Abstract
Utero-placental growth and vascular development are critical for pregnancy establishment that may be altered by various factors including assisted reproductive technologies (ART), nutrition, or others, leading to compromised pregnancy. We hypothesized that placental vascularization and expression of angiogenic factors are altered early in pregnancies after transfer of embryos created using selected ART methods. Pregnancies were achieved through natural mating (NAT), or transfer of embryos from natural mating (NAT-ET), or in vitro fertilization (IVF) or activation (IVA). Placental tissues were collected on day 22 of pregnancy. In maternal caruncles (CAR), vascular cell proliferation was less (P<0.05) for IVA than other groups. Compared to NAT, density of blood vessels was less (P<0.05) for IVF and IVA in fetal membranes (FM), and for NAT-ET, IVF and IVA in CAR. In FM, mRNA expression was decreased (P<0.01–0.08) in NAT-ET, IVF and IVA compared to NAT for vascular endothelial growth factor (VEGF) and its receptor FLT-1, placental growth factor (PGF), neuropilin (NP) 1 and 2, angiopoietin (ANGPT) 1 and 2, endothelial nitric oxide synthase (NOS3), hypoxia inducible factor-1A (HIF1A), fibroblast growth factor (FGF) 2 and its receptor FGFR2. In CAR, mRNA expression was decreased (P<0.01–0.05) in NAT-ET, IVF and IVA compared to NAT for VEGF, FLT-1, PGF, ANGPT1 and TEK. Decreased mRNA expression for 12 of 14 angiogenic factors across FM and CAR in NAT-ET, IVF and IVA pregnancies was associated with reduced placental vascular development, which would lead to poor placental function and compromised fetal and placental growth and development.
Introduction
Placental vascularization is initiated and established early in pregnancy and supports early embryonic survival and subsequent fetal growth and development (Grazul-Bilska et al. 2010, 2011, 2013). Thus, the importance of placental vascular development during early pregnancy has long been recognized (Reynolds et al. 2010, 2013). During early pregnancy, extensive angiogenesis in maternal and fetal placental tissues progresses, accompanied by a marked increase in uterine and umbilical blood flows (Greiss & Anderson 1970, Reynolds 1986, Reynolds & Redmer 1995). Placental angiogenesis is tightly regulated by numerous growth and angiogenic factors (Mayhew et al. 2003, 2004a,b, Redmer et al. 2004, Grazul-Bilska et al. 2010, 2011, Reynolds et al. 2010, 2013).
The pattern of placental vascularization and expression of factors that influence angiogenesis during early pregnancy after natural breeding has been established for sheep (Grazul-Bilska et al. 2010, 2011). However, very limited data concerning placental vascularization in pregnancies established after transfer of embryos obtained through assisted reproductive technologies (ART) or pregnancies affected by environmental factors during early stages of development are available. For later stages of pregnancy, it has been demonstrated that such factors as maternal age, inadequate nutrition, environmental pollutants and others are associated with decreased vascularization and/or blood flow in the placenta of several species (Redmer et al. 2004, Reynolds et al. 2006, 2013, Rennie et al. 2011). Comparison of placental development at several stages of natural pregnancies with those achieved by various ART has shown differences in fetal size, placental and fetal growth, placental steroid metabolism, global DNA methylation, and expression of selected genes in several species (Barnes 2000, Cai et al. 2006, Grazul-Bilska et al. 2006, 2013, Allen et al. 2008, Collier et al. 2009, Delle Piane et al. 2010, Sellers Lopez et al. 2010, Esh-Broder et al. 2011, Tomic & Tomic 2011, Ptak et al. 2013). In addition, placental vascular development is dramatically altered at term in pregnancies resulting from ART in sheep (Palmieri et al. 2007) and cattle (Hill et al. 2000, Miles et al. 2004, Miglino et al. 2007), and such alterations have been shown to occur as early as day 70 of pregnancy in cattle (Miles et al. 2005). Furthermore, reduced placental vascular development and increased vascular resistance during early pregnancy have been associated with early embryonic mortality (Meegdes et al. 1988; Bassil et al. 1995).
Beyond their immediate effects on pregnancy establishment, factors influencing placental vascular development have a dramatic impact on fetal growth and development, and therefore on birth weight as well as postnatal survival and growth (Reynolds & Redmer 2001; Reynolds et al. 2010, 2013). Moreover, it has been shown that such postnatal effects impact not only the postnatal period but also life-long health and productivity in mammals, including humans and livestock (Nathanielsz 2006, Barker 2007, Vonnahme & Lemley 2011, Reynolds & Caton 2012).
We hypothesized that placental vascularization and/or expression of factors involved in the regulation of angiogenesis would be altered very early (2–4 weeks after fertilization) in pregnancies achieved through transfer of embryos from various sources, including those from ART, compared with natural pregnancies. To test this hypothesis, we established pregnancies using a control group that was naturally mated (NAT), as well as three groups involving: (i) follicle stimulating hormone (FSH)-treatment to induce multiple follicle development (Stenbak et al. 2001, Grazul-Bilska et al. 2007) combined with natural mating, embryo flushing and embryo transfer (ET) to synchronized recipients (NAT-ET); (ii) transfer of embryos obtained through IVF of oocytes collected from FSH-treated ewes; and (iii) transfer of embryos obtained through in vitro activation (IVA; i.e., parthenotes – clones containing only maternal genes) of oocytes collected from FSH-treated donors, as described before (Grazul-Bilska et al. 2013). Therefore, the aim of this study was to determine vascularization (e.g., vascular labeling index [LI] and the density of blood vessels) and the expression of mRNA for 14 factors involved in the regulation of angiogenesis in fetal and maternal placenta on day 22 of gestation in four pregnancy types: NAT, NAT-ET, IVF and IVA in sheep.
Materials and Methods
Animals and Tissue Collection
The North Dakota State University Institutional Animal Care and Use Committee approved all animal procedures used in this study. Animal use, experimental design and methodology are described in detail in a recently published paper (Grazul-Bilska et al. 2013). Tissue samples for this and previous (Grazul-Bilska et al. 2013) studies were collected from the same animals, but different measurements were performed. Briefly, estrus was synchronized using a CIDR device for 14 days (MWI, Boise, ID, USA) for adult ewes (n=67; Western Range ewes, primarily Rambouillet, Targhee and Columbia crossbred) randomly assigned to be naturally mated, or to serve as donors or recipients. Twenty four hours after CIDR removal, ewes (n=10) assigned to the NAT group were exposed to a fertile ram. For donor ewes in the NAT-ET, IVF and IVA groups, estrus was checked twice daily using a vasectomized ram. Donor ewes (n=3) for the NAT-ET group were treated twice daily with FSH-P (Sioux Biochemical, Sioux Center, IA, USA): on day 13 following estrus (day 0), 5 mg/injection, day14, 4 mg/injection, and day15, 3 mg/injection as described before (Stenbak et al. 2001; Grazul-Bilska et al. 2006, 2012). Donor ewes (n=22) for the IVF and IVA groups were treated with FSH-P on days 13 and 14 after estrus, as described above. On Day 15 of the estrous cycle, ewes from the NAT-ET group were exposed to a fertile ram for 48 h, but for the ewes in the IVF and IVA groups, ovaries were collected. Then, the oocytes were isolated, matured for 24 h, and fertilized or activated in vitro. To accomplish this, cumulus oocyte complexes (COC) were isolated from visible surface antral follicles >3 mm in diameter and incubated overnight in maturation medium (TCM199; Sigma, St. Louis, MO, USA; up to 30 COC/0.5 ml in a 4-well Nunc culture dish) supplemented with 10% fetal bovine serum (FBS, Sigma), ovine FSH [5 μg/mL; oFSH-RP-1; NIAMDD-NIH, Bethesda, MD, USA], ovine LH [5 μg/mL; oLH-26; NIADDK-NIH], estradiol −17β [1 μg/mL; Sigma], glutamine [2 mM; Sigma], sodium pyruvate [0.25 mM; Sigma], epidermal growth factor [10 ng/mL; Sigma,] and penicillin/streptomycin [100 units/mL penicillin and 100 μg/mL streptomycin; Gibco, Grand Island, NY, USA]). After denuding oocytes of cumulus cells, half of the oocytes from each ewe were used for IVF and the other half for IVA.
