Abstract
Objective
To determine patterns of satiety responsiveness and its relationship to eating in the absence of hunger (EAH), in a cohort of adolescents. We also assessed whether sex, BMI and duration of breastfeeding, during infancy, predicted satiety responsiveness and eating behavior at 16 years.
Methods
Adolescents (n=576) from a longitudinal cohort, that began as an iron deficiency anemia preventive trial, participated in an unlimited breakfast after an overnight fast, and reported satiety response on a visual analogue scale after the meal, followed by an EAH procedure. Height, weight and body composition were measured before breakfast. Latent profile analysis generated profiles that captured individual differences in satiety responsiveness. Multivariable regressions, adjusted for potential confounders, evaluated the association between: 1) satiety responsiveness and EAH, and 2) breastfeeding in infancy, satiety responsiveness and EAH in adolescence.
Results
Participants were on average 16.7-years-old, 48% female, 37% overweight/obese and 76% were breastfed as the sole source of milk for < 6 months. We found three latent profiles of satiety responsiveness: 1. “responsive” (49%); 2. “not responsive” (41%); 3. “still hungry” (10%). Participants in the “not responsive” or “still hungry” profile were more likely to eat during the EAH procedure (OR=2.5, 95%CI 1.8–3.6). Being breastfed for < 6 months was related to higher odds of being in the “not responsive” or “still hungry” profile (OR 1.8, 95%CI 1.2–2.6) and EAH (OR=2.2, 95% CI 1.4–3.3). Satiety responsiveness was not influenced by sex and overweight/obesity.
Conclusion
After an ad libitum meal, we found varied satiety responses, which related to EAH. Furthermore, shorter breastfeeding duration was associated with poorer satiety response and higher consumption during an EAH procedure. Understanding if breastfeeding influences the development of satiety responsiveness and eating behavior may be important in an era characterized by abundant calorie-dense foods and a plethora of environmental cues promoting consumption.
Keywords: eating in the absence of hunger, breastfeeding, visual analogue scale, obesity, satiety responsiveness, latent profile analysis
Introduction
Obesity and its comorbid metabolic and cardiovascular risks often begin during childhood and adolescence (1). Over the last 50 years, dramatic worldwide changes in the environment related to eating and physical activity behavior have led to the well-known obesity epidemic (2). Studying the development of eating behaviors may be particularly important in understanding the impact of an obesigenic environment. Eating when not hungry has become common, encouraged by inexpensive, readily accessible, highly palatable, energy-dense processed foods; increased portion size; omnipresent food advertisements; and the tendency to associate eating with other kinds of pleasures (i.e. toys as presents associated with food) (3–5). These external signals, which promote eating even immediately after a meal, may counteract the internal signals that inhibit eating behavior and regulate satiety. Despite the many environmental cues that stimulate overeating, some individuals exhibit eating behaviors that avoid overconsumption (6), suggesting inter-individual differences in eating regulatory mechanisms.
Eating regulation develops rapidly in infancy and early childhood and may set the stage for lifelong caloric balance and a tendency to prefer certain foods and flavors (7). Sophisticated biological systems that promote energy balance regulate human eating behaviors. Neurophysiological control of eating regulation involves the hypothalamus and brain stem interacting with the gastrointestinal system, pancreas, and adipose tissue through neuroendocrine feedback loops. In spite of elaborate systems of control, there is considerable individual variation in body weight homeostasis, energy expenditure, and regulation of energy balance that could relate to genetic or epigenetic factors (7, 8).
Breastfeeding is an important early environmental exposure that could influence the development of eating regulation and obesity (9, 10). Chile has increased rates of exclusive breastfeeding in recent decades. In 2008, the prevalence of exclusive breastfeeding for 6 months was 49%, compared to only 16% in 1993 (11). Recent research in Chile indicates that while breastfeeding may continue past one year, length of exclusive breastfeeding may be related to duration of paid maternity leave (12). Accumulating evidence suggests that breastfeeding may have a modest, protective effect for the development of obesity, with some studies showing a dose-dependent effect (13). However, several experimental studies resulting in increased breastfeeding did not find decreased obesity prevalence (14–16). In Chile, recent research has not found a relationship between breastfeeding and childhood obesity at 4 or 7 years (12, 17). Furthermore, associations seen in observational studies are reduced when adjusting for confounding factors (18, 19). Disentangling the effects of breastfeeding on obesity from confounding factors is difficult especially because the extent and duration of breastfeeding are socially determined in almost all settings. Nonetheless, the potential protective effect of longer breastfeeding could be explained in part by modulation of eating regulation (7, 20, 21). The finely tuned experience of receiving adequate, but not excessive, calories in response to suckling could be involved in the development of energy regulation and eating behavior (21, 22). Overall, the mechanisms relating breastfeeding and eating regulation are still unclear.
Eating regulation can be assessed at different levels: hormonal, neural activity, perception of satiety, and eating behavior. Satiety responsiveness, defined here as the perception of internal satiety cues, can be measured by self-report after a meal. One instrument, the Visual Analogue Scale (VAS), was first described by Jordan et al. (23) and is widely used to quantify satiety response (24, 25). Responses on the VAS predict later calorie intake (25, 26) and have been associated with neural activity in appetite regulatory centers (27). Eating behavior, on the other hand, can be evaluated using direct observation, choice tests, questionnaires, and experimental approaches (28, 29). One method, described by Birch and Fisher, ‘eating in the absence of hunger’ (EAH) (30) has been used in diverse settings with children of various ages. In the EAH procedure an ad libitum snack and alternative activities (e.g. magazines) are presented to a participant after a meal; caloric intake at a snack of appetizing treats is tabulated to assess eating behavior when hunger is not a factor (31). Eating more during an EAH procedure has been associated with unhealthy eating, child adiposity, genetic predisposition to obesity and prior exposure to certain parental feeding practices (31–33).
