Abstract
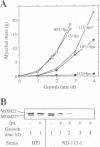
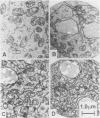
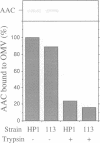
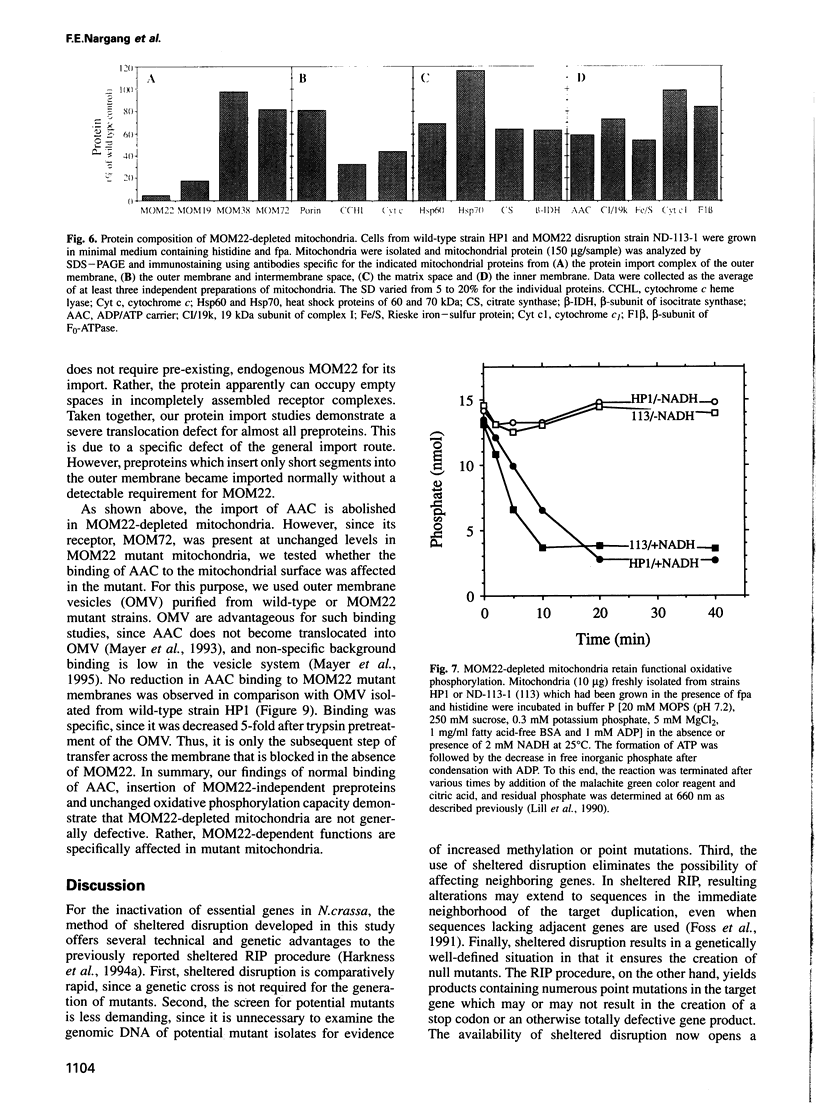
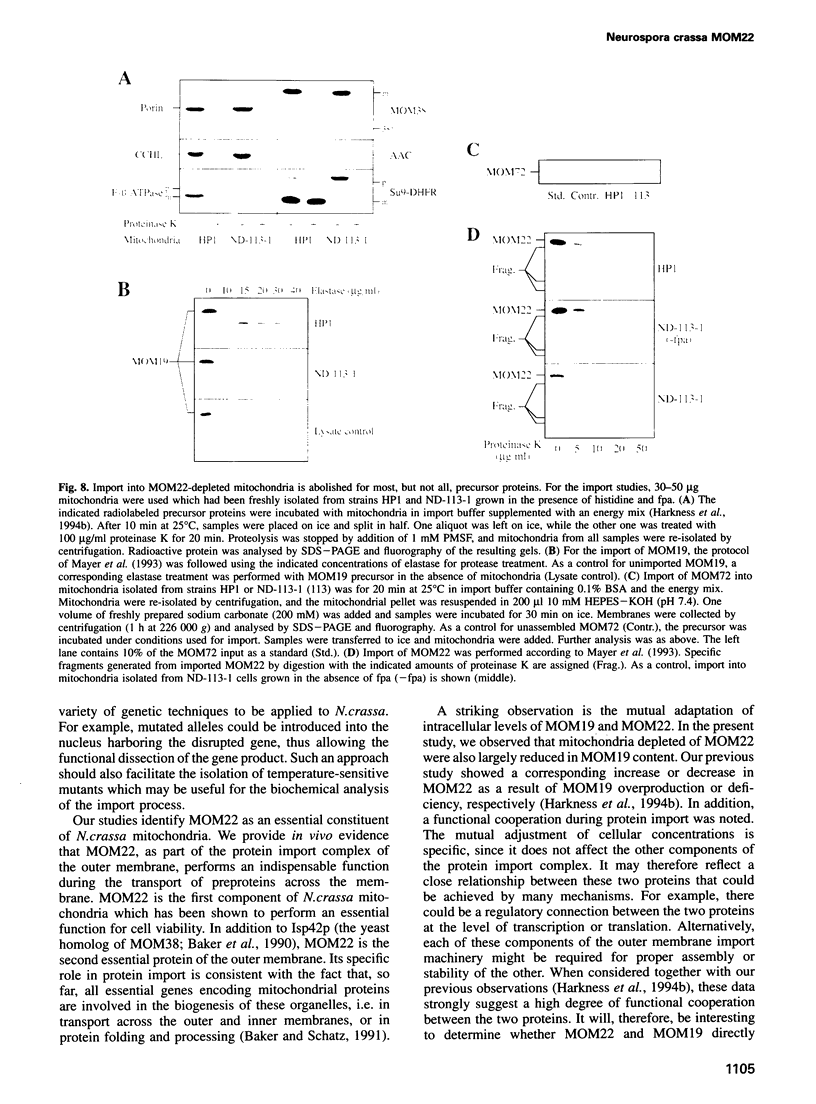
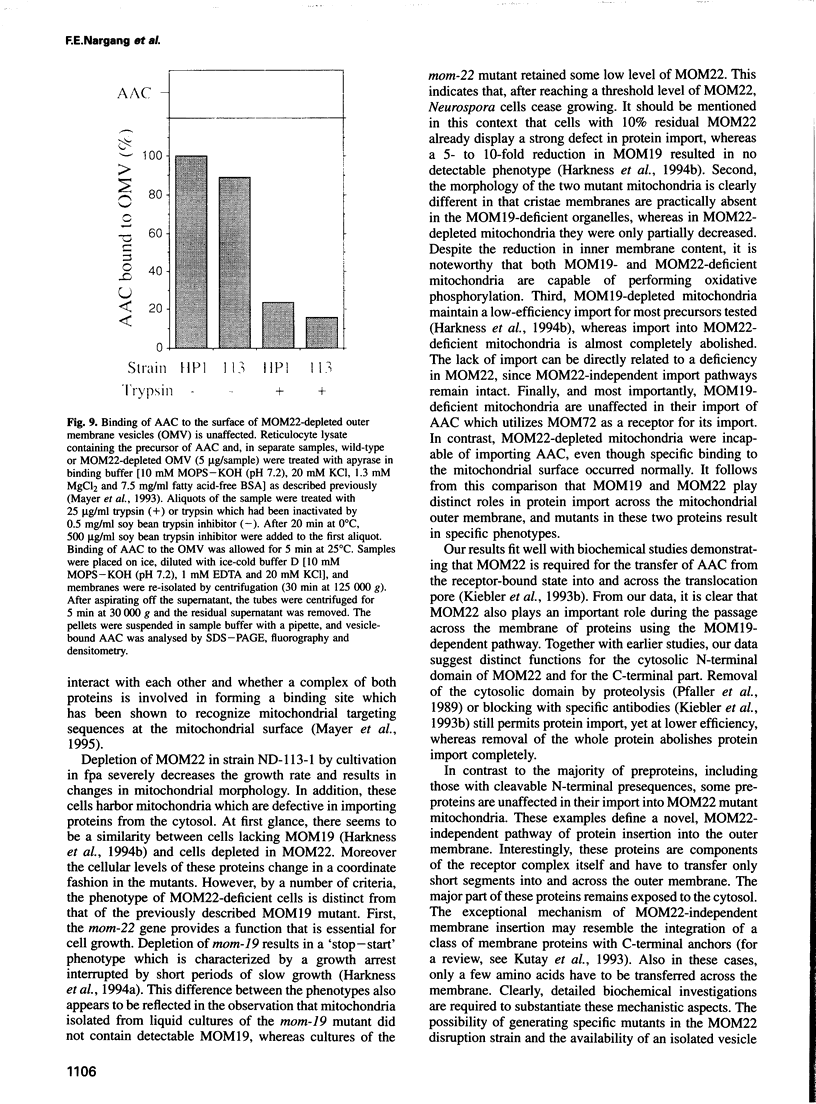
MOM22 is a component of the protein import complex of the mitochondrial outer membrane of Neurospora crassa. Using the newly developed procedure of 'sheltered disruption', we created a heterokaryotic strain harboring two nuclei, one with a null allele of the mom-22 gene and the other with a wild-type allele. Homokaryons bearing the mom-22 disruption could not be isolated, suggesting that mom-22 is an essential gene. The mutant nucleus can be forced to predominate in the heterokaryon through the use of specific nutritional and inhibitor resistance markers. Cultivation of the heterokaryon under conditions favoring the mutant nucleus resulted in selective depletion of MOM22. MOM22-depleted cells did not grow and contained mitochondria with an altered morphology and protein composition. Protein import into isolated, MOM22-depleted mitochondria was abolished for most precursor proteins destined for all subcompartments. In contrast, precursors of MOM19, MOM22 and MOM72 became inserted normally into the outer membrane, defining a novel MOM22-independent import pathway which remained intact in mutant mitochondria. Furthermore, the specific binding of the ADP/ATP carrier to the outer membrane was unaffected, but subsequent transport across the outer membrane did not occur. Our data show that MOM22 is an essential component of Neurospora cells specifically required for the biogenesis of mitochondria.
Full text
PDF
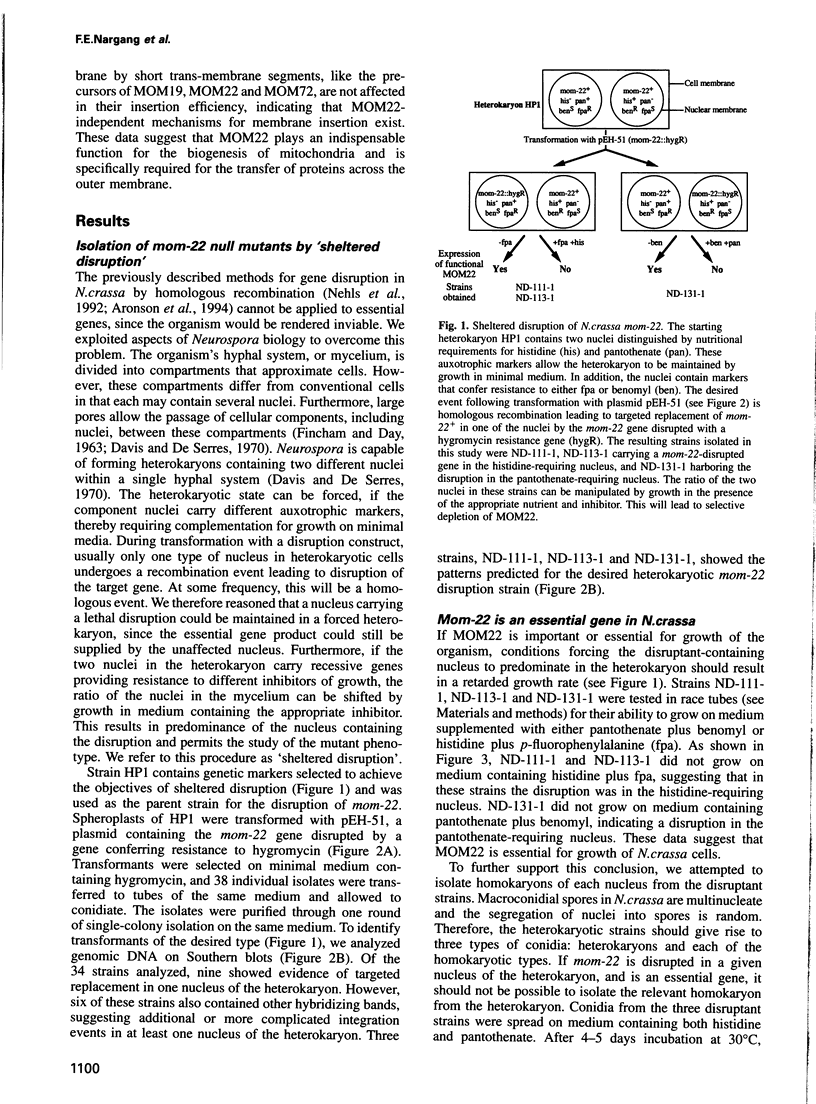


