Abstract
Fetal and perinatal exposure to selective serotonin (5-HT) reuptake inhibitors (SSRIs) has been reported to alter childhood behavior, while transient early exposure in rodents is reported to alter their behavior and decrease brain extracellular 5-HT in adulthood. Since 5-HT2A/2C receptor-mediated neurotransmission can involve G-protein coupled activation of cytosolic phospholipase A2 (cPLA2), releasing arachidonic acid (ARA) from synaptic membrane phospholipid, we hypothesized that transient postnatal exposure to fluoxetine would decrease brain ARA metabolism in adult mice. Brain ARA incorporation coefficients k* and rates Jin were quantitatively imaged following intravenous [1-14C]ARA infusion of unanesthetized adult mice that had been injected daily with fluoxetine (10 mg/kg i.p.) or saline during postnatal days P4–P21. Expression of brain ARA metabolic enzymes and other relevant markers also was measured. On neuroimaging, k* and Jin was decreased widely in early fluoxetine- compared to saline-treated adult mice. Of the enzymes measured, cPLA2 activity was unchanged, while Ca2+-independent iPLA2 activity was increased. There was a significant 74% reduced protein level of cytochrome P450 (CYP) 4A, which can convert ARA to 20-HETE. Reduced brain ARA metabolism in adult mice transiently exposed to postnatal fluoxetine, and a 74% reduction in CYP4A protein, suggest long-term effects independent of drug presence in brain ARA metabolism, and in CYP4A metabolites. Comparable changes in humans might contribute to reported altered behavior following early SSRI.
Keywords: fluoxetine, arachidonic acid, mouse, neurodevelopment, postnatal, neuroimaging, serotonin, SSRI, Cytochrome P450 4A, metabolism, brain
Introduction
Selective serotonin (5-HT) reuptake inhibitors (SSRIs) such as fluoxetine are approved for treating depression, anxiety and personality disorders in women during pregnancy and lactation, and in children and adolescents. Their use during pregnancy has been associated with premature birth, neonatal cardiovascular abnormalities, pulmonary hypertension, and disturbed behavior, and increased risk for autism spectrum disorder in offspring [1, 2]. In addition, infants may be at risk because of exposure to SSRIs via breast milk [3]. SSRIs also were reported to increase suicidality in pediatric patients [4], but a recent review called this into question [5]. In contrast, several studies suggest that antidepressant use during pregnancy has no major long-term effects on neurodevelopment and behavior in the offspring [6, 7], thus the issue remains controversial.
Nevertheless, adult rodents that were exposed transiently (P1 to P21) to an SSRI show decreased brain extracellular 5-HT [8], increased density of the presynaptic 5-HT reuptake transporter (5-HTT) [9, 10], and structurally abnormal serotonergic neurons [11] and dendritic spines [12]. The early-exposed adult rodents also demonstrate depressive-like behaviors [13–16] and, less consistently, anxiety-like behaviors [13, 17]. The P1 to P21 postnatal period in rodents coincides with a brain growth spurt, rapid dendritic and axonal outgrowth, synaptogenesis and myelination, and peak establishment of neural connections and susceptibility to xenobiotics [18–20]. It corresponds to the period of maturation of the human brain monoaminergic system, which begins during the third trimester of pregnancy and continues through the first 2–3 years of life [13, 21].
Changes in behavior and brain integrity in adult rodents following transient SSRI exposure may be related to disturbed arachidonic acid (ARA, 20:4 n-6) neurotransmission and metabolism, since ARA is released from synaptic membrane phospholipid during neurotransmission involving 5-HT2A/2C receptors [22–25]. As a second messenger, ARA can modify multiple aspects of brain function and structure, and it is a precursor of a large number of bioactive eicosanoid products within the brain ARA metabolic cascade [26, 27].
We have developed a method to measure brain ARA signaling in unanesthetized rodents, which involves infusing radiolabeled ARA intravenously and using quantitative autoradiography to quantify regional brain radioactivity, representing tracer ARA incorporation into synaptic membrane phospholipid [25, 28]. A mathematical model is used to calculate ARA incorporation coefficients and rates, k* and Jin, respectively [26, 29]. Since the brain ARA lost by metabolism cannot be synthesized de novo from 2-carbon fragments, or elongated significantly (< 1%) from its shorter chain polyunsaturated precursor within brain, linoleic acid (18:2n-6) [30], Jin stoichiometrically equals ARA metabolic loss [31].
In the present study, we used our in vivo infusion method to determine whether transient postnatal exposure of rats to fluoxetine would alter brain k* and Jin for ARA during adulthood. We thought changes in these parameters might occur because of the changes noted above in serotonin synaptic markers in the adult brain of perinatally exposed rodents. We also measured expression of other ARA metabolic markers, including phospholipase A2 (PLA2) enzymes involved in neurotransmission, downstream ARA oxidizing enzymes, and 5-HTT. Part of this work has been published in abstract form [32].
Methods
Animals
Experiments were conducted following the Guide for the Care and Use of Laboratory Animals (National Institute of Health Publication 86–23), under a protocol approved by the Animal Care and Use Committee of the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Untimed pregnant C57BL/6 mice (Charles River Laboratories International, Wilmington, MA, USA) were housed until they gave birth. Postnatal drug or saline was administered as reported [13]. Briefly, on postnatal day 4 (P4), male pups were assigned randomly to a saline (bacteriostatic 0.9% NaCl injection, USP, Hospira, Lake Forest, IL, USA; i.p.) group, to a fluoxetine hydrochloride (10.0 mg/kg, i.p., dissolved in saline; Sigma-Aldrich, St. Louis, MO, USA) treatment group, given at a therapeutically equivalent human dose [13]. Mice were injected once per day, using a sterile insulin syringe (29G × ½, 0.3 ml), from P4 to P21. This regimen produces therapeutically relevant plasma concentrations of fluoxetine (360 ± 123 ng/ml) and its metabolite norfluoxetine (708 ± 168 ng/ml) at P22 [13].
Lactating dams and pups had free access to water and food (Rodent NIH-07, Ziegler Bros, Gardens, PA, USA). This diet contains soybean, alfalfa, and fishmeal, and has 5% crude fat by weight. By gas-liquid chromatography, the chow contains (as % of total fatty acids) 30.6% saturated, 33.5% monounsaturated, 47.1% linoleic, 4.9% α-linolenic, 0.27% ARA, 1.68% eicosapentaenoic, and 2.2% docosahexaenoic acid (DHA, 22:6n-3) [33]. At weaning (P28), dams were removed, and the pups were sexed and housed in separate cages (3 to 5 mice per cage) until adulthood (P90–P120).
Surgical Procedures and Tracer Infusion
Between P90 and P120, male mice that had been treated postnatally with saline or fluoxetine were anesthetized with isoflurane (2–3% v/v in O2), and polyethylene PE 10 catheters were implanted into the right femoral artery and vein [33]. A mouse was allowed to recover from anesthesia for 3 h with its hindquarters loosely wrapped and taped to a wooden block in a sound-dampened, temperature-controlled chamber. During recovery, body temperature was maintained at 37°C with a rectal probe and a heating element (TACT-2DF Temperature controller, Physitemp Instruments, Clifton, NJ, USA). Then [1-14C]ARA (45 μl, 300 μCi/ml 53 mCi/mmol; 99.4% pure, Moravek Biochemicals, Brea, CA) in 5 mM HEPES buffer (pH 7.4), containing 50 mg/ml fatty acid-free bovine serum albumin, was infused through the femoral vein (3 min, 15 μl/min) using a Hamilton syringe and an infusion pump (Harvard Apparatus Model 22, Holliston, MA, USA). Ten arterial blood samples (15–20 μl) were collected at fixed times during infusion (0, 0.25, 1.0, 1.5, 2.0, 2.8, 3.2, 5.0, 10, and 19 min). At 20 min, the mouse was euthanized by an overdose of Nembutal (50 mg/kg, i.v.). Cardiac puncture was performed to rapidly collect 0.5 ml blood, and the mouse was decapitated. The brain was removed, quickly frozen in 2-methylbutane at - 40°C, then stored at - 80°C for later sectioning. Radiotracer purity exceeded 99% by thin layer chromatography and gas-liquid chromatography, and was measured after converting labeled ARA to its methyl ester with 1% sulfuric acid in anhydrous methanol.