We were interested in describing eating regulation in a cohort of Chilean adolescents followed since infancy based on satiety responsiveness, assessed using the VAS, and eating behavior, assessed using an EAH procedure. We also asked whether individual factors, including breastfeeding in infancy, were related to satiety responsiveness or EAH. We posed 3 research questions: 1. Do participants have distinctive satiety responsiveness profiles?; 2. Does satiety responsiveness relate to EAH?; and 3. Are sex, BMI, or breastfeeding history linked to satiety responsiveness or EAH?
Subjects and methods
We studied 576 post-pubertal Chilean adolescents (16- to 17-years-old) who were evaluated as part of a larger longitudinal study aimed at assessing biopsychosocial determinants of obesity and cardiovascular risk. The participants belonged to a cohort of 1657 participants who were enrolled as infants in an iron deficiency anemia preventive trial (34). For the current wave of data collection, consent for participation was provided by parents and assent by the adolescents. The study was approved by the Institutional Review Boards of the University of California, San Diego for the adolescent wave and by the University of Michigan and the University of Chile, Institute of Nutrition and Food Technology (INTA) for all waves of the study.
Infancy Wave (1991–1996)
From 1991–1996, healthy infants from 4 working-class neighborhoods in Santiago, Chile were recruited to participate in a randomized controlled trial of iron to prevent iron deficiency anemia (IDA). In the initial years of the study, at 6 months, infants without IDA who were taking ≥ 250 ml of formula/cow milk were randomized to receive either supplemental iron using high- or low-iron formula. Part way through the study, random assignment was changed to high-iron supplementation or usual nutrition. Infants taking < 250 ml of formula/cow milk were randomized to vitamins with or without iron. The trial was designed so as not to affect the extent of breastfeeding. However, due to a secular increase in breastfeeding during the study period, and the fact that the “no iron” condition was added later in the study, the study groups differed related to duration of breastfeeding, with the longest breastfeeding in the no-added-iron group. Additional details on the randomization procedures, and the feeding differences by study group, have been previously described (34).
All but 5 infants in this sample were initially breastfed. When the infants were 4-months-old, the mothers reported the date of the infant’s first bottle if they were already supplementing breastfeeding with formula/cow milk. For those exclusively breastfeeding at 4 months, the date of the first bottle was reported prospectively. Introduction of the first bottle corresponded to introduction of formula/cow milk, since giving breast milk in bottles was not customary in Chile (34). We computed the duration of breastfeeding as the sole source of milk (BF) and categorized as < 6 months (yes vs no). We chose this cut off because in Chile for more than 20 years, pediatricians have recommended a minimum of 6 months exclusive BF. We did not collect data on introduction of complementary food. However, in Chile at that time, pureed fruits and cereals were typically introduced after 4 months, with pureed meats and vegetables after 6 months, and legumes and eggs after 9 months (35). We have no data on consumption of other beverages, such as water or fruit juices. In our study, BF is not equivalent to exclusive breastfeeding; infants breastfeeding as their sole source of milk may have received water or juice and almost certainly received complementary foods after 4 – 6 months.
Adolescent Wave (2008–2012)
After an overnight fast, adolescents were measured at INTA without shoes, wearing underwear, in the Frankfurt position. We determined body mass index (BMI) z-score using World Health Organization standards. Participants were classified as normal weight (z-score ≥ −2 to < 1) and overweight/obese (z-score ≥ 1). Lean mass was assessed using Lunar Prodigy Dual Energy X-Ray Absorptiometry scan. All participants were measured according to standard protocols on the same machine calibrated every other day.
After anthropometry and the body composition measurement, adolescents were offered a breakfast tray, including, juice, fruit cup, sandwich (choice of ham and cheese or butter and marmalade), flavored milk (choice of chocolate or strawberry), and tea or coffee. The meal was consumed ad libitum. Participants were not required to eat, but could request additional food or drinks. Up to 4 adolescents were assessed during the same morning and start time was recorded. A research physician accompanied each participant during the breakfast with no other participants, friends, or family members present. At the end of the meal, the researcher verbally confirmed that the participant was not hungry and had eaten to fullness; breakfast duration was recorded. Trained nutrition staff determined calories and nutritional composition of energy consumed based on manufacturer’s information. Exactly 20 minutes after breakfast, the participants were asked to report their satiety responsiveness using the VAS (24). The 10-point scale asks: 1) how hungry do you feel? 2) how satisfied do you feel? 3) how full do you feel? 4) how much do you think you could eat? 5) would you like to eat something sweet? 6) would you like to eat something salty? 7) would you like to eat something savory? 8) would you like to eat something “fatty”? In addition, we asked 5 questions, regarding the palatability of breakfast. All questions were translated into Spanish and pilot-tested with Chilean adolescents to ensure that the respondents understood and felt comfortable answering the questions.