For IVF, capacitated semen pooled from 5 Western range crossbreed rams frozen and stored in liquid nitrogen tank (−197 C) was thawed and viable sperm were separated in the modified sperm washing media (Irvine Scientific, Santa Ana, CA, USA) using the swim up technique (Grazul-Bilska et al. 2006, 2012). Oocytes were cultured in fertilization medium prepared in our laboratory consisting of synthetic oviductal fluid (SOF), 2% heat-inactivated ovine serum collected on day 0–1 of the estrous cycle and 1% penicillin/streptomycin in the presence of capacitated sperm (0.5 to 1 × 106 sperm/ml) for 24 h followed by incubation (at 39°C, 5% O2, 5% CO2 and 90% N2) in culture medium until ET (Grazul-Bilska et al. 2003, 2006, 2012, 2013). Culture medium consisted of SOF supplemented with bovine serum albumin (BSA; 8 mg/ml; Sigma), glutamine (1 mM), MEM non-essential amino acids (0.01 ml/ml, vol/vol; Sigma), BME amino acids (0.02 ml/ml, vol;/vol; Sigma) and penicillin/streptomycin (100 units/mL penicillin and 100 μg/mL streptomycin). For IVA, oocytes were incubated for 5 min in TCM199 medium containing 2% FBS and ionomycin (2.5 μM; Sigma) followed by a 3-h incubation with 6-dimethylaminopurine (DMAP; 2 mM; Sigma). In vitro activated oocytes were then transferred to culture medium and incubated until ET (Grazul-Bilska et al. 2003, 2006, 2013).
For the NAT-ET group, on Day 5 post-mating (Day 1 = day of mating), embryos were flushed, evaluated using a stereomicroscope, and then transferred to synchronized recipients (3 embryos at morula stage from the same donor/recipient; n=9). For the IVF and IVA groups, in vitro-generated embryos at morula stage were transferred on Day 5 after fertilization or activation (Day 1 = day of fertilization or activation) to synchronized recipient ewes (3 embryos from the same donor/recipient; n= 10 and 13, respectively). On Day 22 after mating, fertilization or activation, fetuses and utero-placental tissues were collected from NAT, NAT-ET, IVF and IVA groups (n = 8, 7, 8, and 7 ewes, respectively). Pregnancy rates for NAT, NAT-ET, IVF and IVA groups were 80%, 78%, 80% and 54%, respectively.
Cross sections (approximately 0.5-cm thick) of the entire gravid uterus including fetal membranes (FM, chorioallantois) were fixed by immersion in formalin for Ki67 detection or Carnoy’s solution for smooth muscle cell actin (SMCA) detection, and then embedded in paraffin. For total cellular RNA extraction, FM (fetal placenta) and caruncular (CAR, maternal placenta) tissues were dissected from an area close to the embryo, snap-frozen in isopentane super-cooled in liquid nitrogen, and stored at −70 C. We chose Day 22 for tissue collection because in our previous experiments we demonstrated that between Days 20–22, major changes in cell proliferation, vascularization and expression of angiogenic factors occurred in fetal and maternal placenta in pregnancies achieved through natural mating (Grazul-Bilska et al. 2010, 2011). In addition, by Day 20 to 22 after mating placentation is already initiated in sheep (Igwebuike 2009).
Immunohistochemistry
Immunohistochemical procedures were performed as described by Grazul-Bilska et al. (2010, 2011, 2013). Briefly, paraffin-embedded uterine tissues containing FM were sectioned at 4 μm and mounted onto glass slides. Sections were rinsed several times in PBS containing Triton-X100 (0.3%, v/v) and then were treated for 20 min with blocking buffer [PBS containing normal horse serum (2%, vol/vol)] followed by overnight incubation at 4° C with specific primary antibody for Ki67 (a marker of proliferating cells; 1:500; mouse monoclonal; Vector Laboratories, Burlingame, CA, USA) or SMCA (a marker of pericytes and vascular smooth muscle cells and thus blood vessels; 1:150; mouse monoclonal; Oncogene Research Products; San Diego, CA, USA). Primary antibodies were detected using secondary anti-mouse antibody coupled to peroxidase (ImPress Kit; Vector Laboratories). For Ki67 staining, the sections were then counterstained with hematoxylin and periodic acid-Schiff’s reagent (H and PAS) to visualize cell nuclei as well as basement membranes and thus blood vessels (Reynolds & Redmer 1992). For SMCA staining, the sections were counterstained with nuclear fast red (Sigma) to visualize cell nuclei. Control sections were incubated with normal mouse IgG (4 μg/mL) in place of primary antibody.
Image analysis
For each tissue section, images were taken at 600x (Ki67 staining) or 200x (SMCA staining) magnification using an Eclipse E600 Nikon microscope and digital camera (Nikon Instruments Inc., Melville, NY, USA) or Zeiss Imager M2 epifluorescence microscope equipped with Zeiss piezo automated stage and AxioCam HRm camera (Carl Zeiss International, Jena, Germany). Image analysis (Image-Pro Plus, Media Cybernetics, Inc., Bethesda, MD, USA) was performed for images of 5–10 randomly chosen fields of CAR to determine vascular cell proliferation based on Ki67 staining, while images of 5–40 randomly chosen fields from areas containing FM or CAR were used to determine the density of blood vessels based on SMCA staining, as described previously (Borowicz et al. 2007, Grazul-Bilska et al. 2010, 2011, 2013). The LI was calculated as the percentage (%) of proliferating Ki67-positive cells out of the total number of cells within blood vessels which were marked with H and PAS/CAR tissue area.
Quantitative Real-Time RT-PCR
All procedures for determining the expression of mRNA of genes in ovine tissues by RT-PCR along with sequences for 14 factors involved in the regulation of angiogenesis have been reported previously (Redmer et al. 2005; Grazul-Bilska et al. 2010, 2011). Briefly, snap-frozen CAR and FM tissues were homogenized in Tri-Reagent (Molecular Research Center; Cincinnati, OH, USA) and RNA was extracted according to the manufacturer’s specifications. The quality and quantity of total RNA were determined via capillary electrophoresis using the Agilent 2100 Bioanalyzer (Agilent Technologies, Wilmington, DE, USA). Real-time RT-PCR reagents, probes, and primers were purchased from and used as recommended by Applied Biosystems (Foster City, CA, USA). For each sample, 30 ng total RNA was reverse transcribed in triplicate 20-μl reactions using random hexamers. Sequence-specific Taqman probes and primers were designed using the Primer Express Software from Applied Biosystems. The ABI PRISM 7000 was used for detection of sequences amplified at 60°C typically for 40 or 45 cycles (Applied Biosystems). Quantification was determined from a relative standard curve of dilutions of the cDNA generated from tcRNA pooled from placentomes collected on day 130 of pregnancy. Expression of each gene was normalized to expression of 18S ribosomal RNA (rRNA) in a multiplex reaction using the human 18S pre-developed assay reagent (PDAR) from Applied Biosystems. The PDAR solution, which is primer limited and contains a VIC-labeled probe, was further adjusted using one-fourth the normal amount, so that it would not interfere with amplification of the FAM-labeled gene of interest. Standard curves were also generated with the multiplex solution, and the quantity of 18S rRNA and the gene of interest were determined using each specific standard curve. The concentrations of mRNA were then normalized to 18S rRNA by dividing each of the mRNA values by their corresponding 18S rRNA value.
Statistical Analysis
Data were analyzed using the general linear models (GLM) procedure of SAS (SAS Inst. 2010) with the main effect of embryo origin (i.e., NAT, NAT-ET, IVF or IVA), and presented as means ± SEM. When the F-test was significant (P<0.05), differences between specific group means were evaluated using the least significant differences test (Kirk 1982). In addition, PROC CORR of SAS was used to calculate simple linear correlations between specific variables.
Results
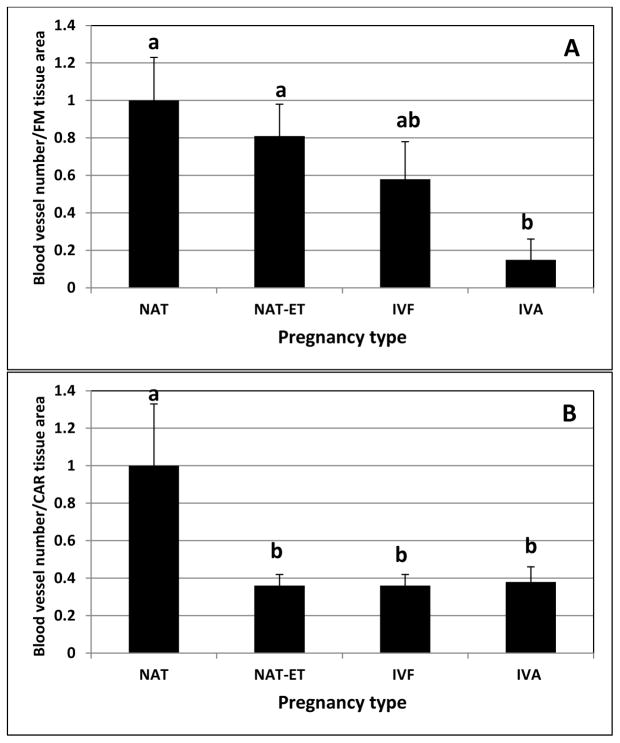
In all pregnancy groups, Ki67 was detected in the nuclei of proliferating cells in blood vessels and other compartments of CAR (Fig. 1), and SMCA was detected in the blood vessels in FM and CAR (Fig. 2). Labeling index of vascular cells and the density of blood vessels in CAR and/or FM were affected by embryo origin. For CAR, the vascular LI was less (P<0.04) in the IVA group compared to any of the other groups, which were similar to each other (3.2±1.2 vs. 9.5±1.3%). In FM, the density of blood vessels was less (P<0.01) in IVA than in NAT or NAT-ET, and was intermediate in IVF (Fig. 3A). In CAR, the density of blood vessels was less (P<0.01) in NAT-ET, IVF and IVA than NAT (Fig. 3B). In NAT, the density of blood vessels was 39.1±3.5/106 μm2 and 39.4±4.3/106 μm2 in FM and CAR, respectively.
