Abstract
Background
Recent studies suggest higher cumulative HIV viraemia exposure measured as viraemia copy-years (VCY) is associated with increased all-cause mortality. The objectives of this study are (a) report the association between VCY and all-cause mortality, and (b) assess associations between common patient characteristics and VCY.
Methods
Analyses were based on patients recruited to the Australian HIV Observational Database (AHOD) who had received ≥ 24 weeks of antiretroviral therapy (ART). We established VCY after 1, 3, 5 and 10 years of ART by calculating the area under the plasma viral load time-series. We used survival methods to determine the association between high VCY and all-cause mortality. We used multivariable mixed-effect models to determine predictors of VCY. We compared a baseline information model with a time-updated model to evaluate discrimination of patients with high VCY.
Results
Of the 3021 AHOD participants that initiated ART, 2073(69%), 1667(55%), 1267(42%) and 638(21%) were eligible for analysis at 1, 3, 5, 10 years of ART respectively. Multivariable adjusted hazard ratio (HR) association between all-cause mortality and high VCY was statistically significant, HR 1.52(1.09, 2.13), p-value=0.01. Predicting high VCY after one-year of ART for a time-updated model compared to a baseline information only model, the area under the sensitivity/specificity curve (AUC) was 0.92 vs. 0.84; and at 10 years of ART, AUC: 0.87 vs. 0.61 respectively.
Conclusion
A high cumulative measure of viral load after initiating ART is associated with increased risk of all-cause mortality. Identifying patients with high VCY is improved by incorporating time-updated information.
Keywords: viraemia copy-years, viremia copy-years, community viral load, HIV mortality, long-term antiretroviral therapy
Introduction
A number of different but related trends are resulting in an increased proportion of persons living with HIV being treated at an earlier time. These include better medicines, some evidence of clinical benefit to individual patients as a result of earlier treatment, and the public health benefits of a test and treat philosophy aimed at lower rates of community transmission of HIV [1,2]. The potential success of a treatment as prevention strategy rests entirely on high levels of treatment efficacy facilitating a reduction of the “community viral load” or overall transmission virus pool. Outside of the tightly controlled, yet successful environment of randomized clinical trials [3] or the theoretical realms of mathematical modeling [4], there are conflicting evidence of treatment as prevention reducing overall HIV incidence at a community level [5,6]. Following these observations and the likelihood of similar scale up in other regional programs, surveillance to identify subsets of the treated population who experience viral blips and rebounds while receiving antiretroviral therapy (ART) will become increasingly important. A single cross sectional measure of the proportion of the treated population having detectable plasma HIV viral load (pVL) does not capture the length of time with detectable circulating virus (an indication of transmission risk [7]) or capture subsets of the populations with continual blips and rebounds while receiving ART. A cumulative viral load measure might better capture these occurrences [8].
Monitoring response to ART in resource-rich settings is largely carried out through a series of periodic laboratory tests, typically plasma HIV viral load and CD4 cell counts. Whilst conventional cross-sectional studies of pVL have proven an invaluable tool for monitoring response to therapy as well as in translational, clinical and prevention research [9], until recently, the pVL measure was rarely examined as a serially collected biomarker. There is a growing body of evidence to suggest that an assessment of an individual’s overall HIV burden using a cumulative metric, e.g. viraemia copy-years, is better able to predict important clinical outcomes such as mortality compared with selected cross-sectional pVL measurements and time-updated CD4 cell counts [9–12]. At an individual patient level, identifying new additional risk factors contributing to mortality risk is an important step to understand the current drivers of the commonly observed excess mortality in HIV populations [13–16]. Furthermore at a population level evaluating the cumulative viral burden within a population might be useful for identifying population subgroups contributing to the virus transmission pool while receiving ART.
To date there has been very limited research assessing the determinants of cumulative viral burden as reflected in viraemia copy-years. An understanding of the characteristics of people living with HIV at risk for experiencing a greater HIV burden after initiation of ART might be clinically relevant and useful for targeted interventions for reducing the number patients at risk of high cumulative viraemia exposure. The objectives of this analysis are, (1) report the association between increasing viraemia copy-years and all-cause mortality, (2) assess associations between common patient characteristics and viraemia copy-years, and finally explore any differences in determinants when considering two sets of risk factors, (i) factors known at ART initiation, and (ii) factors known at ART initiation and their time-updated equivalents.
Methods
Analyses were based on patients recruited to the Australian HIV Observational Database (AHOD), an ongoing longitudinal cohort study for which a comprehensive description has been previously described elsewhere [17]. Briefly, AHOD data are collected from 29 sites throughout Australia including hospital tertiary referral centres, sexual health clinics and general medical practices with a special interest in HIV. Data for AHOD are collected every 6 months on a core set of demographic and clinical variables including sex, age, HIV exposure, hepatitis B virus surface antigen (HBsAg), hepatitis C virus antibody (HCV), CD4 and CD8 cell counts, plasma HIV viral load, antiretroviral treatment history, AIDS illnesses and date and cause of death. Data collection commenced in 1999 and retrospective clinical data collected prior to the establishment of the cohort are provided where available. Data are transferred electronically to the Kirby Institute and are subjected to quality control and quality assurance procedures. Written informed consent was obtained at time of cohort enrolment. Ethics approval for AHOD was granted to all participating sites by relevant Human Research Ethics Committees (HREC).
The data selected for this analysis includes all data collected from participants in AHOD who commenced their first ART regimen after 1st January 1996. We defined ART as a combination of 3 or more different antiretroviral agents. Patients were included in the analysis if they received at least 24 weeks of ART and had a known CD4 cell count and plasma HIV-RNA viral load at ART initiation and subsequently at 24 weeks of therapy. We selected the closest CD4 cell count to ART initiation date within a 6-month prior and 1-month post window of starting ART. Similarly for pVL at ART initiation, the measurement closest to ART initiation date within a 12-month prior and 1-week post ART window was selected. CD4 and pVL at 24 weeks of ART were taken as the values recorded on the closest measurement date to 24 week ART initiation within a window of ±3 months. Furthermore, patients were included in the analysis only if they recorded at least three pVL measurements following 24 weeks of ART. Patents were censored at the first instance where the time between sequential pVL measurements was greater than 1-year.