After completing these questions, each participant was individually invited into a room furnished with teen magazines and a variety of snacks and beverages (soft drinks, cookies, crackers, potato chips, chocolate, ice cream and candy) for the EAH procedure (10, 27). Both breakfast contents and snack items were chosen based on pilot testing. Participants were not told that they would be offered snacks after breakfast. A research staff member invited participants to help themselves to a magazine or something to eat or drink, as they wished. After 20 minutes (time recorded), another member of the research team escorted the participant from the waiting room to complete the assessment. The snacks were weighed before and after the EAH procedure for determination of calories, fat, protein, and carbohydrate consumed.
Statistical Analysis
Latent Profile Analysis
Latent profile analysis (LPA) is an innovative person-centered statistical approach, which can be used to identify unmeasured membership among participants using continuous observed variables to examine varying constellations of response patterns (36–38). The primary objective of LPA is to find groups of individuals who are similar using a categorical latent variable. LPA categorizes people into separate groups based on measurement theory (i.e., true and error scores) and can quantify the extent to which indicators are not perfectly related to latent profile (i.e., measurement error).
LPA was used to generate distinctive profiles that captured individual differences in satiety responsiveness. We used 6 of the 8 VAS items (continuous variables) as latent indicators, omitting 2 items (“how satisfied do you feel? and “how full do you feel”) as responses were virtually identical to those from the question “how hungry do you feel?” The first step involved fitting a one-profile model to the data to establish a baseline model. Next, models with successively increasing numbers of profiles were tested. To determine the best fitting model, we used the following fit indices: Akaike Information Criterion (39), the Bayesian Information Criterion (40), and the adjusted BIC (41). For these fit indices, smaller values indicate better fitting models and are useful when comparing 2 or more models. Lastly, an entropy value approaching 1.0 was used to indicate a clear distinction between profiles (42). In each step, LPA assigned participants to their most likely profile, based on posterior probabilities for each respondent, which resulted in an observed variable that could be associated with other variables (37). LPA analyses were performed using the MPlus 6 software (Muthen & Muthen).
Bivariate and Multivariable Regressions
ANOVA and chi-square tests were used to examine overall differences between groups (sex, weight status, BF and profile). Post-hoc analyses (Bonferroni and multiple chi-square tests) were used to determine significant differences between profiles. In addition, in order to account for the influence of body size in caloric intake, energy consumption at breakfast and EAH snack was adjusted for lean mass and height, using univariate general linear models. We used binary logistic regression to assess whether profile membership (reference=“responsive” profile) was associated with eating during the EAH procedure. Binary logistic regression was also used to determine whether sex, overweight/obesity status, and BF for < 6 months were associated with latent profile (reference=“responsive” profile) and EAH (reference=did not eat EAH snack).
Because of the original study design and the differences in feeding by supplementation group, we controlled for iron supplementation group and IDA in infancy. Thus, all multivariable models were adjusted for sex, overweight/obesity status, iron supplementation and IDA in infancy. Additionally we tested the association of the following covariates with satiety response profile and EAH: caloric intake at breakfast; lean mass; height; assessment start time; usual breakfast consumption (yes vs no); and maternal education. None of these variables were significantly related to the outcomes and were thus removed from final models to achieve parsimony. P-values of < 0.05 were considered statistically significant. Analyses were performed using SPSS 19.
Results
Descriptive information for the sample is presented in Table 1. Participants were on average 16.7±0.2-years old, 48% female, 38% overweight/obese and 76% had been breastfed as the sole source of milk for < than 6 months. All participants ate or drank at the provided breakfast. Twenty minutes after the breakfast, 48% of the participants ate or drank during the EAH procedure. Caloric intake at breakfast and EAH snack (among those who ate) did not significantly differ by sex or weight status after adjusting for lean mass and height (data not shown).
Table 1.
Descriptive statistics, overall and by breastfeeding group†
| Overall n=576 | BF < 6 months n=433‡ | BF ≥ 6 months n=139‡ | |
|---|---|---|---|
| Age at assessment* | 16.7 (0.2) | 16.7 (0.2) | 16.7 (0.1) |
| Female | 48% | 48% | 47% |
| BMI z-score | 0.6 (1.1) | 0.6 (1.1) | 0.8 (1.2) |
| Overweight/obese | 38% | 37% | 40% |
| Age at first bottle (days)* | 112 (96) | 67.5 (52.9) | 249.4 (63.4) |
| Breakfast energy intake (kcal) | 624 (258) | 615 (276) | 654 (185) |
| Breakfast palatability | |||
| How did the breakfast look?a | 9.5 (0.8) | 9.5 (0.8) | 9.6 (0.6) |
| How did the breakfast smell?a | 9.3 (1.2) | 9.2 (1.1) | 9.3 (1.1) |
| How did the breakfast taste?a | 9.5 (0.9) | 9.4 (0.8) | 9.4 (1.2) |