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akins R. A., Lambowitz A. M. General method for cloning Neurospora crassa nuclear genes by complementation of mutants. Mol Cell Biol. 1985 Sep;5(9):2272–2278. doi: 10.1128/mcb.5.9.2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronson B. D., Lindgren K. M., Dunlap J. C., Loros J. J. An efficient method for gene disruption in Neurospora crassa. Mol Gen Genet. 1994 Feb;242(4):490–494. doi: 10.1007/BF00281802. [DOI] [PubMed] [Google Scholar]
- Austin B., Hall R. M., Tyler B. M. Optimized vectors and selection for transformation of Neurospora crassa and Aspergillus nidulans to bleomycin and phleomycin resistance. Gene. 1990 Sep 1;93(1):157–162. doi: 10.1016/0378-1119(90)90152-h. [DOI] [PubMed] [Google Scholar]
- Baker K. P., Schaniel A., Vestweber D., Schatz G. A yeast mitochondrial outer membrane protein essential for protein import and cell viability. Nature. 1990 Dec 13;348(6302):605–609. doi: 10.1038/348605a0. [DOI] [PubMed] [Google Scholar]
- Baker K. P., Schatz G. Mitochondrial proteins essential for viability mediate protein import into yeast mitochondria. Nature. 1991 Jan 17;349(6306):205–208. doi: 10.1038/349205a0. [DOI] [PubMed] [Google Scholar]
- DeBusk R. M., DeBusk A. G. Physiological and regulatory properties of the general amino acid transport system of Neurospora crassa. J Bacteriol. 1980 Jul;143(1):188–197. doi: 10.1128/jb.143.1.188-197.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foss E. J., Garrett P. W., Kinsey J. A., Selker E. U. Specificity of repeat-induced point mutation (RIP) in Neurospora: sensitivity of non-Neurospora sequences, a natural diverged tandem duplication, and unique DNA adjacent to a duplicated region. Genetics. 