Abstract
OBJECTIVE
To examine for the first time the associations between pro-inflammatory cytokines and obesity-related metabolic biomarkers in, exclusively prepubertal, otherwise healthy obese and non-obese Black and White children, 7–9 years of age.
DESIGN AND METHODS
Body mass index (BMI), homeostasis model assessment-estimated insulin resistance, visceral adipose tissue and subcutaneous adipose tissue (SAT (magnetic resonance imaging)); total body fat (dual-energy X-ray absorptiometry), ectopic, intrahepatic lipid (IHL) and intramyocellular lipid (IMCL) fat (proton magnetic resonance spectroscopy) and serum levels of interleukin (IL)-1, IL-6, IL-8, tumor necrosis factor alpha (TNF-α) and monocyte chemoattractant protein-1 were measured in 40 obese and non-obese children. Relationships between inflammatory cytokines and obesity were assessed by analysis of variance and Spearman’s rank correlation.
RESULTS
Significant inverse correlations were found between BMI z-score, SAT, total BF, and IHL and levels of TNF-α (Spearman’s ρ= − 0.36, − 0.39, − 0.43 and − 0.39, respectively; P<0.05). Levels of IL-8 were significantly and inversely correlated with IMCL (− 0.39; P =0. 03) and remained significant after adjusting for race. IMCL was inversely associated with TNF-α only after adjusting for race (− 0.37; P = 0.04).
CONCLUSIONS
Relationships between pro-inflammatory and metabolic markers commonly observed in adults are reversed in healthy, Black and White children before puberty. Prospective studies are warranted to determine how these inverse relationships modify chronic disease risk later in life.
Keywords: inflammation, metabolic syndrome, cytokines, prepubertal youth
INTRODUCTION
Obesity has reached epidemic proportions. The World Health Organization projects there will be more than 2 billion overweight and more than 700 million obese adults by 2015 (reviewed in Rojas et al.1). This observation is apparent in all ethnicities; however, in the United States, minority populations and those from disadvantaged backgrounds are most affected.2 Of more concern is that similar trends are found in young prepubertal children whose obesity perpetuates into adolescence.3–6 Remarkably, more than half of overweight adolescents will carry this condition into adulthood.5 Researchers have identified numerous factors to explain the increased rate of obesity in children, but higher intake of calories and lack of physical activity are among the most influential. For instance, it is well known that increased caloric consumption without an equivalent increase in energy expenditure leads to the accumulation and expansion of adipose tissue. The presence of abdominal adiposity (visceral adipose tissue (VAT)), specifically, in conjunction with altered lipid profiles, blood pressure and abnormal blood glucose, referred to as the metabolic syndrome, is a well-established precursor of type 2 diabetes and cardiovascular disease.1 In adults, increased VAT is followed by macrophage infiltration leading to a state of chronic low-grade inflammation.7–9
This sequence of events has lead researchers to identify obesity as an inflammatory condition,10 which is associated with increased production of cytokines such as interleukin (IL)-1, IL-6, IL-8, tumor necrosis factor α (TNF-α) and monocyte chemoattractant protein-1 (MCP-1), among others. These pro-inflammatory cytokines are linked to various diseases and share obesity as a common feature, for example, cardiovascular disease, asthma and type 2 diabetes.11–17 Obese adults demonstrate increased levels of circulating pro-inflammatory cytokines when compared with lean counterparts;18 one previously identified source of these inflammatory markers is VAT. Although previously classified as metabolically inactive, VAT is now known to produce adipokines (cytokines secreted by adipose tissue), which are either negatively or positively associated with obesity-related metabolic disorders leading to insulin resistance (IR) and type 2 diabetes.19,20 Research in adults also identifies ectopic fat (intrahepatic (IHL) and intramyocellular (IMCL) lipids) as markers of adipose tissue pathology contributing to IR and an adverse metabolic phenotype.21 More specifically, IHL is directly involved in the induction of IR, independent of other fat compartments.22 However, because IHL is highly associated with VAT, the secretion of pro-inflammatory cytokines may lead to oxidative damage and impaired insulin receptor signaling.23–25
In young children before pubertal development, especially minority youth who are at the highest risk for obesity and metabolic disease, the relationship of adiposity and inflammation is unclear. Interestingly, in younger children and adolescents, these associations are contradictory.12,26 Specifically, the relationship between TNF-α and IR is not as clear, or even opposite in children, which is dissimilar to results observed in adults.13 It is widely known that IR appears during puberty and that insulin secretion is modulated by age;27 thus, associations commonly observed in adults may not be evident in young, prepubertal children. Therefore, understanding the relationship between obesity-related inflammation and metabolic and cardiovascular disease in developing youth remains challenging. In an effort to better understand the role of inflammation in the development of obesity and IR over the life span, we determined the associations of circulating pro-inflammatory cytokines, IL-1β, IL-6, IL-8,TNF-α and MCP-1, with body mass index (BMI), BMI z-score (z_BMI) and obesity-related metabolic biomarkers: IR, visceral adiposity (VAT); total body, ectopic fat (IHL and IMCL), using state-of-the-art objective measurement techniques in otherwise healthy, obese and non-obese Black and White prepubertal children, 7–9 years of age.
