Abstract
A recombinant hepatitis E vaccine, Hecolin®, has been proven safe and effective in healthy adults. As hepatitis B surface antigen (HBsAg) positive individuals have a higher risk of poor prognosis after super-infection with hepatitis E virus (HEV), the safety and immunogenicity of Hecolin® in this population should be assessed. The present study is an extending analysis of data from a large randomized controlled clinical trial of Hecolin®. Healthy participants (n = 14,065) without current or previous evidence of chronic liver disease were randomized to receive Hecolin® or placebo (hepatitis B vaccine) and donated their blood samples before vaccination and subsequently over 31 mo. Most of the adverse events were mild and comparable between participants with and without baseline hepatitis B surface antigen (HBsAg). No vaccine-related serious adverse events were reported. Rates of serious adverse events in HBsAg (+) or HBsAg (-) participants were also comparable between both groups. Almost all participants in the Hecolin® group seroconverted to anti-HEV one month after full vaccination. The antibody response rates and levels were similar in HBsAg (+) and HBsAg (-) participants (98.38%, 19.32 Wu/mL vs. 98.69%, 19.00 Wu/mL). The two-year antibody dynamics of HBsAg (+) participants overlapped perfectly with those of HBsAg (-) participants. In conclusion, the safety and immunogenicity of Hecolin® for HBsAg (+) adults is very similar to that for the general population.
Keywords: HBsAg, HEV239, Hecolin®, hepatitis E, immunogenicity, safety, vaccine
Introduction
Hepatitis E virus (HEV) is one of the leading causes of acute hepatitis.1 An estimated one third of the world’s population living in developing countries has been infected with HEV,2 and reports of autochthonous hepatitis E cases are increasing in developed countries.3-7 Recently, a striking spectrum of serious complications has been reported, including “acute-on-chronic” liver failure, extra-hepatic manifestations, and chronic hepatitis. These complications are mainly associated with autochthonous hepatitis E in developed countries.8,9 Acute-on-chronic disease, which refers to hepatitis with the rapid appearance of signs of liver failure, ascites and encephalopathy in a person with pre-existing liver disease, is usually more severe than acute hepatitis and sometimes progresses to fulminant hepatic failure, with a fatality rate of up to 70%.10,11 Therefore, it is imperative for people with a higher risk of chronic liver disease to become protected against HEV infection.
Hepatitis B virus (HBV) accounts for most of the chronic liver diseases (CLDs) in China. Therefore, the safety and immunogenicity data of the hepatitis E vaccine in HBV carriers will be important to the overall vaccine strategy. In a large randomized controlled phase III clinical trial of a recombinant HEV vaccine, HEV239 with the commercial name Hecolin®, demonstrated excellent safety, immunogenicity and efficacy in the general healthy Chinese adult population,12 which led to the first commercialization of the hepatitis E vaccine.13 The trial involved 112 604 healthy adults from 11 townships. Among them 14 065 participants from 2 townships donated their blood samples before and after the vaccination for immunogenicity evaluation. These participants were analyzed according to their baseline HBV status to evaluate the safety and immunogenicity of Hecolin® in HBsAg (+) adults.
Results
Baseline characteristics of the participants
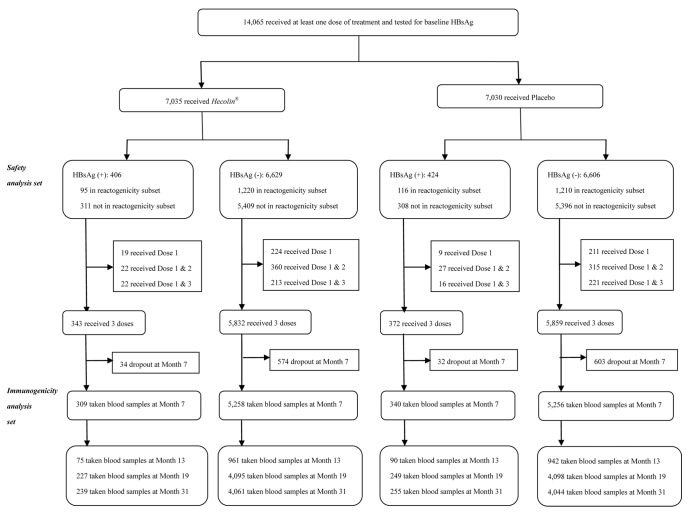
As shown in Figure 1, 14 065 participants received at least one dose of Hecolin® or placebo and were tested for HBsAg before vaccination (Safety Analysis Set). Among them, 830 (5.9%) participants were positive for HBsAg. In the Hecolin® group, 309 HBsAg (+) and 5258 HBsAg (-) participants received three vaccine doses and donated their blood samples at month 7 according to the protocol (Immunogenicity Analysis Set). The compliance rates for HBsAg(+) and HBsAg(-) participants were similar for the Hecolin® and placebo groups (p > 0.05). A subset of 2641 participants was selected for active surveillance of adverse events (reactogenicity subset): 95 HBsAg (+) and 1,220 HBsAg (-) participants were in the Hecolin® group, while 116 and 1210 participants were in the placebo group. The baseline characteristics were generally comparable for the HBsAg (+) and HBsAg (-) populations (Table 1).