| Taste in my mouth after the meal?a | 9.2 (1.3) | 9.1 (1.2) | 9.1 (1.5) |
| How tasty was the meal?b | 9.1 (1.2) | 9.1 (1.2) | 9.2 (1.1) |
| VAS | |||
| How hungry do you feel?c* | 1.2 (1.9) | 1.2 (2.0) | 0.8 (1.5) |
| How satisfied do you feel?d* | 7.8 (2.1) | 7.6 (2.1) | 8.1 (1.9) |
| How full do you feele* | 7.8 (2.3) | 7.6 (2.2) | 8.1 (2.1) |
| How much do you think you can eat?f | 4.2 (3.2) | 4.5 (3.1) | 3.2 (2.9) |
| Would you like to eat something sweet?g | 3.4 (3.3) | 3.5 (3.2) | 2.8 (3.3) |
| Would you like to eat something salty?g* | 3.0 (3.1) | 3.1 (3.1) | 2.3 (2.8) |
| Would you like to eat something savoury?g* | 4.2 (3.3) | 4.4 (3.3) | 3.4 (3.1) |
| Would you like to eat something fatty?g* | 1.7 (2.2) | 1.7 (2.2) | 1.4 (1.9) |
| EAH behavior | |||
| Ate EAH snack* | 48% | 54% | 34% |
| Snack energy intake (kcal)h* | 220 (175) | 234 (179) | 151 (132) |
Values are either frequency or mean (SD)
BF missing for 4 participants
p<0.05 for comparisons between BF groups
0=“bad”, 10=“good”
0=“not tasty at all”, 10=“very tasty”
0=“not hungry at all”, 10=“never been more hungry”
0=“completely empty”, 10=“cannot eat another bite”
0=“not at all full”, 10= “totally full”
0=“nothing at all”,10=“a lot “
0=“not at all”, 10=“very much”
Among those who ate EAH snack (n=277)
Abbreviations: BMI, body mass index; BF, breastfeeding as the sole source of milk; VAS, visual analogue scale; EAH, eating in the absence of hunger
As shown in Table 1, participants who were BF < 6 months versus ≥ 6 months had a similar sex distribution, BMI z-score, and proportion of overweight/obese. Breakfast palatability did not differ by BF group, however, response to VAS did. Participants who were BF < 6 months reported being hungrier, less satisfied, less full and more likely to eat something salty, savoury and fatty, compared to those who were BF ≥ 6 months. Breakfast intake did not differ by BF group even after adjusting for lean mass and height (data not shown). Among those who ate the EAH snack, caloric intake did differ, with adolescents with a shorter BF experience eating more calories on average compared to those with longer BF. This difference remained after adjusting for lean mass and height.
Satiety responsiveness profiles
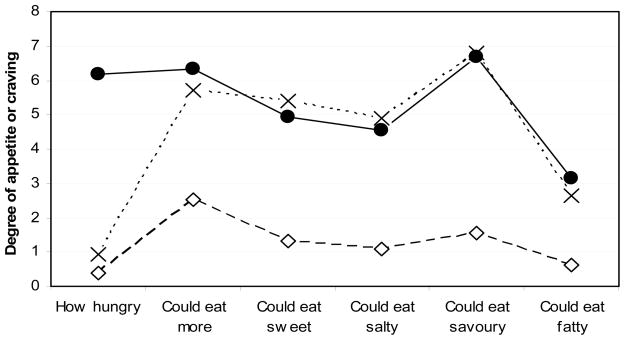
Fit indices for the LPA indicated a 3-group model of satiety responsiveness as the best fit. Profile 1 was labeled “responsive” because it included persons who reported not being hungry and not being able to eat more (49%). Profile 2 was labeled “not responsive” as persons reported not being hungry, but being able to eat more (41%). Profile 3 was labeled “still hungry”, as participants said they were still hungry and could eat more (10%). Figure 1 illustrates the mean VAS item score by profile.
Figure 1.
Visual analogue scale (VAS) responses by satiety responsiveness profile (n=576)†
†Values represent average of scores in each question.
Dashed line and empty diamonds= Profile 1: “responsive” (n=284)
Dashed line and crosses= Profile 2: “not responsive” (n=237)
Continuous line and filled circles= Profile 3: “still hungry” (n=55).
Satiety responsiveness and EAH
Table 2 shows the results of bivariate analyses. Fewer participants from the “responsive” profile ate during the EAH procedure compared to those in the “not responsive” and “still hungry” profiles: 37% vs. 59% and 67%, respectively (p<0.05). Among participants who ate during the procedure, we found significant differences in EAH snack energy intake by profile. In multivariable analysis, compared to “responsive” participants, the other profiles—”not responsive” or “still hungry”—were 2.5 times (95% CI 1.8–3.6) more likely to eat the EAH snack, controlling for covariates.
Table 2.
Bivariate associations between satiety responsiveness profile and background characteristics, breastfeeding duration, and adolescent eating behavior†
| “Responsive” n=284 | “Not Responsive” n=237 | “Still Hungry” n=55 | |
|---|---|---|---|
| Background characteristics | |||
| Age at assessment* | 16.7 (0.2)a | 16.8 (0.2)a,b | 16.9 (0.3)b |
| Female* | 52%a | 43%b | 46%a,b |
| Overweight/obese | 41% | 35% | 31% |
| Duration of breastfeeding as the sole source of milk | |||
| Age at first bottle (days)* | 125 (101) | 106 (91) | 76 (82) |
| BF < 6 months* | 70%a | 78%a,b | 90%b |
| Breakfast behavior | |||
| Breakfast energy intake (kcal) | 636 (242) | 620 (262) | 581 (313) |
| Protein (% of energy) | 13 (3) | 13 (4) | 13 (3) |
| Fat (% of energy) | 32 (8) | 31 (7) | 31 (10) |
| Carbohydrate (% of energy) | 56 (10) | 57 (10) | 57 (13) |
| Duration (minutes) | 19 (6) | 18 (7) | 18 (8) |
| EAH behavior | |||
| Ate EAH snack* | 37%a | 59%b | 67%b |
| Snack energy intake (kcal)‡* | 169 (151)a | 242 (179)b | 279 (192)b |
| Protein (% of energy)‡ | 6 (4) | 6 (4) | 6 (2) |
| Fat (% of energy)‡ | 40 (19) | 44 (19) | 40 (19) |
| Carbohydrate (% of energy)‡ | 55 (25) | 51 (21) | 55 (20) |
| Duration (minutes) | 21 (3) | 21 (5) | 21 (1) |
Values are either frequency or mean (SD)
p<0.05 for overall differences: ANOVA (continuous variables) or Chi-square (categorical variables)
Values with different superscript letters are significantly different (Bonferroni and multiple chi-square tests)
Among those who ate EAH snack (n=277)
Abbreviations: BF, breastfeeding as the sole source of milk; EAH, eating in the absence of hunger
Influence of sex, BMI and BF in satiety responsiveness and EAH
In bivariate analysis, satiety responsiveness profile did differ by sex and BF, but not overweight/obesity status (Table 2).