MATERIALS AND METHODS
Study population
The characteristics of the children in the MET (Mechanisms for the Metabolic Syndrome in Prepubertal Youth) study have been described previously.28,29 Briefly, exclusively prepubertal obese and non-obese children, 7–9 years of age, were recruited in the study from May 2006 to March 2010. The original study included Black, White, Asian, Pacific Islander and Hispanic prepubertal youth; however, only Black and White prepubertal youth were considered for the present study as there was only one Pacific Islander and one Hispanic child enrolled. A pre-enrollment process was carried out by phone interview with parents/guardians of the candidates. Final eligibility was determined after a complete medical examination and screening blood test that included a comprehensive metabolic panel, a complete blood count and a Tanner staging pediatrician examination to confirm prepubertal status.30 Exclusion criteria included Tanner ≥ 2, cardiovascular disease or liver disease, being born from a mother with gestational diabetes and/or family history of type 1 or type 2 diabetes. Parents/guardians read, understood and provided a signed written consent of the children’s participation, and children gave a written approval before any procedure. Institutional review boards from the Louisiana State University Health Sciences Center, Children’s Hospital in New Orleans, LA, Women’s Hospital and the Pennington Biomedical Research Center in Baton Rouge, LA approved the study.
Anthropometrics and metabolic parameters
Anthropometric measurements were determined as previously described.28,29 Briefly, BMI was determined by the ratio weight (kg)/height (m2), and this was used to obtain the z_BMI, based on age, gender and race along with other criteria.31 IR was estimated by the homeostasis model assessment-estimated insulin resistance (HOMA-IR) based on previously published methods;32 VAT and subcutaneous adipose tissue (SAT) were determined by magnetic resonance imaging (MRI) in the fourth through fifth lumbar vertebrae area. A trained technician acquired spin-echo T1 weighted (TR=500; TE=20) images. The manual trackball technique was used to define adipose tissue. The MRI fat signal between the skin and abdominal muscle walls and intra-abdominal adipose tissue area, along with signals from intraperitoneal, mesenteric and omental depots were used to calculate SAT as suggested previously.32–34 Total body fat (BF) was measured by dual-energy X-ray absorptiometry. Ectopic fat (IHL and intramyocellular lipids (IMCL)) were assessed by proton magnetic resonance spectroscopy as detailed previously.29,35
Circulating levels of pro and anti-inflammatory molecules
Fasting serum levels (pg ml−1) of IL-1β, IL-6, IL-8, TNF-α, and MCP-1, and adiponectin were measured using Milliplex Map Kit (Millipore Corporation, Billerica, MA, USA), as recommended by the manufacturer. Briefly, 25 µl of a 1:100 dilution of serum was mixed with immunobeads (supplied in the kit) and incubated overnight at 4 °C, washed twice with a buffer to remove unbound products and incubated with a detection antibody for 1 h at room temperature (20–25 °C) with agitation. Finally, streptavidin-phycoerythrin was added and the samples were incubated 30 min at room temperature, washed twice and the fluorescence was detected on a Bio-Plex system (Bio-Rad, Hercules, CA, USA). The unknown samples (sera) were analyzed in duplicate using a standard curve of known concentrations of each one of the tested molecules. The run included negative and control samples. To visualize the correlations, scatterplots of pairs of markers with significant Spearman’s correlation were constructed using the logarithmic values of the TNF-α, IHL and IL-8 measurements. Owing to limits in the availability of these pediatric participant samples obtained from an ancillary study,36 we were unable to confirm the results by enzyme-linked immunosorbent assay, but our experience shows a strong correlation between the two techniques (Milliplex and enzyme-linked immunosorbent assay).37
Statistical analyses
Statistical analyses were carried out in SAS 9.3 (SAS Institute, Cary, NC, USA). Tests were conducted at 0.05 significance level; no adjustments for multiple testing were performed. For each analysis, effective sample sizes are reported as data are not available for all patients on all measured variables, as previously explained.28,29 Race and obesity status differences in obesity parameters were evaluated by analysis of variance with Satterthwaite correction for unequal variances. The strength of associations between inflammatory cytokines and obesity parameters were assessed by Spearman’s rank correlations. As this was a multiracial cohort of males and females, the potential confounding effect of sex and race were accounted for by corresponding partial correlations.
RESULTS
Demographic, anthropometric and metabolic data were available for 118 Black and White children in the original MET study; inflammatory markers are available in a subgroup of 40 of these children. Analyses were carried out on most complete data (Tables 1 and 2). As expected and as we previously reported,28 we observed very striking differences in metabolic parameters when comparisons were made between obese (z_BMI ≥ 2) and non-obese (z_BMI <2) children (Table 1), as all parameters except HOMA-IR and EMCL were significantly higher in the obese children. No differences in metabolic parameters were observed between racial groups (Table 3).