Figure 1. Trial Profile.
Table 1. Baseline demographic characteristics of the HBsAg (+) and HBsAg (-) participants.
| Vaccine | Placebo | P value# | |||
|---|---|---|---|---|---|
| HBsAg (+) | HBsAg (-) | HBsAg (+) | HBsAg (-) | ||
| All participants (Safety analysis set) | 406 | 6629 | 424 | 6606 | |
| Age (SD) | 43.95 (10.99) | 44.39 (11.31) | 45.31 (10.41) | 44.36 (11.36) | P1 = 0.4375, P2 = 0.0668 |
| Men (%) | 186 (45.81) | 2711 (40.90) | 199 (46.93) | 2739 (41.46) | P1 = 0. 0507, P2 = 0.7461 |
| Positive anti-HEV (prevalence) | 183 (45.07) | 3158 (48.03) | 211 (49.76) | 3060 (46.32) | P1 = 0. 3150, P2 = 0.1762 |
| GMC (95%CI) (Wu/mL)* | 0.53 (0.44–0.63) | 0.54 (0.51–0.56) | 0.65 (0.55–0.77) | 0.52 (0.50–0.54) | P1 = 0.8915, P2 = 0.0936 |
| Immunogenicity analysis set | 309 | 5258 | 340 | 5256 | |
| Age (SD) | 44.73 (10.87) | 45.25 (10.74) | 46.02 (9.93) | 45.20 (10.87) | P1 = 0.4102, P2 = 0.1150 |
| Men (%) | 135 (43.69) | 2036 (38.72) | 146 (42.94) | 2059 (39.17) | P1 = 0.0819, P2 = 0.8476 |
| Reactogenicity subset | 95 | 1220 | 116 | 1210 | |
| Age (SD) | 42.92 (11.90) | 44.83 (11.17) | 44.89 (11.23) | 44.95 (11.09) | P1 = 0.1097, P2 = 0.2181 |
| Men (%) | 45 (47.37) | 478 (39.18) | 59 (50.86) | 501 (41.40) | P1 = 0.1163, P2 = 0.6135 |
GMC: Geometric mean concentration of anti-HEV IgG. #P1-Between Hecolin® Group HBsAg (+) and Hecolin® Group HBsAg (-); P2-Between Hecolin® Group HBsAg (+) and Control Group HBsAg (+).
Safety profile
Most adverse events were mild. Participants in the reactogenicity subset were regularly interviewed by investigators after the receipt of each vaccine dose to assess adverse events (AEs) (Table 2). In the reactogenicity subset of the Hecolin® group, 28 HBsAg (+) participants reported solicited adverse events within 72 h. Among them, 11 (11.6%) reported solicited local adverse events, and 21 (22.1%) reported solicited systemic adverse events. The solicited adverse event rates were similar between HBsAg (+) and HBsAg (-) participants in the Hecolin® group (p > 0.05). The HBsAg (+) population in the Hecolin® group reported a higher rate of solicited local AEs than those in the placebo group, which may be due to the higher antigen content of Hecolin®, and in agreement with a previous report.12 Rates of serious adverse events (SAE) were not significantly different between HBsAg (+) and HBsAg (-) participants in both groups. No vaccine related SAE occurred in any of the subjects during the study.