Adolescents who were BF < 6 months, compared to ≥ 6 months, were 1.8 times (95% CI 1.2–2.6) more likely to be in the “not responsive” or “still hungry” profiles, adjusting for covariates (Table 3). Multivariable results showed no significant association of sex or overweight/obesity status in this relationship.
Table 3.
Final logistic regression models assessing correlates of eating EAH snack or satiety responsiveness profile†
| Outcome
|
||
|---|---|---|
| “not responsifve” or “still hungry” profilea | Ate EAH snackb | |
|
| ||
| OR (95% CI) | OR (95% CI) | |
| BF < 6 months | 1.8 (1.2–2.6) | 2.2 (1.4–3.3) |
| Female | 1.4 (1.0–1.9) | 1.0 (0.7–1.4) |
| Overweight/obese | 1.3 (0.9–1.8) | 0.9 (0.6–1.3) |
All models adjusted for iron supplementation group and IDA in infancy, according to original study design
Reference=“responsive” profile
Reference=did not eat EAH snack
Bold denotes statistically significant result (p<0.05)
Abbreviations: EAH, eating in the absence of hunger; OR, odds ratio; BF, breastfeeding at the sole source of milk
In multivariable regression, adolescents who were BF for < 6 months were 2.2 times (95% CI 1.4–3.3) more likely to eat during the EAH snack, compared to those who had BF for a longer duration (Table 3).
Discussion
In a cohort of healthy Chilean adolescents, almost all indicated low levels of hunger after an ad libitum breakfast, however, many reported that they could still eat more. In fact, almost half accepted tasty snacks 20 minutes after the breakfast. We identified 3 latent profiles of satiety responsiveness: “responsive”, “not responsive” and “still hungry”. Breakfast calories did not differ by profile, but fewer adolescents from the “responsive” profile ate during the EAH compared to those in the other 2 profiles. Furthermore, those from the “not responsive” or “still hungry” profiles who ate during the EAH consumed significantly more those from the “responsive” profile. In multivariable analysis, sex and overweight/obesity status did not differ by satiety responsiveness. However, the experience of being BF for < 6 months was associated with being classified as “not responsive” or “still hungry” and, for those who did consume the EAH snack, eating more calories. In addition, duration of BF was negatively associated with adjusted caloric consumption during the EAH procedure.
We were surprised that a small group of participants (10%) said they were hungry, even though, 20 minutes before at the breakfast, they had consumed as much as they desired. This group did not differ from the others in intake at breakfast or overweight/obesity status or sex. We do not know whether they actually remained hungry after breakfast despite having refused additional food when offered, or if they began to feel hungry in the short time between finishing breakfast and answering the VAS. It is worth noting that this group cannot be considered to have eaten in the absence of hunger since they reported being “still hungry”. Nonetheless, this profile raises interesting questions about how individuals perceive, express and respond to satiety and hunger.
Several studies involving children have found associations between BMI and intake during an EAH assessment (30, 32, 43). We expected weight status would influence caloric intake, but this was not supported by our results. It is possible that all adolescents, but especially those were overweight/obese, may have altered their eating behavior and ratings on the VAS in favor of behavior that they perceived to be socially desirable. Furthermore, as the study was performed at a well-known nutritional research institute, it is possible that the participants’ perception of the research center and the goals of our research may have biased their responses to testing.
As the participants had been followed since infancy, we were able to assess associations between length of BF, satiety responsiveness or EAH behavior 16 years later. Adolescents who had BF < 6 months had significantly higher odds of being “not responsive” or “still hungry” and eating at the EAH snack. Despite the fact that several studies, including the current analysis, have found no long-term effect of breastfeeding on nutritional status (12, 14, 15, 17), our findings suggest better satiety responsiveness as a possible long-lasting effect of feeding patterns in infancy. Longer breastfeeding as the sole source of milk could have an effect through the finely tuned positive feedback loop between infant demand and maternal milk production (20, 44).