Table 1.
Differences in anthropometrics and metabolic parameters in children by obesity status and P-values after adjusting for race (N = 37)
| Non-obese (n = 27) | Obese (n = 9) | P-valuea | ||
|---|---|---|---|---|
| Mean ± s.e.m. | Crude | Race adjusted | ||
| z_BMI | 0.97 ± 0.11 | 2.37 ± 0.087 | 0.0001 | 0.0001 |
| Body fat (%) | 7.86 ± 0.53 | 18.17 ± 1.88 | 0.0004 | 0.0009 |
| HOMA-IR (mmol µl−2) | 0.61 ± 0.1 | 1.36 ± 0.37 | 0.0832 | 0.2395 |
| VAT (cm2) | 13.122 ± 1.218 | 19.64601 ± 2.203 | 0.0222 | 0.0331 |
| SAT (cm2) | 94.377 ± 10.850 | 281.7344 ± 33.644 | 0.0004 | 0.0001 |
| IHL (%) | 0.005 ± 0.0009 | 0.014 ± 0.0024 | 0.0072 | 0.0060 |
| IMCL (%) | 0.004 ± 0.0004 | 0.006 ± 0.0004 | 0.0003 | 0.0001 |
| EMCL (%) | 0.009 ± 0.001 | 0.012 ± 0.002 | 0.0895 | 0.1072 |
Abbreviations: ANOVA, analysis of variance; BMI, body mass index; EMCL, extramyocellular lipids; HOMA-IR, homeostasis model assessment-estimated insulin resistance; IHL, intrahepatic lipid; IMCL, intramyocellular lipid; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue.
One-way/two-way ANOVA.
Table 2.
Association of anthropometric and metabolic parameters with inflammatory markers; crude and race adjusted (Spearman’s correlations)
| IL-8 | TNF-α | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Crude | Race adjusted | Crude | Race adjusted | |||||||
| N | r | P | r | P | n | r | P | r | P | |
| z_BMI | 35 | −0.3174 | 0.0521 | −0.2370 | 0.1772 | 35 | −0.3590 | 0.0269 | −0.4034 | 0.0180 |
| Body fat (%) | 38 | −0.1465 | 0.3940 | −0.3057 | 0.0657 | 38 | −0.4246 | 0.0098 | −0.3794 | 0.0206 |
| HOMA-IR (mmol µl−2) | 29 | −0.0260 | 0.8876 | −0.0389 | 0.8441 | 29 | −0.1617 | 0.3766 | −0.2007 | 0.3059 |
| VAT (cm2) | 29 | −0.1339 | 0.4883 | −0.10245 | 0.6039 | 29 | −0.0332 | 0.864 | −0.00653 | 0.9737 |
| SAT (cm2) | 29 | −0.3359 | 0.0748 | −0.31306 | 0.1048 | 29 | −0.3895 | 0.0367 | −0.37422 | 0.0498 |
| IHL (%) | 30 | −0.1535 | 0.4179 | −0.1664 | 0.3884 | 30 | −0.3864 | 0.0349 | −0.3897 | 0.0367 |
| IMCL (%) | 31 | −0.3923 | 0.0264 | −0.3757 | 0.0408 | 31 | −0.3324 | 0.0630 | −0.3700 | 0.0442 |
| EMCL (%) | 31 | −0.0064 | 0.9722 | −0.0618 | 0.7457 | 31 | −0.1340 | 0.4645 | −0.0891 | 0.6398 |
Abbreviations: BMI, body mass index; EMCL, extramyocellular lipids; HOMA-IR, homeostasis model assessment-estimated insulin resistance; IHL, intrahepatic lipid; IL, interleukin; IMCL, intramyocellular lipid; SAT, subcutaneous adipose tissue; TNF-α, tumor necrosis factor alpha; VAT, visceral adipose tissue.
P-value is for no association test.
Table 3.
Anthropometric and metabolic parameters by race for the subset of children in the MET study (N = 40) with data on inflammatory markers
| Blacks (n = 13) | Whites (n = 27) | P-valuea | |
|---|---|---|---|
| Mean ± s.e.m. | |||
| z_BMI | 1.311 ± 0.248 | 1.061 ± 0.181 | 0.4235 |
| Body fat (%) | 10.9 ± 2.432 | 9.88 ± 0.817 | 0.6971 |
| HOMA-IR (mmol µl−2) | 0.956 ± 0.322 | 0.701 ± 0.0105 | 0.4671 |
| VAT (cm2) | 16.152 ± 1.438 | 14.884 ± 1.479 | 0.5470 |
| SAT (cm2) | 233.6 ± 75.248 | 131.4 ± 15.689 | 0.2367 |
| IHL (%) | 0.006 ± 0.002 | 0.008 ± 0.001 | 0.546 |
| IMCL (%) | 0.005 ± 0.001 | 0.004 ± 0.0003 | 0.2385 |
| EMCL (%) | 0.012 ± 0.002 | 0.009 ± 0.001 | 0.1589 |
Abbreviations: ANOVA, analysis of variance; BMI, body mass index; EMCL, extramyocellular lipids; IHL, intrahepatic lipid; IMCL, intramyocellular lipid; MET Study, Mechanisms for the Metabolic Syndrome in Prepubertal Youth Study; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue.
