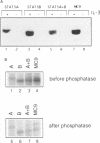
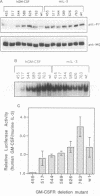
Abstract
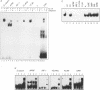
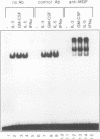
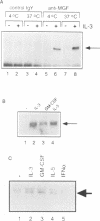
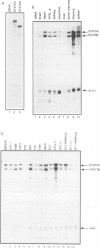
Interleukin-3 (IL-3) is an important regulator of hemopoiesis and considerable effort has been directed towards the study of its mechanism of signal transduction. In this paper, we describe the first molecular identification of a STAT transcription factor that is activated by IL-3. STATs exist in a cytoplasmic, transcriptionally inactive form which, in response to extracellular signals, become tyrosine phosphorylated and translocate to the nucleus where they bind to specific DNA elements. Several of these DNA elements were found which bind proteins in an IL-3-responsive manner. Analysis of these bandshift complexes with available antibodies to the known STATs suggests that IL-3 activates the DNA-binding ability of STAT5, a protein which was originally characterized as a prolactin-responsive transcription factor in sheep. IL-5 and granulocyte-macrophage colony stimulating factor (GM-CSF), which share a common signaling receptor subunit with IL-3, also activate STAT5. Unexpectedly, two murine STAT5 homologs, 96% identical to each other at the amino acid level, were isolated and IL-3-dependent GAS binding could be reconstituted in COS cells transfected with IL-3 receptor and either STAT5 cDNA. In IL-3-dependent hemopoietic cells, both forms of STAT5 are expressed and activated in response to IL-3.
Full text
PDF
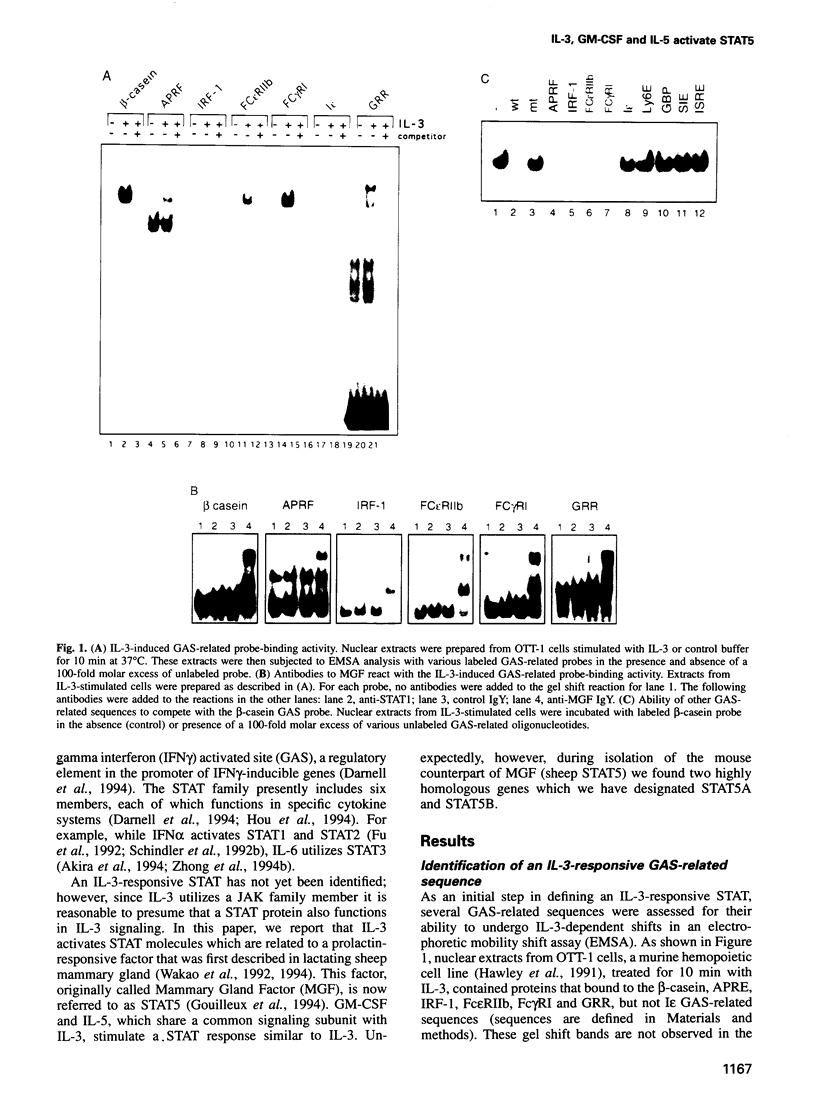

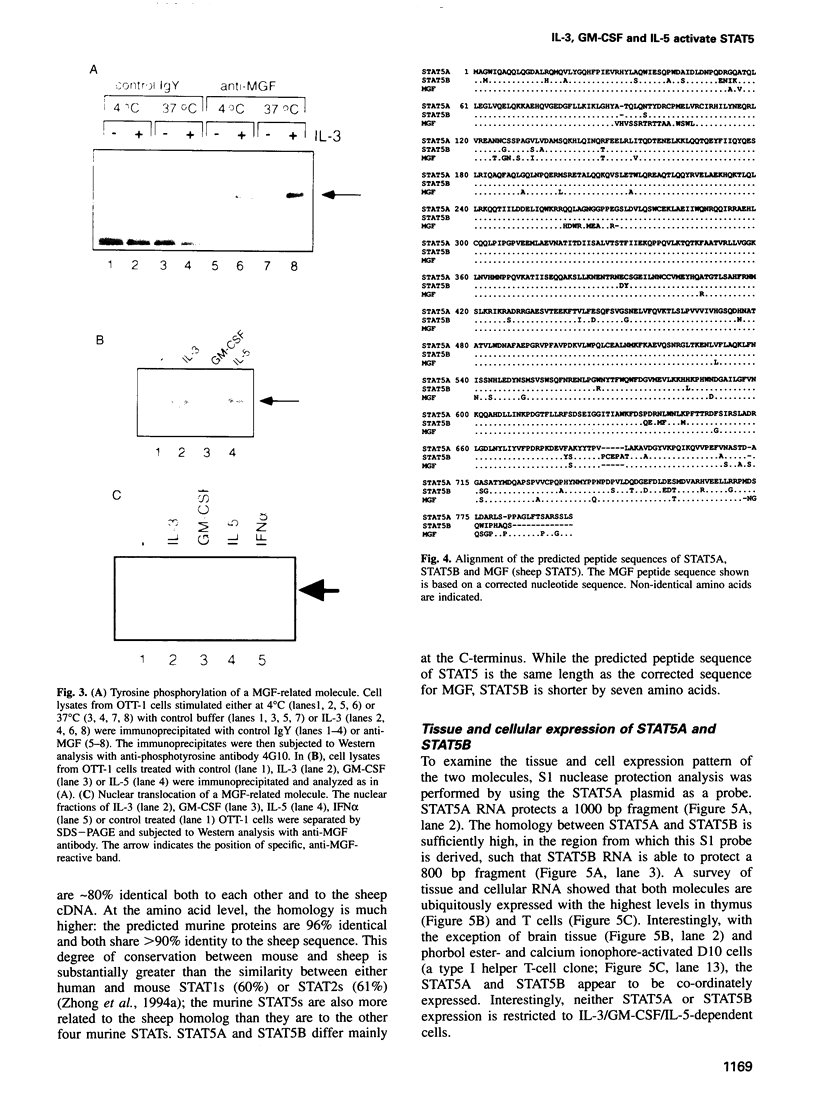



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akira S., Nishio Y., Inoue M., Wang X. J., Wei S., Matsusaka T., Yoshida K., Sudo T., Naruto M., Kishimoto T. Molecular cloning of APRF, a novel IFN-stimulated gene factor 3 p91-related transcription factor involved in the gp130-mediated signaling pathway. Cell. 1994 Apr 8;77(1):63–71. doi: 10.1016/0092-8674(94)90235-6. [DOI] [PubMed] [Google Scholar]
- Arai K. I., Lee F., Miyajima A., Miyatake S., Arai N., Yokota T. Cytokines: coordinators of immune and inflammatory responses. Annu Rev Biochem. 1990;59:783–836. doi: 10.1146/annurev.bi.59.070190.004031. [DOI] [PubMed] [Google Scholar]