Our study has some limitations. Generalizability is limited because we studied adolescents from a low- to middle-income urban cohort in Santiago, Chile. Socioeconomic status (SES), culture and past experiences with food are expected to influence satisfaction after eating and desire to eat. SES and culture are factors that have also been related to breastfeeding duration; however, we did not find an influence of SES within this cohort, possibly because of the limited range of SES. Another limitation is the possibility of bias related to social desirability. Our participants have been assessed at INTA multiple times since infancy. Their comfort with this research setting may have counteracted any tendency to alter their responses based on their perception of what the investigators wanted. Thus, the degree/direction of bias cannot be determined. Additionally, it is possible that some adolescents chose not to eat at the EAH procedure because they did not find the snacks appetizing. Future studies could require all foods be tasted and palatability rated by each participant, as other EAH procedures have done (31, 32). That our EAH procedure was conducted after a breakfast meal, rather than lunch, may also have influenced intake and our ability to compare our findings to other EAH procedures (30, 32). However, a mid-morning snack is common in Chilean schools, thus it is likely adolescent participants were used to being exposed to snacks at that time of the day. We also note that breastfeeding and the measurement of satiety responsiveness and EAH occurred more than 15 years apart. Clearly, other intervening exposures could have played a role. Finally, our findings must be considered in light of the fact that the study began as a randomized preventive trial of iron deficiency anemia. Thus, we controlled for the possible influence of randomized assignment to iron supplementation or usual nutrition as well as IDA in the first year.
This study has several noteworthy strengths. While caution is advised in inferring causation, strengths of our study include prospectively collected breastfeeding data in infancy, which minimizes recall bias and temporal precedence of breastfeeding related to eating regulation in adolescence. In addition, we assessed EAH using a well-validated approach. Our study also adds important information about eating behavior from an understudied context. To our knowledge, our study is the first use of an EAH procedure in a Latin American country. Chile is a country that has undergone rapid economic and cultural changes associated with nutrition resulting in accelerating increases in obesity (42). The prevalence of childhood obesity increased in Chile twice as quickly as it did in the U.S., making this an important setting in which to assess eating behavior.
The development of eating behavior is of critical importance in a worldwide epidemic of child and adolescent obesity. Environmental modulation (e.g., restricting the availability of energy-dense foods) could be an important strategy to tackle unhealthy eating behavior. Future research could examine biological determinants related to how adolescents make choices about when and what to eat (e.g. brain-based reward systems, hormonal regulators of energy balance, and the interaction between environment and genetic influences).
We found varied satiety responses, which related to EAH, among our sample of Chilean adolescents. Breastfeeding duration was associated with poorer satiety response and higher consumption during an EAH procedure. An important proportion of Chilean adolescents showed unhealthy satiety responsiveness and in fact they did eat when exposed to a permissive environment. Our finding that the extent of breastfeeding in infancy related to perception of hunger and desire to eat and to calories consumed during an EAH procedure 16 years later, suggests that breastfeeding may possibly play an important role in the development of eating regulation.
Acknowledgments
Funding: National Institutes of Health, Heart, Lung, And Blood Institute (HL088530, PI: Gahagan), the National Institute of Child Health and Human Development (HD14122, PI: Lozoff, and HD33487, PI: Lozoff and Gahagan).
We thank all participants and their families for their trust and ongoing participation. We also thank the research and health professionals who have worked on the different aspects of the longitudinal project over the last 21 years. This study was supported by grants from the National Institutes of Health, Heart, Lung, And Blood Institute (HL088530, PI: Gahagan), the National Institute of Child Health and Human Development (HD14122, PI: Lozoff, and HD33487, PI: Lozoff and Gahagan), and the National Center of Minority Health & Health Disparities supported the participation of Vanessa Hoyos (Grant # 5-T32-MD-0001425-15, administered by the Center for Human Growth & Development of the University of Michigan). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of interest
The authors do not have any conflicts of interest to report.
References
- 1.Daniels SR, Jacobson MS, McCrindle BW, Eckel RH, Sanner BM. American Heart Association Childhood Obesity Research Summit Report. Circulation. 2009;119(15):e489–517. doi: 10.1161/CIRCULATIONAHA.109.192216. Epub 2009/04/01. [DOI] [PubMed] [Google Scholar]