One-way ANOVA.
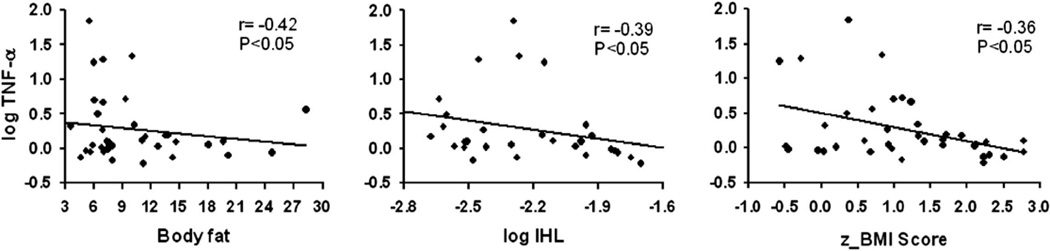
We next examined the correlation between several inflammatory markers, that is, IL-1β, IL-6, IL-8, TNF-α and MCP-1, with obesity (z_BMI) and obesity-related metabolic biomarkers, that is, HOMA-IR, VAT, SAT, BF, IHL and IMCL. As can be observed in Table 2, significant inverse correlations were found between z_BMI, SAT, BF and IHL with the levels of TNF-α (−0.36, −0.39, −0.42 and 0.39, respectively; P < 0.05) and remained significant after adjusting for race. However, no significant relationships were observed between HOMA-IR and VAT and TNF- α. Initially, the relationship between IMCL and TNF-α was not significant. However, after adjusting for race, we observed an inverse association between IMCL and TNF-α (− 0.37; P = 0.04). Conversely, the levels of IL-8 were significantly and inversely correlated with IMCL and remained significant after adjusting for race (− 0.39; P = 0.03). Figure 1 shows data for pairs of markers with significant correlations between z_BMI, BF and IHL with TNF-α. Adjustment for gender had no effect on the results. No other significant correlations were detected.
Figure 1.
Scatterplots of pairs of markers with significant Spearman’s correlation in Table 1. Logarithmic scale values of TNF-α are used to test the correlations with BF, IHL and z_BMI. Lines are estimated linear regressions by least squares in order to visualize the inverse correlation between markers.
DISCUSSION
In this study, we document that traditional relationships between inflammatory (IL-8 and TNF-α) and obesity and metabolic (z_BMI, HOMA-IR, VAT, SAT, BF, IHL and IMCL) biomarkers usually observed in adults and adolescents38 are not evident in healthy, Black and White obese and non-obese children before the onset of puberty. In addition, unlike adults, we found that these pro-inflammatory and metabolic factors did not differ between Blacks and Whites, with the exception of the relationship between TNF-α and IMCL, which was confounded by race. In adults, ectopic fat is shown to increase the secretion of pro-inflammatory adipokines (reviewed in Drouet et al.39 and Galic et al.40). Animal models have shown that obesity may result in damage of the myocardial cells and decreased efficiency of contractions in heart muscle under hypoxic conditions.41 The same animal models have shown that the transport of glucose to the skeletal muscle is significantly reduced in rats fed with a high-fat diet.42 In addition, excess accumulation of fat on blood vessels reduces their contractile activity.43 In contrast, anti-inflammatory biomarkers such as adiponectin prevent cell damage, promote insulin sensitivity and stimulate β-oxidation of fatty acids.5 Thus, the role of inflammatory adipokines in adults is deleterious and leads to adverse cellular consequences. However, in young children, especially as they mature from pre- to postpubertal stages of development, compensatory mechanisms as a result of normal growth may temporarily increase inflammation. In this case, the presence of these biomarkers may actually serve to preserve glucose homeostasis through paradoxical mechanism(s) that are not well understood. In contrast, it is possible that at an early age, the presence of an existing inflammatory environment is crucial for defending the body against infection, allergies and other insults. The chronic nature of this inflammatory environment, beneficial during developmental stages, however, may lead to undesirable immune responses associated with disease later in life (that is, age-related antagonistic pleiotropy).44 Previous research suggests that levels of inflammatory markers, that is IL-6, alter in response to stress during periods of development in youth.45 This individual IL-6 response to stress is directly dependent on the presence of specific genotypes in the human IL-6 gene promoter.44 Interestingly, mouse models of stress show that early life responses to induced stress in mice are reduced, perhaps due to an immature hypothalamic pituitary-adrenal axis.46 However, it remains to be determined whether the immature hypothalamic pituitary-adrenal axis explains the decreased response to stress in humans early in life, although some studies suggest that this may be the case (reviewed in Gunnar et al.47). Thus, it may be that in this cross-sectional examination, we were only able to document an acute inflammatory response, which may actually be beneficial to the conservation of metabolic homeostasis.8 In this instance, resident populations of leukocytes become activated to temporarily suppress lipolytic signals, an event often observed during lipid flux between lean and obese states even in adults. Furthermore, because of natural developmental activity, alterations in body composition, including adipose tissue, during the transition from childhood to puberty, may help explain differences in the type and level of inflammatory and/or anti-inflammatory molecules