Table 2. Adverse reactions/events after vaccination with Hecolin® in the HBsAg (+) and HBsAg (-) participants.
| Vaccine | Placebo | P value* | |||
|---|---|---|---|---|---|
| HBsAg (+) | HBsAg (-) | HBsAg (+) | HBsAg (-) | ||
| Participants in the reactogenicity subset | 95 | 1,220 | 116 | 1210 | |
| Solicited local adverse events within 72 h | |||||
| All | 11 (11.58%) | 166 (13.61%) | 5 (4.31%) | 89 (7.36%) | P1 = 0.5770, P2 = 0.0472 |
| ≥ Grade 3 | 0 (0.00%) | 2 (0.16%) | 0 (0.00%) | 0 (0.00%) | P1 = 1.0000, P2 = 1.0000 |
| Solicited systemic adverse events within 72 h | |||||
| All | 21 (22.11%) | 246 (20.16%) | 13 (11.21%) | 250 (20.66) | P1 = 0.6505, P2 = 0.0322 |
| ≥ Grade 3 | 0 (0.00%) | 7 (0.57%) | 1 (0.86%) | 3 (0.25%) | P1 = 1.0000, P2 = 1.0000 |
| Participants not in the reactogenicity subset | 311 | 5409 | 308 | 5396 | |
| Solicited local adverse events within 72 h | |||||
| All | 6 (1.93%) | 106 (1.96%) | 2 (0.65%) | 53 (0.98%) | P1 = 0.9699, P2 = 0.2859 |
| ≥ Grade 3 | 1 (0.32%) | 10 (0.18%) | 0 (0.00%) | 5 (0.09%) | P1 = 0.4596, P2 = 1.0000 |
| Solicited systemic adverse events within 72 h | |||||
| All | 4 (1.29%) | 96 (1.77%) | 3 (0.97%) | 91 (1.69%) | P1 = 0.5226, P2 = 1.0000 |
| ≥ Grade 3 | 1 (0.32%) | 7 (0.13%) | 0 (0.00%) | 9 (0.17%) | P1 = 0.3608, P2 = 1.0000 |
| Participants in the whole vaccinated cohort | 406 | 6629 | 424 | 6606 | |
| Unsolicited events within 30 d | |||||
| All | 39 (9.61%) | 707 (10.67%) | 40 (9.43%) | 748 (11.32%) | P1 = 0.5010, P2 = 0.9328 |
| ≥ Grade 3 | 2 (0.49%) | 84 (1.27%) | 3 (0.71%) | 80 (1.21%) | P1 = 0.2407, P2 = 1.0000 |
| SAEs during the period from month 0 to month 31 | |||||
| All | 29 (7.14%) | 366 (5.52%) | 25 (5.90%) | 359 (5.43%) | P1 = 0.1683, P2 = 0.4667 |
P1-Between Hecolin® Group HBsAg (+) and Hecolin® Group HBsAg (-); P2-Between Hecolin® Group HBsAg (+) and Control Group HBsAg (+).
Immunologic studies
Those participants who received all 3 doses of Hecolin® according to the protocol and had their anti-HEV IgG level tested at month 0 and month 7 were included in the immunogenicity analysis set (Fig. 1 and Table 3). At month 7, 98.38% and 98.69% of the baseline HBsAg (+) or HBsAg (-) participants showed positive seroconversion, respectively (p = 0.6063). The antibody level of anti-HEV IgG at month 7 in the HBsAg (+) cohort was 19.32 Wu/mL (95%CI, 17.68–21.12), a concentration comparable to that in the HBsAg (-) cohort (19.00 Wu/mL, 95%CI 18.59–19.42) (Table 3). The antibody response for each age group was similar for HBsAg (+) or HBsAg (-) participants (Fig. 2). Antibody dynamics after vaccination were very similar for HBsAg (+) or HBsAg (-) participants, regardless of their baseline anti-HEV status (Fig. 3).
