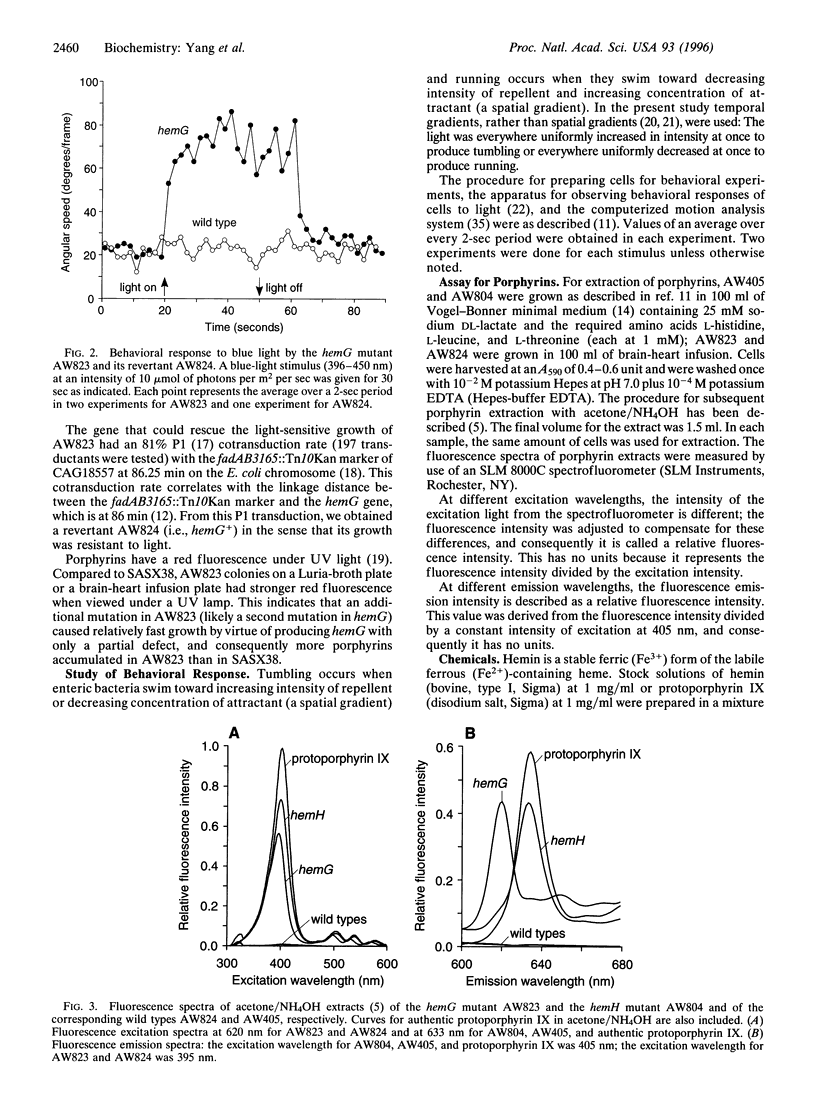
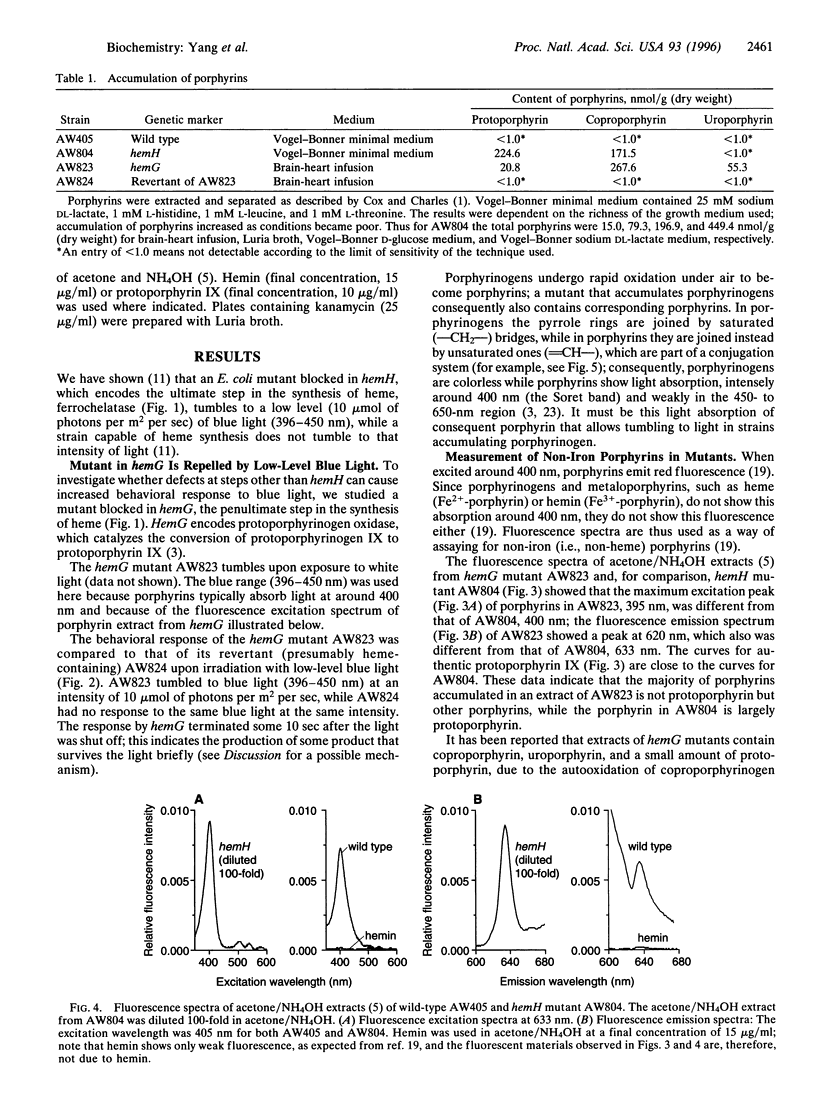
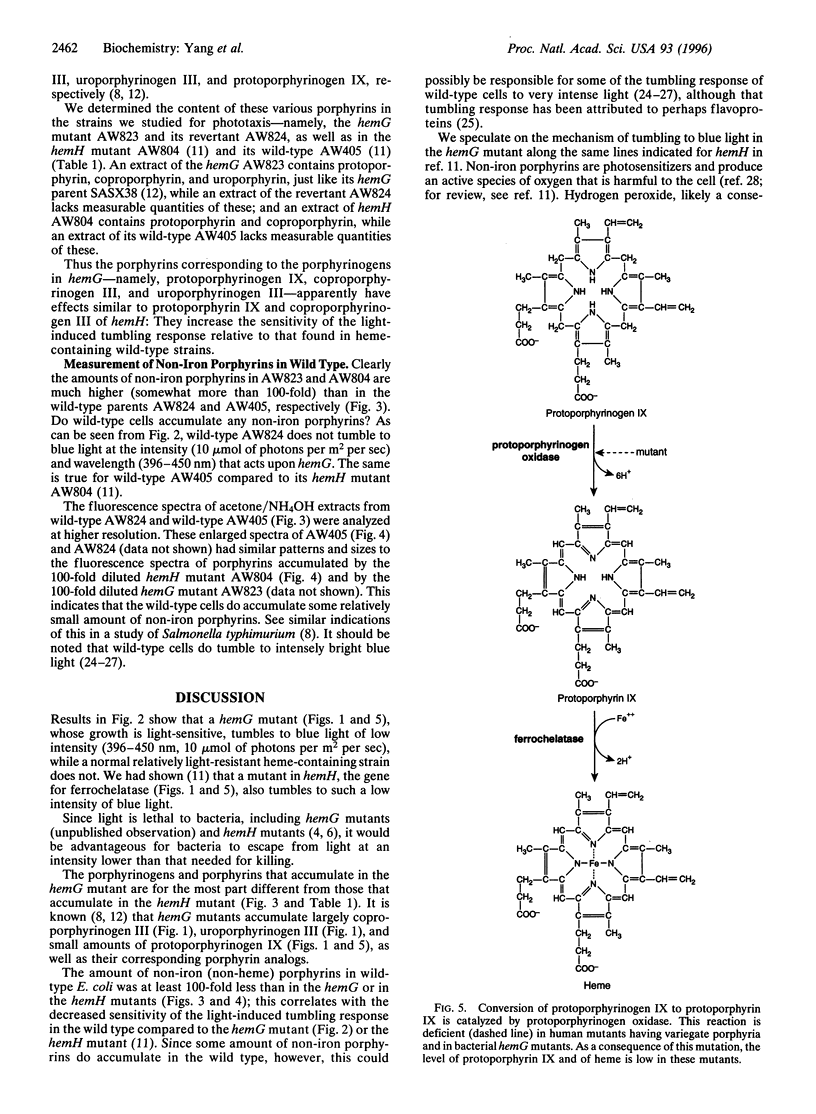
Abstract
Previously we showed that an Escherichia coli hemH mutant, defective in the ultimate step of heme synthesis, ferrochelatase, is somewhat better than 100-fold more sensitive than its wild-type parent in tumbling to blue light. Here we explore the effect of a hemG mutant, defective in the penultimate step, protoporphyrinogen oxidase. We found that a hemG mutant also is somewhat better than 100-fold more sensitive in tumbling to blue light compared to its wild-type parent. The amount of non-iron porphyrins accumulated in hemG or hemH mutants was more than 100-fold greater than in wild type. The nature of these accumulated porphyrins is described. When heme was present, as in the wild type, the non-iron (non-heme) porphyrins were maintained at a relatively low concentration and tumbling to blue light at an intensity effective for hemG or hemH did not occur. The function of tumbling to light is most likely to allow escape from the lethality of intense light.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong J. B., Adler J., Dahl M. M. Nonchemotactic mutants of Escherichia coli. J Bacteriol. 1967 Jan;93(1):390–398. doi: 10.1128/jb.93.1.390-398.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg H. C., Brown D. A. Chemotaxis in Escherichia coli analysed by three-dimensional tracking. Nature. 1972 Oct 27;239(5374):500–504. doi: 10.1038/239500a0. [DOI] [PubMed] [Google Scholar]
- Cox R., Charles H. P. Porphyrin-accumulating mutants of Escherichia coli. J Bacteriol. 1973 Jan;113(1):122–132. doi: 10.1128/jb.113.1.122-132.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frustaci J. M., O'Brian M. R. The Escherichia coli visA gene encodes ferrochelatase, the final enzyme of the heme biosynthetic pathway. J Bacteriol. 1993 Apr;175(7):2154–2156. doi: 10.1128/jb.175.7.2154-2156.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu J., Goldstein B. D., Harber L. C. Photoreactions associated with in vitro hemolysis in erythropoietic protoporphyria. Photochem Photobiol. 1971 Jan;13(1):67–77. doi: 10.1111/j.1751-1097.1971.tb06092.x. [DOI] [PubMed] [Google Scholar]