1991 Apr;127(4):711–717. doi: 10.1093/genetics/127.4.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gessert S. F., Kim J. H., Nargang F. E., Weiss R. L. A polyprotein precursor of two mitochondrial enzymes in Neurospora crassa. Gene structure and precursor processing. J Biol Chem. 1994 Mar 18;269(11):8189–8203. [PubMed] [Google Scholar]
- Hannavy K., Rospert S., Schatz G. Protein import into mitochondria: a paradigm for the translocation of polypeptides across membranes. Curr Opin Cell Biol. 1993 Aug;5(4):694–700. doi: 10.1016/0955-0674(93)90142-d. [DOI] [PubMed] [Google Scholar]
- Harkness T. A., Metzenberg R. L., Schneider H., Lill R., Neupert W., Nargang F. E. Inactivation of the Neurospora crassa gene encoding the mitochondrial protein import receptor MOM19 by the technique of "sheltered RIP". Genetics. 1994 Jan;136(1):107–118. doi: 10.1093/genetics/136.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkness T. A., Nargang F. E., van der Klei I., Neupert W., Lill R. A crucial role of the mitochondrial protein import receptor MOM19 for the biogenesis of mitochondria. J Cell Biol. 1994 Mar;124(5):637–648. doi: 10.1083/jcb.124.5.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil P., Pfanner N. Insertion of MOM22 into the mitochondrial outer membrane strictly depends on surface receptors. FEBS Lett. 1993 Apr 26;321(2-3):197–200. doi: 10.1016/0014-5793(93)80107-6. [DOI] [PubMed] [Google Scholar]
- Kiebler M., Becker K., Pfanner N., Neupert W. Mitochondrial protein import: specific recognition and membrane translocation of preproteins. J Membr Biol. 1993 Sep;135(3):191–207. doi: 10.1007/BF00211091. [DOI] [PubMed] [Google Scholar]