Viraemia Copy Years (VCY) after initiating ART
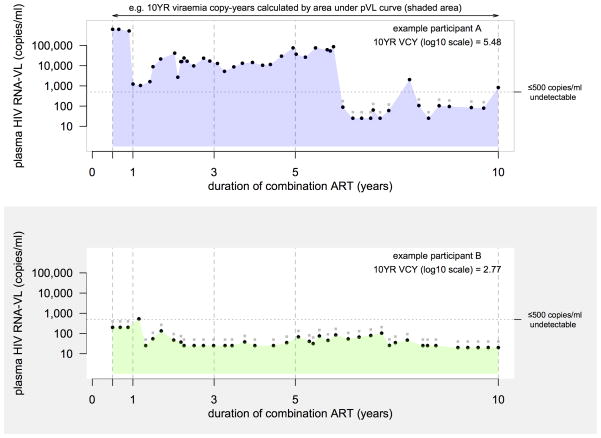
For each patient, we determined VCY by calculating the area under the pVL time series using the trapezoidal rule (Figure 1). VCY is a monotonic increasing measure with time capturing the cumulative exposure of circulating HIV viraemia, a measure akin to smoking-pack-years. A person with a VCY of 10,000 copy-years at 1 year of ART is equivalent of being exposed to 10,000 copies/ml of pVL each day for a year, or 1,000 copies/ml pVL each day for 10 years. For consistency with the current VCY literature, we applied a set of pVL boundary constraints on the maximum and minimum values. Over time there have been many varying levels of assay sensitivity for detecting circulating viraemia. In the early era of treatment, assessment of pVL was limited by an assay sensitivity detection limit of 500 copies/ml. Recent periods have seen assay sensitivity levels as low as 20 copies/ml. Through most of our observed time period (1996 – 2011) we were completely unable to determine a pVL value of 0 copies/ml value, therefore we assumed persistent circulating viraemia below the detection limit and took this value to be the midpoint between the detection limit and 0 [9]. For example, if reported pVL is <500 copies/ml, we recode this figure as 250 copies/ml. Similarly if the reported pVL is <20 copies/ml, we recode this value as 10 copies/ml. At the other end of the measurement scale, due to assay sensitivity limits, we set pVL values >1,000,000 copies to be 1,000,000 copies/ml.
Figure 1.
Example calculation of 10 year viraemia copy-years after initiating ART. Black dots indicate the pVL value used to calculate the VCY, grey dots indicate pVL value assay detection limit of record.
Association of VCY and survival
Building on previous modeling work evaluating long-term survival in AHOD [15], we used multivariable Cox proportional hazards to assess the association between all-cause mortality and, (a) time-updated per-log increase in VCY, and (b) VCY>105 copy-years (referred henceforth as high VCY). We assessed statistical significance at an α=0.05 threshold. The survival models were adjusted for time-updated age, mode of HIV exposure, time-updated AIDS defining illness, HBVsAg positive (ever), time-updated CD4 cell count, time-updated pVL and time-updated ART regimen number. We used Kaplan-Meier curves to assess the association between all-cause mortality and time-updated high VCY stratified by CD4 cell count grouping (0–150, 150–350, >350 cells/μL) at ART initiation. We started counting person-years from 6-months post ART initiation and used a log-rank test to assess the equivalence of survival distributions.
Determinants of VCY
We used an a priori adjusted multivariable maximum likelihood linear mixed effect models to assess determinants of VCY. We simultaneously adjusted for within patient and between patient variations through the specification of a random intercept and slope coefficient. We log10 transformed our response variable (VCY) to impose a multiplicative interpretation of the β-coefficient parameter estimate. In modelling log10(VCY), a β-coefficient estimate of less than 0 is equivalent to a percentage reduction on the real scale, i.e. a difference of −0.5 on the log10 scale is equivalent to a 68% reduction on the real scale. Similarly, a β-coefficient greater than 0 is equivalent to a factor increase on the real scale, i.e. 0.5 on the log10 scale is equivalent to a 3.2 factor increase on the real scale.
As a sensitivity analysis we recalculated all β-coefficient parameter estimates from data based on recent treatment periods, i.e. participants who initiated therapy in the post-SMART (Strategic Management of ART - evaluating scheduled treatment interruptions [18]) era following 1st January 2006. All statistical modeling calculations were performed using R version 3.02 [19] and the statistical packages lme4 [20] and survival [21].
We assessed baseline (time of ART initiation) and time updated determinants of VCY. We evaluated the following patient characteristics; age at ART initiation (<30, 30–40, 40–50, >50 years old); sex; HIV exposure (men who have sex with men, heterosexual, injecting drug user, other, unknown); patient care setting (general practitioner, sexual health clinic, hospital clinic); hepatitis B coinfection, hepatitis C coinfection; time-updated AIDS illness. We examined the following treatment related baseline and time-updated factors; treatment naïve prior to initiating ART; year of ART initiation; initial ART anchor agent, 2 N(t)TRI + (NNRTI, PI, Other); time-updated number of regimen modifications (0, 1–2, 3–5, 6–10, >10); time-updated total time (percentage of follow up) of treatment interruption (0%, 0.1–5%, 5.1–20%, 20.1–50%, >50%). We also evaluated the following immunological and virological related baseline and time-updated factors; CD4 cell count at ART initiation (0–200, 201–350, 351–500, >500 cells/μL); pVL at ART initiation (100–104, 104–105, >105 copies/ml); time-updated CD4 cell count (0–200, 201–350, 351–500, >500 cells/μL).
We compared the model fits between baseline factors and baseline factors plus time updated factors using standard model fit measures, AIC and BIC. To assess significant differences between the models fits, we perform an analysis of variance likelihood ratio test. We determined the adequacy of model fit by comparing at each time point (1, 3, 5 or 10 years of ART) the predicted mean VCY versus observed mean VCY split by ordered observed VCY deciles. The ordered statistics approach visually evaluates the mean VCY model prediction spread along the whole VCY distribution, including the tails/extreme ends of the VCY distribution. We further evaluated the models by measuring the ability of each model to identify patients with high VCY. We used a higher area under the sensitivity/specificity curve metric to deduce better discriminatory performance.
