Abstract
We report a new technique for high-resolution in vivo imaging of myelinated axons in the brain, spinal cord and peripheral nerve that requires no fluorescent labeling. This method, based on spectral confocal reflectance microscopy (SCoRe), uses a conventional laser scanning confocal system to generate images by merging the simultaneously reflected signals from multiple lasers of different wavelengths. Striking color patterns unique to individual myelinated fibers are generated that facilitate their tracing in dense axonal areas. These patterns highlight nodes of Ranvier and Schmidt-Lanterman incisures and can be used to detect various myelin pathologies. Using SCoRe we performed chronic brain imaging up to 400 μm deep, capturing for the first time de novo myelination of mouse cortical axons in vivo. We also established the feasibility of imaging myelinated axons in the human cerebral cortex. SCoRe adds a powerful component to the evolving toolbox for imaging myelination in living animals and potentially in humans.
Keywords: Spectral confocal reflectance microscopy, two photon microscopy, myelin, demyelination, in vivo imaging, cortex, spinal cord, sciatic nerve, human brain, multiple sclerosis, oligodendrocyte, neuropathy, node of Ranvier, Schmidt-Lanterman incisures
Introduction
Myelin is a complex cellular structure that plays critical roles in action potential propagation, axonal insulation and trophic support1 and is a potential site of experience-dependent neural plasticity2,3. Oligodendrocytes and Schwann cells, the myelin producing cells, are affected in a variety of pathologies involving the brain, spinal cord and peripheral nerves4,5.
Imaging techniques such as electron and confocal fluorescence microscopy have been invaluable in furthering the cellular understanding of myelin development, plasticity and pathology. Diffusion tensor magnetic resonance imaging (DTI), has allowed longitudinal studies of cerebral white matter tracts in animal models and humans6, and genetically encoded fluorescent reporters have allowed imaging of oligodendrocytes in living organisms7,8. Methods have also been developed for label-free imaging of myelinated fibers using coherent anti-Stokes Raman scattering (CARS)9–11, optical coherence (OCM) 12 or third harmonic generation (THG)13,14 microscopy.
We developed a powerful yet easy to implement method for high-resolution label-free in vivo imaging of myelinated axons using a conventional laser scanning confocal microscope with tunable wavelength detection capabilities. This method is based on spectral confocal reflectance microscopy (SCoRe) and takes advantage of the high refractive index of lipid-rich myelin15,16. Reflection signals are obtained using multiple confocal lasers, which individually generate images of discontinuous segments but when merged, constitute contiguous myelinated axon images.
Using SCoRe we imaged longitudinally for the first time, fine changes in axonal myelination in the living mouse cortex. We were able to track Schmidt-Lanterman incisures and nodes of Ranvier in vivo in normal and pathological conditions. We also implemented SCoRe concurrently with confocal fluorescence or two-photon microscopy to image the interactions between axons, oligodendrocytes, and other cell types such as astrocytes and microglia. Finally, we demonstrate in a postmortem human cortical explant that SCoRe can be used for high-resolution imaging of cortical myelinated axons directly through the pial surface.
Results
In vivo imaging of myelinated axons with SCoRe microscopy
We first applied single-wavelength confocal reflectance microscopy through a thinned skull in an anesthetized mouse (Fig. 1). We noticed a reflective network pattern, that although patchy (Fig. 1c), was reminiscent of cortical layer I axons17. When using different laser wavelengths, the images remained patchy but did not fully overlap (Fig. 1c). Interestingly, simultaneous imaging with multiple wavelengths gave a complementary reflection pattern that when composited and pseudocolored monochromatically (Fig. 1c–f), appeared as a continuous image, revealing that these processes projected for long distances (Fig. 1b and Supplementary Video 1). Similar wavelengths (476 and 488 nm) tended to generate reflective patches at similar locations along the fiber, while more distant wavelengths (488, 561 and 633 nm) produced less overlapping patches (Fig. 1g–h). Furthermore, the merged images appeared contiguous when using only 488, 561 and 633 nm lasers, and additional wavelengths (Figure 1d–f) or images captured with a white light broadband laser (data not shown), did not lead to more contiguous images. Therefore, we used those lasers for all subsequent experiments.
Figure 1. In vivo imaging of mouse cortex using spectral confocal reflectance microscopy (SCoRe).
(a) Diagram depicting the imaging and optical setup of SCoRe. Three laser wavelengths are emitted simultaneously and reflect off structures in the mouse cortex. Out of focus light is rejected by the pinhole within the microscope, and the reflected light is separated by a prism into three separate photodetectors. (b) Simultaneous imaging of brain vasculature with intravenous injection of fluorescent dextran (red) and combined-wavelength image of reflective fibers (cyan) in the somatosensory cortex. Z-projection over 15 μm. 100 μm scale bar. (c) High magnification images of monochromatic reflective signal captured with the 458, 476 488, 514, 561, and 633 nm lasers and then merged as indicated (bottom panels). 5 μm scale bar. (d–f) Comparison of all lasers to 488, 561 and 633 shows that these three are sufficient for full fiber detection. 5 μm scale bar. (g) Graph showing the reflection intensity along the axon boxed in f demonstrating that lasers of wavelengths 488, 561 and 633 nm have some overlapping but mostly non-overlapping reflection peaks. (h) Graph showing the reflection pattern of similar wavelengths (476 and 488 nm) is mostly overlapping. (i) SCoRe Z-projection (magenta) captured from a Thy1-YFP mouse showing a YFP-labeled axon (green) that is reflective (arrows), however most YFP-labeled axons and all dendrites are not reflective. 50 μm scale bar (experiments were replicated 3 times in n=15 mice).
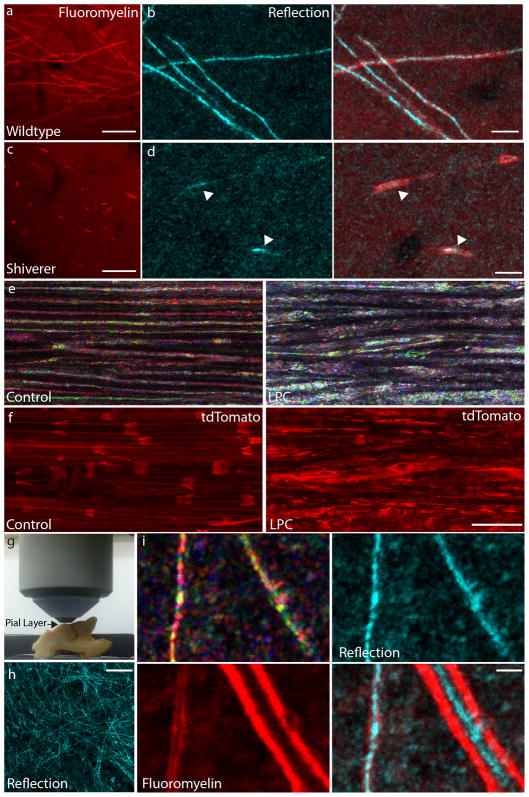
To better characterize these reflective fibers, we combined in vivo SCoRe with confocal fluorescence microscopy. Using mice that express yellow fluorescent protein in a subset of layer V cortical pyramidal neurons (Thy1-YFP), we found that none of the fluorescent dendrites were reflective, while some reflective processes co-localized with YFP (Fig. 1i and Supplementary Video 1) suggesting that they represented myelinated axons, given myelin’s known reflective properties15,16. To confirm this, we labeled myelin through a craniotomy with Fluoromyelin (FM) dye and found that it robustly labeled the myelin sheath of superficial cortical axons as evidenced by their tubular appearance (Fig. 2a). We found that 100% of FM-labeled fibers were reflective (Fig. 2a), while YFP-labeled dendrites, unmyelinated axons (FM-negative) (Fig. 2b), astrocytes and microglia (Supplementary Fig. 1) produced no reflectance. Furthermore, FM-negative axonal segments, such as at some axonal bifurcations (Fig. 2c), which lack myelin18,19, were not reflective, even though the axon was normal as evidenced by intact YFP. Additionally, while imaging peripheral nerves in vivo with SCoRe, we found that individual FM-labeled axons lacked reflection at non-myelinated nodes of Ranvier (Figs. 2d, and 4d–f, i–j). These data strongly suggested that myelin is the source of fiber reflection in both cortex and peripheral nerves.
Figure 2. SCoRe signal is dependent on myelination.