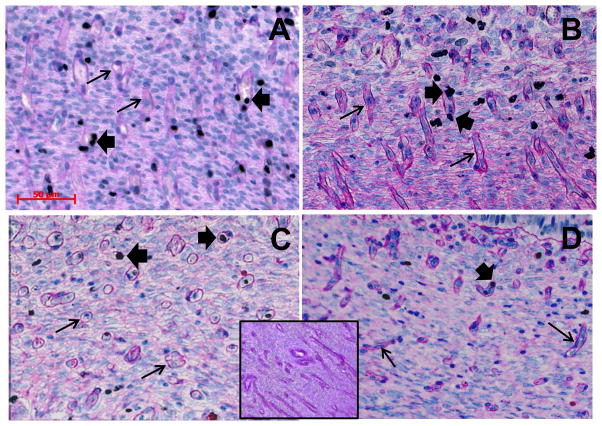
Fig. 1.
Representative photomicrographs of immunohistochemical detection of Ki67 (blackish nuclei, large arrows) followed by hematoxylin (bluish nuclei) and PAS (dark pink staining of basement membranes) staining in CAR from NAT (A), NAT-ET (B), IVF (C) and IVA (D) groups on Day 22 of pregnancy. Small arrows indicate examples of blood vessels, and large arrows point to examples of proliferating cells (Ki67-positive nuclei) within blood vessels. Bar on A (valid for B–C) = 50 μm. In inset in C/D, note the lack of positive Ki67 staining in the control sections in which non-specific mouse IgG was used in place of the primary antibody.
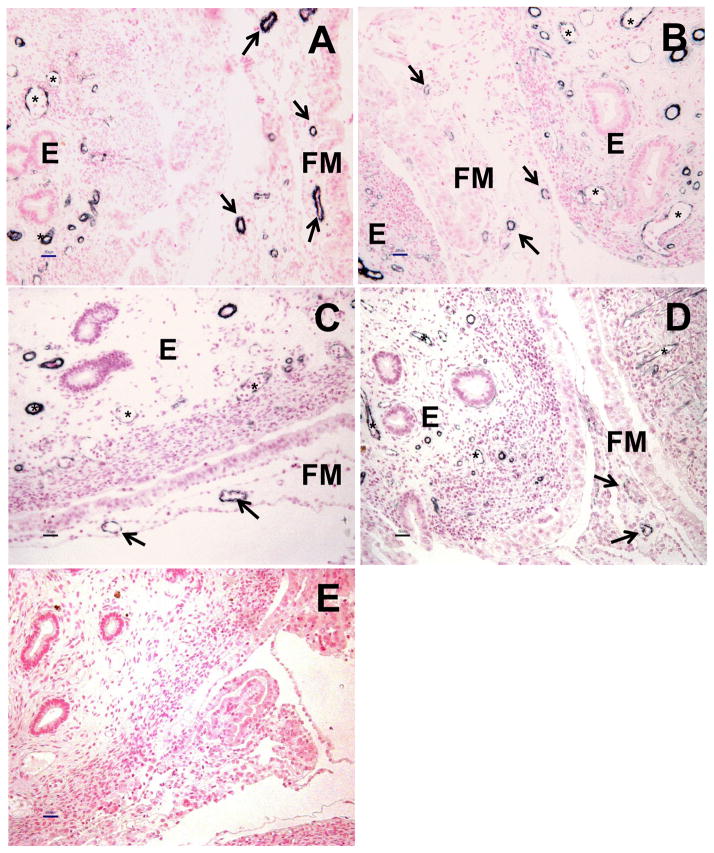
Fig. 2.
Representative photomicrographs of immunohistochemical detection of SMCA (blackish staining) followed by fast red (pinkish cell nuclei) staining in FM and endometrial (E) CAR from NAT (A), NAT-ET (B), IVF (C) and IVA (D) groups on Day 22 of pregnancy. Arrows indicate blood vessels in FM and stars in E of CAR in A–D. Bar = 50 μm for A–E. In E, note the lack of positive SMCA staining in the control sections in which non-specific mouse IgG was used in place of the primary antibody.
Fig. 3.
The density of blood vessels (based on SMCA staining) in FM (A) and CAR (B) from NAT, NAT-ET, IVF and IVA groups on Day 22 of pregnancy. Data are expressed as a fold-change compared to the NAT group, which was arbitrarily set as 1. In NAT, the density of blood vessels was 39.1±3.5/106 μm2 and 39.4±4.3/106 μm2 in FM and CAR, respectively. Means ± SEM with different superscript letters differ within tissue (a,bP < 0.01).
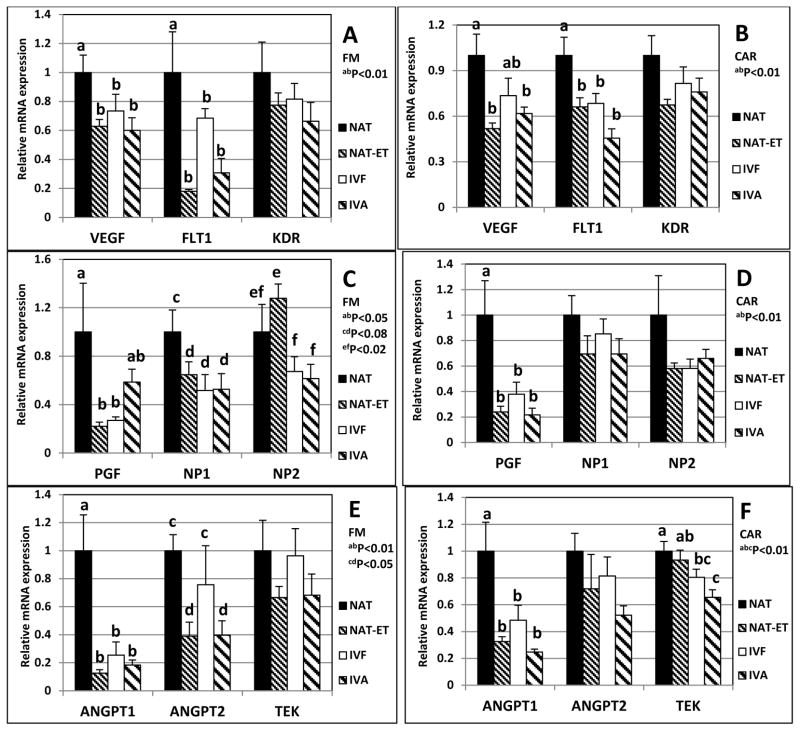
In all pregnancy types, in FM and CAR, mRNA were detected for the following: vascular endothelial growth factor (VEGF) and its receptors, FLT-1 and KDR; placental growth factor (PGF); neuropilin (NP) 1 and 2; the angiopoietins (ANGPT-1 and ANGPT-2) and their receptor, TEK; endothelial nitric oxide synthase (NOS3) and its receptor soluble guanylate kinase (GUCY1B3); hypoxia inducible factor-1A (HIF1A); and fibroblast growth factor (FGF) 2 and its receptor FGFR2 (Fig. 4).
Fig. 4.
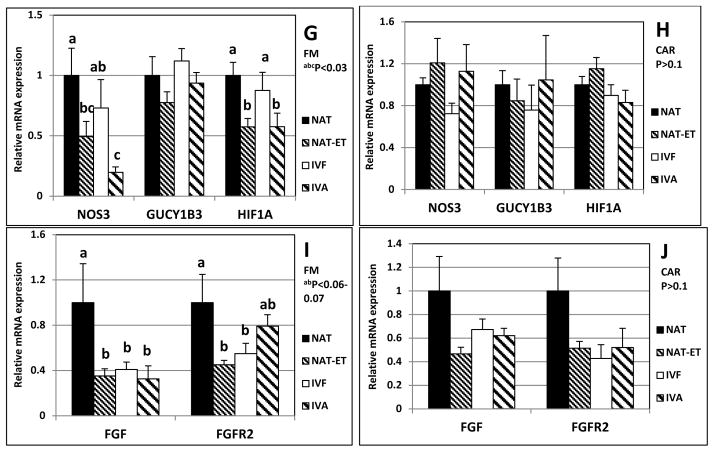
Expression of mRNA for VEGF, FLT1, KDR (A and B), PGF, NP1, NP2 (C and D), ANGPT1, ANGPT2, TEK (E and F), NOS3, GUCY1B3, HIF1A (G and H), FGF2 and FGFR2 (I and J) in FM (left column; A, C, E, G and I) or CAR (right column; B, D, F, H and J) from NAT, NAT-ET, IVF and IVA groups on Day 22 of pregnancy. Data are expressed as fold change compared to NAT control arbitrarily set as 1. Means ± SEM with different superscript letters differ within a specific gene (a,b,c,d,e,fP < 0.01–0.08).
