Abstract
Background
The relationship between anaemia and undiagnosed tuberculosis (TB) in patients living with HIV in sub-Saharan Africa is incompletely defined. We assessed the prevalence of TB among those with HIV-related anaemia and evaluated new means of rapid TB diagnosis.
Methodology
Blood haemoglobin levels were measured in unselected antiretroviral treatment (ART)-naïve patients in Cape Town, South Africa, and anaemia was classified according to WHO criteria. All patients were screened for TB by testing paired sputum samples using liquid culture (reference standard), fluorescence microscopy and Xpert MTB/RIF. Urine samples were tested for lipoarabinomannan (LAM) using the Determine TB-LAM diagnostic assay.
Results
Of 602 adults screened, 485 had complete results. Normal haemoglobin levels were found in 44.5% (n=216) of patients and mild, moderate or severe anaemia were present in 24.9% (n=121), 25.4% (n=123) and 5.2% (n=25) of patients, respectively. Culture-confirmed pulmonary TB was diagnosed in 8.8% (19/216) of those without anaemia compared to 16.5% (20/121), 26.0% (32/123) and 40.0% (10/25) with mild, moderate or severe anaemia, respectively (p<0.001). Anaemia was a strong independent predictor of TB. The sensitivities of diagnostic assays were much higher among those with moderate/severe anaemia compared to those with no/mild anaemia using sputum microscopy (42.9% vs 15.4%); urine LAM (54.8% vs 0%); sputum microscopy plus urine LAM (71.4% vs 15.4%) and sputum Xpert (73.8% vs 41.0%) (P<0.01 for all).
Conclusions
A very high prevalence of undiagnosed TB was found in patients with moderate or severe anaemia. Such patients should be prioritized for routine microbiological investigation using rapid diagnostic assays.
Keywords: HIV, AIDS, TB, tuberculosis, anaemia, haemoglobin, Africa, diagnosis, Xpert, LAM
Background
Anaemia is the most common haematologic abnormality associated with HIV.1–3 It is frequently observed among people living with HIV (PLWH), especially among those with evidence of advanced HIV disease.4 Clinical consequences associated with HIV-associated anaemia include severe fatigue,5 poorer quality of life for PLWH 6,7 and possibly an increased rate of HIV disease progression.2,8,9 Anaemia is independently associated with increased mortality risk 10,11 and remains a common problem among ART-naïve patients in sub-Saharan Africa.12
Anaemia may also be related to HIV-associated tuberculosis (TB), which remains the leading cause of death among PLWH worldwide.13 Anaemia is an independent predictor of early incident TB among those initiating ART in sub-Saharan Africa 12,14 and is also associated with increased mortality in those with HIV-associated TB.15–18 For these reasons, some have recommended routine TB investigations among PLWH with low haemoglobin levels living in high TB incidence settings, before starting ART.19
We conducted this study to further assess whether there is a need to investigate for TB in PLWH who are enrolling for ART and are found to be anaemic. In this study of ART-naïve patients enrolling in a large community-based clinic in Cape Town, South Africa, we report on the prevalence of anaemia, the prevalence of active TB and the relationship between the two. We also evaluated novel tools and approaches to diagnosis of TB in those with anaemia to identify effective means of systematic screening in this patient group.
Methods
Patient characteristics
We have previously described in detail the ART service in Gugulethu township in Cape Town, and the high TB burden of TB among its patients.20–23 Those eligible for this study were ART-naïve, HIV-infected adults, who were without a current TB diagnosis and were consecutively recruited from among patients newly referred to the clinic for initiation of ART between March 2010 and April 2011. Written informed consent was provided by all patients and the study was approved by the research ethics committees of the University of Cape Town and the London School of Hygiene & Tropical Medicine, UK.
At the first visit to the clinic, patients completed standard symptom screening that included the World Health Organization (WHO) symptom screen (the presence of more than one of the following symptoms: cough of any duration, fever, weight loss or night sweats) for HIV-associated TB.24 They were clinically characterized and clinical samples were obtained. Whenever possible, two sputum samples were obtained; the first sample was a spot specimen followed by a second sample.25 Urine samples were collected and stored at −20ºC. Blood CD4 cell counts and plasma viral load was measured. Haemoglobin levels were determined using ADVIA 2120 hematology analyzer (Siemens Healthcare Diagnostics, Erlangen, Germany). Chest radiographs were obtained when possible and were reported by a reader experienced in using the Chest Radiographic Reading and Recording System (CRRS).26
Laboratory procedures
Sputum samples were processed in an accredited laboratory according to standardized protocols with external quality assurance procedures. Decontaminated and centrifuged sputum samples were examined for acid-fast bacilli (AFB) using auramine O fluorescent stain, tested using the Xpert MTB/RIF assay and cultured in liquid media as previously described.27–29 Positive cultures were speciated. Determine TB-LAM lateral-flow assay was used to retrospectively analyse defrosted frozen urine samples for the presence of lipoarabinomannan (LAM). A test band equal to or greater than the intensity of the weakest band on the reference card (grade 1) defined a positive LAM result. Technologists blinded to the outcomes of the other assays read the results of all tests.
Patient outcomes
Patients were followed up within the routine ART service. Those diagnosed as having TB were referred to local TB treatment clinics. ART service patient records were reviewed to determine vital status at 90 days.
Definitions and analysis
Mycobacterium tuberculosis cultured from one or more sputum samples was used to define TB cases. Using haemoglobin values prior to ART initiation, all patients were categorized according to WHO criteria 30 as having: no anaemia ( ≥13.0 g/dL for men, ≥12.0 g/dL for non-pregnant females and ≥11.0 g/dL for pregnant females), mild anaemia (11.0–12.9 g/dL for men, 11.0–11.9 g/dL for non-pregnant females, 10.0–10.9 g/dL for pregnant females), moderate anaemia (8.0–10.9 g/dL for males and non-pregnant females and 7.0–9.9 g/dL for pregnant females) or severe anaemia (<8.0 g/dL for males and non-pregnant females and <7.0 g/dL for pregnant females ).