(a) In vivo staining of cortical myelin with Fluoromyelin (FM) (red) labels only the reflective fibers (cyan). 10 μm scale bar. (b) In vivo FM staining in the cortex of a Thy1-YFP mouse showing that YFP-labeled axons (green), that are FM negative and therefore unmyelinated axons (arrowhead), are not reflective. Dendrites (arrow), which are never myelinated, are also not reflective. However, all FM positive segments are reflective (cyan). 10 μm scale bar. (c) Example of an axonal bifurcation in a Thy1-YFP mouse imaged in vivo demonstrating that specific parts of an axon that are FM positive are also reflective, however the unmyelinated, FM negative portions of the same axon are not reflective (arrow). 3 μm scale bar. (d) In vivo staining and imaging of myelin in the sciatic nerve with FM (red), reveals the location of nodes of Ranvier (arrows) which lack reflection (cyan). 15 μm scale bar (experiments were replicated 3 times in n=13 mice).
Figure 4. Multicolor reflection spectrum reveals distinct myelin structures in the spinal cord and sciatic nerve in vivo.
(a–b) Multicolor SCoRe images captured from the spinal cord (a) and sciatic nerve (b) showing that individual fibers reflect different colors but have a predominant color consistency along each axon. 25 μm scale bars. (c) Two differentially reflecting axons in the sciatic nerve at high-resolution. 10 μm scale bar. (d–f) In vivo SCoRe and fluorescence images captured from an mT/mG mouse expressing tdTomato in myelin sheaths (red) (d) showing Schmidt-Lanterman incisures (arrows) and nodes of Ranvier (arrowheads) (e). Combined reflection image in cyan is shown in composite with tdTomato in (f). 20 μm scale bar in d–f. (g–h) High magnification image of two Schmidt-Lanterman incisures showing SCoRe vertical interference pattern and fluorescent tdTomato (white) overlay (g) and SCoRe alone (h). (i–j) High magnification images of two Schmidt-Lanterman incisures and one node of Ranvier showing all reflected lasers (i) and combined reflection (cyan) with tdTomato fluorescence (red) (j). 4 μm scale bar in g–j (experiments were replicated 3 times in n= 8 mice for spinal cord, n=10 mice for sciatic nerve).
Curiously, even though myelin is the source of reflectance, we observed that when viewed in the XY plane, the signal appeared to arise from the center of the myelinated tube, where the axon is located, rather than from the sides of the tube as seen with FM labeling (Fig. 2). To resolve this apparent contradiction, we analyzed orthogonal (XZ) views of individual axons in the sciatic nerve from Thy1-YFP mice (Supplementary Fig. 2). In the XZ plane, it became apparent that the reflection originates above and below the YFP-labeled axon, generating an hourglass shape, with little reflection observed at the center of the axon (Supplementary Fig. 2a–b, f–g), demonstrating that the source of reflection is indeed the surrounding myelin. This was simulated experimentally by imaging a pulled glass capillary similar in size to an axon, filled with fluorescently-labeled agarose. We found a strikingly similar hourglass reflection shape with the outer and inner glass surfaces being most reflective (Supplementary Fig. 2c–d). This strongly suggests that only the portion of light reflecting at a particular angle of incidence is captured by the confocal detectors (Supplementary Fig. 2e), resulting in the “axonal” appearance in XY projections. Furthermore, measurements of the outer diameter obtained from XZ reflection images, matched almost perfectly those obtained from the XY confocal fluorescent images of FM myelin labeling (Supplementary Figs. 2g, and 3). This not only provides additional confirmation that the reflection signal originates in the myelin sheaths but also constitutes an accurate means for measuring outer fiber diameter in vivo in a label-free fashion.
Although, with SCoRe, the wavelengths used are shorter and light collection less efficient than with two-photon microscopy, we were able to detect cortical myelinated axons as deep as 400 μm, even with low laser intensities (~300μW at the sample) (Supplementary Video 2). This is likely explained by the fact that myelin is highly reflective, making light scattering and collection efficiency less critical for SCoRe compared to fluorescence imaging.
Combined use of SCoRe with fluorescence microscopy
To further characterize the reflection signal, we implemented SCoRe simultaneously with fluorescence microscopy in fluorescent reporter-expressing transgenic mice under control of the proteolipid protein promoter (PLPDsRed)20. In these mice, single oligodendrocytes can be visualized in the superficial cortex with either two-photon (Supplementary Fig. 4 a–b) or confocal (Supplementary Fig. 4 c–g) microscopy in vivo. However, although their fluorescent processes can be clearly detected, it is not possible to accurately trace individual myelinated axons, because oligodendrocytes have a large number of branches that provide myelin for only a single internode.
Combining SCoRe and confocal fluorescence microscopy in vivo allowed us to precisely trace individual myelinated fibers for long distances spanning several internodes. Oligodendrocyte processes were seen running along reflective fibers but several processes especially those close to the oligodendrocyte cell bodies did not produce any reflection, suggesting that they do not form compact myelin sheaths (Supplementary Fig. 4d,g). Thus SCoRe provides unique information about the status of myelin compaction which to our knowledge is not possible with other optical methods. The combination of SCoRe and fluorescence imaging constitutes a powerful tool set for studying oligodendrocyte interactions with axons, formation of a compact myelin sheath, oligodendrocyte injury, myelin degeneration and regeneration.
Distinct patterns of cortical myelin dynamics in neonates and adults
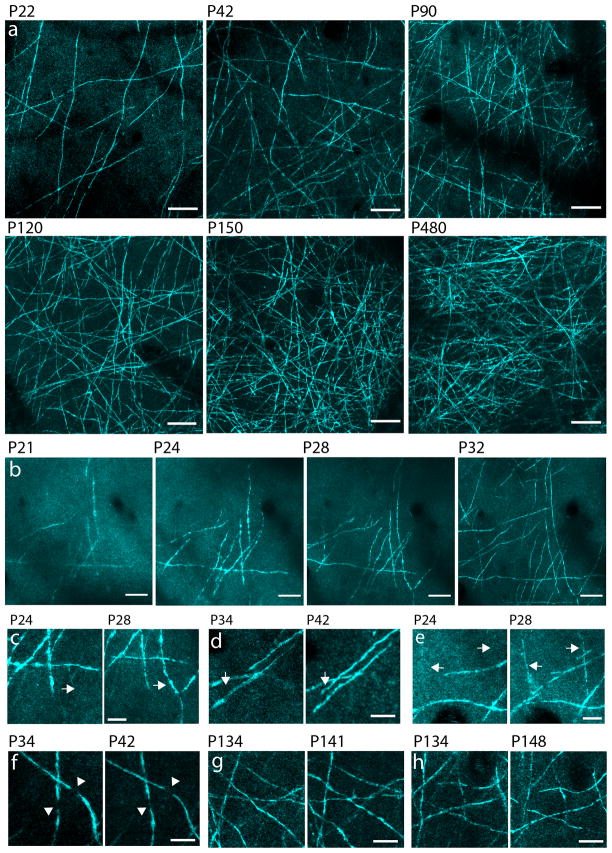
We used SCoRe for time-lapse imaging of layers I–II cortical myelin in mice of various ages. Consistent with the known time course of cortical myelin development22, mice younger than 2 weeks, had neither reflective nor FM stained axons (data not shown) but around P18–21, reflective fibers that appeared as patches (~200 μm in diameter), were first detected (Fig 3 and Supplementary Fig. 5), closely resembling the morphology of individual myelinating oligodendrocytes23 (Supplementary Fig. 5). At subsequent developmental stages and into adulthood we found a steady increase in the density of reflective axons (Fig. 3a).
Figure 3. Transcranial time-lapse imaging of the mouse cortex reveals progressive age-dependent myelination.
(a) In vivo SCoRe z-projections taken from the mouse somatosensory cortex at various postnatal ages. (b) Images of the same cortical region captured through a thinned skull over four time points showing the appearance of new reflecting fibers. 20 μm scale bars in a–b. (c–e) High magnification z-projections of specific axons that were myelinated between imaging sessions (arrows) at the ages indicated, demonstrating that SCoRe reveals new myelin formation in vivo. (f–h) Z-projections showing repeated imaging of suspected nodes of Ranvier (f) (arrowheads) and stable myelinated axons (g–h) in older animals. 10 μm scale bars in c–h (experiments were replicated 3 times in n=9 mice).
Repeated imaging of the same regions over weeks revealed cortical sub-regions that became myelinated, likely due to the maturation of individual oligodendrocytes (Fig. 3b) and also individual axons which became newly myelinated (Fig. 3c–e), stable unmyelinated regions consistent with nodes of Ranvier (Fig. 3f), and unchanged myelinated regions in older animals (Fig. 3g–h). These examples show how fine myelin dynamics can be investigated during development and in the adult using SCoRe.