Compared to NAT, mRNA expression in FM was less (P<0.01) for VEGF and FLT1 in NAT-ET, IVF and IVA, but KDR mRNA expression was similar for all groups (Fig. 4A).
Compared to NAT, VEGF mRNA expression in CAR was less (P<0.01) in NAT-ET and IVA, whereas IVF was intermediate (Fig. 4B). For FLT-1 mRNA in CAR, expression was less (P<0.01) in NAT-ET, IVF, and IVA, compared with NAT, but KDR mRNA expression was similar for all groups (Fig. 4B).
Compared to NAT, mRNA expression in FM was less for PGF (P<0.05) in NAT-ET and IVF, but was intermediate for IVA (Fig. 4C). Expression of mRNA in FM was less for NP1 (P<0.08) in NAT-ET, IVF and IVA compared with NAT (Fig. 4C). In addition, in FM, mRNA expression for NP2 was greater in NAT-ET (P<0.02) than IVF and IVA, and NAT was intermediate (Fig. 4C).
Compared to NAT, PGF mRNA expression in CAR was less (P<0.01) in NAT-ET, IVF and IVA, but mRNA expression for NP1 and 2 was similar for all groups (Fig. 4D).
Compared to NAT, mRNA expression in FM was less (P<0.01) for ANGPT-1 in NAT-ET, IVF, and IVA (Fig. 4E). Expression of ANGPT2 mRNA in FM was less (P<0.05) in NAT-ET and IVA but not IVF (Fig. 4E). Expression of TEK mRNA was similar in FM of all groups (Fig. 4E).
Compared to NAT, mRNA expression in CAR was less (P<0.01) for ANGPT-1 in NAT-ET, IVF and IVA, but ANGPT-2 mRNA expression was similar for all groups (Fig. 4F). Compared to NAT, TEK mRNA expression in CAR was less (P<0.01) for IVF and IVA, but was intermediate in NAT-ET (Fig. 4F).
Compared to NAT, mRNA expression in FM was less (P<0.03) for NOS3 and HIF1A in NAT-ET and IVA, but in the IVF group was either intermediate (NOS3) or similar (HIF1A) to that of the NAT group (Fig. 4G). In FM, mRNA expression for GUCY1B3 was similar for all groups (Fig. 4G).
In CAR, mRNA expression of NOS3, GUCY1B3 and HIF1A (Fig. 4H) and for FGF2 and FGFR2 (Fig. 4J) was similar for all groups.
Compared to NAT, mRNA expression in FM was less (P<0.06) for FGF2 in NAT-ET, IVF and IVA, and for FGFR2 in FM was less (P<0.07) in NAT-ET and IVF but intermediate for IVA (Fig. 4I).
Supplementary Tables 1 and 2 present correlation coefficients for mRNA expression among factors involved in the regulation of angiogenesis and the density of blood vessels in FM and CAR, respectively. For FM, the VEGF system members were correlated with the ANGPT and FGF systems and with eNOS; there were 39 statistically significant correlations among these factors. In addition, for FM, ANGPT2 and eNOS were correlated with blood vessel density. For CAR, the VEGF system members were correlated with the ANGPT and FGF systems, and several factors (n=8) were correlated with the density of blood vessels. For CAR, there were 48 statistically significant correlations among these factors.
Discussion
The present study demonstrated that embryo origin and application of selected ART methods affect placental vascular development and mRNA expression of factors involved in the regulation of angiogenesis in fetal and placental tissues at a specific time point (Day 22) in early pregnancy. In fact, several measurements of vascularization/angiogenesis were decreased after transfer of embryos of different origin. Interestingly, altered blood flow, vascular development and/or expression of selected angiogenic and/or other growth factors have been demonstrated in the placenta of mid to late pregnancies compromised by factors causing fetal growth restrictions in several species (Reynolds et al. 2006, 2013, Vonnahme & Lemley 2011, Gourvas et al. 2012).
In the present study, decreased vascular cell proliferation in maternal placenta was observed only in the IVA group. This demonstrates that when embryos possess only maternal genes, cell proliferation in blood vessels of CAR appears to be altered. Thus, input of the paternal genome seems to be critical for blood vessel growth and development in the maternal placenta. Previously, we have demonstrated that the total cell proliferation in fetal and maternal placental tissues for the same pregnancy types was decreased in NAT-ET, IVF and IVA groups compared to NAT (Grazul-Bilska et al. 2013). Thus, it seems that embryo origin has limited effects on placental blood vessel growth, but has more extensive effects on overall placental tissue growth at the early stages of pregnancy. Therefore, we hypothesize that the mechanisms of regulation of blood vessel cell proliferation differ from those involved in the regulation of proliferation of other cell types in the placenta.
A reduced density of blood vessels in FM or CAR was observed in NAT-ET, IVF or IVA groups in this study. This may be associated with reduced overall growth measured by cell proliferation in FM and CAR reported before for the same animal models (Grazul-Bilska et al. 2013). In addition, the IVA group had the lowest density of blood vessels in FM, and a very low vascular cell proliferation in CAR. These data indicate that in the absence of the paternal genome, fetal-maternal interactions are altered causing profoundly reduced vascularization in the maternal placenta.
Reduced vascular density in maternal and fetal placenta in pregnancies achieved after transfer of embryos created through IVF or oocyte activation reported in this study indicates that angiogenesis was impaired during early pregnancy. Therefore, application of ART may lead to inadequate placental vascularization and thus blood flow at least during early gestation, and eventually may contribute to compromised pregnancy.
Numerous factors are involved in the regulation of vascular function and growth in the placenta, including members of the VEGF, ANGPT, FGF, HIF families, and the NO system (Patan 2000, Reynolds & Redmer 1995, 2001; Zygmunt et al. 2003, Demir et al. 2007, Burton et al. 2009, Reynolds et al. 2010). Expression and function of these factors depend on stage of pregnancy, maternal age, and environmental and other factors (Reynolds et al. 2010, 2013). In the present study, the mRNA expression of 12 out of 14 evaluated factors involved in the regulation of angiogenesis was decreased in FM and/or CAR in NAT-ET, IVF and/or IVA groups compared to the NAT control. Expression of mRNA for only two factors in placental tissues, KDR and GUCY1B3, remained unaffected by embryo origin in the present study.
Factors belonging to the VEGF family, including VEGF, FLT1 and PGF along with NP1 and 2, which are involved in the VEGF and PGF signaling, and also members belonging to the ANGPT family are recognized as the major angiogenic factors in placental and other tissues (Ahmed & Perkins 2000, Reynolds & Redmer 2001, Neufeld et al. 2002, Wulff et al. 2003, Reynolds et al. 2005, 2010, Chaballe et al. 2011, Koch et al. 2011). Expression of these factors has been demonstrated in maternal and/or fetal placenta from early to late pregnancy in sheep and other species (Reynolds & Redmer 2001, Borowicz et al. 2007, Grazul-Bilska et al. 2010, 2011, Reynolds et al. 2010, 2013). In our study, mRNA expression for several members of the VEGF family and NP1 or 2 was reduced in pregnancies achieved using ART. Furthermore, in compromised pregnancies such as those involving IUGR, pre-eclampsia or delivery of small-for-gestational age neonates, altered placental expression of members of the VEGF or ANGPT families, vascularization, plasma concentration of PGF and VEGF, or a balance between various angiogenic and/or antiangiogenic factors from mid to late stages have all been reported for humans and other species (Ahmed & Perkins, 2000, Regnault et al. 2002, Wulff et al. 2003, Redmer et al. 2005, 2009, Arroyo & Winn 2008, Erez et al. 2008). Thus, expression of members of the VEGF and ANGPT families are altered in specific pregnancy disorders at mid to late stages and early pregnancies achieved by transfer of embryos created through ART or oocyte activation. This indicates that changes in expression and possibly function of these factors may contribute to pregnancy disorders.
In FM, but not in CAR, expression of NOS3 and FGF2 mRNA, but not GUCY1B3 or FGFR2 was affected by embryo origin in this study. Furthermore, expression of HIF1A mRNA in CAR but not FM was affected by embryo origin. Members of the NO and FGF systems, and HIF1A are expressed throughout pregnancy in the placenta of several species (Reynolds & Redmer, 2001; Kwon et al. 2004; Grazul-Bilska et al. 2010, 2011; Patel at al. 2010; Reynolds et al. 2010; Krause et al. 2011). It has been demonstrated that placental eNOS and/or HIF1A expression was reduced or altered in human preeclampsia at term (Rajakumar et al. 2007), and also in an ovine model of IUGR at mid to late stages (Galan et al. 2001, Ziebell et al. 2007). This indicates that expression and likely function of these factors are affected in compromised pregnancies, and therefore may contribute to abnormal placental and/or fetal development.