Chemical Analysis
Arterial blood samples (20 μl) were collected before, during and after [1-14C]ARA infusion and were centrifuged (18,000 g for 30 s). Total lipids were extracted from 5 μl plasma with chloroform:methanol (1 ml, 2:1, v/v) and KCl (0.5 ml, 0.1M), using a modified Folch procedure [34]. The lower organic phase (100 μl) of the extract was used to measure the labeled unesterified ARA concentration by scintillation spectrometry (Model 2200CA, Packard Instruments, Downers Grove, IL, USA). As reported, 95–98% of total plasma and brain radioactivity at 5 min was radiolabeled ARA [35].
Concentrations of unesterified unlabeled fatty acids were determined in the arterial plasma (100–150 μl) collected by cardiac puncture. Total lipids were extracted [34] and separated by thin layer chromatography on 60 silica gel plates (Whatman, Clifton, NJ, USA), using the solvent system heptane: diethylether: glacial acetic acid (60:40:3, v/v/v). Unesterified fatty acids were scraped from the plate and methylated with 1% H2SO4 in anhydrous methanol (3 h at 70°C), then separated by gas-liquid chromatography and quantified using an internal standard, heptadecanoic acid (17:0).
Quantitative Autoradiography and Calculations
Twenty-mm thick sections were cut from the left hemisphere and then placed with [14C]methylmethacrylate standards (Amersham, Arlington Heights, IL, USA) on Ektascan C/RA film (Eastman Kodak, Rochester, NY, USA) for 4 weeks. Radioactivity (nCi/g brain wet weight) in 92 identified regions was measured bilaterally six times by quantitative densitometry (NIH Image program 1.62). Regional incorporation coefficients of unesterified plasma ARA into brain phospholipids, k* (ml/s/g brain), were calculated as [29]:
| Eq. 1 |
(20) (nCi/g brain wet wt.) is brain radioactivity 20 min after beginning infusion, (nCi/ml plasma) is labeled plasma unesterified ARA, and t (min) is time after beginning infusion. Integrated plasma radioactivity (input function) was determined by trapezoidal integration and used to calculate k* for each experiment. Regional rates of incorporation of unesterified ARA from plasma into brain phospholipid, Jin (nmol/s/g), were calculated as:
| (Eq. 2) |
where cplasma is the plasma concentration (nmol/ml) of unlabeled unesterified ARA. These rates stoichiometrically equal regions rates of ARA metabolic loss [26, 36].
Preparation of cytosolic and membrane fractions from whole brains
In separate experiments, non-infused mice were anesthetized with Nembutal (50 mg/kg, i.p.) and decapitated. Brains (n = 10 saline, n = 12 fluoxetine) were removed quickly and frozen in 2-methylbutane at −40°C on dry ice, then stored at −80°C. Half-brains were homogenized using a Tenbroeck tissue grinder on ice (Kontes Glass, Vineland, NJ, USA) in 2 vol of cold buffer containing 10 mM HEPES, pH 7.5, 1 mM EDTA, 0.34 M sucrose and protease inhibitor cocktail tablet (Complete, Roche, Mannheim, Germany).
Homogenates were centrifuged at 14,000 g for 20 min, then at 100,000 g for 1 h at 4°C (Beckman L8-M Ultracentrifuge, Fullerton, CA USA). The supernatant corresponded to the cytosolic fraction. The pellet then was resuspended in 0.2 M Tris, pH 7.4 containing 0.2% Triton X-100 and protease inhibitors (Sigma-Aldrich), incubated on ice for 1 h with occasional stirring, and finally centrifuged at 100,000 g for 1 h at 4°C. The soluble supernatant was used as the membrane fraction. Protein concentrations of both fractions were determined by the Bradford method [37].
Whole brain PLA2 activities
Cytosolic fractions were assayed for Ca2+-dependent ARA selective cytosolic cPLA2 and Ca2+-independent docosahexaenoic acid (DHA) selective iPLA2 activities, as described [38]. Activity of secretory sPLA2 was measured using a sPLA2 assay kit (Cayman, Ann Arbor, MI, USA).
Western blotting
Proteins (25 μg) from membrane and cytosolic fractions were separated using 4–20% sodium dodecyl sulfate polyacrylamide gel (Bio-Rad, Hercules, CA, USA) and transferred to a nitrocellulose membrane. After blocking (Blocking buffer, Sigma-Aldrich), blots were incubated overnight at 4°C with primary antibody diluted in blocking buffer [anti-iPLA2-VIA (1:500), anti-cyclooxygenase (COX)-2 (1:200), anti-5-lipoxygenase (LOX) (1:500), anti-12-LOX (1:500), anti-cytochrome P 450 epoxygenase (CYP)2C9 (1:1000) and anti-5-HTT antibodies (1:500) (Abcam, Cambridge, MA, USA), anti-COX-1 (1:200), anti-15-LOX (1:200), anti-CYP4A (1:200) (Santa-Cruz, CA, USA) and anti-membrane prostaglandin E2 synthase (mPGES) (1:500) (Cayman, Ann Arbor, MI, USA)]. After washing, blots were incubated (90 min, room temperature) with the appropriate horseradish peroxidase-conjugated secondary antibodies (Bio-Rad) and peroxidase activity was determined by chemiluminescence using the SuperSignal West Pico reagents (Thermo Fisher Scientific, Rockford, IL, USA). The relative protein expression was determined after normalization with the β-actin protein (Sigma-Aldrich) using the ImageJ software, and expressed as per cent of the saline-treated group (control). Due to limited success with survival, n = 9 for the membrane fraction from saline mice, and n = 8 for the saline group in iPLA2, COX-2 and cytosolic 5-HTT expression assays.
Statistical analysis
Data are reported as mean ± SD or SEM. An unpaired two-tailed t-test was used to compare means between fluoxetine- and saline-treated mice, with statistical significance tested at p ≤ *0.05, **0.02 or ***0.01. For k* and Jin, corrections for multiple comparisons across regions were not made because this study is exploratory and its goal was to screen for patterns of regional changes in brain ARA incorporation [39, 40]. Adjustments for multiple comparisons with large bodies of observation, as found in neuroimaging of multiple regions, leads to a too ready rejection of the null hypothesis [41].
Results
Body weight and arterial plasma input function
Fluoxetine injection from P4 to P21 significantly reduced body weight. At P12, fluoxetine-treated mice weighed 11% less than saline-treated mice (5.90 ± 0.50 g, n = 11 vs. 6.60 ± 0.50 g, n = 7; p = 0.012), and the decrease was maintained until P21 (p = 0.04). Body weight also was reduced at P90–P120 (−13%; p = 0.003).