The spectral reflectance highlights unique myelin features
The reflectance signal from various lasers not only produced a contiguous myelinated axonal image (Fig. 1c–f), but at high zoom appeared as a spectral speckled pattern (Fig. 1c). Remarkably, despite this heterogeneous speckle, in the spinal cord and sciatic nerve, individual axons also had a predominant reflectance color that allowed us to distinguish them from adjacent axons (Fig. 4 and Supplementary Video 3) and could be used for identification of axons over time as the pattern changed minimally over days and was not significantly affected by axial sample rotations (Supplementary Fig. 6). The reflection signal is likely to originate from changes in refractive index between the mostly aqueous neural tissue24 and the lipid-rich myelin, while the multicolor reflection is likely due to thin-film interference25, which leads to constructive and destructive interference of particular wavelengths, depending on differences in the thickness of the reflective surface (See Supplementary Fig. 2). Therefore, the number, thickness, and relative composition of the membranous myelin layers may have a direct impact on the reflected spectrum. The most likely explanation for the differences in the overall color of adjacent axons in the peripheral nerve and spinal cord is the large inter-axonal variability in diameter and degree of myelination in contrast with the more homogeneous axons in the superficial cortex. Regardless of the precise mechanism, these colors allow the identification and tracing of individual axons over long distances in peripheral nerves and spinal cord (Fig. 4).
High-resolution imaging in the sciatic nerve revealed a peculiar periodic vertical multicolor reflection pattern at irregular intervals of approximately 20–60μm (Fig. 4d–j). To investigate its cellular origin, we reasoned that mT/mG transgenic mice, which express tdTomato in cell membranes, would be useful for simultaneously imaging myelin and its reflection in peripheral nerves. Indeed, we observed highly detailed images of myelin layers (Supplementary Fig. 3) and noticed the typical oblique appearance of Schmidt-Lanterman incisures (SLI)26 (Fig. 4d). Remarkably, these periodic vertical reflection areas completely colocalized with fluorescent SLIs. These incisures are cytoplasmic channels, within the otherwise compact myelin that are critical for molecular flow along myelin layers26. To our knowledge, SCoRe is the only label-free technique that can unambiguously image these structures in vivo, opening the possibility of studying them in the context of a variety of pathologies.
Imaging myelin pathology and the human brain with SCoRe
To determine if SCoRe is able to detect myelin defects, we used shiverer mice, a well-known model in which mutation of the myelin basic protein (MBP) gene prevents the formation of compact myelin in the CNS. In vivo imaging of the cortex of shiverer mice showed a marked paucity of reflective fibers (Fig. 5), with occasional small scattered segments of reflectance but no continuous axons (Fig. 5d). To test if these were areas lacking myelin, we labeled the cortex by topical application of Fluoromyelin (FM). Indeed, shiverer mice had severely reduced labeling, but the small scattered areas of reflection uniformly colocalized with FM labeling (Fig. 5d). Imaging on brain slices from shiverer mice confirmed the dramatic reduction in the reflection signal in the corpus callosum (Supplementary Fig. 7). This demonstrates unambiguously that the reflectance signal is dependent on the presence of compact myelin and shows that SCoRe is a sensitive method for detecting central myelination defects.
Figure 5. Myelin pathology and human myelinated axons imaged with SCoRe.
(a–d) Images captured through a cranial window in P35 wildtype (n=3 mice, 5 replicates) (a–b) and congenitally hypomyelinated shiverer mouse (n=2 mice, 4 replicates) (c–d). In shiverer, we saw small patches of Fluoromyelin (FM)-labeled myelinated axon segments (c), which we never observed in wildtype mice (a). These patches were also reflective only in the region that was FM-labeled (d, arrowheads). 25 μm scale bar in a,c, 5 μm in b,d. (e–f) Images acquired in vivo from the sciatic nerve of an mT/mG mouse with membrane bound tdTomato before (left) and after (right) intraneural injection of the demyelinating agent lysophosphatidylcholine (LPC), showing an acute change in the reflected spectrum (e) and in the tdTomato fluorescence distribution (f). 30 μm scale bar in e–f (n=3 mice, 3 replicates). (g) Photograph showing the setup for SCoRe imaging of the cortex through the pial surface in a fixed human brain explant. (h) Z-projection reflection image obtained from the human brain explant. 30 μm scale bar. (i) High-magnification multicolor (top left) and combined (cyan) images of two myelinated reflective fibers demonstrated by Fluoromyelin labeling (red). 3 μm scale bar.
We also imaged the sciatic nerve of shiverer mice as these have been reported to have alterations in the number of Schmidt-Lanterman incisures27. Indeed, we were able to show that these mice have increased density of SLIs (Supplementary Fig. 7), demonstrating that SCoRe is able to detect in vivo very subtle peripheral myelin changes. In addition, we were able to demonstrate rapid changes in the multicolor pattern after exposure to the demyelinating agent lysophosphatidylcholine (LPC), Dimethyl sulfoxide (DMSO) or a hypotonic solution (Fig. 5 e–f and Supplementary Fig. 8) and also detected changes in myelination during axonal degeneration and regeneration after sciatic nerve crush (Supplementary Fig. 8). Thus, SCoRe can be used to detect a variety of myelin pathologies in vivo.
Finally, we imaged a paraformaldehyde fixed postmortem human brain explant to determine if myelinated fibers could be detected in the human cortex. We oriented the tissue to image the surface of the cortex mimicking the situation for in vivo imaging of mouse brain (Fig. 5g). Consistent with mouse images, we saw a highly reflective network of axons (Fig. 5h) that colocalized with Fluoromyelin labeling (Fig. 5i), confirming these were indeed myelinated axons. These data show the feasibility of imaging myelinated axons in human tissue with high resolution, low laser power and no dye administration.
Discussion
In vivo optical imaging has been invaluable for understanding the plasticity of cells in the nervous system during development, aging and pathology28–30. We have developed a technique that allows label-free high-resolution in vivo imaging of myelinated axons using spectral confocal reflectance microscopy (SCoRe). Because SCoRe only requires a confocal microscope with tunable wavelength detection capabilities, which is routinely used throughout the world, this technique could have wide applications in preclinical animal studies of myelin pathologies. In addition, SCoRe has the potential to be adapted as a tool for in vivo human peripheral and cortical myelin imaging.
Although the precise mechanism for the multicolor reflection is not clear, it likely relates to the principle of thin-film interference25. Similar to dichroic mirrors with alternating layers of optical coatings of different refractive indices, myelin is composed of many layers of lipid-rich sheaths. This layered structure leads to constructive and destructive light interference, reinforcing certain reflected wavelengths and suppressing others (Supplementary Fig. 2). The thickness and number of layers may determine the wavelengths that are preferentially reflected, with further variability due to focal irregularities in myelin sheath thickness, lipid composition and other local cellular variables. Interestingly, we found that in addition to the focal color patchiness, individual axons displayed a unique overall color signature. These unique color features of individual axons in the spinal cord and peripheral nerve facilitated their identification and tracing during time-lapse imaging despite the high density of adjacent processes (Figs. 4 and Supplementary Fig. 6), analogous to methods like Brainbow32 or Diolistic labeling33. We also found in peripheral nerve axons a striking periodic multicolor reflection pattern derived from Schmidt-Lanterman incisures; which can now be studied in vivo.
Several methods are currently available for in vivo imaging of myelin and myelin producing cells. Confocal and two photon microscopy has been used with genetically encoded fluorescent markers in zebra fish oligodendrocytes7. Using these fluorescent reporters, however, it is not easy to trace individual myelinated axons or determine when oligodendrocyte cellular processes near an axon have established a mature compact myelin sheath. Furthermore, although fluorescent dyes such as Fluoromyelin can be useful for in vivo imaging, labeling with these dyes is not consistent, making them unsuitable for longitudinal imaging. SCoRe, however, provides a uniform traceable image of the axon, is very sensitive to the presence of a compact myelin sheath and allows repeated transcranial imaging of fine structural myelin changes (Supplementary Fig. 6 and Fig. 3).
While label-free methods such as OCM, CARS and THG have great potential, they require complex setups or rarely available equipment, whereas SCoRe uses a conventional confocal microscope making it easy to implement. Furthermore, because SCoRe is highly sensitive to myelin, it requires light levels that are substantially lower (on the order of 200–400 μW at the sample) than those used for conventional confocal fluorescence or two photon microscopy. Thus, this technique can be used for repeated imaging at high zooms with virtually no photo-toxicity or thermal injury (Fig. 3), making it ideal for in vivo use. Longer wavelength Ti:sapphire lasers, can also be used (data not shown), which would in theory allow it to achieve greater penetration than two photon microscopy as both incident and reflected lights would be infrared and thus less scattering.