The NO system members interact with members of VEGF, ANGPT and FGF families, and they also play a regulatory role in placental angiogenesis, remodeling and immunosuppression (Purcell et al. 1999, Ahmed & Perkins 2000, Reynolds & Redmer 2001, Mata-Greenwood et al. 2008, Grazul-Bilska et al. 2010, 2011, Reynolds et al. 2010, Krause et al. 2011). The HIF1A and other members of the HIF family are involved in regulation of placental growth, remodeling, differentiation, transport and vascularization acting as key mediators of placental development and function and are therefore likely to be important contributors to both normal and adverse pregnancy outcomes (Pringle et al. 2010). Reduced expression of selected genes mentioned above may contribute to inadequate vascularization and blood flow to the fetus.
Reduced expression of several key factors regulating tissue growth and also vascular function in the placenta, including PGF, HIF1A, FGF2 and their receptors (Shimizu et al. 2012) likely resulted in decreased placental cell proliferation and fetal size observed in our previous study using the same animal models (Grazul-Bilska et al. 2013). In addition, it has been recently demonstrated that expression of several imprinted genes (e.g., IGF2, H19 and PEG1) was less in FM in pregnancies achieved after ET of embryos created in vitro compared to natural early pregnancies (Ptak et al. 2013). This indicates that impaired tissue growth is associated with changes in expression of selected growth factors, as we observed in this study, and likely with imprinted genes.
Numerous factors involved in the regulation of angiogenesis interact with each other in order to control blood vessel function and development (Reynolds & Redmer 2001, Borowicz et al. 2007, Reynolds et al. 2010, 2013, Grazul-Bilska et al. 2010), which is reflected by significant correlations among these factors reported for FM and CAR in this study (supplementary Tables 1 and 2). Furthermore, correlations between 8 (out of 14) evaluated factors with the density of blood vessels in CAR observed in our study indicate functional associations between mRNA expression and blood vessel growth. The density of blood vessels in FM was correlated with expression of 2 factors which indicates a different pattern of blood vessel growth and its regulation in fetal vs. maternal placenta.
In the present study, tissues were collected only at one stage of early pregnancy (Day 22) after transfer of embryos of different origin and application of selected ART including estrus synchronization using CIDR devices, FSH-treatment, IVF or IVA and ET. These procedures were associated with a decrease in expression of several genes involved in the regulation of angiogenesis in FM and/or CAR. Thus, selected ART methods, including estrus synchronization, superovulatory treatments and ET with or without IVF or IVA, which are used in animal production and human reproductive medicine, may have some negative effects on fetal and placental growth and function during pregnancy (Sinclair 2008, Laprise 2009). In addition, it has been recently demonstrated that a specific estrus synchronization protocol may affect CAR vascular development during early pregnancy in sheep (Ruiz-Gonzalez et al. 2013), and FSH-superovulatory treatment affected gene expression in bovine granulosa cells which may lead to altered function (Dias et al., 2013). Conversely, in several experiments in which we used a similar estrus synchronization and/or FSH treatment protocol as in this study, the rates of fertilization or blastocyst formation, and the development of fetuses or offspring in sheep seemed to be normal (Grazul-Bilska et al. 2003, 2006, 2013). Although we and others observed changes in placental tissue growth, vascularization, expression of selected genes and global methylation after application of ART during early pregnancy (Grazul-Bilska et al. 2013, Ptak et al. 2013, Ruiz-Gonzalez et al. 2013), some compensatory mechanisms likely exist to minimize adverse effects of ART during early pregnancy and allow for normal fetal and placental growth and function throughout pregnancy and postnatal period. Alternatively, since pregnancies resulting from various ART, including IVF and SCNT, may exhibit poor placental development and vascularization as well as abnormal/altered fetal growth and development at different stages of gestation (Hoffert et al. 2005, Farin et al., 2006, Bauersachs et al. 2009, Collier et al., 2009, Mansouri-Attia et al. 2009; Ptak et al. 2013), the effects observed during early pregnancy in the present study could have long-term consequences for pregnancy outcome. To determine long-term consequences of ART application, additional studies should be undertaken using offspring created through use of selected ART.
As we have discussed in our previous studies using different embryo origin and also ART (Grazul-Bilska et al. 2013), the embryo affects uterine function and has an active role in initiation of pregnancy, and in turn, the uterus affects fetal growth and development (Reynolds & Redmer 1995, 2001, Spencer et al. 2008, Barnea 2004, Ostrup et al. 2011, Grazul-Bilska et al. 2013). In fact, abnormal embryo/fetal-maternal communication, endometrial remodeling, reduced vascularization, altered expression of angiogenic factors and other problems during the peri-implantation and later pregnancy periods have been observed for pregnancies achieved after transfer of embryos created through somatic cell nuclear transfer (SCNT) in animal models (Hill et al. 2000, Loi et al. 2005, Miles et al. 2004, 2005, Fletcher et al. 2007, Miglino et al. 2007, Palmieri et al. 2007, 2008, Campos et al. 2010). However, in pregnancies resulting from the transfer of embryos created through IVF, changes in endometrial vascularization, remodeling, and function were less pronounced than after transfer of embryos from SCNT (Hill et al. 2000, Miles et al. 2004, 2005, Miglino et al. 2007, Palmieri et al. 2007, 2008, Mansouri-Attia et al. 2009, Hoffert et al. 2005). As we discussed before (Grazul-Bilska et al., 2013), endometrial tissues possess mechanisms to adapt to embryos of different origin, which may serve as a biological sensor to meet embryonic demands or adaptation to environmental conditions (Fleming et al. 2004, Borowicz & Reynolds 2010, Coan et al. 2010). This idea is in line with our hypothesis that during pregnancy some compensatory mechanisms may exist to reverse negative effects of embryo manipulations on placental and fetal growth.
In summary, in this study, transfer of embryos of different origins and application of ART decreased vascular cell proliferation in CAR, the density of blood vessels in FM and CAR, and expression of several factors involved in the regulation of placental angiogenesis on Day 22 of pregnancy. Thus, embryo origin may have specific effects on vascular growth, and likely function, in the ovine placenta and fetus through regulation of tissue growth and angiogenesis, as well as other mechanisms such as epigenetic processes. Since relatively few studies have focused on evaluation of selected processes in the placenta during early gestation without or with application of ART in any species, these data provide novel information concerning fetal and placental vascular growth/cell proliferation and angiogenesis in relation to embryo origin. Furthermore, these data provide a foundation for determining the expression of specific factors regulating vascular development and function of placental and embryonic tissues in pregnancies after application of ART. In addition, these data will help us to better understand placental regulatory mechanisms in compromised pregnancies, and to identify strategies for rescuing such pregnancies.
Supplementary Material
Acknowledgments
Funding
This project was supported by USDA grant (2007-01215) to LP Reynolds and AT Grazul-Bilska, NIH grant (HL64141) to LP Reynolds and DA Redmer, Hatch Projects ND01712 to AT Grazul-Bilska and ND1727 to LP Reynolds and DA Redmer, and NSF MRI-R2-ARRA (0959512) grant to AT Grazul-Bilska.
The authors acknowledge Dr. Kimberly Vonnahme, Ms. Tammi Neville, Mr. James D. Kirsch, Mr. Kim C. Kraft, Mr. Robert Weigl, Mr. Terry Skunberg and other members of our laboratories and department for their assistance, and Dr. Jodie Haring for critical review of this manuscript.