- Argetsinger L. S., Campbell G. S., Yang X., Witthuhn B. A., Silvennoinen O., Ihle J. N., Carter-Su C. Identification of JAK2 as a growth hormone receptor-associated tyrosine kinase. Cell. 1993 Jul 30;74(2):237–244. doi: 10.1016/0092-8674(93)90415-m. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Spliced early mRNAs of simian virus 40. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1274–1278. doi: 10.1073/pnas.75.3.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell J. E., Jr, Kerr I. M., Stark G. R. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994 Jun 3;264(5164):1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- Duronio V., Clark-Lewis I., Federsppiel B., Wieler J. S., Schrader J. W. Tyrosine phosphorylation of receptor beta subunits and common substrates in response to interleukin-3 and granulocyte-macrophage colony-stimulating factor. J Biol Chem. 1992 Oct 25;267(30):21856–21863. [PubMed] [Google Scholar]
- Fu X. Y., Schindler C., Improta T., Aebersold R., Darnell J. E., Jr The proteins of ISGF-3, the interferon alpha-induced transcriptional activator, define a gene family involved in signal transduction. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7840–7843. doi: 10.1073/pnas.89.16.7840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X. Y., Zhang J. J. Transcription factor p91 interacts with the epidermal growth factor receptor and mediates activation of the c-fos gene promoter. Cell. 1993 Sep 24;74(6):1135–1145. doi: 10.1016/0092-8674(93)90734-8. [DOI] [PubMed] [Google Scholar]
- Gorman D. M., Itoh N., Kitamura T., Schreurs J., Yonehara S., Yahara I., Arai K., Miyajima A. Cloning and expression of a gene encoding an interleukin 3 receptor-like protein: identification of another member of the cytokine receptor gene family. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5459–5463. doi: 10.1073/pnas.87.14.5459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouilleux F., Wakao H., Mundt M., Groner B. Prolactin induces phosphorylation of Tyr694 of Stat5 (MGF), a prerequisite for DNA binding and induction of transcription. EMBO J. 1994 Sep 15;13(18):4361–4369. doi: 10.1002/j.1460-2075.1994.tb06756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenlund A. C., Farrar M. A., Viviano B. L., Schreiber R. D. Ligand-induced IFN gamma receptor tyrosine phosphorylation couples the receptor to its signal transduction system (p91). EMBO J. 1994 Apr 1;13(7):1591–1600. doi: 10.1002/j.1460-2075.1994.tb06422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara T., Miyajima A. Two distinct functional high affinity receptors for mouse interleukin-3 (IL-3). EMBO J. 1992 May;11(5):1875–1884. doi: 10.1002/j.1460-2075.1992.tb05239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley T. S., McLeish W. A., Hawley R. G. Establishment of a novel factor-dependent myeloid cell line from primary cultures of mouse bone marrow. Cytokine. 1991 Jan;3(1):60–71. doi: 10.1016/1043-4666(91)90011-2. [DOI] [PubMed] [Google Scholar]
- Heyworth C. M., Dexter T. M., Kan O., Whetton A. D. The role of hemopoietic growth factors in self-renewal and differentiation of IL-3-dependent multipotential stem cells. Growth Factors. 1990;2(2-3):197–211. doi: 10.3109/08977199009071506. [DOI] [PubMed] [Google Scholar]
- Hou J., Schindler U., Henzel W. J., Ho T. C., Brasseur M., McKnight S. L. An interleukin-4-induced transcription factor: IL-4 Stat. Science. 1994 Sep 16;265(5179):1701–1706. doi: 10.1126/science.8085155. [DOI] [PubMed] [Google Scholar]
- Ihle J. N., Witthuhn B. A., Quelle F. W., Yamamoto K., Thierfelder W. E., Kreider B., Silvennoinen O. Signaling by the cytokine receptor superfamily: JAKs and STATs. Trends Biochem Sci. 1994 May;19(5):222–227. doi: 10.1016/0968-0004(94)90026-4. [DOI] [PubMed] [Google Scholar]
- Isfort R. J., Ihle J. N. Multiple hematopoietic growth factors signal through tyrosine phosphorylation. Growth Factors. 1990;2(2-3):213–220. doi: 10.3109/08977199009071507. [DOI] [PubMed] [Google Scholar]
- Isfort R. J., Stevens D., May W. S., Ihle J. N. Interleukin 3 binds to a 140-kDa phosphotyrosine-containing cell surface protein. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7982–7986. doi: 10.1073/pnas.85.21.7982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh N., Yonehara S., Schreurs J., Gorman D. M., Maruyama K., Ishii A., Yahara I., Arai K., Miyajima A. Cloning of an interleukin-3 receptor gene: a member of a distinct receptor gene family. Science. 1990 Jan 19;247(4940):324–327. doi: 10.1126/science.2404337. [DOI] [PubMed] [Google Scholar]
- Koyasu S., Tojo A., Miyajima A., Akiyama T., Kasuga M., Urabe A., Schreurs J., Arai K., Takaku F., Yahara I. Interleukin 3-specific tyrosine phosphorylation of a membrane glycoprotein of Mr 150,000 in multi-factor-dependent myeloid cell lines. EMBO J. 1987 Dec 20;6(13):3979–3984. doi: 10.1002/j.1460-2075.1987.tb02740.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larner A. C., David M., Feldman G. M., Igarashi K., Hackett R. H., Webb D. S., Sweitzer S. M., Petricoin E. F., 3rd, Finbloom D. S. Tyrosine phosphorylation of DNA binding proteins by multiple cytokines. Science. 1993 Sep 24;261(5129):1730–1733. doi: 10.1126/science.8378773. [DOI] [PubMed] [Google Scholar]