Fluoxetine treatment did not affect steady-state plasma radioactivity during the 3-min [1-14C]ARA infusion in the adult mice. Integrated plasma radioactivity did not differ between fluoxetine- and saline-treated mice (153,587 ± 20,044 (n = 11) vs. 144,630 ± 15,953 (n = 7) nCi × s/ml ± SD).
Plasma concentrations of unlabeled unesterified fatty acids
There was no statistically significant difference in the plasma concentration of any of eight measured unlabeled unesterified fatty acids between fluoxetine- and saline-treated mice (data not shown), including ARA (4.82 ± 1.67 (n = 11 fluoxetine) vs. 5.77 ± 2.07 (n = 7 saline) nmol/ml plasma).
Regional brain ARA incorporation coefficients, k*
Figure 1 presents color-coded autoradiographs of coronal brain slices from saline- and fluoxetine-treated mice, illustrating regional incorporation coefficients k* for ARA, obtained using Eq. 1. Compared to saline, fluoxetine-treated mice showed widespread decreases in k*. Statistically significant 17% to 29% (average 21 ± 3%) reductions were found in 24 of 92 regions (33%), 7 regions at p < 0.01, 8 regions at p < 0.02, and 9 regions at p < 0.05 (Table 1). Affected were gray matter regions of the neocortex, diencephalon, mesencephalon and rhombencephalon (Table 1). No significant change was noted in white matter, while k* in the choroid plexus was significantly reduced by 23%. Chronic fluoxetine did not elevate k* significantly in any brain region.
Fig. 1.
Autoradiographs showing ARA incorporation coefficients k* in coronal brain sections of saline- and fluoxetine-treated mice. Values of k* are color-coded. Abbreviations: ACg, anterior cingulate cortex; Aud, auditory cortex; CPu, caudate putamen; Hipp, hippocampus; IPC, interpeduncular nucleus; Mot, motor cortex; SN, substantia nigra; Thal, thalamus; Vis, visual cortex.
Table 1.
Postnatal fluoxetine exposure decreases baseline arachidonic acid incorporation coefficients k* in adult mice.
| Brain Region | Saline (n = 7) | Fluoxetine (n = 12) |
|---|---|---|
| k*, (ml/s/g) × 10−4 | ||
| Telencephalon | ||
| Prefrontal cortex layer I | 7.85 ± 1.13 | 7.44 ± 2.05 |
| Prefrontal cortex layer IV | 8.72 ± 1.12 | 7.84 ± 2.17 |
| Primary olfactory cortex | 8.18 ± 0.99 | 7.21 ± 2.21 |
| Frontal cortex (10) | ||
| Layer I | 8.40 ± 1.37 | 7.30 ± 2.13 |
| Layer IV | 9.27 ± 1.50 | 8.08 ± 2.51 |
| Frontal cortex (8) | ||
| Layer I | 9.03 ± 1.23 | 7.50 ± 2.22 |
| Layer IV | 9.52 ± 1.37 | 8.12 ± 2.32 |
| Pyriform cortex | 7.93 ± 1.05 | 6.41 ± 1.67* |
| Anterior cingulate cortex | 11.48 ± 1.33 | 9.30 ± 1.87** |
| Motor cortex | ||
| Layer I | 8.22 ± 1.36 | 7.17 ± 1.56 |
| Layer II – III | 9.38 ± 1.10 | 7.84 ± 1.97 |
| Layer IV | 11.07 ± 0.70 | 9.10 ± 2.45 |
| Layer V | 10.93 ± 1.04 | 8.80 ± 2.19 |
| Layer VI | 10.22 ± 1.14 | 8.11 ± 1.79** |
| Somatosensory cortex | ||
| Layer I | 8.80 ± 1.49 | 7.30 ± 1.85 |
| Layer II–III | 9.96 ± 1.32 | 8.13 ± 1.97 |
| Layer IV | 11.55 ± 0.95 | 9.72 ± 2.35 |
| Layer V | 11.15 ± 1.06 | 8.88 ± 1.91** |
| Layer VI | 10.29 ± 1.07 | 8.30 ± 1.70** |
| Auditory cortex | ||
| Layer I | 8.14 ± 1.34 | 7.02 ± 2.13 |
| Layer IV | 9.37 ± 1.67 | 8.01 ± 2.41 |
| Layer VI | 8.88 ± 1.64 | 7.03 ± 1.90 |
| Visual cortex | ||
| Layer I | 8.29 ± 1.60 | 6.87 ± 1.94 |
| Layer IV | 9.84 ± 1.28 | 7.75 ± 2.19 |
| Layer VI | 8.93 ± 1.19 | 7.08 ± 2.13 |
| Preoptic area (LPO/MPO) | 8.52 ± 1.04 | 6.74 ± 1.49** |
| Suprachiasmatic nu | 9.37 ± 2.08 | 6.65 ± 1.69*** |
| Globus pallidus | 8.89 ± 0.84 | 7.96 ± 2.09 |
| Bed nu stria terminalis | 8.28 ± 1.04 | 6.65 ± 1.56*** |
| Olfactory tubercle | 7.79 ± 1.45 | 6.99 ± 1.90 |
| Diagonal band Dorsal | 9.70 ± 1.32 | 7.37 ± 1.85*** |
| Ventral | 9.24 ± 0.85 | 7.01 ± 1.88*** |
| Amygdala basolat/med | 6.78 ± 1.69 | 6.16 ± 1.71 |
| Hippocampus CA1 | 6.70 ± 1.73 | 6.19 ± 2.09 |
| CA2 | 7.30 ± 1.75 | 6.59 ± 2.57 |
| CA3 | 7.67 ± 1.63 | 7.01 ± 2.35 |
| Dentate gyrus | 8.55 ± 1.81 | 7.56 ± 2.54 |
| SLM | 11.17 ± 2.63 | 9.61 ± 3.21 |
| Accumbens nucleus | 8.35 ± 1.08 | 7.26 ± 2.08 |
| Caudate putamen | ||
| Dorsal | 9.23 ± 0.77 | 7.27 ± 1.86** |
| Ventral | 9.21 ± 0.99 | 7.46 ± 1.96* |
| Lateral | 9.36 ± 0.80 | 7.66 ± 1.99* |
| Medial | 9.21 ± 0.80 | 7.18 ± 1.76** |
| Septal nucleus lateral | 7.96 ± 0.65 | 6.40 ± 1.57* |
| medial | 9.26 ± 0.95 | 7.54 ± 1.90* |
| Diencephalon | ||
| Habenular nu lateral | 14.35 ± 2.94 | 12.66 ± 4.18 |
| Habenular nu medial | 13.54 ± 2.97 | 12.19 ± 4.45 |
| Lateral geniculate nu dorsal | 12.08 ± 1.78 | 9.38 ± 3.31 |
| Medial geniculate nu | 13.21 ± 2.05 | 9.97 ± 2.66 |
| Thalamus | ||
| Ventroposterior lateral nu | 12.21 ± 2.16 | 9.40 ± 3.44 |
| Ventroposterior medial nu | 11.84 ± 2.13 | 9.08 ± 2.96* |
| Paratenial nu | 11.94 ± 1.05 | 9.92 ± 2.65 |