Footnotes
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
References
- Ahmed A, Perkins J. Angiogenesis and intrauterine growth restriction. Baillieres Best Practice and Research Clinical Obstetrics and Gynaecology. 2000;14:981–998. doi: 10.1053/beog.2000.0139. [DOI] [PubMed] [Google Scholar]
- Allen C, Bowdin S, Harrison RF, Sutcliffe AG, Brueton L, Kirby G, Kirkman-Brown J, Barrett C, Reardon W, Maher E. Pregnancy and perinatal outcomes after assisted reproduction: a comparative study. Irish Journal of Medical Science. 2008;177:233–241. doi: 10.1007/s11845-008-0172-9. [DOI] [PubMed] [Google Scholar]
- Arroyo JA, Winn VD. Vasculogenesis and angiogenesis in the IUGR placenta. Seminars in Perinatology. 2008;32:172–177. doi: 10.1053/j.semperi.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Barker DJ. The origins of the developmental origins theory. Journal of Internal Medicine. 2007;261:412–417. doi: 10.1111/j.1365-2796.2007.01809.x. [DOI] [PubMed] [Google Scholar]
- Barnea ER. Insight into early pregnancy events: the emerging role of the embryo. American Journal of Reproductive Immunology. 2004;51:319–322. doi: 10.1111/j.1600-0897.2004.00159.x. [DOI] [PubMed] [Google Scholar]
- Barnes FL. The effects of the early uterine environment on the subsequent development of embryo and fetus. Theriogenology. 2000;53:649–658. doi: 10.1016/s0093-691x(99)00264-2. [DOI] [PubMed] [Google Scholar]
- Bassil JP, Magritte J, Roth M, Nisolle J, Donnez, Gordts S. Uterine vascularity during stimulation and its correlation with implantation in in-vitro fertilization. Human Reproduction. 1995;10:1497–1501. doi: 10.1093/humrep/10.6.1497. [DOI] [PubMed] [Google Scholar]
- Bauersachs S, Ulbrich SE, Zakhartchenko V, Minten M, Reichenbach M, Reichenbach HD, Blum H, Spencer TE, Wolf E. The endometrium responds differently to cloned versus fertilized embryos. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:5681–5686. doi: 10.1073/pnas.0811841106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borowicz PP, Reynolds LP. Placental programming: More may still be less. Perspective. Journal of Physiology. 2010;588:393. doi: 10.1113/jphysiol.2009.185983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borowicz PP, Arnold DR, Johnson ML, Grazul-Bilska AT, Redmer DA, Reynolds LP. Placental growth throughout the last two-thirds of pregnancy in sheep: vascular development and angiogenic factor expression. Biology of Reproduction. 2007;76:259–267. doi: 10.1095/biolreprod.106.054684. [DOI] [PubMed] [Google Scholar]
- Burton GJ, Charnock-Jones DS, Jauniaux E. Regulation of vascular growth and function in the human placenta. Reproduction. 2009;138:895–902. doi: 10.1530/REP-09-0092. [DOI] [PubMed] [Google Scholar]
- Cai LY, Izumi S, Koido S, Uchida N, Suzuki T, Matsubayashi H, Sugi T, Shida N, Kikuchi K, Yoshikata K. Abnormal placental cord insertion may induce intrauterine growth restriction in IVF-twin pregnancies. Human Reproduction. 2006;21:1285–1290. doi: 10.1093/humrep/dei494. [DOI] [PubMed] [Google Scholar]
- Campos DB, Papa PC, Marques JEB, Garbelotti F, Fatima LA, Artoni LP, Birgel EH, Meirelles FV, Buratini J, Leiser R, Pfarrer C. Somatic cell nuclear transfer is associated with altered expression of angiogenic factor system in bovine placentomes at term. Genetic and Molecular Research. 2010;9:309–323. doi: 10.4238/vol9-1gmr729. [DOI] [PubMed] [Google Scholar]
- Chaballe L, Schoenen J, Franzen R. Placental growth factor: a tissue modelling factor with therapeutic potentials in neurology? Acta Neurologica Belgica. 2011;111:10–17. [PubMed] [Google Scholar]
- Coan PM, Vaughan OR, Sekita Y, Finn SL, Burton GJ, Constancia M, Fowden AL. Adaptations in placental phenotype support fetal growth during undernutrition of pregnant mice. Journal of Physiology. 2010;588:527–538. doi: 10.1113/jphysiol.2009.181214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier AC, Miyagi SJ, Yamauchi Y, Ward MA. Assisted reproduction technologies impair placental steroid metabolism. Journal of Steroid Biochemistry and Molecular Biology. 2009;116:21–28. doi: 10.1016/j.jsbmb.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delle Piane L, Lin W, Liu X, Donjacour A, Minasi P, Revelli A, Maltepe E, Rinaudo PF. Effect of the method of conception and embryo transfer procedure on mid-gestation placenta and fetal development in an IVF mouse model. Human Reproduction. 2010;25:2039–2046. doi: 10.1093/humrep/deq165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demir R, Seval Y, Huppertz B. Vasculogenesis and angiogenesis in the early human placenta. Acta Histochemica. 2007;109:257–265. doi: 10.1016/j.acthis.2007.02.008. [DOI] [PubMed] [Google Scholar]
- Dias FC, Khan MI, Sirard MA, Adams GP, Singh J. Differential gene expression of granulosa cells after ovarian superstimulation in beef cattle. Reproduction. 2013;146:181–91. doi: 10.1530/REP-13-0114. [DOI] [PubMed] [Google Scholar]
- Esh-Broder E, Ariel I, Abas-Bashir N, Bdolah Y, Celnikier DH. Placenta accreta is associated with IVF pregnancies: a retrospective chart review. BJOG: An International Journal of Obstetrics and Gynaecology. 2011;118:1084–1089. doi: 10.1111/j.1471-0528.2011.02976.x. [DOI] [PubMed] [Google Scholar]
- Erez O, Romero R, Espinoza J, Fu W, Todem D, Kusanovic JP, Gotsch F, Edwin S, Nien JK, Chaiworapongsa T, Mittal P, Mazaki-Tovi S, Than NG, Gomez R, Hassan SS. The change in concentrations of angiogenic and anti-angiogenic factors in maternal plasma between the first and second trimesters in risk assessment for the subsequent development of preeclampsia and small-for-gestational age. Journal of Maternal-Fetal and Neonatal Medicine. 2008;21:279–287. doi: 10.1080/14767050802034545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farin PW, Piedrahita JA, Farin CE. Errors in development of fetuses and placentas from in vitro-produced bovine embryos. Theriogenology. 2006;65:178–191. doi: 10.1016/j.theriogenology.2005.09.022. [DOI] [PubMed] [Google Scholar]
- Fleming TP, Kwong WY, Porter R, Ursell E, Fesenko I, Wilkins A, Miller DJ, Watkins AJ, Eckert JJ. The embryo and its future. Biology of Reproduction. 2004;71:1046–1054. doi: 10.1095/biolreprod.104.030957. [DOI] [PubMed] [Google Scholar]
- Fletcher CJ, Roberts CT, Hartwich KM, Walker SK, McMillen IC. Somatic cell nuclear transfer in the sheep induces placental defects that likely precede fetal demise. Reproduction. 2007;133:243–255. doi: 10.1530/rep.1.01203. [DOI] [PubMed] [Google Scholar]
- Galan HL, Regnault TR, Le Cras TD, Tyson RW, Anthony RV, Wilkening RB, Abman SH. Cotyledon and binucleate cell nitric oxide synthase expression in an ovine model of fetal growth restriction. Journal of Applied Physiology. 2001;90:2420–2426. doi: 10.1152/jappl.2001.90.6.2420. [DOI] [PubMed] [Google Scholar]
- Gourvas V, Dalpa E, Konstantinidou A, Vrachnis N, Spandidos DA, Sifakis S. Angiogenic factors in placentas from pregnancies complicated by fetal growth restriction. Molecular Medicine Report. 2012;6:23–27. doi: 10.3892/mmr.2012.898. [DOI] [PubMed] [Google Scholar]
- Grazul-Bilska AT, Borowczyk E, Bilski JJ, Reynolds LP, Redmer DA, Caton JS, Vonnahme KA. Overfeeding and underfeeding have detrimental effects on oocyte quality measured by in vitro fertilization and early embryonic development in sheep. Domestic Animal Endocrinology. 2012;43:289–98. doi: 10.1016/j.domaniend.2012.05.001. [DOI] [PubMed] [Google Scholar]
- Grazul-Bilska AT, Borowicz PP, Johnson ML, Minten MA, Bilski JJ, Wroblewski R, Redmer DA, Reynolds LP. Placental development during early pregnancy in sheep: vascular growth and expression of angiogenic factors in maternal placenta. Reproduction. 2010;140:165–174. doi: 10.1530/REP-09-0548. [DOI] [PubMed] [Google Scholar]
- Grazul-Bilska AT, Choi JT, Bilski JJ, Weigl RM, Kirsch JD, Kraft KC, Reynolds LP, Redmer DA. Effects of epidermal growth factor on early embryonic development after in vitro fertilization of oocytes collected from ewes treated with follicle stimulating hormone. Theriogenology. 2003;59:1453–1461. doi: 10.1016/s0093-691x(02)01192-5. [DOI] [PubMed] [Google Scholar]