Using SCoRe, we made several novel observations in vivo: we tracked longitudinally for the first time newly formed myelinated structures in the living mammalian brain and found that myelination progresses rapidly over days (Fig. 3) but change is confined to isolated micro-regions (Fig. 3 and Supplementary Fig. 5), likely representing territories of single oligodendrocytes. Second, we found areas lacking myelination at axonal bifurcations in the cortex, an under-investigated phenomenon18 that can now be studied in vivo. Third, we imaged for the first time longitudinally in a mouse, Schmidt-Lanterman incisures (Fig. 4) and nodes of Ranvier (Figs. 3 and 4). Fourth, by concurrently using SCoRe with fluorescence imaging, we showed that a substantial number of proximal oligodendrocyte processes do not form a compact myelin sheath.
Future modifications of SCoRe with infrared laser excitation and detection capabilities36 or fiber-optic coupling, would increase the imaging depth, and may eventually allow imaging of human brain, spinal cord and peripheral nerve. For example, recently it has become possible to generate myelin with engrafted neural stem cells in humans with severe leukodystrophies35,37. Additionally, it has been documented that subpial cortical demyelination is one of the earliest pathological events in multiple sclerosis38,39. SCoRe, which allows high resolution imaging in the intact cortex, could potentially be used for tracking the formation of myelin after engraftment or its degeneration in severe demyelinating disorders or traumatic brain injury. An approach based on SCoRe could also be applied for imaging peripheral nerves instead of tissue biopsy in various polyneuropathies. Thus, SCoRe is a powerful technique that adds significant capabilities to the toolbox for in vivo imaging of the central and peripheral nervous systems in animal models and potentially in humans.
Online Methods
Animals
All animal procedures were approved by and carried out in accordance with Yale University IACUC guidelines and were performed on both male and female mice aged P15–P480 as indicated in the text. Mouse lines used included Thy1-YFP line H40 (Jackson Labs # 003782), CX3CR1-GFP41 (Jackson Labs #005582), MBPshi (Jackson Labs #001428), mT/mG42 (Jackson Labs #007576) and C57BL/6 (Jackson Labs #000664), PLPDsRed20. Postmortem human tissue was a deidentified tissue sample from a deceased individual from the Northwestern Alzheimer Disease Center IRB-approved Tissue Bank. No investigator blinding for group allocation was necessary for the experiments described in this study. No statistical methods were used to predetermine sample size. Randomization and a power analysis were not necessary for this study.
Spectral Confocal Reflectance Microscopy
We used a Leica SP5 confocal microscope with a water immersion objective (Leica 20x, 1.0 NA), using 458, 476, 488, 514, 561, and 633 nm laser wavelength outputs sent through an Acousto-Optical Tunable Filter (AOTF) and a 30/70 partially reflective mirror. The reflected light was collected using three photodetectors set to collect light through narrow bands defined by prism and mirror-sliders, centered around the laser wavelengths, 486–491nm, 559–564nm, and 631–636nm respectively. The channels from each photodetector were then considered independently, additively combined into one channel, or shown as a color composite with 488 as blue, 561 as green, and 633 as red. For detailed spectral analysis we also used a broadband white light laser (Leica SP8 confocal microscope, with a multi-immersion objective 20x, 0.75 NA). Laser intensities for SCoRe ranged from 200–400 μW at the sample depending on the preparation used (thin skull vs. cranial window), the tissue being imaged, and the specific laser as longer wavelength lasers required less power for sufficient signal and penetration. Images were analyzed using NIH ImageJ. 3D reconstructions were created with Imaris imaging software (Bitplane Scientific Software).
Two-photon Imaging
We used a mode locked MaiTai tunable laser (Spectra Physics) with a two photon microscope (Prairie Technologies) tuned to 1040nm for imaging of DsRed. Images were taken with a water immersion objective (Leica 20x, 1.0 NA) at depths up to 300 μm below the pial surface.
In vivo imaging of the mouse cortex
The thin skull procedure was used for acute and chronic trans-cranial imaging as described previously17 while the cranial window procedure was used for acute imaging sessions with dye labeling. Briefly, mice were fully anesthetized using isoflurane (MBP shiverer) or Ketamine/Xylazine, and the scalp was shaved and sterilized. A midline scalp incision was made, and a custom made metal plate was affixed to the skull using cyanoacrylate. An area no more than 1mm was thinned with a high speed drill and a microsurgical blade to a thickness of 20–30 μm, or removed along with the underlying dura for the cranial window. For fluorescent myelin labeling, Fluoromyelin (Life Technologies) was applied directly to the exposed cortex in a 50% dilution in PBS from stock solution for 45 minutes and then washed thoroughly. Occasionally, we observed reflective fibers that were partially labeled with Fluoromyelin which was likely due to regional variation in dye penetration resulting in incomplete dye labeling. Cortical vasculature was visualized with intravenous injection of 70,000 MW Texas red dextran (Life Technologies). For astrocyte labeling 50 μM sulforhodamine 10143 dissolved in PBS was applied for 20 minutes to the exposed cortex and then washed thoroughly. A #0 glass coverslip cut to size was placed over the cranial window and glued in place using cyanoacrylate.
In vivo imaging of the sciatic nerve
Mice were fully anesthetized using Ketamine/Xylazine and the skin was thoroughly shaved and sterilized on the lower back and upper thigh. A small incision was made in the skin above the plane between the vastus lateralis and the biceps femoris muscles. The skin was gently dissected from the underlying musculature and the sciatic nerve was exposed by separating the vastus lateralis and biceps femoris and separated from the surrounding connective tissue. A custom made metal rod was used to gently elevate the separated nerve to immobilize it for imaging. After imaging, the nerve was lowered back into its original location and the incision was sutured. In some cases, fine #5 forceps were used to cause a controlled crush injury of the nerve. The nerve was pinched for 20 seconds to cause a reproducible crush without severing the nerve. The nerve was imaged before, immediately after, and at one subsequent time point (5–10 days). Otherwise, for acute myelin damage, 1 μL of 2.5% lysophosphatidylcholine (LPC, Sigma Aldrich), 100% DMSO, or 100% double distilled water was injected into the sciatic nerve with a small pulled glass micropipette. Additionally, myelin within the sciatic nerve was sometimes labeled with a direct injection of 0.5–2 μL of Fluoromyelin using a glass electrode This was necessary because the dyes would not diffuse across the surrounding epineurium.
In some cases when projecting images for figure display (Figure 4g and j), it was necessary to merge single 1 μm z-sections that were 3 μm apart, because the best alternating interference pattern signal comes from the top of the axon while the ideal fluorescence incisure signal comes from the middle of the axon.
In vivo imaging of the spinal cord
Mice were fully anesthetized using Ketamine/Xylazine and the back of the mouse was shaved and sterilized with alcohol and betadine. A dorsal midline incision of ~1.5cm was made over vertebrae T10 to L2 and the muscles surrounding the spinous and transverse processes were gently removed to expose the underlying vertebrae. Two standard sterilized staples were used as small anchors and were glued with surgical grade cyanoacrylate to the pedicles of the vertebrae then further secured with dental cement44. A small custom shaped metal rod was placed in the dental cement to serve as an anchor point to immobilize the animal during imaging sessions. Next, a laminectomy was performed on 1 vertebra using a small set of dissecting scissors. 1% low melting agarose was applied to the exposed spinal cord and then a #0 glass coverslip was secured on top of the agarose with cyanoacrylate and then dental cement. After the dental cement had dried the animal was secured to a custom built holder for imaging. In some cases 1 μL of Fluoromyelin dye was injected into the spinal cord to label myelin.
Human explant imaging
A 4% paraformaldehyde fixed explant of human brain (entorhinal cortex) was oriented to perform SCoRe imaging through the pial surface as shown in Figure 5g. Laser intensities necessary for optimal SCoRe signal were similar to those used for in vivo mouse imaging (200–400 μW at the sample) with a 20x 1.0 NA water immersion objective. Fluoromyelin was applied to the cortical surface for 20 minutes to label myelin within the superficial cortex and then washed thoroughly with PBS.
Glass micropipette imaging
Pulled glass micropipettes (diameters ranging from 2–20μm) were filled with 1% agarose containing Alexa 488 dye and then immersed in PBS for imaging. Pipettes were imaged with SCoRe and fluorescence with identical settings to those used for in vivo SCoRe imaging of both the cortex and sciatic nerve.
Supplementary Material
Supplementary Figure 1. Simultaneous imaging of myelinated axons, microglia and astrocytes. in vivo SCoRe (cyan) and confocal fluorescence images captured through a cranial window after topical application of sulforhodamine 101 (red) to label astrocytes in a Thy1-YFP (green) (a–d) or CX3CR1GFP (green) transgenic mouse (e–h) demonstrating implementation of SCoRe with confocal fluorescence imaging in vivo and also showing that astrocytes, dendrites, and microglia are not reflective. 30 μm scale bars (experiments were replicated 5 times in n=3 mice).