Results
Of the 3021 AHOD patients who initiated ART and followed for at least 6 months while receiving ART, 2073 (69%), 1667 (55%), 1267 (42%) and 638 (21%) were eligible for analysis at 1, 3, 5, 10 years of ART respectively. During the analysis period there were a total of 142 deaths reported from 15,385 person years of follow up while receiving ART (crude mortality rate: 9.2 [7.8, 10.8] per 1000 person-years). A total of 652 persons had a gap of more than 1-year between subsequent pVL measurements (rate: 4.2 [3.9, 4.6] per 100 person-years). The patient characteristics of the analysis population are outlined in Table 1. There are minimal differences proportionally between each factor over duration of ART. AHOD participants are typically men who have sex with men, middle aged, hepatitis B and C negative, were treatment naïve prior to initiating ART in 1996–2004 and commenced therapy with a CD4 cell count <350 cell/μL. Of the AHOD participants that initiated ART and were excluded from the analysis due to missing CD4/pVL measurements, there are minimal differences between patient characteristics when comparing to participants included in the analysis at time point 1.
Table 1.
Analysis study population patient characteristics by duration of ART.
| Duration of combination ART
|
Excluded Population3 (n=998) | ||||||
|---|---|---|---|---|---|---|---|
| Factor | Level | 1 year (n=2073) | 3 years (n=1667) | 5 years (n=1267) | 10 years (n=638) | P2 | |
| Age at ART initiation (years) | <30 | 241 (12) | 172 (10) | 130 (10) | 65 (10) | 0.94 | 136 (13) |
| 30 to 39 | 801 (39) | 664 (40) | 497 (39) | 242 (38) | 396 (40) | ||
| 40 to 50 | 631 (30) | 510 (31) | 394 (31) | 206 (32) | 316 (32) | ||
| >50 | 400 (19) | 321 (19) | 246 (19) | 125 (20) | 150 (15) | ||
|
| |||||||
| Sex | Male/Transgender | 1955 (94) | 1573 (94) | 1191 (94) | 602 (94) | 0.98 | 936 (94) |
| Female | 118 (6) | 94 (6) | 76 (6) | 36 (6) | 62 (6) | ||
|
| |||||||
| Mode of Exposure | MSM | 1572 (76) | 1268 (76) | 963 (76) | 502 (79) | 0.93 | 715 (72) |
| Heterosexual | 201 (10) | 154 (9) | 116 (9) | 44 (7) | 102 (10) | ||
| Injecting drug use | 107 (5) | 81 (5) | 64 (5) | 33 (5) | 68 (7) | ||
| Blood Products/Other | 176 (8) | 147 (9) | 113 (9) | 53 (8) | 101 (10) | ||
| Unknown | 17 (1) | 17 (1) | 11 (1) | 6 (1) | 12 (1) | ||
|
| |||||||
| Patient care setting | General Practice | 748 (36) | 579 (35) | 424 (33) | 184 (29) | <0.01 | 402 (40) |
| Hospital | 586 (28) | 486 (29) | 391 (31) | 232 (36) | 144 (15) | ||
| Sexual Health Clinic | 739 (36) | 602 (36) | 452 (36) | 222 (35) | 452 (45) | ||
|
| |||||||
| Hepatitis B (HBsAg) | Yes | 89 (4) | 77 (5) | 56 (4) | 29 (5) | 0.97 | 52 (5) |
| No | 1703 (82) | 1388 (83) | 1086 (86) | 567 (89) | 774 (78) | ||
| Not reported | 281 (14) | 202 (12) | 125 (10) | 42 (7) | 172 (17) | ||
|
| |||||||
| Hepatitis C (HCV-antibody) | Yes | 223 (11) | 178 (11) | 144 (11) | 77 (12) | 0.75 | 115 (12) |
| No | 1668 (80) | 1352 (81) | 1043 (82) | 529 (83) | 758 (76) | ||
| Not reported | 182 (9) | 137 (8) | 80 (6) | 32 (5) | 125 (13) | ||
|
| |||||||
| PreART AIDS illness | Yes | 343 (17) | 288 (17) | 230 (18) | 135 (21) | 0.06 | 142 (14) |
| No | 1730 (83) | 1379 (83) | 1037 (82) | 503 (79) | 856 (86) | ||
|
| |||||||
| Prior ARV exposure at ART start | Yes | 549 (26) | 499 (30) | 432 (34) | 261 (41) | <0.01 | 455 (46) |
| No | 1524 (74) | 1168 (70) | 835 (66) | 377 (59) | 543 (54) | ||
|
| |||||||
| Year of ART start | 1996–2001 | 1394 (67) | 1242 (75) | 1040 (82) | 636 (100) | <0.01 | 648 (65) |
| 2001–2005 | 281 (14) | 243 (15) | 188 (15) | 2 (0) | 85 (9) | ||
| 2006–2012 | 398 (19) | 182 (10) | 39 (3) | 0 | 265 (26) | ||
|
| |||||||
| ART anchor agent1 | NNRTI | 850 (41) | 614 (37) | 406 (32) | 153 (24) | <0.01 | 399 (40) |
| PI | 993 (48) | 874 (52) | 712 (56) | 428 (67) | 440 (44) | ||
| II/Other | 230 (11) | 179 (11) | 149 (12) | 57 (9) | 159 (16) | ||
|
| |||||||
| CD4 cell count at ART initiation (cells/μL) | 0 to 200 | 629 (30) | 534 (32) | 405 (32) | 208 (33) | 0.90 | 149 (32) |
| 201 to 350 | 556 (27) | 429 (26) | 334 (26) | 164 (26) | 133 (28) | ||
| 351 to 500 | 430 (21) | 332 (20) | 256 (20) | 139 (22) | 100 (21) | ||
| >500 | 458 (22) | 372 (22) | 272 (22) | 127 (20) | 89 (19) | ||
|
| |||||||
| pVL at ART initiation (copies/ml) | 0–104 | 927 (45) | 719 (43) | 533 (42) | 288 (45) | 0.47 | 317 (56) |
| 104–105 | 635 (31) | 512 (31) | 384 (30) | 180 (28) | 134 (24) | ||
| >105 | 511 (25) | 436 (26) | 350 (28) | 170 (27) | 114 (20) | ||
|
| |||||||
| Number of ART regimen changes | mean | 0.8 | 2.1 | 3.3 | 6.1 | <0.01 | - |
| median(q1-q3) | 0(0–1) | 1(0–3) | 2(1–5) | 5(3–8) | |||
|
| |||||||
| Percentage of time | mean | 4.5 | 7.1 | 9 | 9.6 | <0.01 | - |
| ART interruption (%) | median(q1-q3) | 0.0(0.0–0.0) | 0.0(0.0–1.3) | 0.0(0.0–7.0) | 0.8(0.0–13.3) | ||
Initial ART regimen was classified as 2 N(t)RTI’s + (NNRTI or PI or Other); N(t)RTI – nucleos(t)ide reverse transcriptase inhibitors; NNRTI – non-nucleoside reverse transcriptase inhibitors; PI – protease inhibitors.