- Grazul-Bilska AT, Johnson ML, Borowicz PP, Baranko L, Redmer DA, Reynolds LP. Placental development during early pregnancy in sheep: Effects of embryo origin on fetal and placental growth, and global methylation. Theriogenology. 2013;79:94–102. doi: 10.1016/j.theriogenology.2012.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grazul-Bilska AT, Johnson ML, Borowicz PP, Minten M, Wroblewski R, Coupe LR, Redmer DA, Reynolds LP. Placental development during early pregnancy in sheep: Cell proliferation, global methylation and angiogenesis in fetal placenta. Reproduction. 2011;141:529–540. doi: 10.1530/REP-10-0505. [DOI] [PubMed] [Google Scholar]
- Grazul-Bilska AT, Kirsch JD, Bilski JJ, Kraft KC, Windorski EJ, Luther JS, Vonnahme KA, Reynolds LP, Redmer DA. Superovulation in sheep: Number and weight of the corpora lutea and serum progesterone. Sheep Goat Research Journal. 2007;22:26–31. [Google Scholar]
- Grazul-Bilska AT, Pant D, Luther JS, Choi JT, Borowicz P, Navanukraw C, Kirsch JD, Kraft KC, Weigl RM, Redmer DA, Reynolds LP. Pregnancy rates and gravid uterine parameters in single, twin and triplet pregnancies in naturally bred ewes and ewes after transfer of in vitro produced embryos. Animal Reproduction Science. 2006;92:268–283. doi: 10.1016/j.anireprosci.2005.06.013. [DOI] [PubMed] [Google Scholar]
- Greiss FC, Anderson SG. Uterine blood flow during early ovine pregnancy. American Journal of Obstetrics and Gynecology. 1970;106:30. doi: 10.1016/0002-9378(70)90123-7. [DOI] [PubMed] [Google Scholar]
- Hoffert KA, Batchelder CA, Bertolini M, Moyer AL, Famula TR, Anderson DL, Anderson GB. Measures of maternal-fetal interaction in day-30 bovine pregnancies derived from nuclear transfer. Cloning Stem Cells. 2005;7:289–305. doi: 10.1089/clo.2005.7.289. [DOI] [PubMed] [Google Scholar]
- Hill JR, Burghardt RC, Jones K, Long CR, Looney CR, Shin T, Spencer TE, Thompson JA, Winger QA, Westhusin ME. Evidence for placental abnormality as the major cause of mortality in first-trimester somatic cell cloned bovine fetuses. Biology of Reproduction. 2000;63:1787–1794. doi: 10.1095/biolreprod63.6.1787. [DOI] [PubMed] [Google Scholar]
- Igwebuike UM. A review of uterine structural modifications that influence conceptus implantation and development in sheep and goats. Animal Reproduction Sciences. 2009;112:1–7. doi: 10.1016/j.anireprosci.2008.12.010. [DOI] [PubMed] [Google Scholar]
- Kirk RE. Experimental Design: Procedures for the Behavioral Sciences. 2. Belmont, CA: Brooks/Cole; 1982. [Google Scholar]
- Koch S, Tugues S, Li X, Gualandi L, Claesson-Welsh L. Signal transduction by vascular endothelial growth factor receptors. Biochemical Journal. 2011;437:169–183. doi: 10.1042/BJ20110301. [DOI] [PubMed] [Google Scholar]
- Krause BJ, Hanson MA, Casanello P. Role of nitric oxide in placental vascular development and function. Placenta. 2011;32:797–805. doi: 10.1016/j.placenta.2011.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon H, Wu G, Meininger CJ, Bazer FW, Spencer TE. Developmental changes in nitric oxide synthesis in the ovine placenta. Biology of Reproduction. 2004;70:679–86. doi: 10.1095/biolreprod.103.023184. [DOI] [PubMed] [Google Scholar]
- Laprise SL. Implications of epigenetics and genomic imprinting in assisted reproductive technologies. Molecular Reproduction and Development. 2009;76:1006–1018. doi: 10.1002/mrd.21058. [DOI] [PubMed] [Google Scholar]
- Loi P, Clinton M, Vackova I, Fulka J, Jr, Feil R, Palmieri C, Della Salda L, Ptak G. Placental abnormalities associated with post-natal mortality in sheep somatic cell clones. Theriogenology. 2005;65:1110–1121. doi: 10.1016/j.theriogenology.2005.07.016. [DOI] [PubMed] [Google Scholar]
- Mansouri-Attia N, Sandra O, Aubert J, Degrelle S, Everts RE, Giraud-Delville C, Heyman Y, Galio L, Hue I, Yang X, Tian XC, Lewin HA, Renard JP. Endometrium as an early sensor of in vitro embryo manipulation technologies. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:5687–5692. doi: 10.1073/pnas.0812722106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mata-Greenwood E, Liao WX, Zheng J, Chen DB. Differential activation of multiple signalling pathways dictates eNOS upregulation by FGF2 but not VEGF in placental artery endothelial cells. Placenta. 2008;29:708–717. doi: 10.1016/j.placenta.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayhew TM, Ohadike C, Baher PN, Crocker IP, Mitchell C, Ong SS. Stereological investigation of placental morphology in pregnancies complicated by pre-eclampsia with and without intrauterine growth restriction. Placenta. 2003;24:219–116. doi: 10.1053/plac.2002.0900. [DOI] [PubMed] [Google Scholar]
- Mayhew TM, Charnock-Jones DS, Kaufmann P. Aspects of human fetoplacental vasculogenesis and angiogenesis. III. Changes in complicated pregnancies. Placenta. 2004a;25:127–139. doi: 10.1016/j.placenta.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Mayhew TM, Wijesekara J, Baker PN, Ong SS. Short Communication: Morphometric evidence that villous development and fetoplacental angiogenesis are compromised by intrauterine growth restriction but not by pre-eclampsia. Placenta. 2004b;25:829–833. doi: 10.1016/j.placenta.2004.04.011. [DOI] [PubMed] [Google Scholar]
- Meegdes HL, Ingenhoes MR, Peeters LLH, Exalto N. Early pregnancy wastage: relationship between chorionic vascularization and embryonic development. Fertility and Sterility. 1988;49:216–220. doi: 10.1016/s0015-0282(16)59704-0. [DOI] [PubMed] [Google Scholar]
- Miglino MA, Pereira FTV, Visintin JA, Garcia JM, Meirelles FV, Rumpf R, Ambrosio CE, Papa PC, Santos TC, Carvalho AF, Leiser R, Carter AM. Placentation in cloned cattle: Structure and microvascular architecture. Theriogenology. 2007;68:604–617. doi: 10.1016/j.theriogenology.2007.04.060. [DOI] [PubMed] [Google Scholar]
- Miles JR, Farin CE, Rodriguez KF, Alexander JE, Farin PW. Angiogenesis and morphometry of bovine placentas in late gestation from embryos produced in vivo or in vitro. Biology of Reproduction. 2004;71:1919–1926. doi: 10.1095/biolreprod.104.031427. [DOI] [PubMed] [Google Scholar]
- Miles JR, Farin CE, Rodriguez KF, Alexander JE, Farin PW. Effects of embryo culture on angiogenesis and morphometry of bovine placentas during early gestation. Biology of Reproduction. 2005;73:663–671. doi: 10.1095/biolreprod.105.040808. [DOI] [PubMed] [Google Scholar]
- Nathanielsz PW. Animal models that elucidate basic principles of the developmental origins of adult diseases. Institute for Laboratory Animal Research Journal. 2006;47:73–82. doi: 10.1093/ilar.47.1.73. [DOI] [PubMed] [Google Scholar]
- Neufeld G, Kessler O, Herzog Y. The interaction of Neuropilin-1 and Neuropilin-2 with tyrosine-kinase receptors for VEGF. Advances in Experimental Medicine and Biology. 2002;515:81–90. doi: 10.1007/978-1-4615-0119-0_7. [DOI] [PubMed] [Google Scholar]
- Ostrup E, Hyttel P, Ostrup O. Embryo-maternal communication: signalling before and during placentation in cattle and pig. Reproduction Fertility Development. 2011;23:964–975. doi: 10.1071/RD11140. [DOI] [PubMed] [Google Scholar]
- Palmieri C, Loi P, Ptak G, Della Salda L. Review paper: a review of the pathology of abnormal placentae of somatic cell nuclear transfer clone pregnancies in cattle, sheep, and mice. Veterinary Pathology. 2008;45:865–880. doi: 10.1354/vp.45-6-865. [DOI] [PubMed] [Google Scholar]
- Palmieri C, Loi P, Reynolds LP, Ptak G, Della Salda L. Placental abnormalities in ovine somatic cell clones at term: A light and electron microscopic investigation. Placenta. 2007;28:577–584. doi: 10.1016/j.placenta.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Patan S. Vasculogenesis and angiogenesis as mechanisms of vascular network formation, growth and remodeling. Journal of Neuro-oncology. 2000;50:1–15. doi: 10.1023/a:1006493130855. [DOI] [PubMed] [Google Scholar]
- Patel J, Landers K, Mortimer RH, Richard K. Regulation of hypoxia inducible factors (HIF) in hypoxia and normoxia during placental development. Placenta. 2010;31:951–957. doi: 10.1016/j.placenta.2010.08.008. [DOI] [PubMed] [Google Scholar]