secreted.48,49 These fluctuations may partly explain our finding that SAT, but not VAT, was inversely associated with TNF-α in our cohort of healthy, prepubertal obese and non-obese youth. In a similar investigation in overweight and obese prepubertal youth (n = 30), Maffeis et al.50 reported significant relationships between VAT and C-reactive protein but no significant association between VAT or SAT and TNF-α, although they did find a significant inverse relationship between insulin sensitivity and SAT but not VAT. It is important to note, however, that all of the participants in Maffeis’ study were overweight or obese, whereas our cohort was predominantly non-obese. In a study of 16 prepubertal non-obese, children, which examined adipogenic capacity of VAT and SAT samples via biopsy, TNF-α blocked the differentiation of both VAT and AT precursor cell; however, this effect was more pronounced in SAT cells.51 Thus, examining inflammatory markers and adipose tissue before puberty when children are destined toward rapid growth may be counterintuitive owing to fluctuations in lipid stores related to growth of organ and muscle tissues, and this may be even more evident in non-obese versus obese youth.
Conversely, the developmental dilemma may actually be explained through further examination of ectopic fat stores in liver and skeletal muscle. Perhaps, fluctuating lipid levels within liver and muscle cells in developing youth may contribute to the maintenance of a healthy metabolic function by augmenting the toxic inflammatory consequences of excessive VAT, especially in healthy, physically active children. Elevated IMCL, in particular, is often observed after exercise training in insulin-sensitive-trained athletes.52 This increase is met with a corresponding increase in the pro-inflammatory cytokine, IL-6 (but not TNF-α) even in the absence of muscle damage. Levels then decline during the post-exercise period.53 However, it is important to note that alterations in IMCL following short-term exercise training are specific to the metabolic status of the population studied.54 Bajpeyi et al.54 recently reported that individuals with type 2 diabetes experienced a 35% reduction in IMCL after 10 consecutive days of exercise, whereas IMCL levels were unchanged in both obese and lean adults. In the current cross-sectional analysis, we observed both obese and non-obese developing children during varied seasons. Several of these children, especially those who were non-obese, may have been actively engaged in exercise training as part of sports and recreational pursuits at the time of our examination. Thus, physical activity and training status may need to be considered when evaluating relationships between inflammatory and metabolic markers in prepubertal, non-obese youth who may engage in daily exercise. This may be especially relevant in Black children, who are at increased risk for obesity and report low levels of physical activity in comparison with White youth. Regardless, these unexpected findings provide compelling evidence to inform clinical practice, especially in severely obese prepubertal children with related inflammatory conditions such as asthma, hepatic steatosis and IR.
In conclusion, we report here that the relationships between pro-inflammatory and metabolic markers commonly observed in adults are reversed in healthy, Black and White obese and non-obese children before puberty. One limitation of our study is the low number of participants due to the challenges of implementing the study in such young children (for example, prepubertal, 7–9 years old). Despite the benefits of including accurate, state-of-the-art methodologies (for example, VAT and SAT by MRI and ectopic fat by proton magnetic resonance spectroscopy, BF by dual-energy X-ray absorptiometry and so on) to assess obesity and metabolic biomarkers, one unfortunate consequence was the amount of missing data. Missing data occurred more frequently in tests that, as we expected, were more difficult for young children in this age group to successfully complete as opposed to simpler, more routine measurements (for example, waist circumference, BMI and so on). Notwithstanding, we believe that our inclusion of such advanced measurements and methods are warranted as currently there are no studies that have simultaneously examined pro- and anti-inflammatory markers and, VAT, SAT and ectopic fat by MRI/proton magnetic resonance spectroscopy in a sample of otherwise healthy, exclusively prepubertal (<2 Tanner) obese and non-obese Black and White children, 7–9 years of age.
Regardless and despite this limitation, statistically significant inverse correlations were observed between the parameters studied, which were not anticipated; thus, prospective studies are warranted to unravel this complex sequence in developing children to determine mechanisms by which these observations modify the risk of cardiovascular disease and type 2 diabetes later in life.