Table 3. Immunogenicity of Hecolin® in HBsAg (+) and HBsAg (-) participants.
| Anti-HEV IgG status | HBsAg (+) | HBsAg (-) | P value |
|---|---|---|---|
| Baseline | |||
| Number | 309 | 5,258 | |
| No. +ve (rate, %)& | 144 (46.60) | 2,515 (47.83) | 0.6740 |
| GMC (95% CI, Wu/ml)* | 0.53 (0.43, 0.65) | 0.54 (0.51, 0.56) | 0.8752 |
| Antibody response | |||
| Seroconversion rate (95% CI, %) | |||
| All | 98.38 (96.26,99.47) | 98.69 (98.34,98.98) | 0.6053 |
| Baseline anti-HEV (+) | 96.53 (92.08, 98.86) | 97.46 (96.76, 98.03) | 0.4207 |
| Baseline anti-HEV (-) | 100.00 (97.79, 100.00) | 99.82 (99.58, 99.94) | 1.0000 |
| GMC on 7 mo (95%CI, Wu/ml)* | |||
| All | 19.32 (17.68, 21.12) | 19.00 (18.59, 19.42) | 0.7271 |
| Baseline anti-HEV (+) | 25.33 (22.74, 28.21) | 24.71 (24.14, 25.29) | 0.6283 |
| Baseline anti-HEV (-) | 15.25 (13.42, 17.34) | 14.94 (14.44, 15.45) | 0.7712 |
&No of participants who had baseline positive anti-HEV IgG, the ratio in the parenthesis is the positive rate in this group. *GMC, geometric mean concentration.
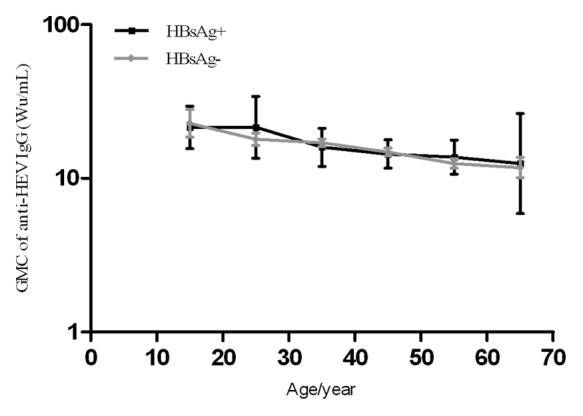
Figure 2. Anti-HEV IgG levels of HBsAg (+) and HBsAg (-) participants at month 7 by age. Baseline anti-HEV (-) participants who received all 3 doses of Hecolin® according to the protocol were analyzed. Solid black line: HBsAg (+) participants; solid gray lines: HBsAg (-) participants. 95% confidence intervals (CI) are shown. GMC, geometric mean concentration.
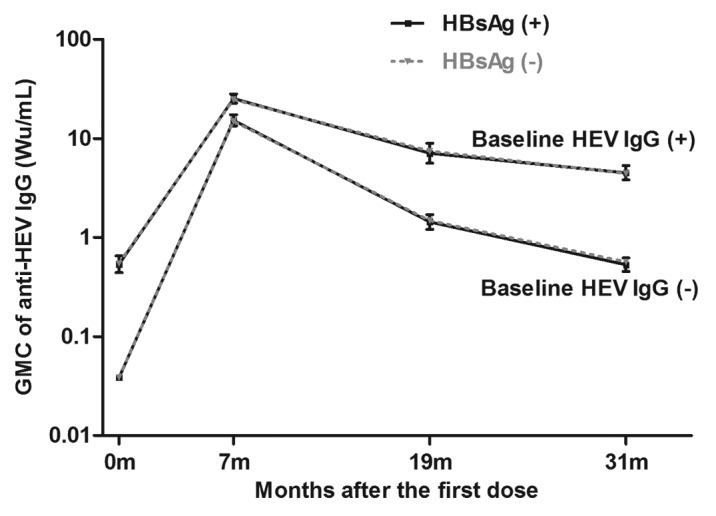
Figure 3. Anti-HEV dynamics after Hecolin® vaccination. Participants who received Hecolin® according to the protocol and were tested for anti-HEV at month 0 and month 7 (immunogenicity analysis set) were included. Solid black line: HBsAg (+) participants; dashed gray lines: HBsAg (-) participants. GMC, geometric mean concentration. 95% CI is shown.
Discussion
In this analysis of the randomized controlled clinical trial of the hepatitis E vaccine Hecolin®, the vaccine was proven to be highly immunogenic and well tolerated for both HBsAg positive participants and persons without HBV infection. This finding is important for the use of the vaccine to prevent the injury of HEV super-infection in HBV carriers.