- Pringle KG, Kind KL, Sferruzzi-Perri AN, Thompson JG, Roberts CT. Beyond oxygen: complex regulation and activity of hypoxia inducible factors in pregnancy. Human Reproduction Update. 2010;16:415–431. doi: 10.1093/humupd/dmp046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptak GE, D’Agostino A, Toschi P, Fidanza A, Zacchini F, Czernik M, Monaco F, Loi P. Post-implantation mortality of in vitro produced embryos is associated with DNA methyltransferase 1 dysfunction in sheep placenta. Human Reproduction. 2013;28:298–305. doi: 10.1093/humrep/des397. [DOI] [PubMed] [Google Scholar]
- Purcell TL, Given R, Chwalisz K, Garfield RE. Nitric oxide synthase distribution during implantation in the mouse. Molecular Human Reproduction. 1999;5:467–475. doi: 10.1093/molehr/5.5.467. [DOI] [PubMed] [Google Scholar]
- Rajakumar A, Jeyabalan A, Markovic N, Ness R, Gilmour C, Conrad KP. Placental HIF-1 alpha, HIF-2 alpha, membrane and soluble VEGF receptor-1 proteins are not increased in normotensive pregnancies complicated by late-onset intrauterine growth restriction. American Journal of Physiology- Regulatory Integrative and Comparative Physiology. 2007;293:766–774. doi: 10.1152/ajpregu.00097.2007. [DOI] [PubMed] [Google Scholar]
- Redmer DA, Wallace JM, Reynolds LP. Effect of nutrient intake during pregnancy on fetal and placental growth and vascular development. Domestic Animal Endocrinology. 2004;27:199–217. doi: 10.1016/j.domaniend.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Redmer DA, Aitken RP, Milne JS, Reynolds LP, Wallace JM. Influence of maternal nutrition on messenger RNA expression of placental angiogenic factors and their receptors at mid-gestation in adolescent sheep. Biology of Reproduction. 2005;72:1004–1009. doi: 10.1095/biolreprod.104.037234. [DOI] [PubMed] [Google Scholar]
- Redmer DA, Luther J, Milne J, Aitken R, Johnson M, Borowicz P, Borowicz M, Reynolds LP, Wallace J. Fetoplacental growth and vascular development in overnourished adolescent sheep at day 50, 90 and 130 of gestation. Reproduction. 2009;137:749–757. doi: 10.1530/REP-08-0516. [DOI] [PubMed] [Google Scholar]
- Regnault TR, Orbus RJ, de Vrijer B, Davidsen ML, Galan HL, Wilkening RB, Anthony RV. Placental expression of VEGF, PlGF and their receptors in a model of placental insufficiency-intrauterine growth restriction (PI-IUGR) Placenta. 2002;23:132–144. doi: 10.1053/plac.2001.0757. [DOI] [PubMed] [Google Scholar]
- Rennie MY, Detmar J, Whiteley KJ, Yang J, Jurisicova A, Adamson SL, Sled JG. Vessel tortuousity and reduced vascularization in the fetoplacental arterial tree after maternal exposure to polycyclic aromatic hydrocarbons. American Journal of Physiology-Heart and Circulatory Physiology. 2011;300:675–684. doi: 10.1152/ajpheart.00510.2010. [DOI] [PubMed] [Google Scholar]
- Reynolds LP. Utero-ovarian interactions during early pregnancy: Role of conceptus induced vasodilation. Journal of Animal Sciences. 1986;62:47–61. doi: 10.1093/ansci/62.2.47. [DOI] [PubMed] [Google Scholar]
- Reynolds LP, Caton JS. Role of the pre- and post-natal environment in developmental programming of health and productivity. Molecular and Cellular Endocrinology. 2012;354:54–59. doi: 10.1016/j.mce.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds LP, Redmer DA. Growth and microvascular development of the uterus during early pregnancy in ewes. Biology of Reproduction. 1992;47:698–708. doi: 10.1095/biolreprod47.5.698. [DOI] [PubMed] [Google Scholar]
- Reynolds LP, Redmer DA. Utero-placental vascular development and placental function. Journal of Animal Science. 1995;73:1839–1851. doi: 10.2527/1995.7361839x. [DOI] [PubMed] [Google Scholar]
- Reynolds LP, Redmer DA. Angiogenesis in the placenta. Biology of Reproduction. 2001;64:1033–1040. doi: 10.1095/biolreprod64.4.1033. [DOI] [PubMed] [Google Scholar]
- Reynolds LP, Borowicz PP, Caton JS, Vonnahme KA, Luther JS, Buchanan DS, Hafez SA, Grazul-Bilska AT, Redmer DA. Utero-placental vascular development and placental function: An update. International Journal of Developmental Biology. 2010;54:355–366. doi: 10.1387/ijdb.082799lr. [DOI] [PubMed] [Google Scholar]
- Reynolds LP, Borowicz PP, Vonnahme KA, Johnson ML, Grazul-Bilska AT, Wallace JM, Caton JS, Redmer DA. Animal models of placental angiogenesis. Placenta. 2005;26:689–708. doi: 10.1016/j.placenta.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Reynolds LP, Caton JS, Redmer DA, Grazul-Bilska AT, Vonnahme KA, Borowicz PP, Luther JS, Wallace JM, Wu G, Spencer TE. Evidence for altered placental blood flow and vascularity in compromised pregnancies. Journal of Physiology. 2006;572:51–58. doi: 10.1113/jphysiol.2005.104430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds LP, Vonnahme KA, Lemley CO, Redmer DA, Grazul-Bilska AT, Borowicz PP, Caton JS. Maternal Stress and Placental Vascular Function and Remodeling. Current Vascular Pharmacology. 2013;11:564–593. doi: 10.2174/1570161111311050003. [DOI] [PubMed] [Google Scholar]
- Ruiz-Gonzalez I, Sanchez MA, Garcia-Fernandez RA, Garcia-Palnecia P, Sanchez B, Gonzalez-Bulnes A, Flores JM. Different influence of ovine estrus synchronization treatments on caruncular early angiogenesis. Histology and Histopathology. 2013;28:373–383. doi: 10.14670/HH-28.373. [DOI] [PubMed] [Google Scholar]
- SAS Institute. SAS User’s Guide, Statistics. 5. Cary, NC: Statistical Analysis System Institute; 2010. [Google Scholar]
- Sellers López F, Orozco-Beltran D, Gil-Guillen V, Lozano JM, Palacios A, Bernabeu R. Analysis of placental vascularization by means of 3D Power Doppler in women pregnant following oocyte donation. Reproductive Sciences. 2010;17:754–759. doi: 10.1177/1933719110371013. [DOI] [PubMed] [Google Scholar]
- Shimizu T, Hoshino Y, Miyazaki H, Sato E. Angiogenesis and microvasculature in the female reproductive organs: physiological and pathological implications. Current Pharmaceutical Design. 2012;18:303–309. doi: 10.2174/138161212799040367. [DOI] [PubMed] [Google Scholar]
- Sinclair KD. Assisted reproductive technologies and pregnancy outcomes: mechanistic insights from animal studies. Seminars in Reproductive Medicine. 2008;26:153–161. doi: 10.1055/s-2008-1042954. [DOI] [PubMed] [Google Scholar]
- Spencer TE, Sandra O, Wolf E. Genes involved in conceptus-endometrial interactions in ruminants: insights from reductionism and thoughts on holistic approaches. Reproduction. 2008;135:165–179. doi: 10.1530/REP-07-0327. [DOI] [PubMed] [Google Scholar]
- Stenbak TK, Redmer DA, Berginski HR, Erickson AS, Navanukraw C, Toutges MJ, Bilski JJ, Kirsch JD, Kraft KC, Reynolds LP, Grazul-Bilska AT. Effects of follicle stimulating hormone (FSH) on follicular development, oocyte retrieval, and in vitro fertilization (IVF) in ewes during the breeding season and seasonal anestrus. Theriogenology. 2001;56:51–64. doi: 10.1016/s0093-691x(01)00542-8. [DOI] [PubMed] [Google Scholar]
- Tomic V, Tomic J. Neonatal outcome of IVF singletons versus naturally conceived in women aged 35 years and over. Archives of Gynecology and Obstetrics. 2011;284:1411–1416. doi: 10.1007/s00404-011-1873-2. [DOI] [PubMed] [Google Scholar]
- Tseng JJ, Chou MM. Differential expression of growth-, angiogenesis- and invasion-related factors in the development of placenta accreta. Taiwan Journal of Obstetrics and Gynecology. 2006;45:100–106. doi: 10.1016/S1028-4559(09)60205-9. [DOI] [PubMed] [Google Scholar]
- Vonnahme KA, Lemley CO. Programming the offspring through altered uteroplacental hemodynamics: how maternal environment impacts uterine and umbilical blood flow in cattle, sheep and pigs. Reproduction Fertility and Development. 2011;24:97–104. doi: 10.1071/RD11910. [DOI] [PubMed] [Google Scholar]
- Wulff C, Weigand M, Kreienberg R, Fraser HM. Angiogenesis during primate placentation in health and disease. Reproduction. 2003;126:569–577. doi: 10.1530/rep.0.1260569. [DOI] [PubMed] [Google Scholar]
- Ziebell BT, Galan HL, Anthony RV, Regnault TR, Parker TA, Arroyo JA. Ontogeny of endothelial nitric oxide synthase mRNA in an ovine model of fetal and placental growth restriction. American Journal of Obstetrics and Gynecology. 2007;197:420, e1–5. doi: 10.1016/j.ajog.2007.07.016. [DOI] [PubMed] [Google Scholar]
- Zygmunt M, Herr F, Münstedt K, Lang U, Liang OD. Angiogenesis and vasculogenesis in pregnancy. European Journal of Obstetrics Gynecology & Reproductive Biology. 2003;110:S10–18. doi: 10.1016/s0301-2115(03)00168-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.