- Kiebler M., Keil P., Schneider H., van der Klei I. J., Pfanner N., Neupert W. The mitochondrial receptor complex: a central role of MOM22 in mediating preprotein transfer from receptors to the general insertion pore. Cell. 1993 Aug 13;74(3):483–492. doi: 10.1016/0092-8674(93)80050-o. [DOI] [PubMed] [Google Scholar]
- Kiebler M., Pfaller R., Söllner T., Griffiths G., Horstmann H., Pfanner N., Neupert W. Identification of a mitochondrial receptor complex required for recognition and membrane insertion of precursor proteins. Nature. 1990 Dec 13;348(6302):610–616. doi: 10.1038/348610a0. [DOI] [PubMed] [Google Scholar]
- Kutay U., Hartmann E., Rapoport T. A. A class of membrane proteins with a C-terminal anchor. Trends Cell Biol. 1993 Mar;3(3):72–75. doi: 10.1016/0962-8924(93)90066-a. [DOI] [PubMed] [Google Scholar]
- Lill R., Dowhan W., Wickner W. The ATPase activity of SecA is regulated by acidic phospholipids, SecY, and the leader and mature domains of precursor proteins. Cell. 1990 Jan 26;60(2):271–280. doi: 10.1016/0092-8674(90)90742-w. [DOI] [PubMed] [Google Scholar]
- Mayer A., Lill R., Neupert W. Translocation and insertion of precursor proteins into isolated outer membranes of mitochondria. J Cell Biol. 1993 Jun;121(6):1233–1243. doi: 10.1083/jcb.121.6.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer A., Neupert W., Lill R. Mitochondrial protein import: reversible binding of the presequence at the trans side of the outer membrane drives partial translocation and unfolding. Cell. 1995 Jan 13;80(1):127–137. doi: 10.1016/0092-8674(95)90457-3. [DOI] [PubMed] [Google Scholar]