| Anteroventral nu | 15.26 ± 1.19 | 14.06 ± 3.96 |
| Anteromedial nu | 13.13 ± 1.12 | 10.57 ± 3.02 |
| Reticular nu | 12.91 ± 1.00 | 10.65 ± 2.89 |
| Paraventricular nu | 11.37 ± 1.59 | 9.39 ± 2.52 |
| Parafascicular nu | 11.58 ± 2.15 | 8.82 ± 3.39 |
| Subthalamic nucleus | 11.19 ± 2.00 | 9.42 ± 2.96 |
| Hypothalamus | ||
| Supraoptic nu | 8.54 ± 0.99 | 7.51 ± 1.35 |
| Lateral | 7.99 ± 1.38 | 7.10 ± 1.53 |
| Anterior | 7.85 ± 1.25 | 6.99 ± 1.54 |
| Periventricular | 7.85 ± 1.29 | 7.15 ± 1.40 |
| Arcuate | 8.23 ± 1.75 | 7.22 ± 1.39 |
| Ventromedial | 8.26 ± 1.86 | 7.36 ± 2.08 |
| Posterior | 8.38 ± 2.02 | 6.79 ± 1.64 |
| Mammillary nucleus | 7.54 ± 1.85 | 6.79 ± 1.64 |
| Mesencephalon | ||
| Interpeduncular nucleus | 16.45 ± 2.64 | 13.44 ± 3.59 |
| Substantia nigra | 9.45 ± 0.97 | 7.63 ± 2.24 |
| Pretectal area | 11.51 ± 1.79 | 8.97 ± 2.20* |
| Superior colliculus | 11.23 ± 2.57 | 9.07 ± 1.86 |
| Deep layers | 10.47 ± 1.37 | 8.81 ± 1.79 |
| Inferior colliculus | 19.52 ± 3.54 | 14.52 ± 2.80*** |
| Median Raphe nu | 10.94 ± 3.91 | 9.31 ± 1.44 |
| Dorsal Raphe nu | 10.06 ± 2.47 | 9.24 ± 1.67 |
| Pedunculopontine tegmental nu | 10.64 ± 2.07 | 8.04 ± 1.45*** |
| Rhombencephalon | ||
| Flocculus | 12.89 ± 1.19 | 12.48 ± 2.70 |
| Cerebellar gray matter | 11.73 ± 2.13 | 10.76 ± 2.24 |
| Molecular layer cerebellar gray matter | 12.49 ± 1.99 | 12.17 ± 1.90 |
| Raphe magnus nu | 11.26 ± 3.15 | 8.77 ± 1.37* |
| Raphe pallidus nu | 11.26 ± 2.52 | 8.74 ± 1.55** |
| Locus coeruleus | 12.93 ± 2.84 | 10.96 ± 2.41 |
| Cochlear nu | 13.20 ± 3.97 | 12.05 ± 2.15 |
| Spinal trigeminal nu | 10.31 ± 2.34 | 10.67 ± 1.63 |
| Superior olive | 11.27 ± 2.71 | 9.41 ± 1.57 |
| Medial vestibular nu | 19.29 ± 2.14 | 15.98 ± 2.98* |
| White matter | ||
| Corpus callosum | 6.89 ± 1.13 | 5.48 ± 1.59 |
| Internal Capsule | 5.88 ± 1.44 | 6.15 ± 1.48 |
| Cerebellar white matter | 7.35 ± 0.85 | 6.92 ± 1.34 |
| Non-blood-brain barrier regions | ||
| Subfornical organ | 8.58 ± 0.86 | 7.53 ± 1.53 |
| Median eminence | 7.34 ± 1.84 | 7.28 ± 2.07 |
| Choroid plexus | 25.48 ± 3.08 | 19.62 ± 4.34*** |
Values are mean ± S.D.
Abbreviations: nu, nucleus; lat, lateral; med, medial; SLM, stratum lacunosum-molecule of hippocampus.
Unpaired t-test with P < 0.05*, 0.02**. 0.01***
Regional ARA incorporation rates Jin
Baseline incorporation rates Jin of unlabeled unesterified ARA from plasma into brain were calculated by multiplying individual regional values of k* by the unlabeled unesterified plasma ARA concentration in each experiment (Eq. 2) (Supplementary Table 1). Seventy-eight of the 92 brain regions (85%) showed a statistically significant decreased Jin following fluoxetine compared with saline treatment (p < 0.05), thus much of the brain (Supplementary Figure 1). In saline-treated mice, Jin ranged from 37.7 × 10−4 nmol/s/g in the internal capsule to 161.7 × 10−4 nmol/s/g in the choroid plexus. In fluoxetine-treated mice, Jin ranged from 29.6 × 10−4 nmol/s/g in the internal capsule to 94.5 × 10−4 nmol/s/g in the choroid plexus.
Whole brain enzyme activities and protein levels
Whole brain cytosolic activities of cPLA2 and sPLA2, each of which can release ARA from phospholipid [42], did not differ significantly between fluoxetine- and saline-treated mice (Table 2). DHA-preferring Ca2+-independent iPLA2 [43, 44] activity was significantly increased by 32% (p = 0.004) in fluoxetine-treated mice, but mean iPLA2-VIA protein was unaltered (Table 2). There was no significant change in cytosolic protein levels of COX-1, COX-2, 5-LOX, 12-LOX, 15-LOX, and CYP2C9 or mPGES between treatment groups (Table 3). However, CYP4 A1, A2 and A3 protein levels were reduced by 74% after early fluoxetine treatment (p = 0.004) (Table 2). We quantified these three isoforms together as their molecular weights were too close to be distinguished.
Table 2.
Effects of postnatal fluoxetine exposure on whole-brain enzyme activities and protein levels during adulthood
| Saline (n = 10) | Fluoxetine (n = 12) | |
|---|---|---|
| Activity | ||
| cPLA2 (pmol/min/mg protein) | 5.42 ± 0.41 | 5.70 ± 0.28 |
| sPLA2 (nmol/min/mg protein) | 104.4 ± 7.9 (n = 9) | 123.8 ± 11.6 |
| iPLA2 (pmol/min/mg protein) | 47.1 ± 4.5 (n = 9) | 62.0 ± 2.1**(+31%) |
| Protein (%, fluoxetine/saline) | ||
| iPLA2-VIA | 100.0 ± 15.6 (n = 8) | 108.3 ± 14.4 |
| COX-1 (69 kDa) | 100.0 ± 12.7 | 105.6 ± 17.6 |
| COX-2 (69 kDa) | 100.0 ± 15.9 (n = 8) | 102.5 ± 12.3 |
| 5-LOX (78 kDa) | 100.0 ± 13.5 | 104.5 ± 9.2 |
| 12-LOX (75 kDa) | 100.0 ± 12.3 | 101.9 ± 6.9 |
| 15-LOX (79 kDa) | 100.0 ± 8.6 | 93.7 ± 6.9 |
| mPGES (33 kDa) | 100.0 ± 10.7 (n = 9) | 105.45 ± 6.9 |
| CYP2C9 (59 kDa) | 100.0 ± 8.1 | 79.9 ± 7.1 |
| CYP4A (50-52-54 kDa) | 100.0 ± 20.0 | 25.8 ± 13.3**(−74%) |
Values are mean ± SEM.