Categorical Factors: Chi-Squared Test, Continuous Factors: Analysis of Variance
AHOD participants who initiated ART who were excluded from the analysis due to (i) time on ART <6 months, (ii) missing CD4/pVL measurement at ART initiation, (iii) missing CD4/pvL measurement 24 weeks of ART, and (iv) less than 3 CD4/pVL measurements following post 24 weeks of ART.
VCY after initiating ART
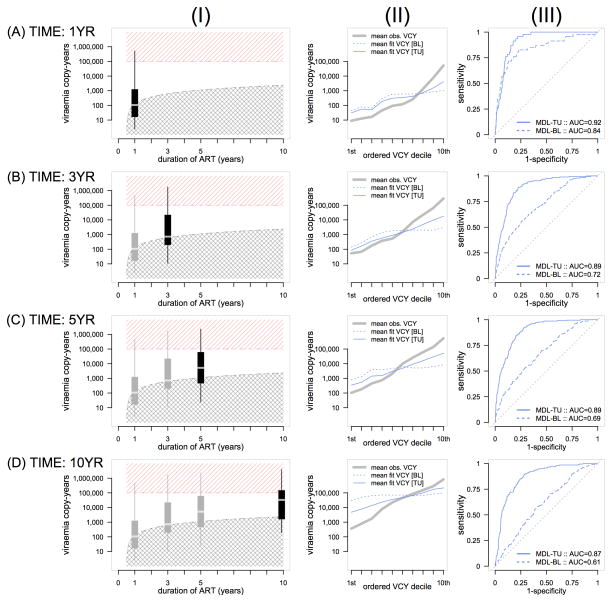
Mean (95% confidence interval) VCY at 1, 3, 5 or 10 years of ART were 204 (182 to 229), 1,862 (1,622 to 2,138), 5,129 (4,467 to 6,026) and 19,953 (16,596 to 24,547) respectively. On the log10 scale, mean (95% confidence interval) VCY at 1, 3, 5 or 10 years of ART were 2.31 (2.26 to 2.36), 3.27 (3.21 to 3.33), 3.71 (3.65 to 3.78) and 4.3 (4.22 to 4.39) log10 respectively. The observed empirical VCY distributions at each time point are shown in Figure 2, column panel (I). The crosshatched shading region represents the threshold of the expected viraemia copy-years for a person who has strictly controlled their pVL below 500 copies/ml over time while receiving ART. The hatched shading region identifies the threshold of high VCY. At 1 and 3 years duration of ART, the median VCY is on the cusp of the threshold indicating that approximately 50% of the population has maintained undetectable levels of pVL. Extending out to 10 years duration of ART, the data indicate that approximately 25% of the population at this time has maintained undetectable levels of pVL while receiving ART.
Figure 2.
(I) Empirical viraemia copy-year distribution by duration of ART, (II) Overall model fit by model type and duration of ART, (III) Sensitivity/Specificity curve of predicting VCY>105 by duration of ART.
Association of VCY and Survival
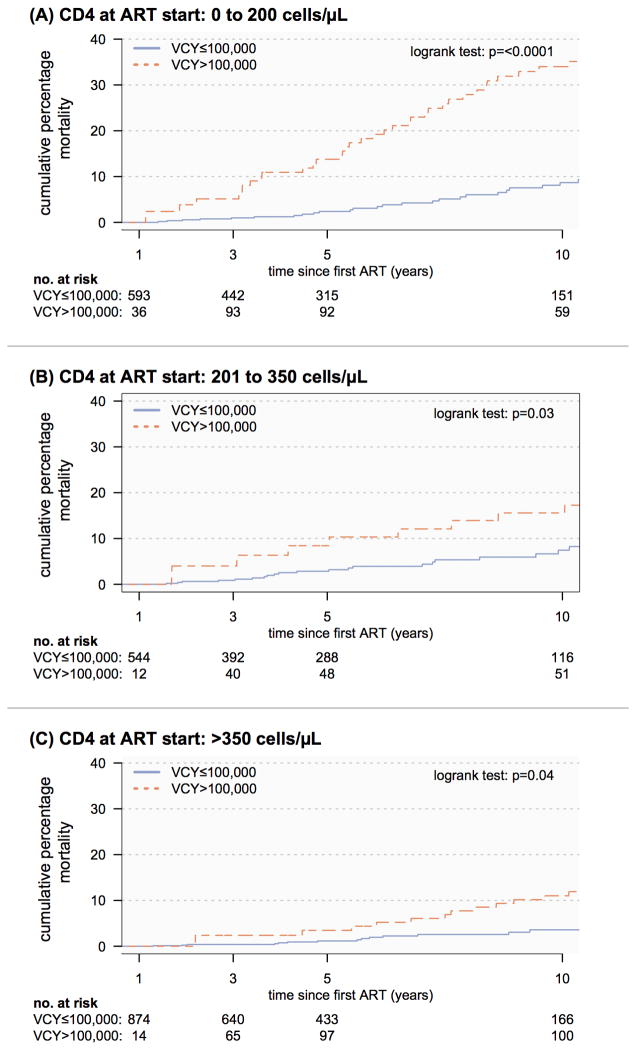
The multivariable adjusted hazard ratio (HR) association between all-cause mortality and time-updated per-log increase in VCY was not statistically significant, HR 1.14 (0.94, 1.38), p-value=0.19. However the multivariable adjusted HR association between all-cause mortality and high VCY was statistically significant, HR 1.52 (1.09, 2.13), p-value=0.01. Supplemental Digital Content 1 illustrates the calculated multivariable HR estimates for the adjustment covariates in the survival models for each VCY characterisation are similar in both magnitude and direction of association. Figure 3 outlines the at-risk population and Kaplan-Meier survival curves of time-updated high/low VCY stratified by CD4 cell count at ART initiation.