ACKNOWLEDGEMENTS
Research relating to this manuscript was supported by NICHD 1R01HD41071-01A2, 1R01HD49046-05, NCMHD P20 MD00481701, NIDDK (NORC) 1P30 DK072476, NIGMS—P20GM103501, R01HL072889, the LSU Health Sciences Center CTRC, LSUHSC IRB, and Children’s Hospital in New Orleans, and the Women’s Hospital and Pennington Biomedical Research Center in Baton Rouge.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- 1.Rojas J, Arraiz N, Aguirre M, Velasco M, Bermudez V. AMPK as target for intervention in childhood and adolescent obesity. J Obes. 2011;2011:252817. doi: 10.1155/2011/252817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity in the United States, 2009–2010. NCHS Data Brief. 2012;82:1–8. [PubMed] [Google Scholar]
- 3.Li S, Chen W, Srinivasan SR, Bond MG, Tang R, Urbina EM, et al. Childhood cardiovascular risk factors and carotid vascular changes in adulthood: the Bogalusa Heart Study. JAMA. 2003;290:2271–2276. doi: 10.1001/jama.290.17.2271. [DOI] [PubMed] [Google Scholar]
- 4.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999–2010. JAMA. 2012;307:483–490. doi: 10.1001/jama.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun SS, Liang R, Huang TT, Daniels SR, Arslanian S, Liu K, et al. Childhood obesity predicts adult metabolic syndrome: the Fels Longitudinal Study. J Pediatr. 2008;152:191–200. doi: 10.1016/j.jpeds.2007.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yach D, Stuckler D, Brownell KD. Epidemiologic and economic consequences of the global epidemics of obesity and diabetes. Nat Med. 2006;12:62–66. doi: 10.1038/nm0106-62. [DOI] [PubMed] [Google Scholar]
- 7.Hotamisligil GS, Erbay E. Nutrient sensing and inflammation in metabolic diseases. Nat Rev Immunol. 2008;8:923–934. doi: 10.1038/nri2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Odegaard JI, Chawla A. Mechanisms of macrophage activation in obesity-induced insulin resistance. Nat Clin Pract Endocrinol Metab. 2008;4:619–626. doi: 10.1038/ncpendmet0976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Das UN. Is obesity an inflammatory condition? Nutrition. 2001;17:953–966. doi: 10.1016/s0899-9007(01)00672-4. [DOI] [PubMed] [Google Scholar]
- 11.Fernandez-Real JM, Broch M, Ricart W, Casamitjana R, Gutierrez C, Vendrell J, et al. Plasma levels of the soluble fraction of tumor necrosis factor receptor 2 and insulin resistance. Diabetes. 1998;47:1757–1762. doi: 10.2337/diabetes.47.11.1757. [DOI] [PubMed] [Google Scholar]
- 12.Gupta A, Ten S, Anhalt H. Serum levels of soluble tumor necrosis factor-alpha receptor 2 are linked to insulin resistance and glucose intolerance in children. J Pediatr Endocrinol Metab. 2005;18:75–82. doi: 10.1515/jpem.2005.18.1.75. [DOI] [PubMed] [Google Scholar]
- 13.Ijzerman RG, Voordouw JJ, Van Weissenbruch MM, Yudkin JS, Serne EH, Delemarre-van de Waal HA, et al. TNF-alpha levels are associated with skin capillary recruitment in humans: a potential explanation for the relationship between TNF-alpha and insulin resistance. Clin Sci. 2006;110:361–368. doi: 10.1042/CS20050314. [DOI] [PubMed] [Google Scholar]
- 14.Kelly AS, Steinberger J, Kaiser DR, Olson TP, Bank AJ, Dengel DR. Oxidative stress and adverse adipokine profile characterize the metabolic syndrome in children. J Cardiometab Syndr. 2006;1:248–252. doi: 10.1111/j.1559-4564.2006.05758.x. [DOI] [PubMed] [Google Scholar]
- 15.Owen CG, Whincup PH, Orfei L, Chou QA, Rudnicka AR, Wathern AK, et al. Is body mass index before middle age related to coronary heart disease risk in later life? Evidence from observational studies. Int J Obes. 2009;33:866–877. doi: 10.1038/ijo.2009.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roemmich JN, Clark PA, Lusk M, Friel A, Weltman A, Epstein LH, et al. Pubertal alterations in growth and body composition. VI. Pubertal insulin resistance: relation to adiposity, body fat distribution and hormone release. Int J Obes Relat Metab Disord. 2002;26:701–709. doi: 10.1038/sj.ijo.0801975. [DOI] [PubMed] [Google Scholar]
- 17.Zinman B, Hanley AJ, Harris SB, Kwan J, Fantus IG. Circulating tumor necrosis factor-alpha concentrations in a native Canadian population with high rates of type 2 diabetes mellitus. J Clin Endocrinol Metab. 1999;84:272–278. doi: 10.1210/jcem.84.1.5405. [DOI] [PubMed] [Google Scholar]