P < 0.01, unpaired t-test
Whole brain 5-HTT protein level
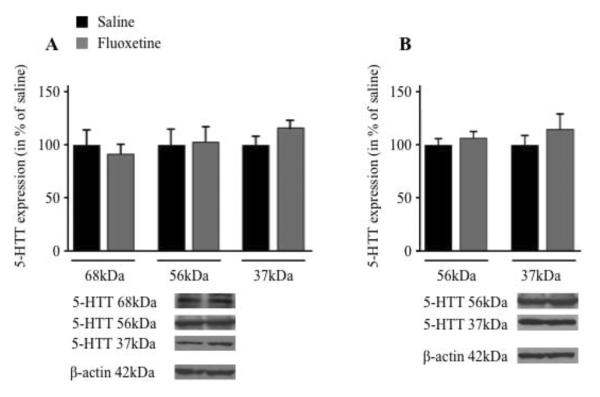
By varying exposure time in Western blotting, three isoforms of 5-HTT were detected in the brain membrane fraction, with molecular weights of 37 kDa, 56 kDa and 68 kDa (Figure 2A), but only the 37 kDa and 56 kDa isoforms were observed in the cytosol (Figure 2B). This expression profile is consistent with a study reporting cleavages of the native 56 kDa 5-HTT protein in rat brain [45]. Postnatal fluoxetine had no significant effect on 5-HTT isoform protein levels in membrane or cytosolic fractions of whole brain.
Fig. 2.
5-HTT protein isoforms in brain membranes and cytosol of fluoxetine and saline treated rats. Proteins were measured in brain membrane (A) or cytosolic (B) fractions by Western blotting (representative immunoblots are displayed, with adjusted exposure time for each isoform). 37 kDa, 56 kDa and 68 kDa isoforms were detected in the membrane fraction and 37 kDa and 56 kDA isoforms were detected in the cytosol. After normalization to β-actin protein, results are expressed as % of the control values (means ± SEM, n = 8–9 for saline and n = 12 for fluoxetine).
Discussion
Transient postnatal fluoxetine, administered daily from P4 to P21, significantly decreased baseline brain ARA incorporation coefficients k* in 24 of 92 measured regions in adult male mice (P90 to P120), as well as ARA incorporation rates, Jin, in 78 regions. Postnatal fluoxetine also significantly increased brain iPLA2 activity and decreased CYP4A protein, but did not change protein levels of other measured markers (iPLA2-VIA, COX-2, 5-LOX, 12-LOX, CYP2C9, COX-1, 15-LOX, or mPGES) or of 5-HTT. Body weight was reduced 13% by postnatal fluoxetine, similar to prior reports [11, 17, 46].
Significantly reduced ARA incorporation coefficients and rates following transient postnatal fluoxetine contrast with increased ARA incorporation in adult 5-HTT knockout mice, and in adult rats treated chronically with fluoxetine, in each case of which brain cPLA2 activity and extracellular 5-HT concentration are increased [33, 35, 47–50]. However, our finding reduced ARA incorporation coefficients and rates in postnatally fluoxetine exposed adult rats corresponds to evidence that extracellular 5-HT is reduced and that cortical 5-HTT is increased in adult mice exposed postnatally to an SSRI [8–10], although we did not find a change in whole brain 5-HTT in the present study. Thus, in each of the three conditions, fluoxetine treatment of adult rats, 5-HTT knockout adult mice, and transient postnatal fluoxetine exposure of rats measured during adulthood (present study), the direction of changes in baseline ARA incorporation corresponds to the direction of change of extracellular 5-HT, suggesting that the baseline ARA signal in each of these three conditions reflects extracellular 5-HT.
Depressed serotonergic transmission appears to be a risk factor for clinical depression. Thus, lowering brain 5-HT level by tryptophan depletion can induce depressive symptoms, and major depression can be ameliorated with SSRIs [51]. In agreement, transient postnatal fluoxetine in rats, which reduces ARA incorporation (this study) and extracellular 5-HT in adulthood, also increases depressive-like behaviors in adulthood [52–54]. The 5-HTT knockout mouse, having elevated extracellular 5-HT [50] and increased ARA metabolism [33] shows mainly anxiety-like behaviors [55], while tests of depression have yielded less consistent results [56, 57].
Decreased baseline brain ARA incorporation coefficients and rates following postnatal fluoxetine in the present study were not associated with reduced activity cPLA2 or of sPLA2, both of which hydrolyze ARA from membrane phospholipid [42]. Activity of iPLA2, which preferentially hydrolyzes DHA from membrane phospholipid [43, 44, 58], was elevated, but iPLA2 VIA protein was not significantly different from control, suggesting post-translation upregulation. Following dietary PUFA manipulation, the change of iPLA2 expression is opposite in direction to changes of cPLA2 and sPLA2 expression [59, 60].
As noted in the Introduction, Jin for ARA stoichiometrically equals the rate of ARA metabolic loss from brain, since ARA cannot be synthesized de novo in vertebrates nor elongated significantly in brain from circulating precursors derived from plasma [31, 36]. On the other hand, k* represents the “avidity” of brain for unesterified plasma ARA, and depends on diffusional and enzymatic processes within the brain deacylation-reacylation cycle [29, 61–63]. Therefore, the significantly decreased values of k* and Jin for ARA of 33% and 85% of the 92 regions studied, respectively, suggest downregulation of brain affinity for ARA and of ARA metabolic loss [64, 65]. Our data also suggest that one pathway that is downregulated involves ARA metabolism by CYP4A, whose protein level was reduced by 74%. CYP4A oxidizes ARA to 20-hydroxyeicosatrienoic acid (20-HETE) [66], which influences cerebrovascular function and can be produced by stimulation of 5-HT1B receptors [67–70]. Future studies might use lipidomic methods [71] to test whether 20-HETE or other ARA metabolites are reduced following postnatal fluoxetine. Additionally, regional expression of enzymes might show differences not evidenced by global analysis.
Long-term effects of transient exposure to fluoxetine during rodent neurodevelopment suggest epigenetic or other neuroplastic mechanisms, and epigenetic changes associated with SSRI administration to both neonatal and adult rats have been reported [72, 73][74]. These potential mechanisms remain to be studied in more detail. Effects are not due to remaining fluoxetine or its metabolites, which have elimination half-lives of less than 15 h [75].
In summary, transient postnatal fluoxetine caused a downregulation of global baseline brain ARA metabolism in adult mice, suggesting long-term quasi-permanent consequences of early SSRI exposure. The reduced ARA metabolism may be related to the reported reduction in extracellular 5-HT, since ARA hydrolysis is coupled to 5-HT2A/2C receptors, and to reduced conversion of unesterified ARA to 20-HETE by CYP4A, whose expression was 74% reduced.
Supplementary Material
Acknowledgements
This work was supported by the Intramural Research Programs of the National Institute on Aging and the National Institute of Mental Health at the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No author has a conflict of interest.