Medians were compared using either Wilcoxon rank-sum tests or Kruskal-Wallis tests where appropriate. Chi-squared tests or Fisher’s exact tests were used as indicated to compare proportions. The sensitivity, specificity, and predictive values of the different TB diagnostic assays were calculated (with corresponding 95% confidence intervals) for patient groups stratified by severity of anaemia. Logistic regression analyses were used to identify factors independently associated with HIV-associated anaemia. All variables in the univariable model meeting a cut-off of p≤0.1 were included in the multivariable model. Statistical tests were 2-sided at α=0.05.
Results
Anaemia diagnoses
Of 602 patients recruited, 485 had complete results of tests done on blood, sputum and urine samples. Among those included in the analysis (n=485), the median age was 33.6 (IQR, 27.9–40.7), 63.5% were female and the median CD4 count was 169 cells/uL (IQR, 96–231). The median blood haemoglobin level was 12.0 g/dL (IQR, 10.6–13.4).
Anaemia was diagnosed in 269 patients (prevalence, 55.5%; 95% CI, 50.9–60.0) and the remaining 216 (44.5%) had normal haemoglobin levels. Anaemia was classified as mild in 121 (24.9%), moderate in 123 (25.4%) and severe in 25 (5.2%). Patients with greater severity of anaemia were more likely to be female, have lower BMI’s and have higher white cell counts and absolute neutrophil counts (Table 1). Such patients also tended to have lower CD4 cell counts, higher HIV viral load, pulmonary TB and either stage 3 or 4 HIV disease at programme enrolment. However, pregnancy was not associated with degree of anaemia.
TABLE 1.
Patient characteristics among all HIV patients independent of TB status (n=485)
| All patients n=485 (100%) |
No anaemia n=216 (44.5%) |
Mild anaemia n=121 (24.9%) |
Moderate anaemia n=123 (25.4%) |
Severe anaemia n=25 (5.2%) |
p-value | |
|---|---|---|---|---|---|---|
| Patient characteristics | ||||||
| Age, median (IQR) | 33.6 (27.9–40.7) | 34.1 (28.5–41.1) | 33.8 (27.9–40.7) | 33.2 (27.7–41.2) | 29.4 (25.8–33.7) | 0.244 |
| Female (%) | 308 (63.5) | 115 (53.2) | 69 (57.0) | 102 (82.9) | 22 (88.0) | <0.001 |
| Pregnant (%) | 20 (4.1) | 6 (2.8) | 8 (6.7) | 6 (4.9) | 0 | 0.293 |
| BMI, median (IQR) | 23.6 (20.9–27.1) | 23.8 (21.3–28.5) | 23.1 (20.7–26.1) | 23.8 (19.8–26.9) | 21.4 (19.7–24.6) | 0.004 |
| Haemotological testsa | ||||||
| Hemoglobin (g/dL) | 12.0 (10.6–13.4) | 13.5 (12.6–14.4) | 11.7 (11.3–12.2) | 10.0 (9.1–10.6) | 7.5 (7.0–7.7) | <0.001 |
| White blood cell count (cells/μL) | 4.9 (3.9–6.1) | 4.8 (3.9–5.8) | 4.4 (3.6–5.8) | 5.3 (4.1–6.6) | 6.5 (4.9–8.2) | 0.003 |
| Absolute neutrophil count (cells/μL) | 2.6 (1.9–3.6) | 2.6 (1.8–3.5) | 2.3 (1.8–3.1) | 3.0 (2.1–4.0) | 3.7 (2.1–5.7) | <0.001 |
| Absolute lymphocyte count (cells/μL) | 1.6 (1.2–2.1) | 1.6 (1.3–2.0) | 1.5 (1.2–2.0) | 1.5 (1.2–2.1) | 1.4 (0.7–2.2) | 0.343 |
| Platelets (platelets/μL) | 265 (211–335) | 257 (203–319) | 260 (210–328) | 303 (232–382) | 273 (178–414) | 0.002 |
| CD4 cell count (cells/μL)b | ||||||
| Median (IQR) | 169 (96–231) | 192 (126–248) | 157 (86–227) | 143 (60–201) | 129 (35–184) | <0.001 |
| CD4 0–99 | 123 (25.5) | 38 (17.7) | 34 (28.1) | 43 (35.3) | 8 (32.0) | 0.002 |
| CD4 100–199 | 181 (37.5) | 77 (35.8) | 46 (38.0) | 47 (38.5) | 11 (44.0) | |
| CD4 ≥200 | 179 (37.1) | 100 (46.5) | 41 (33.9) | 32 (26.2) | 6 (24.0) | |
| Log viral load (copies/ml), Median (IQR) | 4.6 (4.0–5.0) | 4.4 (3.8–4.8) | 4.6 (4.1–5.0) | 4.8 (4.3–5.3) | 5.2 (4.4–5.6) | <0.001 |
| WHO stage at enrolment | ||||||