- 2.Mitchell NS, Catenacci VA, Wyatt HR, Hill JO. Obesity: overview of an epidemic. Psychiatr Clin North Am. 2011;34(4):717–32. doi: 10.1016/j.psc.2011.08.005. Epub 2011/11/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hill JO, Peters JC. Environmental contributions to the obesity epidemic. Science. 1998;280(5368):1371–4. doi: 10.1126/science.280.5368.1371. Epub 1998/06/20. [DOI] [PubMed] [Google Scholar]
- 4.Kral TV, Faith MS. Child eating patterns and weight regulation: a developmental behaviour genetics framework. Acta Paediatr Suppl. 2007;96(454):29–34. doi: 10.1111/j.1651-2227.2007.00167.x. Epub 2007/02/23. [DOI] [PubMed] [Google Scholar]
- 5.Monteiro CA, Levy RB, Claro RM, de Castro IR, Cannon G. Increasing consumption of ultra-processed foods and likely impact on human health: evidence from Brazil. Public Health Nutr. 14(1):5–13. doi: 10.1017/S1368980010003241. Epub 2011/01/08. [DOI] [PubMed] [Google Scholar]
- 6.van Strien T, Herman CP, Verheijden MW. Eating style, overeating and weight gain. A prospective 2-year follow-up study in a representative Dutch sample. Appetite. 59(3):782–9. doi: 10.1016/j.appet.2012.08.009. Epub 2012/08/25. [DOI] [PubMed] [Google Scholar]
- 7.Gahagan S. Development of eating behavior: biology and context. J Dev Behav Pediatr. 2012;33(3):261–71. doi: 10.1097/DBP.0b013e31824a7baa. Epub 2012/04/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harrold JA, Dovey TM, Blundell JE, Halford JC. CNS regulation of appetite. Neuropharmacology. 63(1):3–17. doi: 10.1016/j.neuropharm.2012.01.007. Epub 2012/02/09. [DOI] [PubMed] [Google Scholar]
- 9.Bartok CJ, Ventura AK. Mechanisms underlying the association between breastfeeding and obesity. Int J Pediatr Obes. 2009;4(4):196–204. doi: 10.3109/17477160902763309. Epub 2009/11/20. [DOI] [PubMed] [Google Scholar]
- 10.Birch LL, Fisher JO. Development of eating behaviors among children and adolescents. Pediatrics. 1998;101(3 Pt 2):539–49. Epub 2002/09/13. [PubMed] [Google Scholar]
- 11.Niño RSG, Atalah E. Determinants of exclusive breastfeeding in health centers in Santiago, Chile. Rev Chil Pedriatr. 2012;83(2):161–9. [Google Scholar]
- 12.Rios-Castillo I, Cerezo S, Corvalan C, Martinez M, Kain J. Risk factors during the prenatal period and the first year of life associated with overweight in 7-year-old low-income Chilean children. Maternal & child nutrition. 2012 doi: 10.1111/mcn.12024. Epub 2012/12/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arenz S, Ruckerl R, Koletzko B, von Kries R. Breast-feeding and childhood obesity--a systematic review. Int J Obes Relat Metab Disord. 2004;28(10):1247–56. doi: 10.1038/sj.ijo.0802758. Epub 2004/08/18. [DOI] [PubMed] [Google Scholar]
- 14.Kramer MS, Matush L, Vanilovich I, Platt RW, Bogdanovich N, Sevkovskaya Z, et al. A randomized breast-feeding promotion intervention did not reduce child obesity in Belarus. J Nutr. 2009;139(2):417S–21S. doi: 10.3945/jn.108.097675. Epub 2008/12/25. [DOI] [PubMed] [Google Scholar]
- 15.Martin RM, Patel R, Kramer MS, Guthrie L, Vilchuck K, Bogdanovich N, et al. Effects of promoting longer-term and exclusive breastfeeding on adiposity and insulin-like growth factor-I at age 11.5 years: a randomized trial. JAMA. 2013;309(10):1005–13. doi: 10.1001/jama.2013.167. Epub 2013/03/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morley R, Lucas A. Randomized diet in the neonatal period and growth performance until 7.5–8 y of age in preterm children. Am J Clin Nutr. 2000;71(3):822–8. doi: 10.1093/ajcn/71.3.822. Epub 2000/03/04. [DOI] [PubMed] [Google Scholar]
- 17.Corvalan C, Uauy R, Stein AD, Kain J, Martorell R. Effect of growth on cardiometabolic status at 4 y of age. Am J Clin Nutr. 2009;90(3):547–55. doi: 10.3945/ajcn.2008.27318. Epub 2009/07/31. [DOI] [PubMed] [Google Scholar]
- 18.Anzman SL, Rollins BY, Birch LL. Parental influence on children’s early eating environments and obesity risk: implications for prevention. Int J Obes (Lond) 34(7):1116–24. doi: 10.1038/ijo.2010.43. Epub 2010/03/03. [DOI] [PubMed] [Google Scholar]
- 19.Owen CG, Martin RM, Whincup PH, Smith GD, Cook DG. Effect of infant feeding on the risk of obesity across the life course: a quantitative review of published evidence. Pediatrics. 2005;115(5):1367–77. doi: 10.1542/peds.2004-1176. Epub 2005/05/04. [DOI] [PubMed] [Google Scholar]
- 20.Li R, Fein SB, Grummer-Strawn LM. Do infants fed from bottles lack self-regulation of milk intake compared with directly breastfed infants? Pediatrics. 125(6):e1386–93. doi: 10.1542/peds.2009-2549. Epub 2010/05/12. [DOI] [PubMed] [Google Scholar]
- 21.Disantis KI, Collins BN, Fisher JO, Davey A. Do infants fed directly from the breast have improved appetite regulation and slower growth during early childhood compared with infants fed from a bottle? Int J Behav Nutr Phys Act. 2011;8:89. doi: 10.1186/1479-5868-8-89. Epub 2011/08/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li R, Fein SB, Grummer-Strawn LM. Do infants fed from bottles lack self-regulation of milk intake compared with directly breastfed infants? Pediatrics. 2010;125(6):e1386–93. doi: 10.1542/peds.2009-2549. Epub 2010/05/12. [DOI] [PubMed] [Google Scholar]