Supplementary Figure 2 and discussion: Mechanisms and source of the reflection signal in SCoRe. (a) Orthogonal (XZ) image of a YFP-labeled axon (red) in a Thy1-YFP sciatic nerve imaged in vivo showing the reflection (cyan) originating from the myelin and not the axon. 1 μm scale bar. (b) XY-Image of the same axon shown in a at the top, middle and bottom of the axonal z-stack, showing strong reflection signal at the level of the myelin on both the top and bottom, but with very little reflection at the center of the axon. 1 μm scale bar. (c) Orthogonal (XZ) SCoRe image of a glass micropipette filled with fluorescently labeled agarose (red), showing a similar hourglass pattern to the myelinated axon. 4 μm scale bar. (d) XY-Image of the same pulled glass micropipette shown in c at the top, middle, and bottom, showing the reflection signal coming from the Z section at the level of the glass with very little reflection in the center of the micropipette. 2.5 μm scale bar. (e) Diagram of a possible explanation for the hourglass shape of the reflected signal from a myelinated axon in the orthogonal view, and why the lateral sides of the myelin cannot be imaged. Incident laser light (black arrows) is reflected off of the myelin at different angles (blue arrows). Light that is reflected at an oblique angle by the lateral sides of the myelin is rejected by the confocal pinhole and cannot be detected. Only the light reflected from the blue-highlighted portion of each layer of myelin can be detected. As the circumference of the myelin decreases, the area which reflects back into the pinhole decreases. Therefore, as the diameter of the myelin sheath decreases the reflected signal detected similarly decreases, creating the hourglass shape. This phenomenon also occurs on the bottom layers of myelin (not shown for clarity). Because the lateral sides of the myelin are not imaged, the reflection may appear to coincide with the axon if examined in the XY plane. (f) Orthogonal SCoRe image (cyan) of a YFP-labeled axon (green) stained with Fluoromyelin (red) demonstrating that the reflection comes from the myelin. 4 μm scale bar. (g) Orthogonal images from two different size axons imaged in the sciatic nerve in vivo, demonstrating that the reflection signal can be used to obtain the total fiber diameter when measured in the orthogonal view. 4 μm scale bar. (h) Examples of a pulled glass micropipette and its reflective spectrum. Glass micropipettes of 1–2 μm diameter (similar to axonal diameter) were imaged with SCoRe, and the relative intensities of the blue, green, and red reflection were graphed along the length of the electrode (with the electrode decreasing in size). (i) A diagram of the properties of the thin film interference principle (modified from 25). An incident ray of light (top left) traveling through a medium of refractive index n1 at angle θ1 will reflect off and also pass through at the interface of a new medium with higher refractive index n2. Subsequently, a similar reflection will happen on the bottom end of the latter medium at angle θ2. If the height of the second medium, d, is close to the wavelengths of the incident light, then constructive or destructive interference will take place, with the wavelength of greatest constructive interference (λ) occurring at integer multiples (m) given by the equation below at left. In the case of confocal microscopes, the incident light from the excitation lasers is essentially coming vertically, so that θ2=0, which collapses the equation as seen on the right. Here we have depicted the incident ray in green, the reflection off the top of the higher n medium in red, and the reflection off the bottom in blue. In (j), we diagram how the incident light in our confocal microscope may be affected by the myelin surrounding an axon. The fibers we are imaging are in aqueous environments and the lipid rich myelin acts as the higher-refractive index medium. In this case, its thickness would be the determinant of which wavelengths constructively or destructively interfere (diagramed on the left). However, there may be more complex interactions, because myelin itself is layer upon layer of alternating lipids and proteins. In this case, the relative amount of protein, the density of lipids, and amount of residual cytosol in the myelin may be the determining factors for interference (diagrammed on the right).
Supplementary Figure 3. SCoRe allows label-free measurement of myelinated fiber diameter in vivo. Sciatic nerves of mT/mG mice were injected in vivo with Fluoromyelin (FM, green), which labels compact myelin, and a single myelinated axon near a node of Ranvier (star) is shown. The tdTomato (a, red) can be seen most brightly in cytosolic compartments of the Schwann cell, namely the inner (little arrow) and outer (arrowhead) cytoplasmic tongues, along with Schmidt-Lanterman incisures (large arrows). FM (b, green) binds to compact myelin and can be seen in composite (c) to fill the space between the inner and outer cytoplasmic tongues labeled by tdTomato, but does not highlight Schmidt-Lanterman incisures in contrast to mT/mG mice. 10 μm scale bar. (d) In vivo orthogonal (XZ) images of three axons that were stained with FM (red) and imaged with SCoRe (cyan) demonstrating that total fiber diameter can be determined using SCoRe alone. 4 μm scale bar. (e) Graph showing the raw diameter measurements along 15 points of the three axons depicted in panel d demonstrating that the measured diameter with reflection and with FM is consistent along individual axons. (f) Graph showing the average diameters measured from 15 points along each axon using both reflection and FM imaging from 12 separate axons (error bars = standard deviation). When the ratio between reflection and FM (refl : fm) is calculated, it is consistently found to be 1.1 ± 0.06 instead of 1, likely due to the lower axial resolution in the orthogonal view. These data demonstrate that SCoRe can be used in vivo to easily determine the total diameter of individual myelinated fibers (data acquired from n=4 mice, 4 replicates).
Supplementary Figure 4. In vivo imaging of cortical oligodendrocytes and myelin. (a–b) In vivo two-photon images of cortical oligodendrocytes imaged in a P40 transgenic mouse with the proteolipid protein (PLP) promoter driving expression of DsRed fluorescent protein (PLPDsRed). 100 μm scale bar in a and 50 μm scale bar in b. (c) In vivo confocal image taken from the cortex of a P40 PLPDsRed transgenic mouse (red) and imaged with SCoRe (cyan). 100 μm scale bar. (d–g) High magnification images of single DsRed expressing oligodendrocytes imaged with fluorescence and SCoRe from P40 (d–e) and P32 (f–g) PLPDsRed mice demonstrating that proximal non-myelinating oligodendrocyte processes are not reflective (arrows in g) and that reflection stops at individual locations along an axon where DsRed expression stops, likely representing the end of a single internode (arrowheads in f). These data demonstrate the combination of SCoRe and fluorescence oligodendrocyte imaging is complimentary, especially since many transgenic mice do not label 100% of the oligodendrocytes and fluorescent proteins are not able to fully diffuse into all compact myelin membranes, making the identification of myelin sheaths by fluorescence imaging ambiguous. 20 μm scale bar in d–e, 5 μm scale bars in f–g (experiments were replicated 5 times in n=3 mice).
Supplementary Figure 5. In vivo imaging of myelinated micro-regions during postnatal development in the mouse cortex. (a) Low magnification SCoRe image captured in a P21 mouse through a cranial window showing patches of reflective fibers with specific domains. 100 μm scale bar. (b–g) High magnification images showing individual patches of reflective fibers with morphology reminiscent of individual mature oligodendrocyte territories. 50 μm scale bars (experiments were replicated 4 times in n=3 mice).
Supplementary Figure 6. Individual axon colors are retained over days and after axial rotation in the sciatic nerve. (a) In vivo low magnification images of YFP expression imaged in a sciatic nerve of a Thy1-YFP mouse on two consecutive days. 100 μm scale bar. (b) In vivo images showing SCoRe and YFP fluorescence in the same axons (area outlined in a) on two consecutive days showing that individual axons retain their unique SCoRe color signature over multiple imaging sessions. 25 μm scale bar. (c) In vivo images showing the same 3 axons on two separate days demonstrating stable reflective properties of both axons and Schmidt-Lanterman incisures (arrows). 10 μm scale bar. (d) A sciatic nerve was imaged and a representative color of each axon is shown above. (e) The same nerve rotated axially and re-imaged. Some axons were obscured by epineurium reflection after the rotation (white). The same axons can be seen in orthogonal views (f–g, respectively). The axons retain their reflective spectrum even though their relative positions to other axons, the connective tissue, and the lens has changed, proving that these relative positions are not the determining factor for the reflected spectrum. In (h), we show a diagrammatic representation of the change in position of the axons relative to the objective lens before (left) and after (right) axial rotation (experiments were replicated 3 times in n=3 mice).