| 1 or 2 | 325 (67.0) | 167 (77.3) | 84 (69.4) | 65 (52.9) | 9 (36.0) | <0.001 |
| 3 or 4 | 160 (33.0) | 49 (22.7) | 37 (30.6) | 58 (47.2) | 16 (64.0) | |
| Tuberculosis | 0.920 | |||||
| History of previous TB (%) | 130 (26.8) | 55 (25.5) | 35 (28.9) | 33 (26.8) | 7 (28.0) | |
| Positive WHO symptom screen (%) | 333 (68.7) | 142 (67.0) | 83 (70.9) | 87 (66.4) | 21 (84.0) | 0.312 |
| Prevalence of current culture-confirmed TB (%) | 81 (16.7) | 19 (8.8) | 20 (16.5) | 32 (26.0) | 10 (40.0) | <0.001 |
Median (IQR)
n=483
Prevalence of TB among patients with HIV-associated anaemia
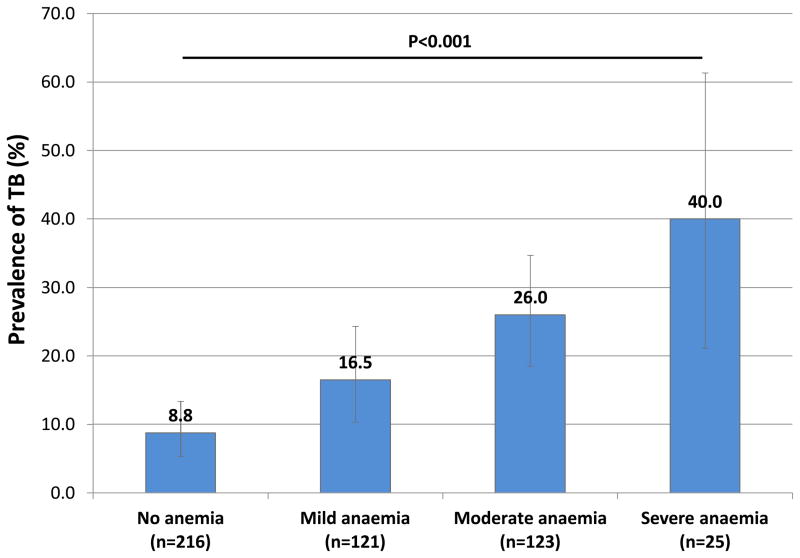
Overall, 81 patients had culture-positive TB diagnosed (prevalence, 16.7%; 95% CI, 13.5–20.3) and 404 (83.3%) patients had negative sputum cultures. Among the 269 patients with any degree of anaemia, 62 had TB (prevalence, 23.0%; 95% CI, 18.2–28.5). The prevalence of TB was strongly and directly correlated with degree of anaemia (Figure 1). However, the prevalence of TB did not differ by gender, as 33.3% (95%CI, 15.6–55.3) of men with moderate or severe anaemia had TB compared to 27.4% (95%CI, 19.8–36.2) among females with moderate or severe anaemia (p=0.556). A positive WHO symptom screen was found in 66 (81.5%) of overall patients with TB and in 88.1% of the sub-set of TB patients with either moderate or severe anaemia compared to 75.0% among those with no or mild anaemia (p=0.0094).
Figure 1.
The proportion (with 95% confidence intervals) of HIV-infected patients (n=485) who have TB, stratified by degree of anaemia.
Risk factors for HIV-associated anaemia
We next used logistic regression to define whether TB was an independent risk factor associated with anaemia. Both univariable and multivariable analyses demonstrated that a number of variables were associated with moderate or severe anaemia (Table 2) TB remained strongly associated with moderate and severe anaemia after adjustment for all other variables.
TABLE 2.
Univariable and multivariable analysis of factors associated with moderate or severe anemia among HIV-infected patients (n=485)
| Risk factor | Unadjusted OR (95%CI) | P-value | Adjusted OR (95%CI) | P-value |
|---|---|---|---|---|
| Age (years) -for every 1 unit increase | 1.00 (0.97–1.02) | 0.6804 | - | - |
| Female gender | 4.30 (2.64–6.99) | <0.0001 | 8.00 (4.56–14.05) | <0.0001 |
| Pregnancy | 0.97 (0.37–2.58) | 0.9542 | - | - |
| Tuberculosis | 3.03 (1.86–4.94) | <0.0001 | 2.62 (1.49–4.64) | 0.0009 |
| BMI (kg/m2)- for every 1 unit decrease | 1.06 (1.02–1.10) | 0.0017 | 1.11 (1.06–1.16) | <0.0001 |
| CD4 cell counts (cells/uL)- for every 50 unit decrease | 1.20 (1.09–1.33) | 0.0002 | 1.14 (1.01–1.29) | 0.0242 |
| Log viral load (log copies/mL)- for every 1 unit increase | 2.07 (1.53–2.81) | <0.0001 | 1.65 (1.17–2.33) | 0.0030 |
Characteristics and outcomes of patients with HIV-associated TB and anaemia
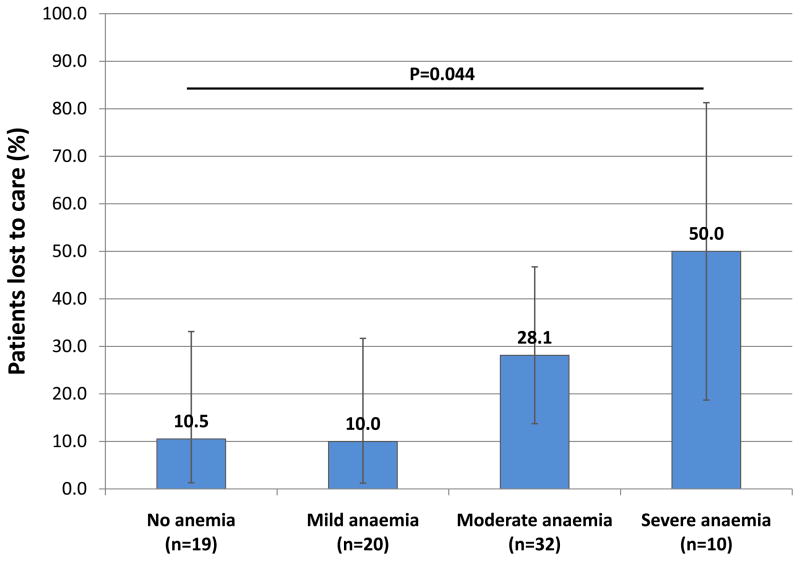
Among patients with HIV-associated TB (n=81), those with lower haemoglobin levels were more likely to be female, have higher absolute neutrophil counts, lower CD4 cell counts and higher HIV viral loads (data not shown). Radiological abnormalities were generally not associated with anaemia with the exception that those with more severe anaemia were more likely to have mediastinal lymphadenopathy. Patients with a greater severity of anaemia were much less likely to be retained in programme after 90 days due to death or loss to follow-up (Figure 2). Patients with TB who died during follow-up (n=5) all had moderate or severe anaemia (p=0.026).