The data suggest that individuals with chronic liver disease should be prioritized for vaccination to prevent serious damage from HEV infection.8-11 However, the exclusion of pre-diagnosed CLD patients in enrollment prevented the direct evaluation of the vaccine’s safety and immunogenicity in this cohort. There are several other limitations to this study. First, the vaccine efficacy in HBsAg (+) participants cannot be directly calculated due to the low infection rate in the area. Second, the baseline hepatocellular status of these HBsAg (+) participants has not been tested, and no data of the fluctuation in the hepatocellular status after each dose and after the full vaccination course were available. Thus some subclinical impact of the vaccine may have been overlooked.
Many reports evidence that acute super-infection of the hepatitis virus, hepatitis A virus, hepatitis B virus or hepatitis E virus, in underlying CLD patients resulted in poor prognosis and a high fatality rate.8-11,14-16 The safety and immunogenicity of the hepatitis A vaccine and the hepatitis B vaccine for CLD patients has been previously demonstrated.17,18 The results precipitated the recommendation that both vaccines should be administered to CLD patients without the corresponding viral infection. The American Association for the Study of Liver Diseases (AASLD) recommends hepatitis A vaccination for all hepatitis B and hepatitis C virus infected patients, regardless of the stage of their liver disease.19,20 Furthermore, both the National Institutes of Health (NIH) and AASLD recommend hepatitis B vaccination for chronic hepatitis C patients who are at risk for hepatitis B infection, whereas the Centers for Disease Control and Prevention (CDC) recommend hepatitis B vaccination for individuals with CLD.21-23 Considering that many “healthy” HBsAg positive individuals suffer from liver damage to some extent,24 the current finding in “healthy” HBsAg (+) participants paves the way for further studies to assess the benefits of Hecolin® in CLD patients.
In conclusion, this study demonstrated that Hecolin® is safe and immunogenic in HBsAg (+) individuals. HBV carriers have a higher risk of progressing into CLD and cirrhosis. In view of the apparent risks posed by acute HEV infection in patients with CLD, we strongly recommend HBV carriers to be vaccinated with the HEV vaccine.
Materials and Methods
Participants
The initial efficacy analysis of the trial has been reported previously.12 In brief, a double-blind, randomized, placebo-controlled trial was conducted from August 2007 through June 2009 in Dongtai City, Jiangsu Province, China.12 Healthy eligible adults aged 16–65 y without current or previous evidence of chronic liver disease were randomly assigned to receive three doses of the Hecolin® vaccine (containing 30 μg recombinant HEV virus-like particles adsorbed in 0.5 mL of alum adjuvant; Xiamen Innovax) or the placebo hepatitis B vaccine (containing 5 μg recombinant HBsAg in 0.5 mL of alum adjuvant; Beijing Tiantan Biologic). Written consent was obtained from each participant. Independent Ethics Committee approval was obtained from the Ethics Committee of the Jiangsu Provincial Center for Disease Control and Prevention.
A total of 112 604 adults participated in the trial, enrolled from 11 townships in Dongtai County. Participants living in two townships (Qingdong and Anfeng) who donated their blood samples on month 0 (baseline), month 7 (one month after the third dose), month 19, and month 31 were evaluated for vaccine safety (Safety Analysis Set). A subset of participants from Qingdong township were visited timely at home to observe their adverse events by investigators (Reactogenicity Subset). Other participants were asked to record any adverse events on diary cards. Any SAEs were recorded throughout the study for all the participants. Participants who received all three doses of vaccines according to the protocol and who donated paired serum samples at month 0 and month 7 were included in the immunogenicity analysis set.
Laboratory measurements
The anti-HEV IgG were tested and quantified with commercialized assays (Beijing Wantai) and expressed in WHO unit per ml (Wu/ml), as previously described.12 Those participants whose serum samples at month 7 contained an anti-HEV IgG level four times higher than baseline were labeled as positive for seroconversion. HBsAg was tested with a commercialized assay (Beijing Wantai) according to the product protocol.
Statistical analyses
A chi-square test or a two-sided Fisher’s exact test was used to compare the rates between different groups. The independent-sample t-test was used to compare mean age and the geometric mean concentration of anti-HEV between groups. Data analysis was performed with the use of SPSS (Statistical Package for the Social Sciences, version 18.0). All reported P values are two-sided with an α value of 0.05.