Supplementary Figure 7. In vivo detection of myelin pathology and differences in the density of Schmidt-Lanterman incisures in shiverer mice. (a) Low magnification image taken from fixed tissue showing the reflection signal in the corpus callosum and cortex. 1 mm scale bar. (b–c) SCoRe images captured from age matched wildtype (b) and shiverer (c) fixed coronal sections taken with the same microscope settings showing a decrease in the reflection signal in the corpus callosum of the hypomyelinating mutant. Asterisk denotes similar background reflection. 100 μm scale bars. (d–e) Example in vivo images taken from wildtype (n=3 mice) (d) and shiverer (n=2 mice) (e) sciatic nerves showing a difference in the density of Schmidt-Lanterman incisures (SLI) (arrowheads) detected with SCoRe. Nodes of Ranvier indicated by asterisk. 15 μm scale bars. (f–g) Quantification showing the differences in the total number of SLIs detected in both wildtype (n=40 axons, 115 SLI) and shiverer (n=40 axons, 176 SLI) sciatic nerves and in relation to individual axon width (experiments were replicated 3 times).
Supplementary Figure 8. Myelin pathology can be detected in the peripheral nerve and spinal cord. (a,d) Low magnification images of YFP-labeled axons in the sciatic nerve of a Thy1-YFP mouse before (a) and 7 days after (d) nerve crush. 100 μm scale bars. (b–c,e–f) High magnification images taken from the regions depicted in the boxes in a and d showing reflection before (b–c) and after (e–f) nerve crush, demonstrating that regenerating axons (red) are not reflective (arrowheads in e–f). 10 μm scale bar. (g–h) SCoRe image captured from an acute spinal cord explant before (g) and after (h) exposure to a hypotonic solution (H20) demonstrating a change in the reflected spectrum after 20 minutes. 70 μm scale bar. (i–j) In vivo sciatic nerve SCoRe images showing a change in the reflected spectrum 30 minutes after exposure to DMSO which changes the phospholipid composition of the membrane. 15 μm scale bar. (k–l) In vivo SCoRe images from a Thy1-YFP (white) sciatic nerve, showing a change in the reflected spectrum 20 minutes after exposure to a hypotonic solution. Note that the YFP-labeled axon was not substantially changed during this interval, demonstrating that SCoRe is sensitive to early myelin injury that cannot be detected with fluorescence imaging of the axon. 10 μm scale bar (experiments were replicated 3 times).
Supplementary Video 1. Three dimensional rendering of a SCoRe image captured in vivo in a Thy1-YFP transgenic mouse. A single YFP-labeled (green) reflective (magenta) axon (arrow) is shown among many non myelinated YFP-labeled axons and dendrites.
Supplementary Video 2. Representative 450 μm z stack of the mouse cortex acquired in vivo with SCoRe. Video demonstrates the depth capabilities of SCoRe which maintains a high signal to noise ratio even at ~400 μm. The decrease in the number of reflected fibers with depth indicates the drop-off in the number of myelinated fibers just below cortical layer 1 projection axons in addition to the change in the orientation of some of the myelinated axons as SCoRe is not able to efficiently detect myelinated axons running orthogonally. Step size 1 μm, depth indicated in upper left corner and video displayed at 10 frames per second.
Supplementary Video 3. Three dimensional rendering of a SCoRe image from the sciatic nerve. Video shows the unique reflected spectrum from individual axons after removing the layers of the highly reflective and disordered signal from the sciatic epineurium.
Acknowledgments
This study was supported by the following Grants: R01AG027855 and R01HL106815 (JG). We would like to thank J. Bewersdorf and D. Toomre for helpful discussions and P. Yuan for critical reading of the manuscript. We thank A. Nishiyama (University of Connecticut, Storrs CT USA) and F. Kirchhoff (University of Saarland, Homburg Germany) for providing PLPDsRed mice. Postmortem human specimen was obtained from the brain bank in the Cognitive Neurology and Alzheimer’s disease Center (CNADC) at Northwestern University (Grant #AG13854).
Footnotes
Contributions
Project initial design and conception (AJS, JG), experimental design (AJS, RAH, JG), experimental execution (AJS, RAH), data analysis (AJS, RAH, JG), manuscript writing (AJS, RAH, JG), project supervision (JG).
References
- 1.Nave KA. Myelination and support of axonal integrity by glia. Nature. 2010;468:244–52. doi: 10.1038/nature09614. [DOI] [PubMed] [Google Scholar]
- 2.Zatorre RJ, Fields RD, Johansen-Berg H. Plasticity in gray and white: neuroimaging changes in brain structure during learning. Nat Neurosci. 2012;15:528–36. doi: 10.1038/nn.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu J, et al. Impaired adult myelination in the prefrontal cortex of socially isolated mice. Nat Neurosci. 2012;15:1621–3. doi: 10.1038/nn.3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Franklin RJM, Ffrench-Constant C. Remyelination in the CNS: from biology to therapy. Nat Rev Neurosci. 2008;9:839–55. doi: 10.1038/nrn2480. [DOI] [PubMed] [Google Scholar]
- 5.Fancy SPJ, Chan JR, Baranzini SE, Franklin RJM, Rowitch DH. Myelin regeneration: a recapitulation of development? Annu Rev Neurosci. 2011;34:21–43. doi: 10.1146/annurev-neuro-061010-113629. [DOI] [PubMed] [Google Scholar]
- 6.Bartzokis G, et al. Multimodal magnetic resonance imaging assessment of white matter aging trajectories over the lifespan of healthy individuals. Biol Psychiatry. 2012;72:1026–34. doi: 10.1016/j.biopsych.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 7.Kirby BB, et al. In vivo time-lapse imaging shows dynamic oligodendrocyte progenitor behavior during zebrafish development. Nat Neurosci. 2006;9:1506–11. doi: 10.1038/nn1803. [DOI] [PubMed] [Google Scholar]
- 8.Kaya F, et al. Live imaging of targeted cell ablation in Xenopus: a new model to study demyelination and repair. J Neurosci. 2012;32:12885–95. doi: 10.1523/JNEUROSCI.2252-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang H, Fu Y, Zickmund P, Shi R, Cheng JX. Coherent anti-stokes Raman scattering imaging of axonal myelin in live spinal tissues. Biophys J. 2005;89:581–91. doi: 10.1529/biophysj.105.061911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fu Y, Huff TB, Wang HW, Wang H, Cheng JX. Ex vivo and in vivo imaging of myelin fibers in mouse brain by coherent anti-Stokes Raman scattering microscopy. Opt Express. 2008;16:19396–409. doi: 10.1364/oe.16.019396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Imitola J, et al. Multimodal coherent anti-Stokes Raman scattering microscopy reveals microglia-associated myelin and axonal dysfunction in multiple sclerosis-like lesions in mice. J Biomed Opt. 2011;16:021109. doi: 10.1117/1.3533312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ben Arous J, et al. Single myelin fiber imaging in living rodents without labeling by deep optical coherence microscopy. J Biomed Opt. 2011;16:116012. doi: 10.1117/1.3650770. [DOI] [PubMed] [Google Scholar]
- 13.Witte S, et al. Label-free live brain imaging and targeted patching with third-harmonic generation microscopy. Proc Natl Acad Sci USA. 2011;108:5970–5. doi: 10.1073/pnas.1018743108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farrar MJ, Wise FW, Fetcho JR, Schaffer CB. In vivo imaging of myelin in the vertebrate central nervous system using third harmonic generation microscopy. Biophys J. 2011;100:1362–71. doi: 10.1016/j.bpj.2011.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Filler TJ, Peuker ET. Reflection contrast microscopy (RCM): a forgotten technique? J Pathol. 2000;190:635–8. doi: 10.1002/(SICI)1096-9896(200004)190:5<635::AID-PATH571>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 16.Xiao J, Levitt JB, Buffenstein R. The use of a novel and simple method of revealing neural fibers to show the regression of the lateral geniculate nucleus in the naked mole-rat (Heterocephalus glaber) Brain Res. 2006;1077:81–9. doi: 10.1016/j.brainres.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 17.Grutzendler J, Kasthuri N, Gan WB. Long-term dendritic spine stability in the adult cortex. Nature. 2002;420:812–6. doi: 10.1038/nature01276. [DOI] [PubMed] [Google Scholar]
- 18.Waxman SG. Ultrastructural observations on branching patterns of central axons. Neurosci Lett. 1975;1:251–6. doi: 10.1016/0304-3940(75)90039-7. [DOI] [PubMed] [Google Scholar]
- 19.Ha H. Axonal bifurcation in the dorsal root ganglion of the cat: a light and electron microscopic study. J Comp Neurol. 1970;140:227–40. doi: 10.1002/cne.901400206. [DOI] [PubMed] [Google Scholar]
- 20.Hirrlinger PG, et al. Expression of reef coral fluorescent proteins in the central nervous system of transgenic mice. Mol Cell Neurosci. 2005;30:291–303. doi: 10.1016/j.mcn.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 21.Yang G, Pan F, Parkhurst CN, Grutzendler J, Gan WB. Thinned-skull cranial window technique for long-term imaging of the cortex in live mice. Nat Protoc. 2010;5:201–8. doi: 10.1038/nprot.2009.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jacobson S. Sequence of myelination in the brain of the albino rat. A Cerebral cortex, thalamus and related structures. J Comp Neurol. 1963;121:5–29. doi: 10.1002/cne.901210103. [DOI] [PubMed] [Google Scholar]