References
- [1].Croen LA, Grether JK, Yoshida CK, Odouli R, Hendrick V. Antidepressant use during pregnancy and childhood autism spectrum disorders. Arch Gen Psychiatry. 2011;68:1104–1112. doi: 10.1001/archgenpsychiatry.2011.73. [DOI] [PubMed] [Google Scholar]
- [2].Olivier JD, Akerud H, Kaihola H, Pawluski JL, Skalkidou A, Hogberg U, Sundstrom-Poromaa I. The effects of maternal depression and maternal selective serotonin reuptake inhibitor exposure on offspring. Frontiers in cellular neuroscience. 2013;7:73. doi: 10.3389/fncel.2013.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Suri R, Stowe ZN, Hendrick V, Hostetter A, Widawski M, Altshuler LL. Estimates of nursing infant daily dose of fluoxetine through breast milk. Biological psychiatry. 2002;52:446–451. doi: 10.1016/s0006-3223(02)01368-9. [DOI] [PubMed] [Google Scholar]
- [4].Hammad TA, Laughren T, Racoosin J. Suicidality in pediatric patients treated with antidepressant drugs. Arch Gen Psychiatry. 2006;63:332–339. doi: 10.1001/archpsyc.63.3.332. [DOI] [PubMed] [Google Scholar]
- [5].Haliburn J. Adolescent suicide and SSRI antidepressants. Australasian psychiatry : bulletin of Royal Australian and New Zealand College of Psychiatrists. 2010;18:587. doi: 10.3109/10398562.2010.502574. [DOI] [PubMed] [Google Scholar]
- [6].Austin MP, Karatas JC, Mishra P, Christl B, Kennedy D, Oei J. Infant neurodevelopment following in utero exposure to antidepressant medication. Acta paediatrica. 2013;102:1054–1059. doi: 10.1111/apa.12379. [DOI] [PubMed] [Google Scholar]
- [7].Riggin L, Frankel Z, Moretti M, Pupco A, Koren G. The fetal safety of fluoxetine: a systematic review and meta-analysis. J Obstet Gynaecol Can. 2013;35:362–369. doi: 10.1016/S1701-2163(15)30965-8. [DOI] [PubMed] [Google Scholar]
- [8].Altieri SC, Yang H, O'Brien H, Hensler JG, Andrews AM. Early exposure to antidepressants does not recapitulate constitutive serotonin transporter deficiency. Neuropsychopharmacology (American College of Neuropsychopharmacology 51st Annual Meeting) 2012;38(Issue S1):S115. [Google Scholar]
- [9].Bock N, Quentin DJ, Huther G, Moll GH, Banaschewski T, Rothenberger A. Very early treatment with fluoxetine and reboxetine causing long-lasting change of the serotonin but not the noradrenaline transporter in the frontal cortex of rats. World J Biol Psychiatry. 2005;6:107–112. doi: 10.1080/15622970510029731. [DOI] [PubMed] [Google Scholar]
- [10].Haskell SE, Hermann GM, Reinking BE, Volk KA, Peotta VA, Zhu V, Roghair RD. Sertraline exposure leads to small left heart syndrome in adult mice. Pediatric research. 2013;73:286–293. doi: 10.1038/pr.2012.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Silva CM, Goncalves L, Manhaes-de-Castro R, Nogueira MI. Postnatal fluoxetine treatment affects the development of serotonergic neurons in rats. Neurosci Lett. 2010;483:179–183. doi: 10.1016/j.neulet.2010.08.003. [DOI] [PubMed] [Google Scholar]
- [12].Zheng J, Xu DF, Li K, Wang HT, Shen PC, Lin M, Cao XH, Wang R. Neonatal exposure to fluoxetine and fluvoxamine alteres spine density in mouse hippocampal CA1 pyramidal neurons. Int J Clin Exp Pathol. 2011;4:162–168. [PMC free article] [PubMed] [Google Scholar]
- [13].Ansorge MS, Zhou M, Lira A, Hen R, Gingrich JA. Early-life blockade of the 5-HT transporter alters emotional behavior in adult mice. Science. 2004;306:879–881. doi: 10.1126/science.1101678. [DOI] [PubMed] [Google Scholar]
- [14].Hansen HH, Sanchez C, Meier E. Neonatal administration of the selective serotonin reuptake inhibitor Lu 10-134-C increases forced swimming-induced immobility in adult rats: a putative animal model of depression? The Journal of pharmacology and experimental therapeutics. 1997;283:1333–1341. [PubMed] [Google Scholar]
- [15].Popa D, Lena C, Alexandre C, Adrien J. Lasting syndrome of depression produced by reduction in serotonin uptake during postnatal development: evidence from sleep, stress, and behavior. J Neurosci. 2008;28:3546–3554. doi: 10.1523/JNEUROSCI.4006-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kim JW, Ahn HS, Baik JH, Yoon BJ. Administration of clomipramine to neonatal mice alters stress response behavior and serotonergic gene expressions in adult mice. Journal of psychopharmacology. 2013;27:171–180. doi: 10.1177/0269881112460107. [DOI] [PubMed] [Google Scholar]
- [17].Karpova NN, Lindholm J, Pruunsild P, Timmusk T, Castren E. Long-lasting behavioural and molecular alterations induced by early postnatal fluoxetine exposure are restored by chronic fluoxetine treatment in adult mice. Eur Neuropsychopharmacol. 2009;19:97–108. doi: 10.1016/j.euroneuro.2008.09.002. [DOI] [PubMed] [Google Scholar]
- [18].Davison A, Dobbings J. Applied Neurochemistry. Blackwell; Oxford, U. K: 1968. [Google Scholar]
- [19].Tabata H, Bell JM, Miller JC, Rapoport SI. Incorporation of plasma palmitate into the brain of the rat during development. Brain Res. 1986;394:1–8. doi: 10.1016/0165-3806(86)90076-3. [DOI] [PubMed] [Google Scholar]
- [20].Alm H, Kultima K, Scholz B, Nilsson A, Andren PE, Fex-Svenningsen A, Dencker L, Stigson M. Exposure to brominated flame retardant PBDE-99 affects cytoskeletal protein expression in the neonatal mouse cerebral cortex. Neurotoxicology. 2008;29:628–637. doi: 10.1016/j.neuro.2008.04.021. [DOI] [PubMed] [Google Scholar]
- [21].Clancy B, Silva-Filho M, Friedlander MJ. Structure and projections of white matter neurons in the postnatal rat visual cortex. The Journal of comparative neurology. 2001;434:233–252. doi: 10.1002/cne.1174. [DOI] [PubMed] [Google Scholar]
- [22].Felder CC, Kanterman RY, Ma AL, Axelrod J. Serotonin stimulates phospholipase A2 and the release of arachidonic acid in hippocampal neurons by a type 2 serotonin receptor that is independent of inositolphospholipid hydrolysis. Proc Natl Acad Sci U S A. 1990;87:2187–2191. doi: 10.1073/pnas.87.6.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Garcia MC, Kim HY. Mobilization of arachidonate and docosahexaenoate by stimulation of the 5-HT2A receptor in rat C6 glioma cells. Brain Res. 1997;768:43–48. doi: 10.1016/s0006-8993(97)00583-0. [DOI] [PubMed] [Google Scholar]
- [24].Basselin M, Ramadan E, Rapoport SI. Imaging brain signal transduction and metabolism via arachidonic and docosahexaenoic acid in animals and humans. Brain Res Bull. 2012;87:154–171. doi: 10.1016/j.brainresbull.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Jones CR, Arai T, Bell JM, Rapoport SI. Preferential in vivo incorporation of [3H]arachidonic acid from blood into rat brain synaptosomal fractions before and after cholinergic stimulation. J. Neurochem. 1996;67:822–829. doi: 10.1046/j.1471-4159.1996.67020822.x. [DOI] [PubMed] [Google Scholar]
