individuals. PLoS One. 2011;6:e19644. doi: 10.1371/journal.pone.0019644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pion M, Serramia MJ, Diaz L, Bryszewska M, Gallart T, García F, et al. Phenotype and functional analysis of human monocytes-derived dendritic cells loaded with a carbosilane dendrimer. Biomaterials. 2010;31:8749–58. doi: 10.1016/j.biomaterials.2010.07.093. [DOI] [PubMed] [Google Scholar]
- 60.Climent N, Munier S, Piqué N, García F, Pavot V, Leon A, et al. Loading dendritic cells from HIV-1 infected patients with PLA-p24 nanoparticles or MVA expressing HIV genes induces HIV-1-specific T cell response. AIDS Vaccine 2012 Conference Boston, USA September 9-12, 2012. [Google Scholar]
- 61.Aline F, Brand D, Pierre J, Roingeard P, Séverine M, Verrier B, et al. Dendritic cells loaded with HIV-1 p24 proteins adsorbed on surfactant-free anionic PLA nanoparticles induce enhanced cellular immune responses against HIV-1 after vaccination. Vaccine. 2009;27:5284–91. doi: 10.1016/j.vaccine.2009.05.028. [DOI] [PubMed] [Google Scholar]
- 62.Guillon C, Mayol K, Terrat C, Compagnon C, Primard C, Charles MH, et al. Formulation of HIV-1 Tat and p24 antigens by PLA nanoparticles or MF59 impacts the breadth, but not the magnitude, of serum and faecal antibody responses in rabbits. Vaccine. 2007;25:7491–501. doi: 10.1016/j.vaccine.2007.08.060. [DOI] [PubMed] [Google Scholar]
- 63.Casanova V, Naval-Macabuhay I, Massanella M, Rodríguez-García M, Blanco J, Gatell JM, et al. Adenosine deaminase enhances the immunogenicity of human dendritic cells from healthy and HIV-infected individuals. PLoS One. 2012;7:e51287. doi: 10.1371/journal.pone.0051287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Blanco J, Valenzuela A, Herrera C, Lluís C, Hovanessian AG, Franco R. The HIV-1 gp120 inhibits the binding of adenosine deaminase to CD26 by a mechanism modulated by CD4 and CXCR4 expression. FEBS Lett. 2000;477:123–8. doi: 10.1016/S0014-5793(00)01751-8. [DOI] [PubMed] [Google Scholar]
- 65.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–65. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Velu V, Titanji K, Zhu B, Husain S, Pladevega A, Lai L, et al. Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature. 2009;458:206–10. doi: 10.1038/nature07662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Van Lint S, Goyvaerts C, Maenhout S, Goethals L, Disy A, Benteyn D, et al. Preclinical evaluation of TriMix and antigen mRNA-based antitumor therapy. Cancer Res. 2012;72:1661–71. doi: 10.1158/0008-5472.CAN-11-2957. [DOI] [PubMed] [Google Scholar]
- 69.Brentjens RJ, Davila ML, Riviere I, Park J, Wang X, Cowell LG, et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med. 2013;5:77ra38. doi: 10.1126/scitranslmed.3005930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, et al. MOPH-TAVEG Investigators Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361:2209–20. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 71.Sandström E, Nilsson C, Hejdeman B, Bråve A, Bratt G, Robb M, et al. HIV Immunogenicity Study 01/02 Team Broad immunogenicity of a multigene, multiclade HIV-1 DNA vaccine boosted with heterologous HIV-1 recombinant modified vaccinia virus Ankara. J Infect Dis. 2008;198:1482–90. doi: 10.1086/592507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Flamar A, Le Grand R, Contreras V, Zurawski S, Dereudre-Bosquet N. M, et al. Targeting HIV Gag p24 To DICR On Dendritic Cells Induces T Cell And Potent And Long-Lasting Antibody Responses In Non-Human Primates. AIDS Vaccine Conference Boston, USA September 9-12, 2012. [Google Scholar]
- 73.Flynn BJ, Kastenmüller K, Wille-Reece U, Tomaras GD, Alam M, Lindsay RW, et al. Immunization with HIV Gag targeted to dendritic cells followed by recombinant New York vaccinia virus induces robust T-cell immunity in nonhuman primates. Proc Natl Acad Sci U S A. 2011;108:7131–6. doi: 10.1073/pnas.1103869108. [DOI] [PMC free article] [PubMed] [Google Scholar]