Figure 2.
The proportion (with 95% confidence intervals) of patients with HIV-associated TB (n=81) who either died or were lost to follow-up within 90 days of ART initiation, stratified by degree of anaemia.
Diagnosis of TB among patients with HIV-associated anaemia
We next assessed the diagnostic accuracy of a range of microbiological assays for TB used during routine systematic screening (Table 3). It was striking that for each diagnostic assay, the sensitivities were significantly greater among those with moderate or severe anaemia compared to those with mild or no anaemia (Table 3). This increment ranged from 27.5% for sputum microscopy to 54.8% for Determine TB-LAM. Sputum Xpert MTB/RIF and Determine TB-LAM each detected a majority of TB cases among those with moderate or severe anaemia, whereas sputum smear microscopy did not. Combining Determine TB-LAM with either sputum smear microscopy or Xpert MTB/RIF increased the diagnostic sensitivity among those with moderate or severe anaemia to more than 70% and 80%, respectively.
TABLE 3.
Diagnostic utility of novel diagnostics for the detection of TB among patients with HIV-associated anaemia; (a) sensitivity among patients with culture-confirmed TB (b) PPV and NPV among the overall cohort (c) specificity among patients without culture confirmed TB
| a) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| TB Prevalence % (95% CI) | Sputum microscopy | Sputum Xpert x1 | LAM | LAM+sputum microscopy | LAM+ SputumXpert | ||||||
| TB patients | Number | Sensitivity (95% CI) | Number | Sensitivity (95% CI) | Number | Sensitivity (95% CI) | Number | Sensitivity (95% CI) | Number | Sensitivity (95% CI) | |
| Overall (n=81) | - | 24 | 30 (20–41) | 47 | 58 (47–69) | 23 | 28 (19–39) | 36 | 44 (33–56) | 50 | 61 (50–72) |
| No anaemia or mild anaemia (n=39) | - | 6 | 15 (6–31) | 16 | 41 (26–58) | 0 | 0 (0–9) | 6 | 15 (6–31) | 16 | 41 (25–57) |
| Moderate or severe anaemia (n=42) | - | 18 | 43 (28–59) | 31 | 74 (58–86) | 23 | 55 (39–70) | 30 | 71 (55–84) | 34 | 81 (66–91) |
| P-value1 | - | - | 0.007 | - | 0.003 | - | <0.001 | - | <0.001 | - | <0.001 |
| b) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall cohort | TB Prevalence % (95% CI) | Sputum microscopy | Sputum Xpert x1 | LAM | LAM+sputum microscopy | LAM+ SputumXpert | |||||
| PPV (95% CI) | NPV (95% CI) | PPV (95% CI) | NPV (95% CI) | PPV (95% CI) | NPV (95% CI) | PPV (95% CI) | NPV (95% CI) | PPV (95% CI) | NPV (95% CI) | ||
| Overall (n=485) | 17 (13–20) | 96 (78–100) | 88 (84–90) | 92 (80–98) | 92 (89–94) | 79 (60–91) | 87 (84–90) | 83.7 (69–93) | 90 (87–92) | 83 (71–91) | 93 (90–95) |
| No anaemia or mild anaemia (n=337) | 12 (8–15) | 100 (52–100) | 90 (86–93) | 84 (60–96) | 93 (89–95) | 0 (0–60) | 88 (84–91) | 60 (27–86) | 90 (86–93) | 70 (47–86) | 93 (89–95) |
| Moderate or severe anaemia (n=148) | 28 (21–36) | 95 (72–100) | 81.4 (73.4–87.5) | 97 (82–100) | 91 (83–95) | 92 (72–98) | 85 (77–90) | 91 (75–98) | 90 (82–94) | 92 (77–98) | 93 (86–97) |
| c) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| TB Prevalence % (95% CI) | Sputum microscopy | Sputum Xpert x1 | LAM | LAM+sputum microscopy | LAM+ SputumXpert | ||||||
| Number | Specificity (95% CI) | Number | Specificity (95% CI) | Number | Specificity (95% CI) | Number | Specificity (95% CI) | Number | Specificity (95% CI) | ||
| Overall (n=404) | - | 403 | 100 (98–100) | 400 | 99 (97–100) | 398 | 99 (97–99) | 403 | 100 (98–100) | 400 | 99 (97–100) |
| No anaemia or mild anemia (n=298) | - | 298 | 100 (99–100) | 295 | 99 (97–100) | 294 | 99 (97–100) | 294 | 99 (97–100) | 291 | 98 (95–99) |
| Moderate or severe anaemia (n=106) | - | 105 | 99 (95–100) | 105 | 99 (95–100) | 104 | 98 (93–100) | 103 | 97 (92–99) | 103 | 97 (92–99) |
P value for comparison of sensitivity of diagnostic assays in those with no anaemia/mild anaemia versus those with moderate/severe anaemia.