- 18.Bruun JM, Verdich C, Toubro S, Astrup A, Richelsen B. Association between measures of insulin sensitivity and circulating levels of interleukin-8, interleukin-6 and tumor necrosis factor-alpha. Effect of weight loss in obese men. Eur J Endocrinol. 2003;148:535–542. doi: 10.1530/eje.0.1480535. [DOI] [PubMed] [Google Scholar]
- 19.Smith SR, Ravussin E. Emerging paradigms for understanding fatness and diabetes risk. Curr Diab Rep. 2002;2:223–230. doi: 10.1007/s11892-002-0087-1. [DOI] [PubMed] [Google Scholar]
- 20.Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, et al. The hormone resistin links obesity to diabetes. Nature. 2001;409:307–312. doi: 10.1038/35053000. [DOI] [PubMed] [Google Scholar]
- 21.Magkos F, Fabbrini E, Mohammed BS, Patterson BW, Klein S. Increased whole-body adiposity without a concomitant increase in liver fat is not associated with augmented metabolic dysfunction. Obesity. 2010;18:1510–1515. doi: 10.1038/oby.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kirchhoff K, Kantartzis K, Machann J, Schick F, Thamer C, Machicao F, et al. Impact of different fat depots on insulin sensitivity: predominant role of liver fat. J Diabetes Sci Technol. 2007;1:753–759. doi: 10.1177/193229680700100521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fabbrini E, Magkos F, Mohammed BS, Pietka T, Abumrad NA, Patterson BW, et al. Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proc Natl Acad Sci USA. 2009;106:15430–15435. doi: 10.1073/pnas.0904944106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gade W, Schmit J, Collins M, Gade J. Beyond obesity: the diagnosis and pathophysiology of metabolic syndrome. Clin Lab Sci. 2010;23:51–61. [PubMed] [Google Scholar]
- 25.Unger RH, Scherer PE. Gluttony, sloth and the metabolic syndrome: a roadmap to lipotoxicity. Trends Endocrinol Metab. 2010;21:345–352. doi: 10.1016/j.tem.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rubin DA, McMurray RG, Harrell JS, Hackney AC, Thorpe DE, Haqq AM. The association between insulin resistance and cytokines in adolescents: the role of weight status and exercise. Metabolism. 2008;57:683–690. doi: 10.1016/j.metabol.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Potau N, Ibanez L, Rique S, Carrascosa A. Pubertal changes in insulin secretion and peripheral insulin sensitivity. Horm Res. 1997;48:219–226. doi: 10.1159/000185519. [DOI] [PubMed] [Google Scholar]
- 28.Bennett B, Larson-Meyer DE, Ravussin E, Volaufova J, Soros A, Cefalu WT, et al. Impaired insulin sensitivity and elevated ectopic fat in healthy obese vs. nonobese prepubertal children. Obesity. 2012;20:371–375. doi: 10.1038/oby.2011.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Larson-Meyer DE, Newcomer BR, Ravussin E, Volaufova J, Bennett B, Chalew S, et al. Intrahepatic and intramyocellular lipids are determinants of insulin resistance in prepubertal children. Diabetologia. 2011;54:869–875. doi: 10.1007/s00125-010-2022-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tanner JM. Growth at Adolescence. 2nd edn. Oxford, UK: Blackwell Scientific; 1962. [Google Scholar]
- 31.Neovius M, Linne Y, Barkeling B, Rossner S. Discrepancies between classification systems of childhood obesity. Obes Rev. 2004;5:105–114. doi: 10.1111/j.1467-789X.2004.00136.x. [DOI] [PubMed] [Google Scholar]
- 32.Cutfield WS, Jefferies CA, Jackson WE, Robinson EM, Hofman PL. Evaluation of HOMA and QUICKI as measures of insulin sensitivity in prepubertal children. Pediatr Diabetes. 2003;4:119–125. doi: 10.1034/j.1399-5448.2003.t01-1-00022.x. [DOI] [PubMed] [Google Scholar]
- 33.Lee S, Janssen I, Ross R. Interindividual variation in abdominal subcutaneous and visceral adipose tissue: influence of measurement site. J Appl Physiol. 2004;97:948–954. doi: 10.1152/japplphysiol.01200.2003. [DOI] [PubMed] [Google Scholar]
- 34.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 35.Larson-Meyer DE, Newcomer BR, VanVrancken-Tompkins CL, Sothern M. Feasibility of assessing liver lipid by proton magnetic resonance spectroscopy in healthy normal and overweight prepubertal children. Diabetes Technol Ther. 2010;12:207–212. doi: 10.1089/dia.2009.0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tompkins CL, Cefalu W, Ravussin E, Goran M, Soros A, Volaufova J, et al. Feasibility of intravenous glucose tolerance testing prior to puberty. Int J Pediatr Obes. 2010;5:51–55. doi: 10.3109/17477160903055937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim SH, Sierra RA, McGee DJ, Zabaleta J. Transcriptional profiling of gastric epithelial cells infected with wild type or arginase-deficient Helicobacter pylori. BMC Microbiol. 