Figure 3.
Kaplan-Meier all-cause mortality survival of low VCY versus high VCY (time updated) stratified by CD4 cell count at ART initiation.
Determinants of VCY after initiating ART
The multivariable model using predictors known only at ART initiation showed the following factors significantly associated with VCY (Table 2): (negative relationship, decreasing VCY), older age (p<0.01), mode of HIV exposure compared to IDU (p=0.02), higher CD4 cell counts at ART initiation (p<0.01), treatment naïve at ART initiation (p<0.01); and (positive relationship, increasing VCY) HCV coinfection (p=0.03), a non-NNRTI anchor initial ART regimens (p<0.01) and higher pVL at ART initiation (p<0.01). The multivariable model using time updated predictors showed the aforementioned factors to be similarly significantly associated with VCY, with the exception of HCV coinfection and the inclusion of a significant negative association between female and VCY. Of the time-updated factors, an increase in the number of regimen changes was strongly associated with increased VCY (p<0.01), as well as increases in the total proportion of treatment interruption time being associated with increased VCY (p<0.01). Time updated increasing CD4 cell counts indicated a strong negative relationship with VCY (p<0.01), i.e. lower VCY was associated with greater time-updated CD4 cell counts, Table 2. The AIC model fit criteria for the baseline factor model was 13773 and for the time-updated model 11050. The analysis of variance likelihood ratio test was significant (p=<0.01), indicating a superior model fit using time-updated information. Similar results were found using data restricted to participants who initiated ART after 1 January 2006 (Supplemental Digital Content 2).
Table 2.
Multivariable β (standard error) parameter estimates^ for modelling viraemia copy-years.
| Factors | Level | No. of Participants [yr=1, yr=5] | MODEL: Baseline
|
MODEL: Time-updated
|
||
|---|---|---|---|---|---|---|
| Estimate (S.E) | p# | Estimate (S.E) | p# | |||
| Age at ART | (per 5 years) | [2073, 1267] | −0.04 (0.01) | <0.01 | −0.03 (0.01) | <0.01 |
|
| ||||||
| Sex | Female | [118, 76] | −0.04 (0.11) | 0.66 | −0.20 (0.08) | 0.02 |
|
| ||||||
| Mode HIV exposure | MSM1 | [1572, 963] | 0.00 | 0.02 | 0.00 | 0.18 |
| Heterosexual | [201, 116] | −0.09 (0.09) | −0.04 (0.07) | |||
| IDU | [107, 64] | 0.21 (0.10) | 0.11 (0.08) | |||
| Other/Blood | [176, 113] | −0.15 (0.08) | −0.07 (0.06) | |||
| Unknown | [17, 11] | 0.27 (0.24) | 0.27 (0.18) | |||
|
| ||||||
| Primary Care Type | GP | [748, 424] | 0.00 | 0.87 | 0.00 | 0.29 |
| Hospital | [586, 391] | −0.01 (0.05) | −0.03 (0.04) | |||
| SHC | [739, 452] | 0.02 (0.05) | −0.06 (0.04) | |||
|
| ||||||
| HBV co-infection | Yes | [89, 56] | −0.01 (0.05) | 0.95 | 0.08 (0.08) | 0.34 |
|
| ||||||
| HCV co-infection | Yes | [223, 144] | 0.16 (0.07) | 0.03 | 0.01 (0.05) | 0.92 |
|
| ||||||
| Prior ARV exposure2 | Yes | [549,432] | 0.46 (0.05) | <0.01 | 0.31 (0.04) | <0.01 |
|
| ||||||
| Initial ART anchor3 | NNRTI | [850, 406] | 0.00 | <0.01 | 0.00 | 0.03 |
| PI | [993, 712] | 0.23 (0.05) | 0.07 (0.04) | |||
| Other | [230, 149] | 0.24 (0.07) | 0.13 (0.06) | |||
|
| ||||||
| CD4 at ART start (cells/μL) | 0 to 200 | [629, 405] | 0.00 | <0.01 | ||
| 201 to 350 | [556, 334] | −0.20 (0.06) | ||||
| 351 to 500 | [430, 256] | −0.16 (0.06) | ||||
| >500 | [458, 272] | 0.01 (0.06) | ||||
|
| ||||||
| pVL at ART start (copies/ml) | 0 to 104 | [927, 533] | 0.00 | <0.01 | 0.00 | <0.01 |
| 104 to 105 | [635, 384] | 0.22 (0.05) | 0.14 (0.04) | |||
| >105 | [511,350] | 0.34 (0.06) | 0.26 (0.04) | |||
|
| ||||||
| Time Updated | ||||||
|
| ||||||
| AIDS illness | Yes | [373, 291] | 0.11 (0.04) | <0.01 | ||
|
| ||||||
| CD4 cell count (cells/μL) | 0 to 200 | [275, 101] | 0.00 | <0.01 | ||
| 201 to 350 | [434, 188] | −0.10 (0.03) | ||||
| 351 to 500 | [510, 273] | −0.18 (0.03) | ||||
| >500 | [854, 705] | −0.30 (0.04) | ||||
|
| ||||||
| Number of ART changes4 | 0 | [1197, 173] | 0.00 | <0.01 | ||
| 1 to 2 | [700, 472] | 0.36 (0.02) | ||||
| 3 to 5 | [159, 392] | 0.57 (0.03) | ||||
| 6 to 10 | [17, 186] | 0.57 (0.04) | ||||
| >10 | [0, 44] | 0.56 (0.07) | ||||
|
| ||||||
| % time ART interruption | 0.0 | [1800, 793] | 0.00 | <0.01 | ||
| 0.1 to 5% | [38, 123] | 0.67 (0.04) | ||||
| 5 to 20% | [65, 168] | 1.10 (0.03) | ||||
| 20 to 50% | [100, 110] | 1.44 (0.04) | ||||
| >50% | [70, 73] | 1.86 (0.05) | ||||
|
| ||||||
| Model Fit Statistics (smaller is better) | ||||||
| AIC | 13773 | 11050 | ||||
| BIC | 13939 | 10982 | ||||
| −LogLikelihood | 6862 | 5491 | <0.01# | |||
Linear mixed model, unstructured correlation structure, adjusted for duration of ART and calendar year of ART initiation
Analysis of Variance - Likelihood ratio test, alpha=0.05
MSM – men who have sex with men.