Both sputum Xpert MTB/RIF and Determine TB-LAM correctly identified TB in all of those who died in the first 90 days of clinical follow-up (n=5) whereas sputum microscopy only diagnosed TB in two of the five cases. The positive predictive value was maintained above 90% for all assays among those with moderate or severe anaemia (Table 3). The specificity of all assays either in isolation or in combination was greater than 98% and 97%, respectively, and did not differ according to the severity of anaemia (Table 3).
Discussion
This study found a high prevalence of anaemia among treatment-naïve patients enrolling to start ART and that a substantial proportion of those with anaemia had underlying TB, which was a strong independent risk factor for anaemia. The prevalence of TB among those with moderate or severe anaemia was so high and their clinical outcomes were so poor that it suggests the need for routine microbiological investigations for TB in this patient group. A majority of TB among those with moderate or severe anaemia could be rapidly diagnosed using Determine TB-LAM and/or Xpert MTB/RIF.
The prevalence of TB among ART-naive patients with anaemia in developing countries is poorly defined and is likely to vary greatly between settings. We carefully documented this relationship in a South African cohort, showing that a higher prevalence of TB was directly associated with lower haemoglobin levels (reaching as high as 40% among those with severe anaemia) and that TB was a strong independent risk factor for HIV-associated anaemia. These data are consistent with the findings of previous studies from Malawi,31 Rwanda 32 and India.33 Multivariable analysis additionally found that while low CD4 cell count, high viral load and low body mass index also demonstrated associations, female sex was the factor most strongly associated with moderate or severe anaemia. This may be explained, for example, by menstrual blood loss and low dietary iron intake.
The mechanisms underlying anaemia in patients with HIV-associated TB remain incompletely defined and are likely to be multiple. The most common mechanism is likely anaemia of chronic disease.34,35 Iron-deficiency anaemia 35,36 may also occur as a result of insufficient dietary intake or blood loss from the gastrointestinal (GI) tract due to mucosal involvement with TB.37 TB may also disseminate to the bone marrow 38,39 and impair all hematopoietic cell lines, including red blood cells. Other reported mechanisms may include autoimmune hemolysis 40,41 and nutritional deficiencies of folate, selenium 42 and rarely vitamin B12 secondary to malabsorption caused by ileal TB involvement.43 Additionally, HIV-associated anaemia is associated with worsening HIV disease parameters, 44 including higher HIV viral load and low CD4 counts 2,45 and the risk for active TB disease increases with greater severity of immunosuppression.
Among those with HIV-associated TB, all deaths occurred among those with moderate or severe anaemia and patients were overall less likely to be retained in care after 3 months in programme. While the outcomes of those lost to follow-up are unknown, it is likely that those not retained in care were at high risk for mortality.46,47 Our findings are consistent with other studies which have demonstrated that lower haemoglobin levels are associated with decreased survival and may independently predict mortality among patients with HIV-associated TB.15–18 Therefore, we suggest that routine investigation for TB among HIV patients with anaemia may not only yield a large number of TB cases, but may identify many of those at greatest risk for significant TB-related morbidity and mortality.
In resource-limited settings, the WHO symptom screen for HIV-associated TB 24 is used to identify those who require further evaluation with a view to possible microbiological screening for TB. However, the sensitivity of this screen is incomplete 24 (as found in the present study). Moreover, due to its very low specificity, symptom screening identifies very large numbers of patients for whom it is simply not feasible to conduct microbiological investigations. However, the association between anaemia and TB was so strong that this might reasonably be used as an absolute indication for microbiological screening. Thus, the presence of anaemia readily identifies a sub-set of patients for whom investigations should be prioritized regardless of the presence or absence of symptoms.
We have previously shown that sputum Xpert MTB/RIF and urine Determine TB-LAM assays have higher diagnostic sensitivity in those with poorer prognostic characteristics, including anaemia.48 In the present study, we have now demonstrated the sensitivities of these assays used alone, together or in combination with smear microscopy among those with anaemia classified according to WHO criteria. The observation that the sensitivities of these diagnostic assays were much higher among those with greater severity of anaemia is likely to be related to the probability that both these factors are associated with more advanced and disseminated disease and mycobacterial load.48 Anaemia may be particularly severe in patients with disseminated HIV-associated TB due to several factors. These include high levels of systemic inflammation (with up-regulation of myelosuppressive pro-inflammatory cytokines) direct involvement of the bone marrow (leading to suppression of haematopoiesis) and gastrointestinal tract (leading to blood loss).
Determine-TB LAM is best prioritized for use in screening for TB among HIV-infected patients with CD4 cell counts <200 cells/μL.49,50 However, in many resource-limited settings, CD4 counts may not be available. Low haemoglobin levels may, however, represent an alternative simple trigger for appropriate TB testing among HIV-infected patients using LAM point-of-care assay. Further studies to assess the diagnostic accuracy and impact of TB screening using this and other rapid diagnostic assays in patients with moderate-to-severe HIV-associated anaemia in resource-limited settings are prudent.
Among patients with moderate or severe anaemia, the sensitivity of LAM point-of-care assay combined with sputum microscopy was very similar to that of a single sputum Xpert cartridge. This has important implications for resource limited settings. While Xpert is being scaled up and is becoming available in several sub-Saharan African countries, its cost and technical requirements will prohibit its implementation in some settings with high HIV-associated TB burdens.51 Microscopy is already widely available in most resource-limited settings and continues to be first line for TB diagnosis in most of these settings. LAM point-of-care assay is rapid, easy to use and inexpensive and may be a very useful add-on test in resource-limited settings to increase the yield of TB diagnoses.