- Ikemi M., Murakami K., Hashimoto M., Murooka Y. Cloning and characterization of genes involved in the biosynthesis of delta-aminolevulinic acid in Escherichia coli. Gene. 1992 Nov 2;121(1):127–132. doi: 10.1016/0378-1119(92)90170-t. [DOI] [PubMed] [Google Scholar]
- Li C., Louise C. J., Shi W., Adler J. Adverse conditions which cause lack of flagella in Escherichia coli. J Bacteriol. 1993 Apr;175(8):2229–2235. doi: 10.1128/jb.175.8.2229-2235.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macnab R. M., Koshland D. E., Jr The gradient-sensing mechanism in bacterial chemotaxis. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2509–2512. doi: 10.1073/pnas.69.9.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macnab R., Koshland D. E., Jr Bacterial motility and chemotaxis: light-induced tumbling response and visualization of individual flagella. J Mol Biol. 1974 Apr 15;84(3):399–406. doi: 10.1016/0022-2836(74)90448-3. [DOI] [PubMed] [Google Scholar]
- Miyamoto K., Kanaya S., Morikawa K., Inokuchi H. Overproduction, purification, and characterization of ferrochelatase from Escherichia coli. J Biochem. 1994 Mar;115(3):545–551. doi: 10.1093/oxfordjournals.jbchem.a124373. [DOI] [PubMed] [Google Scholar]
- Miyamoto K., Nakahigashi K., Nishimura K., Inokuchi H. Isolation and characterization of visible light-sensitive mutants of Escherichia coli K12. J Mol Biol. 1991 Jun 5;219(3):393–398. doi: 10.1016/0022-2836(91)90180-e. [DOI] [PubMed] [Google Scholar]
- Miyamoto K., Nishimura K., Masuda T., Tsuji H., Inokuchi H. Accumulation of protoporphyrin IX in light-sensitive mutants of Escherichia coli. FEBS Lett. 1992 Oct 5;310(3):246–248. doi: 10.1016/0014-5793(92)81341-i. [DOI] [PubMed] [Google Scholar]