Mono/duo ARV exposure prior to initiating ART.
Initial ART regimen was classified as 2 N(t)RTI’s + (NNRTI or PI or Other); N(t)RTI – nucleos(t)ide reverse transcriptase inhibitors; NNRTI – non-nucleoside reverse transcriptase inhibitors; PI – protease inhibitors.
Includes any modification to ART regimen.
Figure 2, column panel (II) shows the overall general model fit of VCY for baseline and time updated models. Both the baseline and time updated models indicate departures from observed to fitted means at the lower and higher deciles of the ordered VCY distribution. Both baseline and time-updated predictive models broadly over-fit the lower VCY mass of the observed empirical distribution and under fit the upper mass of the distribution. However, translating the models to predict a dichotomised outcome (high/low VCY), the time-updated model is indicative of a reasonable discriminatory power. This finding was consistent across 1, 3, 5 and 10 years duration of ART, Figure 2 column panel (III).
Discussion
Our analysis has shown that a high cumulative measure of viral load after ART initiation is associated with all-cause mortality independent of an adjustment for time updated recent plasma viral load and time updated CD4 cell count. Furthermore we have identified several predictors of viraemia copy-years and established that incorporating time-updated information is necessary to adequately predict a marker of high VCY. Our data also demonstrates for a population receiving ART, episodic viral rebound during continual therapy contributes to a large proportion of patients with a high VCY well above the expected VCY of a strictly virologically suppressed population.
The literature examining the association between a measure of cumulative viral load (viraemia copy-years) and all-cause mortality while receiving ART are conflicting. Two separate research groups have demonstrated an increasing risk of mortality with per-log increasing VCY while adjusting for most recent pVL measure, group 1: HR 1.44 (1.07, 1.94) [9]; group 2: RR 1.52 (1.28, 1.82) [12]. Another study found that although VCY is predictive of mortality following ART initiation in a univariate model, following adjustment for most recent pVL, the VCY measure was no longer significantly predictive of mortality [22]. Our results presented in this analysis fit between these reported findings. The HR calculated from our data, which includes adjustment for recent CD4 cell count and recent pVL was smaller in magnitude and not statistically significant. However, the confidence intervals of our HR estimate however overlap with confidence intervals from the previously reported significant associations. Investigating further, the increased mortality association for a dichotomous indicator of high VCY further supports the notion of an increasing mortality risk with increasing VCY.
Our data also contributes to the existing literature through examination of the mortality association of high VCY stratified by CD4 cell count at start of ART initiation (Figure 3). In this instance, independent of baseline ART CD4 cell count, we see that populations with a marker of high VCY experience higher rates of all-cause mortality. Given that VCY in this study is calculated following 24 weeks of ART, the VCY measure calculated here excludes any cumulative virus exposure experienced prior to initiating ART. Based on the established pVL and CD4 relationship [23] for non-treated populations, we would expect that persons initiating ART with low CD4 cell counts (<200 cells/μL) would have a much higher duration of infection, pointing to a higher cumulative viral burden exposure than those with a shorter duration of infection, i.e. who initiate therapy at higher CD4 cell counts (>350 cells/μL). The Kaplan-Meier curves illustrate the absolute importance of aiming for virological control while receiving ART, particularly for those who initiated therapy at lower CD4 cell counts (e.g. persons presenting late into care with advanced HIV infection) where it has been established are at higher risk of death.
There is not a large established base of determinants of cumulative viral burden. A recent small cohort study which directly aimed to evaluate factors associated with 2 year viraemia copy-years found that in a multivariable analysis, the only significant association was clinical visit adherence [8]. The authors reported an increase in VCY for participants who missed scheduled clinical appointments. In our study we were not able to evaluate this association with our data as we do not collect patient visit schedules.
In our assessment of factors associated with VCY, we note two important points. Firstly, we established that incorporating time-updated information, including treatment information, vastly improves discriminatory power to identify populations with increased VCY while receiving ART for up to 10 years. Secondly, to establish the robustness of our determinants and as a sensitivity analysis to isolate changes in treatment management strategies over time, we showed that recalculating associations based on recently treated participants (initial treatment after 1 January 2006) the majority of factor associations qualitatively maintained the same interpretation. However they were mostly not statistically significant, likely due to limited statistical power with a smaller sample sizes. Exceptions to this statement included two determinants, initial ART anchor agent and number of ART modifications. We speculate that the clear change in direction for these factor associations are due to improved second-generation protease inhibitor antiretroviral agents and better understanding of when to switch therapy for patients [1]. For example, first generation protease inhibitors compared to non-nucleoside reverse transcriptase inhibitors have been shown to be less virologically efficacious [24].
The evolution of the empirical VCY distribution over the duration of ART (Figure 2) reiterates the need for continual viral suppression for persons receiving ART, as has been highlighted in the SMART study of treatment interruptions increasing all-cause mortality [18]. At an individual level, our results show that a measure of high cumulative viral burden is important for predicting mortality after adjustment for recent CD4 cell count and recent pVL. At a population level, VCY might also useful as an indicative measure of total community virus burden in a population receiving antiretrovirals. In order for a treatment as prevention strategy to be successful, year-on-year, the majority of the empirical VCY distribution must be lower than an ideal threshold based on an acceptable level of pVL (in this example we have used <500 copies/ml). A recent AHOD study, stratified by treatment initiation calendar year period, examined the cross sectional proportion of participants where pVL was detectable (>400 copies/ml) while receiving ART [25]. The authors reported relatively low proportions of detectable participants while receiving therapy, approximately 13%, however the authors note that HIV diagnoses in Australia are steadily increasing year-on-year over the same analysis period. As a surveillance tool, a measure of population cumulative viral burden which captures duration of time detectable might be useful for evaluating targeted HIV prevention strategies.