- [26].Rapoport SI. Arachidonic acid and the brain. J Nutr. 2008;138:2515–2520. doi: 10.1093/jn/138.12.2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Fitzpatrick F, Soberman R. Regulated formation of eicosanoids. J Clin Invest. 2001;107:1347–1351. doi: 10.1172/JCI13241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].DeGeorge JJ, Noronha JG, Bell JM, Robinson P, Rapoport SI. Intravenous injection of [1-14C]arachidonate to examine regional brain lipid metabolism in unanesthetized rats. J Neurosci Res. 1989;24:413–423. doi: 10.1002/jnr.490240311. [DOI] [PubMed] [Google Scholar]
- [29].Robinson PJ, Noronha J, DeGeorge JJ, Freed LM, Nariai T, Rapoport SI. A quantitative method for measuring regional in vivo fatty-acid incorporation into and turnover within brain phospholipids: Review and critical analysis. Brain Res Brain Res Rev. 1992;17:187–214. doi: 10.1016/0165-0173(92)90016-f. [DOI] [PubMed] [Google Scholar]
- [30].Demar JC, Jr., Ma K, Chang L, Bell JM, Rapoport SI. α-Linolenic acid does not contribute appreciably to docosahexaenoic acid within brain phospholipids of adult rats fed a diet enriched in docosahexaenoic acid. J Neurochem. 2005;94:1063–1076. doi: 10.1111/j.1471-4159.2005.03258.x. [DOI] [PubMed] [Google Scholar]
- [31].Rapoport SI, Chang MC, Spector AA. Delivery and turnover of plasma-derived essential PUFAs in mammalian brain. J Lipid Res. 2001;42:678–685. [PubMed] [Google Scholar]
- [32].Rapoport SI, Blanchard H, Cheon Y, Fox MA, Basselin M, Ramadan E. Transient postnatal fluoxetine administration to mice decreases brain arachidonic acid metabolism in adulthood. Long term effects of an early SSRI. Abstr. 68th Ann Meeting Society Biological Psychiatry; San Francisco, CA. May 16–18, 2013. [Google Scholar]
- [33].Basselin M, Fox M, Chang L, Bell JM, Greenstein D, Chen M, Murphy DL, Rapoport SI. Imaging elevated brain arachidonic acid signaling in unanesthetized serotonin transporter (5-HTT)-deficient mice. Neuropsychopharmacology. 2009;34:1695–1709. doi: 10.1038/npp.2008.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- [35].Lee HJ, Rao JS, Ertley RN, Chang L, Rapoport SI, Bazinet RP. Chronic fluoxetine increases cytosolic phospholipase A2 activity and arachidonic acid turnover in brain phospholipids of the unanesthetized rat. Psychopharmacology (Berl) 2007;190:103–115. doi: 10.1007/s00213-006-0582-1. [DOI] [PubMed] [Google Scholar]
- [36].DeMar JCJ, Lee HJ, Ma K, Chang L, Bell JM, Rapoport SI, Bazinet RP. Brain elongation of linoleic acid is a negligible source of the arachidonate in brain phospholipids of adult rats. Biochim Biophys Acta. 2006;1761:1050–1059. doi: 10.1016/j.bbalip.2006.06.006. [DOI] [PubMed] [Google Scholar]
- [37].Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- [38].Yang HC, Mosior M, Johnson CA, Chen Y, Dennis EA. Group-specific assays that distinguish between the four major types of mammalian phospholipase A2. Anal Biochem. 1999;269:278–288. doi: 10.1006/abio.1999.4053. [DOI] [PubMed] [Google Scholar]
- [39].Eintrei C, Sokoloff L, Smith CB. Effects of diazepam and ketamine administered individually or in combination on regional rates of glucose utilization in rat brain. British journal of anaesthesia. 1999;82:596–602. doi: 10.1093/bja/82.4.596. [DOI] [PubMed] [Google Scholar]
- [40].Landis SC, Amara SG, Asadullah K, Austin CP, Blumenstein R, Bradley EW, Crystal RG, Darnell RB, Ferrante RJ, Fillit H, Finkelstein R, Fisher M, Gendelman HE, Golub RM, Goudreau JL, Gross RA, Gubitz AK, Hesterlee SE, Howells DW, Huguenard J, Kelner K, Koroshetz W, Krainc D, Lazic SE, Levine MS, Macleod MR, McCall JM, Moxley RT, 3rd, Narasimhan K, Noble LJ, Perrin S, Porter JD, Steward O, Unger E, Utz U, Silberberg SD. A call for transparent reporting to optimize the predictive value of preclinical research. Nature. 2012;490:187–191. doi: 10.1038/nature11556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1:43–46. [PubMed] [Google Scholar]
- [42].Six DA, Dennis EA. The expanding superfamily of phospholipase A2 enzymes: classification and characterization. Biochim Biophys Acta. 2000;1488:1–19. doi: 10.1016/s1388-1981(00)00105-0. [DOI] [PubMed] [Google Scholar]
- [43].Basselin M, Rosa AO, Ramadan E, Cheon Y, Chang L, Chen M, Greenstein D, Wohltmann M, Turk J, Rapoport SI. Imaging decreased brain docosahexaenoic acid metabolism and signaling in iPLA2{beta} (VIA)-deficient mice. J Lipid Res. 2010;51:3166–3173. doi: 10.1194/jlr.M008334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Strokin M, Sergeeva M, Reiser G. Docosahexaenoic acid and arachidonic acid release in rat brain astrocytes is mediated by two separate isoforms of phospholipase A2 and is differently regulated by cyclic AMP and Ca2+ Br J Pharmacol. 2003;139:1014–1022. doi: 10.1038/sj.bjp.0705326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Dmitriev AD, Factor MI, Segal OL, Pavlova EV, Massino YS, Smirnova MB, Yakovleva DA, Dmitriev DA, Kizim EA, Kolyaskina GI, Brusov OS. Western blot analysis of human and rat serotonin transporter in platelets and brain using site-specific antibodies: evidence that transporter undergoes endoproteolytic cleavage. Clin Chim Acta. 2005;356:76–94. doi: 10.1016/j.cccn.2004.12.019. [DOI] [PubMed] [Google Scholar]
- [46].Vijayakumar M, Meti BL. Alterations in the levels of monoamines in discrete brain regions of clomipramine-induced animal model of endogenous depression. Neurochemical research. 1999;24:345–349. doi: 10.1023/a:1020992314534. [DOI] [PubMed] [Google Scholar]
- [47].Qu Y, Chang L, Klaff J, Seemann R, Greenstein D, Rapoport SI. Chronic fluoxetine upregulates arachidonic acid incorporation into the brain of unanesthetized rats. Eur Neuropsychopharmacol. 2006;16:561–571. doi: 10.1016/j.euroneuro.2006.01.008. [DOI] [PubMed] [Google Scholar]
- [48].Rao JS, Ertley RN, Lee HJ, Rapoport SI, Bazinet RP. Chronic fluoxetine upregulates activity, protein and mRNA levels of cytosolic phospholipase A(2) in rat frontal cortex. Pharmacogenomics J. 2006;6:413–420. doi: 10.1038/sj.tpj.6500391. [DOI] [PubMed] [Google Scholar]
- [49].Stenfors C, Ross SB. Evidence for involvement of 5-hydroxytryptamine(1B) autoreceptors in the enhancement of serotonin turnover in the mouse brain following repeated treatment with fluoxetine. Life Sci. 2002;71:2867–2880. doi: 10.1016/s0024-3205(02)02138-0. [DOI] [PubMed] [Google Scholar]
- [50].Mathews TA, Fedele DE, Coppelli FM, Avila AM, Murphy DL, Andrews AM. Gene dose-dependent alterations in extraneuronal serotonin but not dopamine in mice with reduced serotonin transporter expression. J Neurosci Methods. 2004;140:169–181. doi: 10.1016/j.jneumeth.2004.05.017. [DOI] [PubMed] [Google Scholar]
- [51].Cowen PJ. Serotonin and depression: pathophysiological mechanism or marketing myth? Trends Pharmacol Sci. 2008;29:433–436. doi: 10.1016/j.tips.2008.05.004. [DOI] [PubMed] [Google Scholar]