Strengths of this study include consecutive enrollment of patients who were well characterized. Sputum induction was used to obtain quality samples and liquid culture was used as the reference gold standard and processed in an accredited laboratory according to standardized protocols and quality assurance procedures. A limitation of this study includes the lack of available red blood cell (RBC) indices or iron studies. With only haemoglobin levels available, we could only classify patients according to the degree of anaemia without being able to further investigate possible underlying mechanisms, such as iron-deficiency or chronic inflammation. An additional limitation is that it was not possible to determine whether anaemia in patients with TB was directly related to their TB disease, attributable to their HIV infection, or was simply a prevalent co-morbidity unrelated to either their TB or HIV disease. Finally, the reference standard was determined by testing paired sputum samples using liquid culture. As extrapulmonary TB is more common in patients with advanced immunodeficiency, this may have underestimated the prevalence of active disease. Sampling multiple sites of disease for extrapulmonary TB may have therefore enhanced the reference standard.
In conclusion, HIV-associated anaemia was common and there was a very high prevalence of undiagnosed TB among those with moderate or severe anaemia that was associated with very poor clinical outcomes. Sputum Xpert MTB/RIF and urine Determine TB-LAM were able to rapidly diagnose TB with useful sensitivity among such patients. PLWH with moderate or severe anaemia in high burden settings should be investigated for TB using rapid microbiological assays regardless of symptoms.
Acknowledgments
Financial Support
SDL is funded by the Wellcome Trust. RW was funded in part by the National Institutes of Health (NIH) through grants RO1 A1058736-01A1 and 5UO1A1069519-02. We are grateful to the Foundation for Innovative New Diagnostics (FIND), Geneva, Switzerland for providing access to the Xpert MTB/RIFassay cartridges with preferential pricing. Alere provided the LAM assays free of charge. None of these sources played any role in the design, conduct, analysis, interpretation or decision to publish these data.
Footnotes
Conflicts of Interest
None to declare.
Authors’ Contributions
SDL and ADK initiated and planned the study. SDL, RW and MV collected the data. SDL and MV ran laboratory assays. ADK did the data analysis. ADK and SDL wrote the paper with input from RW. All authors approved the final version of the manuscript prior to submission.
References
- 1.O’Brien ME, Kupka R, Msamanga GI, Saathoff E, Hunter DJ, Fawzi WW. Anemia is an independent predictor of mortality and immunologic progression of disease among women with HIV in Tanzania. J Acquir Immune Defic Syndr. 2005;40:219–25. doi: 10.1097/01.qai.0000166374.16222.a2. [DOI] [PubMed] [Google Scholar]
- 2.Mocroft A, Kirk O, Barton SE, et al. Anaemia is an independent predictive marker for clinical prognosis in HIV-infected patients from across Europe. EuroSIDA study group. AIDS. 1999;13:943–50. doi: 10.1097/00002030-199905280-00010. [DOI] [PubMed] [Google Scholar]
- 3.Levine AM, Berhane K, Masri-Lavine L, et al. Prevalence and correlates of anemia in a large cohort of HIV-infected women: Women’s Interagency HIV Study. J Acquir Immune Defic Syndr. 2001;26:28–35. doi: 10.1097/00126334-200101010-00004. [DOI] [PubMed] [Google Scholar]
- 4.Belperio PS, Rhew DC. Prevalence and outcomes of anemia in individuals with human immunodeficiency virus: a systematic review of the literature. The American Journal of Medicine. 2004;116:27–43. doi: 10.1016/j.amjmed.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 5.Breitbart W, McDonald MV, Rosenfeld B, Monkman ND, Passik S. Fatigue in ambulatory AIDS patients. J Pain Symptom Manage. 1998;15:159–67. doi: 10.1016/s0885-3924(97)00260-1. [DOI] [PubMed] [Google Scholar]
- 6.Revicki DA, Brown RE, Henry DH, McNeill MV, Rios A, Watson T. Recombinant human erythropoietin and health-related quality of life of AIDS patients with anemia. J Acquir Immune Defic Syndr. 1994;7:474–84. [PubMed] [Google Scholar]
- 7.Abrams DI, Steinhart C, Frascino R. Epoetin alfa therapy for anaemia in HIV-infected patients: impact on quality of life. Int J STD AIDS. 2000;11:659–65. doi: 10.1258/0956462001915020. [DOI] [PubMed] [Google Scholar]
- 8.Morfeldt-månson L, Böttiger B, Nilsson B, Stedingk L-VV. Clinical signs and laboratory markers in predicting progression to AIDS in HIV-1 infected patients. Scand J Infect Dis. 1991;23:443–9. doi: 10.3109/00365549109075092. [DOI] [PubMed] [Google Scholar]
- 9.Moore RD, Creagh-Kirk T, Keruly J, et al. Long-term safety and efficacy of zidovudine in patients with advanced human immunodeficiency virus disease. Arch Intern Med. 1991;151:981. [PubMed] [Google Scholar]
- 10.De Santis GC, Brunetta DM, Vilar FC, et al. Hematological abnormalities in HIV-infected patients. Int J Infect Dis. 2011;15:e808–11. doi: 10.1016/j.ijid.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 11.Sullivan PS, Hanson DL, Chu SY, Jones JL, Ward JW. Epidemiology of anemia in human immunodeficiency virus (HIV)-infected persons: results from the multistate adult and adolescent spectrum of HIV disease surveillance project. Blood. 1998;91:301–8. [PubMed] [Google Scholar]