There are several important limitations of this work worth mentioning. In calculating the association between VCY and all-cause mortality, we did not adjust our estimates for confounding due to patient lost to follow-up, visit frequency, or prior time VCY density using inverse probability weighting as done by Mugavero et al [9]. It is likely that those lost from care are at a greater risk of death, therefore our results might underestimate the all-cause mortality risk. Furthermore, due to the analysis design we are not able to assess any survivor bias due to including only participants that survived to receive 24 weeks of ART. We were also unable to make adjustments for viral blips/rebounds due to lifestyle and behavioral confounders [26]. In this analysis we are not reporting the causality of increased VCY on all-cause mortality, rather, we reporting and confirming previously observed associations using a different set of cohort data. Additionally, in our analysis due to data collection limitations, we were unable to identify and adjust for those patients who ceased ART due to a palliative care management plans but still maintained routine HIV monitoring schedules. We acknowledge that many different analytical methods can be used to identify determinants/associations of VCY, including the detailed modelling of factor-response relationships. Future analyses using robust statistical methods as well as modern model selection procedures might adjust for some impacts of the aforementioned limitations.
In summary, our analysis has shown that a high cumulative measure of viral load after initiating ART is associated with increased risk of all-cause mortality. Furthermore we have identified several determinants of viraemia copy-years, possibly useful for identifying subpopulations with increased HIV viraemic burden. We have also established that incorporating time updated information is necessary to adequately predict a dichotomised marker of high VCY. Finally, our data also demonstrates that for a population receiving ART for up to 10 years, episodic viral blips/rebound during continual antiretroviral therapy shifts the majority of an empirical VCY distribution mass above the theoretical expected VCY threshold for a population that continually achieves virus suppression. This indicates surveillance need for monitoring on treatment pVL levels under a treatment as prevention strategy.
Supplementary Material
Acknowledgments
Funding Source
The Australian HIV Observational Database is funded as part of the Asia Pacific HIV Observational Database, a program of The Foundation for AIDS Research, amfAR, and is supported in part by a grant from the U.S. National Institutes of Health’s National Institute of Allergy and Infectious Diseases (NIAID) (Grant No. U01-AI069907) and by unconditional grants from Merck Sharp & Dohme; Gilead; Bristol-Myers Squibb; Boehringer Ingelheim; Roche; Pfizer; GlaxoSmithKline; Janssen-Cilag.
Australian HIV Observational Database contributors
Asterisks indicate steering committee members in 2013.
New South Wales: D Ellis, General Medical Practice, Coffs Harbour; M Bloch, S Agrawal, T Vincent, Holdsworth House Medical Practice, Darlinghurst; D Allen, JL Little, Holden Street Clinic, Gosford; D Smith, C Mincham, Lismore Sexual Health & AIDS Services, Lismore; D Baker*, V Ieroklis, East Sydney Doctors, Surry Hills; DJ Templeton*, CC O’Connor, S Phan, RPA Sexual Health Clinic, Camperdown; E Jackson, K McCallum, Blue Mountains Sexual Health and HIV Clinic, Katoomba; M Grotowski, S Taylor, Tamworth Sexual Health Service, Tamworth; D Cooper, A Carr, F Lee, K Hesse, K Sinn, R Norris, St Vincent’s Hospital, Darlinghurst; R Finlayson, I Prone, A Patel, Taylor Square Private Clinic, Darlinghurst; R Varma, J Shakeshaft, Nepean Sexual Health and HIV Clinic, Penrith; K Brown, C McGrath, V McGrath, S Halligan, Illawarra Sexual Health Service, Warrawong; L Wray, P Read, H Lu, Sydney Sexual Health Centre, Sydney; D Couldwell, Parramatta Sexual Health Clinic; DE Smith*, V Furner, Albion Street Centre; Clinic 16 – Royal North Shore Hospital, S Fernando; Dubbo Sexual Health Centre, Dubbo; Holdsworth House Medical Practice, Byron Bay, J Chuah*; J Watson*, National Association of People living with HIV/AIDS; C Lawrence*, National Aboriginal Community Controlled Health Organisation; B Mulhall*, Department of Public Health and Community Medicine, University of Sydney; M Law*, K Petoumenos*, S Wright*, H McManus*, C Bendall*, M Boyd*, The Kirby Institute, University of NSW. Northern Territory: N Ryder, R Payne, Communicable Disease Centre, Royal Darwin Hospital, Darwin. Queensland: D Russell, S Doyle-Adams, Cairns Sexual Health Service, Cairns; D Sowden, K Taing, K McGill, Clinic 87, Sunshine Coast-Wide Bay Health Service District, Nambour; D Orth, D Youds, Gladstone Road Medical Centre, Highgate Hill; M Kelly, A Gibson, H Magon, Brisbane Sexual Health and HIV Service, Brisbane; B Dickson*, CaraData. South Australia: W Donohue, O’Brien Street General Practice, Adelaide. Victoria: R Moore, S Edwards, R Liddle, P Locke, Northside Clinic, North Fitzroy; NJ Roth*, H Lau, Prahran Market Clinic, South Yarra; T Read, J Silvers*, W Zeng, Melbourne Sexual Health Centre, Melbourne; J Hoy*, K Watson*, M Bryant, S Price, The Alfred Hospital, Melbourne; I Woolley, M Giles*, T Korman, J Williams*, Monash Medical Centre, Clayton. Western Australia: D Nolan, J Robinson, Department of Clinical Immunology, Royal Perth Hospital, Perth.
Coding of Death Form (CoDe) reviewers:
AHOD reviewers: D Sowden, DJ Templeton, J Hoy, L Wray, K Morwood, T Read, N Roth, I Woolley, M Kelly, K Choong. TAHOD reviewers: PCK Li, MP Lee, S Vanar, S Faridah, A Kamarulzaman, JY Choi, B Vannary, R Ditangco, K Tsukada, S Pujari, A Makane, OT Ng, AJ Sasisopin. Independent reviewers: M Boyd.
Footnotes
The views expressed in this publication do not necessarily represent the position of the Australian Government. The Kirby Institute is affiliated with the Faculty of Medicine, University of New South Wales.