- [52].Vogel G, Neill D, Hagler M, Kors D. A new animal model of endogenous depression: a summary of present findings. Neuroscience and biobehavioral reviews. 1990;14:85–91. doi: 10.1016/s0149-7634(05)80164-2. [DOI] [PubMed] [Google Scholar]
- [53].Velazquez-Moctezuma J, Diaz Ruiz O. Neonatal treatment with clomipramine increased immobility in the forced swim test: an attribute of animal models of depression. Pharmacology, biochemistry, and behavior. 1992;42:737–739. doi: 10.1016/0091-3057(92)90022-8. [DOI] [PubMed] [Google Scholar]
- [54].Ribas VR, Aniceto HK, Martins HA, Ribas KH, Guerra-Ribas Rde M, Fraga Sdo N, Ribeiro-Ribas V, Vasconcelos CM, Viana MT, Manhaes-de-Castro R. Neonatal administration of fluoxetine did not alter the anxiety indicators, but decreased the locomotor activity in adult rats in the elevated plus-maze. Arquivos de neuro-psiquiatria. 2008;66:844–847. doi: 10.1590/s0004-282x2008000600013. [DOI] [PubMed] [Google Scholar]
- [55].Line SJ, Barkus C, Coyle C, Jennings KA, Deacon RM, Lesch KP, Sharp T, Bannerman DM. Opposing alterations in anxiety and species-typical behaviours in serotonin transporter overexpressor and knockout mice. Eur Neuropsychopharmacol. 2011;21:108–116. doi: 10.1016/j.euroneuro.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Holmes A, Murphy DL, Crawley JN. Abnormal behavioral phenotypes of serotonin transporter knockout mice: parallels with human anxiety and depression. Biol Psychiatry. 2003;54:953–959. doi: 10.1016/j.biopsych.2003.09.003. [DOI] [PubMed] [Google Scholar]
- [57].Kalueff AV, Fox MA, Gallagher PS, Murphy DL. Hypolocomotion, anxiety and serotonin syndrome-like behavior contribute to the complex phenotype of serotonin transporter knockout mice. Genes Brain Behav. 2006 doi: 10.1111/j.1601-183X.2006.00270.x. [DOI] [PubMed] [Google Scholar]
- [58].Ramadan E, Rosa AO, Chang L, Chen M, Rapoport SI, Basselin M. Extracellular-derived calcium does not initiate in vivo neurotransmission involving docosahexaenoic acid. J Lipid Res. 2010;51:2334–2340. doi: 10.1194/jlr.M006262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Kim HW, Rao JS, Rapoport SI, Igarashi M. Dietary n-6 PUFA deprivation downregulates arachidonate but upregulates docosahexaenoate metabolizing enzymes in rat brain. Biochim Biophys Acta. 2011;1811:111–117. doi: 10.1016/j.bbalip.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Kim HW, Rao JS, Rapoport SI, Igarashi M. Regulation of rat brain polyunsaturated fatty acid (PUFA) metabolism during graded dietary n-3 PUFA deprivation. Prostaglandins Leukot Essent Fatty Acids. 2011;85:361–368. doi: 10.1016/j.plefa.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Umhau JC, Zhou W, Thada S, Demar J, Hussein N, Bhattacharjee AK, Ma K, Majchrzak-Hong S, Herscovitch P, Salem N, Jr., Urish A, Hibbeln JR, Cunnane SC, Rapoport SI, Hirvonen J. Brain Docosahexaenoic Acid [DHA] Incorporation and Blood Flow Are Increased in Chronic Alcoholics: A Positron Emission Tomography Study Corrected for Cerebral Atrophy. PloS one. 2013;8:e75333. doi: 10.1371/journal.pone.0075333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Lands WEM, Crawford CG. In: Enzymes of membrane phospholipid metabolism. Martonosi A, editor. Plenum; New-York: 1976. [Google Scholar]
- [63].Sun GY, MacQuarrie RA. Deacylation-reacylation of arachidonoyl groups in cerebral phospholipids. Ann N Y Acad Sci. 1989;559:37–55. doi: 10.1111/j.1749-6632.1989.tb22597.x. [DOI] [PubMed] [Google Scholar]
- [64].Rapoport SI. Brain arachidonic and docosahexaenoic acid cascades are selectively altered by drugs, diet and disease. Prostaglandins Leukot Essent Fatty Acids. 2008;79:153–156. doi: 10.1016/j.plefa.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Shimizu T, Wolfe LS. Arachidonic acid cascade and signal transduction. J Neurochem. 1990;55:1–15. doi: 10.1111/j.1471-4159.1990.tb08813.x. [DOI] [PubMed] [Google Scholar]
- [66].Konkel A, Schunck WH. Role of cytochrome P450 enzymes in the bioactivation of polyunsaturated fatty acids. Biochimica et biophysica acta. 2011;1814:210–222. doi: 10.1016/j.bbapap.2010.09.009. [DOI] [PubMed] [Google Scholar]
- [67].Gebremedhin D, Lange AR, Lowry TF, Taheri MR, Birks EK, Hudetz AG, Narayanan J, Falck JR, Okamoto H, Roman RJ, Nithipatikom K, Campbell WB, Harder DR. Production of 20-HETE and its role in autoregulation of cerebral blood flow. Circulation research. 2000;87:60–65. doi: 10.1161/01.res.87.1.60. [DOI] [PubMed] [Google Scholar]
- [68].Stromstedt M, Warner M, Gustafsson JA. Cytochrome P450s of the 4A subfamily in the brain. Journal of neurochemistry. 1994;63:671–676. doi: 10.1046/j.1471-4159.1994.63020671.x. [DOI] [PubMed] [Google Scholar]
- [69].Roman RJ. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev. 2002;82:131–185. doi: 10.1152/physrev.00021.2001. [DOI] [PubMed] [Google Scholar]
- [70].Cambj-Sapunar L, Yu M, Harder DR, Roman RJ. Contribution of 5-hydroxytryptamine1B receptors and 20-hydroxyeiscosatetraenoic acid to fall in cerebral blood flow after subarachnoid hemorrhage. Stroke; a journal of cerebral circulation. 2003;34:1269–1275. doi: 10.1161/01.STR.0000065829.45234.69. [DOI] [PubMed] [Google Scholar]
- [71].Murphy DL, Moya PR. Human serotonin transporter gene (SLC6A4) variants: their contributions to understanding pharmacogenomic and other functional GxG and GxE differences in health and disease. Curr Opin Pharmacol. 2011;11:3–10. doi: 10.1016/j.coph.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Wang Y, Neumann M, Hansen K, Hong SM, Kim S, Noble-Haeusslein LJ, Liu J. Fluoxetine increases hippocampal neurogenesis and induces epigenetic factors but does not improve functional recovery after traumatic brain injury. J Neurotrauma. 2011;28:259–268. doi: 10.1089/neu.2010.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Melas PA, Rogdaki M, Lennartsson A, Bjork K, Qi H, Witasp A, Werme M, Wegener G, Mathe AA, Svenningsson P, Lavebratt C. Antidepressant treatment is associated with epigenetic alterations in the promoter of P11 in a genetic model of depression. Int J Neuropsychopharmacol. 2012;15:669–679. doi: 10.1017/S1461145711000940. [DOI] [PubMed] [Google Scholar]
- [74].Clinton SM, Glover ME, Pugh PC, Cohen J, Akil H. Neonatal SSRI exposure alters neurodevelopment and risk for depression in model rats. Neuropsychopharmacology. 2013;38:S435–S593. [Google Scholar]
- [75].Caccia S, Cappi M, Fracasso C, Garattini S. Influence of dose and route of administration on the kinetics of fluoxetine and its metabolite norfluoxetine in the rat. Psychopharmacology (Berl) 1990;100:509–514. doi: 10.1007/BF02244004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.