- 12.van Rie A, Westreich D, Sanne I. Tuberculosis in patients receiving antiretroviral treatment: incidence, risk factors, and prevention strategies. J Acquir Immune Defic Syndr. 2011;56:349–55. doi: 10.1097/QAI.0b013e3181f9fb39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lawn SD, Zumla AI. Tuberculosis. Lancet. 2011;378:57–72. doi: 10.1016/S0140-6736(10)62173-3. [DOI] [PubMed] [Google Scholar]
- 14.McDermid JM, Hennig BJ, van der Sande M, et al. Host iron redistribution as a risk factor for incident tuberculosis in HIV infection: an 11-year retrospective cohort study. BMC Infect Dis. 2013;13:1–1. doi: 10.1186/1471-2334-13-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mugusi FM, Mehta S, Villamor E, et al. Factors associated with mortality in HIV-infected and uninfected patients with pulmonary tuberculosis. BMC Public Health. 2009;9:409. doi: 10.1186/1471-2458-9-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kourbatova EV, Borodulin BE, Borodulina EA, del Rio C, Blumberg HM, Leonard MK. Risk factors for mortality among adult patients with newly diagnosed tuberculosis in Samara, Russia. Int J Tuberc Lung Dis. 2006;10:1224–30. [PubMed] [Google Scholar]
- 17.Ciglenecki I, Glynn JR, Mwinga A, et al. Population differences in death rates in HIV-positive patients with tuberculosis. Int J Tuberc Lung Dis. 2007;11:1121–8. [PubMed] [Google Scholar]
- 18.Kendon M-A, Knight S, Ross A, Giddy J. Timing of antiretroviral therapy initiation in adults with HIV-associated tuberculosis: Outcomes of therapy in an urban hospital in KwaZulu-Natal. S Afr Med J. 2012;102 doi: 10.7196/samj.5574. [DOI] [PubMed] [Google Scholar]
- 19.Russell EC, Charalambous S, Pemba L, Churchyard GJ, Grant AD, Fielding K. Low haemoglobin predicts early mortality among adults starting antiretroviral therapy in an HIV care programme in South Africa: a cohort study. BMC Public Health. 2010;10:433. doi: 10.1186/1471-2458-10-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lawn SD, Myer L, Bekker L-G, Wood R. Burden of tuberculosis in an antiretroviral treatment programme in sub-Saharan Africa: impact on treatment outcomes and implications for tuberculosis control. AIDS. 2006;20:1605–12. doi: 10.1097/01.aids.0000238406.93249.cd. [DOI] [PubMed] [Google Scholar]
- 21.Lawn SD, Myer L, Orrell C, Bekker L-G, Wood R. Early mortality among adults accessing a community-based antiretroviral service in South Africa: implications for programme design. AIDS. 2005;19:2141–8. doi: 10.1097/01.aids.0000194802.89540.e1. [DOI] [PubMed] [Google Scholar]
- 22.Lawn SD, Myer L, Bekker L-G, Wood R. Tuberculosis-associated immune reconstitution disease: incidence, risk factors and impact in an antiretroviral treatment service in South Africa. AIDS. 2007;21:335–41. doi: 10.1097/QAD.0b013e328011efac. [DOI] [PubMed] [Google Scholar]
- 23.Gupta A, Wood R, Kaplan R, Bekker L-G, Lawn SD. Prevalent and Incident Tuberculosis Are Independent Risk Factors for Mortality among Patients Accessing Antiretroviral Therapy in South Africa. PLoS ONE. 2013;8:e55824. doi: 10.1371/journal.pone.0055824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Getahun H, Kittikraisak W, Heilig CM, et al. Development of a standardized screening rule for tuberculosis in people living with HIV in resource-constrained settings: individual participant data meta-analysis of observational studies. PLoS Med. 2011;8:e1000391. doi: 10.1371/journal.pmed.1000391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lawn SD, Kerkhoff AD, Pahlana P, Vogt M, Wood RR. Diagnostic yield of tuberculosis using sputum induction in HIV-positive patients before antiretroviral therapy. Int J Tuberc Lung Dis. 2012;16:1354–7. doi: 10.5588/ijtld.12.0174. [DOI] [PubMed] [Google Scholar]
- 26.Dawson R, Masuka P, Edwards DJ, et al. Chest radiograph reading and recording system: evaluation for tuberculosis screening in patients with advanced HIV. Int J Tuberc Lung Dis. 2010;14:52–8. [PMC free article] [PubMed] [Google Scholar]
- 27.Lawn SD, Kerkhoff AD, Vogt M, Wood R. Diagnostic accuracy of a low-cost, urine antigen, point-of-care screening assay for HIV-associated pulmonary tuberculosis before antiretroviral therapy: a descriptive study. Lancet Infect Dis. 2012;12:201–9. doi: 10.1016/S1473-3099(11)70251-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lawn SD, Kerkhoff AD, Vogt M, Ghebrekristos Y, Whitelaw A, Wood R. Characteristics and early outcomes of patients with Xpert MTB/RIF-negative pulmonary tuberculosis diagnosed during screening before antiretroviral therapy. Clin Infect Dis. 2012;54:1071–9. doi: 10.1093/cid/cir1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lawn SD, Brooks SV, Kranzer K, et al. Screening for HIV-associated tuberculosis and rifampicin resistance before antiretroviral therapy using the Xpert MTB/RIF assay: a prospective study. PLoS Med. 2011;8:e1001067. doi: 10.1371/journal.pmed.1001067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.World Health Organization. Geneva: Vitamin and Mineral Nutrition Information System. Vol. 2011 Geneva: World Health Organization; 2011. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. (WHO/NMH/NHD/MNM/11.1) http://www.who.int/vmnis/indicators/haemoglobin.pdf. [Google Scholar]