Acknowledgments
This work was supported by the National Major Scientific and Technological Special Project for the “Prevention and Control of Important Infectious Diseases” (2012ZX10002001, 2011ZX10004-903) and the National Major Scientific and Technological Special Project for “Significant New Drug Development” (2013ZX09101017).
Glossary
Abbreviations:
- HBsAg
hepatitis B surface antigen
- HEV
hepatitis E virus
- HBV
hepatitis B virus
- CLD
chronic liver disease
- AE
adverse event
- HCC
hepatocellular carcinoma
- HBsAg
hepatitis B surface antigen
- SAE
serious adverse event
- AASLD
American Association for the Study of Liver Diseases
- Wu
World Health Organization unit
- CI
confidence interval
- GMC
geometric mean concentration
Disclosure of Potential Conflicts of Interest
Xiao-Hui Liu and Meng Guo are currently employees of Xiamen Innovax. The other authors declare that they have no conflicts of interest.
Footnotes
Previously published online: www.landesbioscience.com/journals/vaccines/article/25814
References
- 1.Kamar N, Bendall R, Legrand-Abravanel F, Xia NS, Ijaz S, Izopet J, et al. Hepatitis E. Lancet. 2012;379:2477–88. doi: 10.1016/S0140-6736(11)61849-7. [DOI] [PubMed] [Google Scholar]
- 2.Purcell RH, Emerson SU. Prevention. In: Thomas HC, Lemon S, Zuckerman AJ, eds. Viral hepatitis, 3rd edn. Malden, MA Blackwell Publishing; 2005:635-45. [Google Scholar]
- 3.Dalton HR, Stableforth W, Thurairajah P, Hazeldine S, Remnarace R, Usama W, et al. Autochthonous hepatitis E in Southwest England: natural history, complications and seasonal variation, and hepatitis E virus IgG seroprevalence in blood donors, the elderly and patients with chronic liver disease. Eur J Gastroenterol Hepatol. 2008;20:784–90. doi: 10.1097/MEG.0b013e3282f5195a. [DOI] [PubMed] [Google Scholar]
- 4.Mizuo H, Yazaki Y, Sugawara K, Tsuda F, Takahashi M, Nishizawa T, et al. Possible risk factors for the transmission of hepatitis E virus and for the severe form of hepatitis E acquired locally in Hokkaido, Japan. J Med Virol. 2005;76:341–9. doi: 10.1002/jmv.20364. [DOI] [PubMed] [Google Scholar]
- 5.Mansuy JM, Peron JM, Abravanel F, Poirson H, Dubois M, Miedouge M, et al. Hepatitis E in the south west of France in individuals who have never visited an endemic area. J Med Virol. 2004;74:419–24. doi: 10.1002/jmv.20206. [DOI] [PubMed] [Google Scholar]
- 6.Tsang TH, Denison EK, Williams HV, Venczel LV, Ginsberg MM, Vugia DJ. Acute hepatitis E infection acquired in California. Clin Infect Dis. 2000;30:618–9. doi: 10.1086/313730. [DOI] [PubMed] [Google Scholar]
- 7.Dalton HR, Fellows HJ, Gane EJ, Wong P, Gerred S, Schroeder B, et al. Hepatitis E in new zealand. J Gastroenterol Hepatol. 2007;22:1236–40. doi: 10.1111/j.1440-1746.2007.04894.x. [DOI] [PubMed] [Google Scholar]
- 8.Dalton HR, Hazeldine S, Banks M, Ijaz S, Bendall R. Locally acquired hepatitis E in chronic liver disease. Lancet. 2007;369:1260. doi: 10.1016/S0140-6736(07)60595-9. [DOI] [PubMed] [Google Scholar]
- 9.Péron JM, Bureau C, Poirson H, Mansuy JM, Alric L, Selves J, et al. Fulminant liver failure from acute autochthonous hepatitis E in France: description of seven patients with acute hepatitis E and encephalopathy. J Viral Hepat. 2007;14:298–303. doi: 10.1111/j.1365-2893.2007.00858.x. [DOI] [PubMed] [Google Scholar]