2012;12:175. doi: 10.1186/1471-2180-12-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim JS, Le KA, Mahurkar S, Davis JN, Goran MI. Influence of elevated liver fat on circulating adipocytokines and insulin resistance in obese Hispanic adolescents. Pediatr Obes. 2012;7:158–164. doi: 10.1111/j.2047-6310.2011.00014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Drouet M, Dubuquoy L, Desreumaux P, Bertin B. Visceral fat and gut inflammation. Nutrition. 2012;28:113–117. doi: 10.1016/j.nut.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 40.Galic S, Oakhill JS, Steinberg GR. Adipose tissue as an endocrine organ. Mol Cell Endocrinol. 2010;316:129–139. doi: 10.1016/j.mce.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 41.Kolbina MV, Dolgikh VT, Chesnokov VI. Effect of hypoxia on metabolism and contractile function of the heart in rats with type 2 diabetes mellitus and abdominal obesity. Bull Exp Biol Med. 2004;138:223–225. doi: 10.1007/s10517-005-0004-0. [DOI] [PubMed] [Google Scholar]
- 42.Han DH, Hansen PA, Host HH, Holloszy JO. Insulin resistance of muscle glucose transport in rats fed a high-fat diet: a reevaluation. Diabetes. 1997;46:1761–1767. doi: 10.2337/diab.46.11.1761. [DOI] [PubMed] [Google Scholar]
- 43.Szasz T, Webb RC. Perivascular adipose tissue: more than just structural support. Clin Sci. 2012;122:1–12. doi: 10.1042/CS20110151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cole SW, Arevalo JM, Manu K, Telzer EH, Kiang L, Bower JE, et al. Antagonistic pleiotropy at the human IL6 promoter confers genetic resilience to the pro-inflammatory effects of adverse social conditions in adolescence. Dev Psychol. 2011;47:1173–1180. doi: 10.1037/a0023871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kiecolt-Glaser JK, Preacher KJ, MacCallum RC, Atkinson C, Malarkey WB, Glaser R. Chronic stress and age-related increases in the proinflammatory cytokine IL-6. Proc Natl Acad Sci USA. 2003;100:9090–9095. doi: 10.1073/pnas.1531903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schmidt MV, Enthoven L, van der MM, Levine S, de Kloet ER, Oitzl MS. The postnatal development of the hypothalamic-pituitary-adrenal axis in the mouse. Int J Dev Neurosci. 2003;21:125–132. doi: 10.1016/s0736-5748(03)00030-3. [DOI] [PubMed] [Google Scholar]
- 47.Gunnar M, Quevedo K. The neurobiology of stress and development. Annu Rev Psychol. 2007;58:145–173. doi: 10.1146/annurev.psych.58.110405.085605. [DOI] [PubMed] [Google Scholar]
- 48.Gilsanz V, Smith ML, Goodarzian F, Kim M, Wren TA, Hu HH. Changes in brown adipose tissue in boys and girls during childhood and puberty. J Pediatr. 2012;160:604–609. doi: 10.1016/j.jpeds.2011.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Loomba-Albrecht LA, Styne DM. Effect of puberty on body composition. Curr Opin Endocrinol Diabetes Obes. 2009;16:10–15. doi: 10.1097/med.0b013e328320d54c. [DOI] [PubMed] [Google Scholar]
- 50.Maffeis C, Manfredi R, Trombetta M, Sordelli S, Storti M, Benuzzi T, et al. Insulin sensitivity is correlated with subcutaneous but not visceral body fat in overweight and obese prepubertal children. J Clin Endocrinol Metab. 2008;93:2122–2128. doi: 10.1210/jc.2007-2089. [DOI] [PubMed] [Google Scholar]
- 51.Grohmann M, Sabin M, Holly J, Shield J, Crowne E, Stewart C. Characterization of differentiated subcutaneous and visceral adipose tissue from children: the influences of TNF-alpha and IGF-I. J Lipid Res. 2005;46:93–103. doi: 10.1194/jlr.M400295-JLR200. [DOI] [PubMed] [Google Scholar]
- 52.Dube JJ, Amati F, Stefanovic-Racic M, Toledo FG, Sauers SE, Goodpaster BH. Exercise-induced alterations in intramyocellular lipids and insulin resistance: the athlete’s paradox revisited. Am J Physiol Endocrinol Metab. 2008;294:E882–E888. doi: 10.1152/ajpendo.00769.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Petersen AM, Pedersen BK. The anti-inflammatory effect of exercise. J Appl Physiol. 2005;98:1154–1162. doi: 10.1152/japplphysiol.00164.2004. [DOI] [PubMed] [Google Scholar]
- 54.Bajpeyi S, Reed MA, Molskness S, Newton C, Tanner CJ, McCartney JS, et al. Effect of short-term exercise training on intramyocellular lipid content. Appl Physiol Nutr Metab. 2012;37:822–828. doi: 10.1139/h2012-051. [DOI] [PMC free article] [PubMed] [Google Scholar]