- 31.Lewis DK, Whitty CJM, Walsh AL, et al. Treatable factors associated with severe anaemia in adults admitted to medical wards in Blantyre, Malawi, an area of high HIV seroprevalence. Trans R Soc Trop Med Hyg. 2005;99:561–7. doi: 10.1016/j.trstmh.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 32.Masaisa F, Gahutu JB, Mukiibi J, Delanghe J, Philippé J. Anemia in human immunodeficiency virus-infected and uninfected women in Rwanda. Am J Trop Med Hyg. 2011;84:456–60. doi: 10.4269/ajtmh.2011.10-0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Subbaraman R, Devaleenal B, Selvamuthu P, et al. Factors associated with anaemia in HIV-infected individuals in southern India. Int J STD AIDS. 2009;20:489–92. doi: 10.1258/ijsa.2008.008370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singh KJ, Ahluwalia G, Sharma SK, Saxena R, Chaudhary VP, Anant M. Significance of haematological manifestations in patients with tuberculosis. J Assoc Physicians India. 2001;49:788, 790–4. [PubMed] [Google Scholar]
- 35.Lee SW, Kang YA, Yoon YS, et al. The prevalence and evolution of anemia associated with tuberculosis. J Korean Med Sci. 2006;21:1028–32. doi: 10.3346/jkms.2006.21.6.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Isanaka S, Mugusi F, Urassa W, et al. Iron deficiency and anemia predict mortality in patients with tuberculosis. J Nutr. 2012;142:350–7. doi: 10.3945/jn.111.144287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marshall JB. Tuberculosis of the gastrointestinal tract and peritoneum. Am J Gastroenterol. 1993;88:989–99. [PubMed] [Google Scholar]
- 38.Viallard J-F, Parrens M, Boiron J-M, Texier J, Mercie P, Pellegrin J-L. Reversible myelofibrosis induced by tuberculosis. Clin Infect Dis. 2002;34:1641–3. doi: 10.1086/340524. [DOI] [PubMed] [Google Scholar]
- 39.Hungund BR, Sangolli SS, Bannur HB, et al. Blood and bone marrow findings in tuberculosis in adults-A cross sectional study. Al Ameen J Med Sci. 2012:5. [Google Scholar]
- 40.Kuo PH, Yang PC, Kuo SS, Luh KT. Severe immune hemolytic anemia in disseminated tuberculosis with response to antituberculosis therapy. Chest. 2001;119:1961–3. doi: 10.1378/chest.119.6.1961. [DOI] [PubMed] [Google Scholar]
- 41.Murray HW. Transient autoimmune hemolytic anemia and pulmonary tuberculosis. N Engl J Med. 1978;299:488. doi: 10.1056/nejm197808312990920. [DOI] [PubMed] [Google Scholar]
- 42.van Lettow M, West CE, van der Meer JWM, Wieringa FT, Semba RD. Low plasma selenium concentrations, high plasma human immunodeficiency virus load and high interleukin-6 concentrations are risk factors associated with anemia in adults presenting with pulmonary tuberculosis in Zomba district, Malawi. Eur J Clin Nutr. 2005;59:526–32. doi: 10.1038/sj.ejcn.1602116. [DOI] [PubMed] [Google Scholar]
- 43.Sharma MP, Bhatia V. Abdominal tuberculosis. Indian J Med Res. 2004;120:305–15. [PubMed] [Google Scholar]
- 44.Volberding PA, Levine AM, Dieterich D, et al. Anemia in HIV infection: clinical impact and evidence-based management strategies. Clin Infect Dis. 2004;38:1454–63. doi: 10.1086/383031. [DOI] [PubMed] [Google Scholar]
- 45.Semba RD, Shah N, Klein RS, Mayer KH, Schuman P, Vlahov D. Prevalence and cumulative incidence of and risk factors for anemia in a multicenter cohort study of human immunodeficiency virus–infected and–uninfected women. Clin Infect Dis. 2002;34:260–6. doi: 10.1086/338151. [DOI] [PubMed] [Google Scholar]
- 46.Nglazi MD, Kaplan R, Wood R, Bekker L-G, Lawn SD. Identification of losses to follow-up in acommunity-based antiretroviral therapy clinic inSouth Africa using a computerized pharmacytracking system. BMC Infect Dis. 2010;10:329. doi: 10.1186/1471-2334-10-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fox MP, Brennan A, Maskew M, MacPhail P, Sanne I. Using vital registration data to update mortality among patients lost to follow-up from ART programmes: evidence from the Themba Lethu Clinic, South Africa. Trop Med Int Health. 2010 doi: 10.1111/j.1365-3156.2010.02473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lawn SD, Kerkhoff AD, Vogt M, Wood R. HIV-associated tuberculosis: relationship between disease severity and the sensitivity of new sputum-based and urine-based diagnostic assays. BMC Med. 2013;11:1. doi: 10.1186/1741-7015-11-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lawn SD. Point-of-care detection of lipoarabinomannan (LAM) in urine for diagnosis of HIV-associated tuberculosis: a state of the art review. BMC Infect Dis. 2012;12:1. doi: 10.1186/1471-2334-12-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lawn SD, Dheda K, Kerkhoff AD, et al. Determine TB-LAM lateral flow urine antigen assay for HIV-associated tuberculosis: recommendations on the design and reporting of clinical studies. BMC Infect Dis. 2013;13:1. doi: 10.1186/1471-2334-13-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lawn SD, Mwaba P, Bates M, et al. Advances in tuberculosis diagnostics: the Xpert MTB/RIF assay and future prospects for a point-of-care test. Lancet Infect Dis. 2013;13:349–61. doi: 10.1016/S1473-3099(13)70008-2. [DOI] [PMC free article] [PubMed] [Google Scholar]