- Nakahigashi K., Nishimura K., Miyamoto K., Inokuchi H. Photosensitivity of a protoporphyrin-accumulating, light-sensitive mutant (visA) of Escherichia coli K-12. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10520–10524. doi: 10.1073/pnas.88.23.10520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayashiki T., Nishimura K., Inokuchi H. Cloning and sequencing of a previously unidentified gene that is involved in the biosynthesis of heme in Escherichia coli. Gene. 1995 Feb 3;153(1):67–70. doi: 10.1016/0378-1119(94)00805-3. [DOI] [PubMed] [Google Scholar]
- Nishimura K., Nakayashiki T., Inokuchi H. Cloning and identification of the hemG gene encoding protoporphyrinogen oxidase (PPO) of Escherichia coli K-12. DNA Res. 1995;2(1):1–8. doi: 10.1093/dnares/2.1.1. [DOI] [PubMed] [Google Scholar]
- Nishimura K., Nakayashiki T., Inokuchi H. Cloning and sequencing of the hemE gene encoding uroporphyrinogen III decarboxylase (UPD) from Escherichia coli K-12. Gene. 1993 Oct 29;133(1):109–113. doi: 10.1016/0378-1119(93)90233-s. [DOI] [PubMed] [Google Scholar]
- Nishimura K., Taketani S., Inokuchi H. Cloning of a human cDNA for protoporphyrinogen oxidase by complementation in vivo of a hemG mutant of Escherichia coli. J Biol Chem. 1995 Apr 7;270(14):8076–8080. doi: 10.1074/jbc.270.14.8076. [DOI] [PubMed] [Google Scholar]
- Sager B. M., Sekelsky J. J., Matsumura P., Adler J. Use of a computer to assay motility in bacteria. Anal Biochem. 1988 Sep;173(2):271–277. doi: 10.1016/0003-2697(88)90189-3. [DOI] [PubMed] [Google Scholar]
- Sasarman A., Letowski J., Czaika G., Ramirez V., Nead M. A., Jacobs J. M., Morais R. Nucleotide sequence of the hemG gene involved in the protoporphyrinogen oxidase activity of Escherichia coli K12. Can J Microbiol. 1993 Dec;39(12):1155–1161. doi: 10.1139/m93-174. [DOI] [PubMed] [Google Scholar]