References
- 1.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services; 2013. pp. 1–267. Available at http://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf. [Google Scholar]
- 2.World Health Organisation. The use of antiretroviral drugs for treating and preventing HIV infection. 2013:1–272. Available at http://apps.who.int/iris/bitstream/10665/85321/1/9789241505727_eng.pdf. [PubMed]
- 3.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Prevention of HIV-1 Infection with Early Antiretroviral Therapy. N Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eaton JW, Johnson LF, Salomon JA, Bärnighausen T, Bendavid E, Bershteyn A, et al. HIV Treatment as Prevention: Systematic Comparison of Mathematical Models of the Potential Impact of Antiretroviral Therapy on HIV Incidence in South Africa. PLoS Med. 2012;9:e1001245. doi: 10.1371/journal.pmed.1001245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilson DP. HIV Treatment as Prevention: Natural Experiments Highlight Limits of Antiretroviral Treatment as HIV Prevention. PLoS Med. 2012;9:e1001231. doi: 10.1371/journal.pmed.1001231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tanser F, Bärnighausen T, Grapsa E, Zaidi J, Newell M-L. High coverage of ART associated with decline in risk of HIV acquisition in rural KwaZulu-Natal, South Africa. Science. 2013;339:966–971. doi: 10.1126/science.1228160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Attia S, Egger M, Müller M, Zwahlen M, Low N. Sexual transmission of HIV according to viral load and antiretroviral therapy: systematic review and meta-analysis. AIDS. 2009;23:1397–1404. doi: 10.1097/QAD.0b013e32832b7dca. [DOI] [PubMed] [Google Scholar]
- 8.Mugavero MJ, Amico KR, Westfall AO, Crane HM, Zinski A, Willig JH, et al. Early retention in HIV care and viral load suppression: implications for a test and treat approach to HIV prevention. J Acquir Immune Defic Syndr. 2012;59:86–93. doi: 10.1097/QAI.0b013e318236f7d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mugavero MJ, Napravnik S, Cole SR, Eron JJ, Lau B, Crane HM, et al. Viremia copy-years predicts mortality among treatment-naive HIV-infected patients initiating antiretroviral therapy. Clinical Infectious Diseases. 2011;53:927–935. doi: 10.1093/cid/cir526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cole SR, Napravnik S, Mugavero MJ, Lau B, Eron JJ, Saag MS. Copy-years viremia as a measure of cumulative human immunodeficiency virus viral burden. Am J Epidemiol. 2010;171:198–205. doi: 10.1093/aje/kwp347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zoufaly A, Stellbrink HJ, Heiden der MA, Kollan C, Hoffmann C, van Lunzen J, et al. Cumulative HIV Viremia during Highly Active Antiretroviral Therapy Is a Strong Predictorof AIDS-Related Lymphoma. J Infect Dis. 2009;200:79–87. doi: 10.1086/599313. [DOI] [PubMed] [Google Scholar]
- 12.Brennan A, Maskew M, Sanne I, Fox MP. Viremia Copy-years as a Measure of Viral Load Burden and Associated Mortality Risk among ART Patients. Johannesburg, South Africa: p. 308. [Google Scholar]
- 13.Lewden C, Bouteloup V, De Wit S, Sabin C, Mocroft A, et al. The Collaboration of Observational HIV Epidemiological Research Europe (COHERE) in EuroCoord. All-cause mortality in treated HIV-infected adults with CD4 >=500/mm3 compared with the general population: evidence from a large European observational cohort collaboration. International Journal of Epidemiology. 2012;41:433–445. doi: 10.1093/ije/dyr164. [DOI] [PubMed] [Google Scholar]
- 14.The Antiretroviral Therapy Cohort Collaboration. Mortality of HIV-infected patients starting potent antiretroviral therapy: comparison with the general population in nine industrialized countries. International Journal of Epidemiology. 2009;38:1624–1633. doi: 10.1093/ije/dyp306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McManus H, O’Connor CC, Boyd M, Broom J, Russell D, Watson K, et al. Long-Term Survival in HIV Positive Patients with up to 15 Years of Antiretroviral Therapy. PLoS ONE. 2012;7:e48839. doi: 10.1371/journal.pone.0048839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodger AJ, Lodwick R, Schechter M, Deeks S, Amin J, Gilson R, et al. Mortality in well controlled HIV in the continuous antiretroviral therapy arms of the SMART and ESPRIT trials compared with the general population. AIDS. 2013;27:973–979. doi: 10.1097/QAD.0b013e32835cae9c. [DOI] [PubMed] [Google Scholar]
- 17.Australian HIV Observational Database. Rates of combination antiretroviral treatment change in Australia, 1997–2000. HIV Med. 2002;3:28–36. doi: 10.1046/j.1464-2662.2001.00094.x. [DOI] [PubMed] [Google Scholar]
- 18.El-Sadr WM, Lundgren JD, Neaton JD, Gordin F, Abrams D, et al. Group SFMOATSS. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355:2283–2296. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 19.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2013. URL http://www.R-project.org/ [Google Scholar]
- 20.Bates Douglas, Maechler Martin, Bolker Ben, Walker Steven. lme4: Linear mixed-effects models using Eigen and S4. R package version 1.0–5. 2013 http://CRAN.R-project.org/package=lme4.
- 21.Therneau T. A Package for Survival Analysis in S. R package version 2.37–4. 2013 http://CRAN.R-project.org/package=survival.
- 22.Chirouze C, Journot V, Le Moing V, Bernard L, Taieb A, Katlama C, et al. Viremia Copy-years as a Predictive Marker in HIV-1+ Patients Initiating a Protease Inhibitor-containing HAART. p. 537. [DOI] [PubMed] [Google Scholar]
- 23.Poli G, Pantaleo G, Fauci AS. Immunopathogenesis of Human Immunodeficiency Virus Infection. Clinical Infectious Diseases. 1993:17. [PubMed] [Google Scholar]
- 24.Chou R, Fu R, Huffman LH, Korthuis PT. Initial highly-active antiretroviral therapy with a protease inhibitor versus a non-nucleoside reverse transcriptase inhibitor: discrepancies between direct and indirect meta-analyses. Lancet. 2006;368:1503–1515. doi: 10.1016/S0140-6736(06)69638-4. [DOI] [PubMed] [Google Scholar]
- 25.Law MG, Woolley I, Templeton DJ, Roth N, Chuah J, Mulhall B, et al. Trends in detectable viral load by calendar year in the Australian HIV observational database. J Int AIDS Soc. 2011;14:10. doi: 10.1186/1758-2652-14-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sabin CA, Cooper DA, Collins S, Schechter M. Rating evidence in treatment guidelines. AIDS. 2013;27:1839–1846. doi: 10.1097/qad.0b013e328360d546. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.