- Nehls U., Friedrich T., Schmiede A., Ohnishi T., Weiss H. Characterization of assembly intermediates of NADH:ubiquinone oxidoreductase (complex I) accumulated in Neurospora mitochondria by gene disruption. J Mol Biol. 1992 Oct 20;227(4):1032–1042. doi: 10.1016/0022-2836(92)90519-p. [DOI] [PubMed] [Google Scholar]
- Nicholson D. W., Köhler H., Neupert W. Import of cytochrome c into mitochondria. Cytochrome c heme lyase. Eur J Biochem. 1987 Apr 1;164(1):147–157. doi: 10.1111/j.1432-1033.1987.tb11006.x. [DOI] [PubMed] [Google Scholar]
- Pandit N. N., Russo V. E. Reversible inactivation of a foreign gene, hph, during the asexual cycle in Neurospora crassa transformants. Mol Gen Genet. 1992 Sep;234(3):412–422. doi: 10.1007/BF00538700. [DOI] [PubMed] [Google Scholar]
- Pfaller R., Pfanner N., Neupert W. Mitochondrial protein import. Bypass of proteinaceous surface receptors can occur with low specificity and efficiency. J Biol Chem. 1989 Jan 5;264(1):34–39. [PubMed] [Google Scholar]
- Pfaller R., Steger H. F., Rassow J., Pfanner N., Neupert W. Import pathways of precursor proteins into mitochondria: multiple receptor sites are followed by a common membrane insertion site. J Cell Biol. 1988 Dec;107(6 Pt 2):2483–2490. doi: 10.1083/jcb.107.6.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatz G. The protein import machinery of mitochondria. Protein Sci. 1993 Feb;2(2):141–146. doi: 10.1002/pro.5560020202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider H., Söllner T., Dietmeier K., Eckerskorn C., Lottspeich F., Trülzsch B., Neupert W., Pfanner N. Targeting of the master receptor MOM19 to mitochondria. Science. 1991 Dec 13;254(5038):1659–1662. doi: 10.1126/science.1661031. [DOI] [PubMed] [Google Scholar]