- 23.Chong SYC, et al. Neurite outgrowth inhibitor Nogo-A establishes spatial segregation and extent of oligodendrocyte myelination. Proc Natl Acad Sci USA. 2012;109:1299–304. doi: 10.1073/pnas.1113540109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Binding J, et al. Brain refractive index measured in vivo with high-NA defocus-corrected full-field OCT and consequences for two-photon microscopy. Opt Express. 2011;19:4833–47. doi: 10.1364/OE.19.004833. [DOI] [PubMed] [Google Scholar]
- 25.Macleod HA. Thin-Film Optical Filters Sciences New York. Vol. 668. Institute of Physics Publishing; 2001. [DOI] [Google Scholar]
- 26.Balice-Gordon RJ, Bone LJ, Scherer SS. Functional gap junctions in the schwann cell myelin sheath. J Cell Biol. 1998;142:1095–104. doi: 10.1083/jcb.142.4.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gould RM, Byrd AL, Barbarese E. The number of Schmidt-Lanterman incisures is more than doubled in shiverer PNS myelin sheaths. J Neurocytol. 1995;24:85–98. doi: 10.1007/BF01181552. [DOI] [PubMed] [Google Scholar]
- 28.Lam CK, Yoo T, Hiner B, Liu Z, Grutzendler J. Embolus extravasation is an alternative mechanism for cerebral microvascular recanalization. Nature. 2010;465:478–82. doi: 10.1038/nature09001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davalos D, et al. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8:752–8. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- 30.Harb R, Whiteus C, Freitas C, Grutzendler J. In vivo imaging of cerebral microvascular plasticity from birth to death. J Cereb Blood Flow Metab. 2012 doi: 10.1038/jcbfm.2012.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poliak S, Peles E. The local differentiation of myelinated axons at nodes of Ranvier. Nat Rev Neurosci. 2003;4:968–80. doi: 10.1038/nrn1253. [DOI] [PubMed] [Google Scholar]
- 32.Livet J, et al. Transgenic strategies for combinatorial expression of fluorescent proteins in the nervous system. Nature. 2007;450:56–62. doi: 10.1038/nature06293. [DOI] [PubMed] [Google Scholar]
- 33.Gan WB, Grutzendler J, Wong WT, Wong RO, Lichtman JW. Multicolor “DiOlistic” labeling of the nervous system using lipophilic dye combinations. Neuron. 2000;27:219–25. doi: 10.1016/s0896-6273(00)00031-3. [DOI] [PubMed] [Google Scholar]
- 34.Kirschner Da, Caspar DL. Myelin structure transformed by dimethylsulfoxide. Proc Natl Acad Sci USA. 1975;72:3513–7. doi: 10.1073/pnas.72.9.3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gupta N, et al. Neural stem cell engraftment and myelination in the human brain. Sci Transl Med. 2012;4:155ra137. doi: 10.1126/scitranslmed.3004373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hong G, et al. Multifunctional in vivo vascular imaging using near-infrared II fluorescence. Nature Medicine. 2012;18:1841–1846. doi: 10.1038/nm.2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Windrem MS, et al. Neonatal chimerization with human glial progenitor cells can both remyelinate and rescue the otherwise lethally hypomyelinated shiverer mouse. Cell Stem Cell. 2008;2:553–65. doi: 10.1016/j.stem.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bø L, Vedeler CA, Nyland HI, Trapp BD, Mørk SJ. Subpial demyelination in the cerebral cortex of multiple sclerosis patients. J Neuropathol Exp Neurol. 2003;62:723–32. doi: 10.1093/jnen/62.7.723. [DOI] [PubMed] [Google Scholar]
- 39.Lucchinetti CF, et al. Inflammatory cortical demyelination in early multiple sclerosis. N Engl J Med. 2011;365:2188–97. doi: 10.1056/NEJMoa1100648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feng G, et al. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron. 2000;28:41–51. doi: 10.1016/s0896-6273(00)00084-2. [DOI] [PubMed] [Google Scholar]
- 41.Jung S, et al. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol Cell Biol. 2000;20:4106–4114. doi: 10.1128/mcb.20.11.4106-4114.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. A global double-fluorescent Cre reporter mouse. Genesis. 2007;45:593–605. doi: 10.1002/dvg.20335. [DOI] [PubMed] [Google Scholar]
- 43.Nimmerjahn A, Kirchhoff F, Kerr JND, Helmchen F. Sulforhodamine 101 as a specific marker of astroglia in the neocortex in vivo. Nat methods. 2004;1:31–7. doi: 10.1038/nmeth706. [DOI] [PubMed] [Google Scholar]
- 44.Fenrich KK, et al. Long-term in vivo imaging of normal and pathological mouse spinal cord with subcellular resolution using implanted glass windows. J physiol. 2012;590:3665–75. doi: 10.1113/jphysiol.2012.230532. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Simultaneous imaging of myelinated axons, microglia and astrocytes. in vivo SCoRe (cyan) and confocal fluorescence images captured through a cranial window after topical application of sulforhodamine 101 (red) to label astrocytes in a Thy1-YFP (green) (a–d) or CX3CR1GFP (green) transgenic mouse (e–h) demonstrating implementation of SCoRe with confocal fluorescence imaging in vivo and also showing that astrocytes, dendrites, and microglia are not reflective. 30 μm scale bars (experiments were replicated 5 times in n=3 mice).
Supplementary Figure 2 and discussion: Mechanisms and source of the reflection signal in SCoRe. (a) Orthogonal (XZ) image of a YFP-labeled axon (red) in a Thy1-YFP sciatic nerve imaged in vivo showing the reflection (cyan) originating from the myelin and not the axon. 1 μm scale bar. (b) XY-Image of the same axon shown in a at the top, middle and bottom of the axonal z-stack, showing strong reflection signal at the level of the myelin on both the top and bottom, but with very little reflection at the center of the axon. 1 μm scale bar. (c) Orthogonal (XZ) SCoRe image of a glass micropipette filled with fluorescently labeled agarose (red), showing a similar hourglass pattern to the myelinated axon. 4 μm scale bar. (d) XY-Image of the same pulled glass micropipette shown in c at the top, middle, and bottom, showing the reflection signal coming from the Z section at the level of the glass with very little reflection in the center of the micropipette. 2.5 μm scale bar. (e) Diagram of a possible explanation for the hourglass shape of the reflected signal from a myelinated axon in the orthogonal view, and why the lateral sides of the myelin cannot be imaged. Incident laser light (black arrows) is reflected off of the myelin at different angles (blue arrows). Light that is reflected at an oblique angle by the lateral sides of the myelin is rejected by the confocal pinhole and cannot be detected. Only the light reflected from the blue-highlighted portion of each layer of myelin can be detected. As the circumference of the myelin decreases, the area which reflects back into the pinhole decreases. Therefore, as the diameter of the myelin sheath decreases the reflected signal detected similarly decreases, creating the hourglass shape. This phenomenon also occurs on the bottom layers of myelin (not shown for clarity). Because the lateral sides of the myelin are not imaged, the reflection may appear to coincide with the axon if examined in the XY plane. (f) Orthogonal SCoRe image (cyan) of a YFP-labeled axon (green) stained with Fluoromyelin (red) demonstrating that the reflection comes from the myelin. 4 μm scale bar. (g) Orthogonal images from two different size axons imaged in the sciatic nerve in vivo, demonstrating that the reflection signal can be used to obtain the total fiber diameter when measured in the orthogonal view. 4 μm scale bar. (h) Examples of a pulled glass micropipette and its reflective spectrum. Glass micropipettes of 1–2 μm diameter (similar to axonal diameter) were imaged with SCoRe, and the relative intensities of the blue, green, and red reflection were graphed along the length of the electrode (with the electrode decreasing in size). (i) A diagram of the properties of the thin film interference principle (modified from 25). An incident ray of light (top left) traveling through a medium of refractive index n1 at angle θ1 will reflect off and also pass through at the interface of a new medium with higher refractive index n2. Subsequently, a similar reflection will happen on the bottom end of the latter medium at angle θ2. If the height of the second medium, d, is close to the wavelengths of the incident light, then constructive or destructive interference will take place, with the wavelength of greatest constructive interference (λ) occurring at integer multiples (m) given by the equation below at left. In the case of confocal microscopes, the incident light from the excitation lasers is essentially coming vertically, so that θ2=0, which collapses the equation as seen on the right. Here we have depicted the incident ray in green, the reflection off the top of the higher n medium in red, and the reflection off the bottom in blue. In (j), we diagram how the incident light in our confocal microscope may be affected by the myelin surrounding an axon. The fibers we are imaging are in aqueous environments and the lipid rich myelin acts as the higher-refractive index medium. In this case, its thickness would be the determinant of which wavelengths constructively or destructively interfere (diagramed on the left). However, there may be more complex interactions, because myelin itself is layer upon layer of alternating lipids and proteins. In this case, the relative amount of protein, the density of lipids, and amount of residual cytosol in the myelin may be the determining factors for interference (diagrammed on the right).