- Singer M., Baker T. A., Schnitzler G., Deischel S. M., Goel M., Dove W., Jaacks K. J., Grossman A. D., Erickson J. W., Gross C. A. A collection of strains containing genetically linked alternating antibiotic resistance elements for genetic mapping of Escherichia coli. Microbiol Rev. 1989 Mar;53(1):1–24. doi: 10.1128/mr.53.1.1-24.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundberg S. A., Alam M., Spudich J. L. Excitation signal processing times in Halobacterium halobium phototaxis. Biophys J. 1986 Nov;50(5):895–900. doi: 10.1016/S0006-3495(86)83530-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Săsărman A., Chartrand P., Lavoie M., Tardif D., Proschek R., Lapointe C. Mapping of a new hem gene in Escherichia coli K12. J Gen Microbiol. 1979 Aug;113(2):297–303. doi: 10.1099/00221287-113-2-297. [DOI] [PubMed] [Google Scholar]
- Taylor B. L., Koshland D. E., Jr Intrinsic and extrinsic light responses of Salmonella typhimurium and Escherichia coli. J Bacteriol. 1975 Aug;123(2):557–569. doi: 10.1128/jb.123.2.557-569.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor B. L., Koshland D. E., Jr Perturbation of the chemotactic tumbling of bacteria. J Supramol Struct. 1976;4(3):343–353. doi: 10.1002/jss.400040305. [DOI] [PubMed] [Google Scholar]
- Taylor B. L., Miller J. B., Warrick H. M., Koshland D. E., Jr Electron acceptor taxis and blue light effect on bacterial chemotaxis. J Bacteriol. 1979 Nov;140(2):567–573. doi: 10.1128/jb.140.2.567-573.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Xu K., Delling J., Elliott T. The genes required for heme synthesis in Salmonella typhimurium include those encoding alternative functions for aerobic and anaerobic coproporphyrinogen oxidation. J Bacteriol. 1992 Jun;174(12):3953–3963. doi: 10.1128/jb.174.12.3953-3963.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H., Inokuchi H., Adler J. Phototaxis away from blue light by an Escherichia coli mutant accumulating protoporphyrin IX. Proc Natl Acad Sci U S A. 1995 Aug 1;92(16):7332–7336. doi: 10.1073/pnas.92.16.7332. [DOI] [PMC free article] [PubMed] [Google Scholar]