- Schweizer M., Case M. E., Dykstra C. C., Giles N. H., Kushner S. R. Identification and characterization of recombinant plasmids carrying the complete qa gene cluster from Neurospora crassa including the qa-1+ regulatory gene. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5086–5090. doi: 10.1073/pnas.78.8.5086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segui-Real B., Stuart R. A., Neupert W. Transport of proteins into the various subcompartments of mitochondria. FEBS Lett. 1992 Nov 16;313(1):2–7. doi: 10.1016/0014-5793(92)81171-h. [DOI] [PubMed] [Google Scholar]
- Stadler D. R., Kariya B. Intragenic recombination at the mtr locus of Neurospora with segregation at an unselected site. Genetics. 1969 Oct;63(2):291–316. doi: 10.1093/genetics/63.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söllner T., Griffiths G., Pfaller R., Pfanner N., Neupert W. MOM19, an import receptor for mitochondrial precursor proteins. Cell. 1989 Dec 22;59(6):1061–1070. doi: 10.1016/0092-8674(89)90762-9. [DOI] [PubMed] [Google Scholar]
- Söllner T., Pfaller R., Griffiths G., Pfanner N., Neupert W. A mitochondrial import receptor for the ADP/ATP carrier. Cell. 1990 Jul 13;62(1):107–115. doi: 10.1016/0092-8674(90)90244-9. [DOI] [PubMed] [Google Scholar]
- Söllner T., Rassow J., Pfanner N. Analysis of mitochondrial protein import using translocation intermediates and specific antibodies. Methods Cell Biol. 1991;34:345–358. doi: 10.1016/s0091-679x(08)61689-1. [DOI] [PubMed] [Google Scholar]
- Söllner T., Rassow J., Wiedmann M., Schlossmann J., Keil P., Neupert W., Pfanner N. Mapping of the protein import machinery in the mitochondrial outer membrane by crosslinking of translocation intermediates. Nature. 1992 Jan 2;355(6355):84–87. doi: 10.1038/355084a0. [DOI] [PubMed] [Google Scholar]