- 10.Kumar Acharya S, Kumar Sharma P, Singh R, Kumar Mohanty S, Madan K, Kumar Jha J, et al. Hepatitis E virus (HEV) infection in patients with cirrhosis is associated with rapid decompensation and death. J Hepatol. 2007;46:387–94. doi: 10.1016/j.jhep.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 11.Ramachandran J, Eapen CE, Kang G, Abraham P, Hubert DD, Kurian G, et al. Hepatitis E superinfection produces severe decompensation in patients with chronic liver disease. J Gastroenterol Hepatol. 2004;19:134–8. doi: 10.1111/j.1440-1746.2004.03188.x. [DOI] [PubMed] [Google Scholar]
- 12.Zhu FC, Zhang J, Zhang XF, Zhou C, Wang ZZ, Huang SJ, et al. Efficacy and safety of a recombinant hepatitis E vaccine in healthy adults: a large-scale, randomised, double-blind placebo-controlled, phase 3 trial. Lancet. 2010;376:895–902. doi: 10.1016/S0140-6736(10)61030-6. [DOI] [PubMed] [Google Scholar]
- 13.Park SB. Hepatitis E vaccine debuts. Nature. 2012;491:21–2. doi: 10.1038/491021a. [DOI] [PubMed] [Google Scholar]
- 14.Kumar M, Herrera JL. Importance of hepatitis vaccination in patients with chronic liver disease. South Med J. 2010;103:1223–31. doi: 10.1097/SMJ.0b013e3181fae4e8. [DOI] [PubMed] [Google Scholar]
- 15.Wu JC, Chen CL, Hou MC, Chen TZ, Lee SD, Lo KJ. Multiple viral infection as the most common cause of fulminant and subfulminant viral hepatitis in an area endemic for hepatitis B: application and limitations of the polymerase chain reaction. Hepatology. 1994;19:836–40. doi: 10.1002/hep.1840190406. [DOI] [PubMed] [Google Scholar]
- 16.Wang JY, Lee SD, Tsai YT, Lo KJ, Chiang BN. Fulminant hepatitis A in chronic HBV carrier. Dig Dis Sci. 1986;31:109–11. doi: 10.1007/BF01347921. [DOI] [PubMed] [Google Scholar]
- 17.Lee SD, Chan CY, Yu MI, Lu RH, Chang FY, Lo KJ. Hepatitis B vaccination in patients with chronic hepatitis C. J Med Virol. 1999;59:463–8. doi: 10.1002/(SICI)1096-9071(199912)59:4<463::AID-JMV7>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 18.Keeffe EB, Iwarson S, McMahon BJ, Lindsay KL, Koff RS, Manns M, et al. Safety and immunogenicity of hepatitis A vaccine in patients with chronic liver disease. Hepatology. 1998;27:881–6. doi: 10.1002/hep.510270336. [DOI] [PubMed] [Google Scholar]
- 19.Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50:661–2. doi: 10.1002/hep.23190. [DOI] [PubMed] [Google Scholar]
- 20.Strader DB, Wright T, Thomas DL, Seeff LB, American Association for the Study of Liver Diseases Diagnosis, management, and treatment of hepatitis C. Hepatology. 2004;39:1147–71. doi: 10.1002/hep.20119. [DOI] [PubMed] [Google Scholar]
- 21.NIH Consensus Statement on Management of Hepatitis C: 2002. NIH Consens State Sci Statements. 2002;19:1–46. [PubMed] [Google Scholar]
- 22.Jacobs RJ, Meyerhoff AS, Saab S. Immunization needs of chronic liver disease patients seen in primary care versus specialist settings. Dig Dis Sci. 2005;50:1525–31. doi: 10.1007/s10620-005-2873-5. [DOI] [PubMed] [Google Scholar]
- 23.Arguedas MR, Fallon MB. Prevention in liver disease. Am J Med Sci. 2001;321:145–51. doi: 10.1097/00000441-200102000-00006. [DOI] [PubMed] [Google Scholar]
- 24.Fattovich G, Bortolotti F, Donato F. Natural history of chronic hepatitis B: special emphasis on disease progression and prognostic factors. J Hepatol. 2008;48:335–52. doi: 10.1016/j.jhep.2007.11.011. [DOI] [PubMed] [Google Scholar]