- 23.Jordan HA, Wieland WF, Zebley SPES, AJS Direct Measurement of Food Intake in Man: A Method for the Objective Study of Eating Behavior. Psychomsom Med. 1966;XXVIII(6):836–42. [Google Scholar]
- 24.Flint A, Raben A, Blundell JE, Astrup A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes Relat Metab Disord. 2000;24(1):38–48. doi: 10.1038/sj.ijo.0801083. Epub 2000/03/07. [DOI] [PubMed] [Google Scholar]
- 25.Parker BA, Sturm K, MacIntosh CG, Feinle C, Horowitz M, Chapman IM. Relation between food intake and visual analogue scale ratings of appetite and other sensations in healthy older and young subjects. Eur J Clin Nutr. 2004;58(2):212–8. doi: 10.1038/sj.ejcn.1601768. Epub 2004/01/30. [DOI] [PubMed] [Google Scholar]
- 26.Drapeau V, King N, Hetherington M, Doucet E, Blundell J, Tremblay A. Appetite sensations and satiety quotient: predictors of energy intake and weight loss. Appetite. 2007;48(2):159–66. doi: 10.1016/j.appet.2006.08.002. Epub 2006/10/19. [DOI] [PubMed] [Google Scholar]
- 27.Wang GJ, Volkow ND, Telang F, Jayne M, Ma J, Rao M, et al. Exposure to appetitive food stimuli markedly activates the human brain. Neuroimage. 2004;21(4):1790–7. doi: 10.1016/j.neuroimage.2003.11.026. Epub 2004/03/31. [DOI] [PubMed] [Google Scholar]
- 28.Fisher JO, Birch LL. Restricting access to palatable foods affects children’s behavioral response, food selection, and intake. Am J Clin Nutr. 1999;69(6):1264–72. doi: 10.1093/ajcn/69.6.1264. Epub 1999/06/05. [DOI] [PubMed] [Google Scholar]
- 29.Wardle J, Guthrie CA, Sanderson S, Rapoport L. Development of the Children’s Eating Behaviour Questionnaire. J Child Psychol Psychiatry. 2001;42(7):963–70. doi: 10.1111/1469-7610.00792. Epub 2001/11/06. [DOI] [PubMed] [Google Scholar]
- 30.Fisher JO, Birch LL. Eating in the absence of hunger and overweight in girls from 5 to 7 y of age. Am J Clin Nutr. 2002;76(1):226–31. doi: 10.1093/ajcn/76.1.226. Epub 2002/06/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Birch LL, Fisher JO, Davison KK. Learning to overeat: maternal use of restrictive feeding practices promotes girls’ eating in the absence of hunger. Am J Clin Nutr. 2003;78(2):215–20. doi: 10.1093/ajcn/78.2.215. Epub 2003/07/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shomaker LB, Tanofsky-Kraff M, Zocca JM, Courville A, Kozlosky M, Columbo KM, et al. Eating in the absence of hunger in adolescents: intake after a large-array meal compared with that after a standardized meal. Am J Clin Nutr. 2010;92(4):697–703. doi: 10.3945/ajcn.2010.29812. Epub 2010/08/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kral TV, Moore RH, Stunkard AJ, Berkowitz RI, Stettler N, Stallings VA, et al. Adolescent eating in the absence of hunger and relation to discretionary calorie allowance. J Am Diet Assoc. 2010;110(12):1896–900. doi: 10.1016/j.jada.2010.09.009. Epub 2010/11/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lozoff B, De Andraca I, Castillo M, Smith JB, Walter T, Pino P. Behavioral and developmental effects of preventing iron-deficiency anemia in healthy full-term infants. Pediatrics. 2003;112(4):846–54. Epub 2003/10/03. [PubMed] [Google Scholar]
- 35.Walter T, Pino P, Pizarro F, Lozoff B. Prevention of iron-deficiency anemia: comparison of high- and low-iron formulas in term healthy infants after six months of life. J Pediatr. 1998;132(4):635–40. doi: 10.1016/s0022-3476(98)70352-x. Epub 1998/05/15. [DOI] [PubMed] [Google Scholar]
- 36.Lanza ST, Collins LM, Lemmon DR, Schafer JL. PROC LCA: A SAS Procedure for Latent Class Analysis. Structural equation modeling: a multidisciplinary journal. 2007;14(4):671–94. doi: 10.1080/10705510701575602. Epub 2007/01/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roesch SC, Villodas M, Villodas F. Latent class/profile analysis in maltreatment research: a commentary on Nooner et al., Pears et al., and looking beyond. Child abuse & neglect. 2010;34(3):155–60. doi: 10.1016/j.chiabu.2010.01.003. Epub 2010/03/09. [DOI] [PubMed] [Google Scholar]
- 38.Muthen B, Muthen LK. Integrating person-centered and variable-centered analyses: growth mixture modeling with latent trajectory classes. Alcohol Clin Exp Res. 2000;24(6):882–91. Epub 2000/07/11. [PubMed] [Google Scholar]
- 39.Akaike H. Factor-analysis and AIC. Psychometrika. 1987;52(3):317–32. [Google Scholar]
- 40.Schwarz G. Estimating dimension of a model. Annals of Statistics. 1978;6(2):461–4. [Google Scholar]
- 41.Sclove SL. Application of a model-selection criteria to some problems in multivariate-analysis. Pshychometrika. 1987;52(3):333–43. [Google Scholar]
- 42.Celeux G, Soromenho G. An enthropy criterion for assessing the number of clusters in a mixture model. Journal of Classification. 1996;13(2):195–212. [Google Scholar]
- 43.Moens E, Braet C. Predictors of disinhibited eating in children with and without overweight. Behav Res Ther. 2007;45(6):1357–68. doi: 10.1016/j.brat.2006.10.001. Epub 2006/11/15. [DOI] [PubMed] [Google Scholar]
- 44.Disantis KI, Collins BN, Fisher JO, Davey A. Do infants fed directly from the breast have improved appetite regulation and slower growth during early childhood compared with infants fed from a bottle? Int J Behav Nutr Phys Act. 8:89. doi: 10.1186/1479-5868-8-89. Epub 2011/08/19. [DOI] [PMC free article] [PubMed] [Google Scholar]