Supplementary Figure 3. SCoRe allows label-free measurement of myelinated fiber diameter in vivo. Sciatic nerves of mT/mG mice were injected in vivo with Fluoromyelin (FM, green), which labels compact myelin, and a single myelinated axon near a node of Ranvier (star) is shown. The tdTomato (a, red) can be seen most brightly in cytosolic compartments of the Schwann cell, namely the inner (little arrow) and outer (arrowhead) cytoplasmic tongues, along with Schmidt-Lanterman incisures (large arrows). FM (b, green) binds to compact myelin and can be seen in composite (c) to fill the space between the inner and outer cytoplasmic tongues labeled by tdTomato, but does not highlight Schmidt-Lanterman incisures in contrast to mT/mG mice. 10 μm scale bar. (d) In vivo orthogonal (XZ) images of three axons that were stained with FM (red) and imaged with SCoRe (cyan) demonstrating that total fiber diameter can be determined using SCoRe alone. 4 μm scale bar. (e) Graph showing the raw diameter measurements along 15 points of the three axons depicted in panel d demonstrating that the measured diameter with reflection and with FM is consistent along individual axons. (f) Graph showing the average diameters measured from 15 points along each axon using both reflection and FM imaging from 12 separate axons (error bars = standard deviation). When the ratio between reflection and FM (refl : fm) is calculated, it is consistently found to be 1.1 ± 0.06 instead of 1, likely due to the lower axial resolution in the orthogonal view. These data demonstrate that SCoRe can be used in vivo to easily determine the total diameter of individual myelinated fibers (data acquired from n=4 mice, 4 replicates).
Supplementary Figure 4. In vivo imaging of cortical oligodendrocytes and myelin. (a–b) In vivo two-photon images of cortical oligodendrocytes imaged in a P40 transgenic mouse with the proteolipid protein (PLP) promoter driving expression of DsRed fluorescent protein (PLPDsRed). 100 μm scale bar in a and 50 μm scale bar in b. (c) In vivo confocal image taken from the cortex of a P40 PLPDsRed transgenic mouse (red) and imaged with SCoRe (cyan). 100 μm scale bar. (d–g) High magnification images of single DsRed expressing oligodendrocytes imaged with fluorescence and SCoRe from P40 (d–e) and P32 (f–g) PLPDsRed mice demonstrating that proximal non-myelinating oligodendrocyte processes are not reflective (arrows in g) and that reflection stops at individual locations along an axon where DsRed expression stops, likely representing the end of a single internode (arrowheads in f). These data demonstrate the combination of SCoRe and fluorescence oligodendrocyte imaging is complimentary, especially since many transgenic mice do not label 100% of the oligodendrocytes and fluorescent proteins are not able to fully diffuse into all compact myelin membranes, making the identification of myelin sheaths by fluorescence imaging ambiguous. 20 μm scale bar in d–e, 5 μm scale bars in f–g (experiments were replicated 5 times in n=3 mice).
Supplementary Figure 5. In vivo imaging of myelinated micro-regions during postnatal development in the mouse cortex. (a) Low magnification SCoRe image captured in a P21 mouse through a cranial window showing patches of reflective fibers with specific domains. 100 μm scale bar. (b–g) High magnification images showing individual patches of reflective fibers with morphology reminiscent of individual mature oligodendrocyte territories. 50 μm scale bars (experiments were replicated 4 times in n=3 mice).
Supplementary Figure 6. Individual axon colors are retained over days and after axial rotation in the sciatic nerve. (a) In vivo low magnification images of YFP expression imaged in a sciatic nerve of a Thy1-YFP mouse on two consecutive days. 100 μm scale bar. (b) In vivo images showing SCoRe and YFP fluorescence in the same axons (area outlined in a) on two consecutive days showing that individual axons retain their unique SCoRe color signature over multiple imaging sessions. 25 μm scale bar. (c) In vivo images showing the same 3 axons on two separate days demonstrating stable reflective properties of both axons and Schmidt-Lanterman incisures (arrows). 10 μm scale bar. (d) A sciatic nerve was imaged and a representative color of each axon is shown above. (e) The same nerve rotated axially and re-imaged. Some axons were obscured by epineurium reflection after the rotation (white). The same axons can be seen in orthogonal views (f–g, respectively). The axons retain their reflective spectrum even though their relative positions to other axons, the connective tissue, and the lens has changed, proving that these relative positions are not the determining factor for the reflected spectrum. In (h), we show a diagrammatic representation of the change in position of the axons relative to the objective lens before (left) and after (right) axial rotation (experiments were replicated 3 times in n=3 mice).
Supplementary Figure 7. In vivo detection of myelin pathology and differences in the density of Schmidt-Lanterman incisures in shiverer mice. (a) Low magnification image taken from fixed tissue showing the reflection signal in the corpus callosum and cortex. 1 mm scale bar. (b–c) SCoRe images captured from age matched wildtype (b) and shiverer (c) fixed coronal sections taken with the same microscope settings showing a decrease in the reflection signal in the corpus callosum of the hypomyelinating mutant. Asterisk denotes similar background reflection. 100 μm scale bars. (d–e) Example in vivo images taken from wildtype (n=3 mice) (d) and shiverer (n=2 mice) (e) sciatic nerves showing a difference in the density of Schmidt-Lanterman incisures (SLI) (arrowheads) detected with SCoRe. Nodes of Ranvier indicated by asterisk. 15 μm scale bars. (f–g) Quantification showing the differences in the total number of SLIs detected in both wildtype (n=40 axons, 115 SLI) and shiverer (n=40 axons, 176 SLI) sciatic nerves and in relation to individual axon width (experiments were replicated 3 times).
Supplementary Figure 8. Myelin pathology can be detected in the peripheral nerve and spinal cord. (a,d) Low magnification images of YFP-labeled axons in the sciatic nerve of a Thy1-YFP mouse before (a) and 7 days after (d) nerve crush. 100 μm scale bars. (b–c,e–f) High magnification images taken from the regions depicted in the boxes in a and d showing reflection before (b–c) and after (e–f) nerve crush, demonstrating that regenerating axons (red) are not reflective (arrowheads in e–f). 10 μm scale bar. (g–h) SCoRe image captured from an acute spinal cord explant before (g) and after (h) exposure to a hypotonic solution (H20) demonstrating a change in the reflected spectrum after 20 minutes. 70 μm scale bar. (i–j) In vivo sciatic nerve SCoRe images showing a change in the reflected spectrum 30 minutes after exposure to DMSO which changes the phospholipid composition of the membrane. 15 μm scale bar. (k–l) In vivo SCoRe images from a Thy1-YFP (white) sciatic nerve, showing a change in the reflected spectrum 20 minutes after exposure to a hypotonic solution. Note that the YFP-labeled axon was not substantially changed during this interval, demonstrating that SCoRe is sensitive to early myelin injury that cannot be detected with fluorescence imaging of the axon. 10 μm scale bar (experiments were replicated 3 times).
Supplementary Video 1. Three dimensional rendering of a SCoRe image captured in vivo in a Thy1-YFP transgenic mouse. A single YFP-labeled (green) reflective (magenta) axon (arrow) is shown among many non myelinated YFP-labeled axons and dendrites.
Supplementary Video 2. Representative 450 μm z stack of the mouse cortex acquired in vivo with SCoRe. Video demonstrates the depth capabilities of SCoRe which maintains a high signal to noise ratio even at ~400 μm. The decrease in the number of reflected fibers with depth indicates the drop-off in the number of myelinated fibers just below cortical layer 1 projection axons in addition to the change in the orientation of some of the myelinated axons as SCoRe is not able to efficiently detect myelinated axons running orthogonally. Step size 1 μm, depth indicated in upper left corner and video displayed at 10 frames per second.
Supplementary Video 3. Three dimensional rendering of a SCoRe image from the sciatic nerve. Video shows the unique reflected spectrum from individual axons after removing the layers of the highly reflective and disordered signal from the sciatic epineurium.