- Linnekin D., Evans G., Michiel D., Farrar W. L. Characterization of a 97-kDa phosphotyrosylprotein regulated by multiple cytokines. J Biol Chem. 1992 Nov 25;267(33):23993–23998. [PubMed] [Google Scholar]
- Lütticken C., Wegenka U. M., Yuan J., Buschmann J., Schindler C., Ziemiecki A., Harpur A. G., Wilks A. F., Yasukawa K., Taga T. Association of transcription factor APRF and protein kinase Jak1 with the interleukin-6 signal transducer gp130. Science. 1994 Jan 7;263(5143):89–92. doi: 10.1126/science.8272872. [DOI] [PubMed] [Google Scholar]
- Masuda E. S., Tokumitsu H., Tsuboi A., Shlomai J., Hung P., Arai K., Arai N. The granulocyte-macrophage colony-stimulating factor promoter cis-acting element CLE0 mediates induction signals in T cells and is recognized by factors related to AP1 and NFAT. Mol Cell Biol. 1993 Dec;13(12):7399–7407. doi: 10.1128/mcb.13.12.7399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami Y., Kono T., Yamada K., Kobayashi N., Kawahara A., Perlmutter R. M., Taniguchi T. Association of p56lck with IL-2 receptor beta chain is critical for the IL-2-induced activation of p56lck. EMBO J. 1993 Feb;12(2):759–768. doi: 10.1002/j.1460-2075.1993.tb05710.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyajima A., Hara T., Kitamura T. Common subunits of cytokine receptors and the functional redundancy of cytokines. Trends Biochem Sci. 1992 Oct;17(10):378–382. doi: 10.1016/0968-0004(92)90004-s. [DOI] [PubMed] [Google Scholar]
- Miyajima A., Mui A. L., Ogorochi T., Sakamaki K. Receptors for granulocyte-macrophage colony-stimulating factor, interleukin-3, and interleukin-5. Blood. 1993 Oct 1;82(7):1960–1974. [PubMed] [Google Scholar]
- Miyajima A., Otsu K., Schreurs J., Bond M. W., Abrams J. S., Arai K. Expression of murine and human granulocyte-macrophage colony-stimulating factors in S. cerevisiae: mutagenesis of the potential glycosylation sites. EMBO J. 1986 Jun;5(6):1193–1197. doi: 10.1002/j.1460-2075.1986.tb04346.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyajima A., Schreurs J., Otsu K., Kondo A., Arai K., Maeda S. Use of the silkworm, Bombyx mori, and an insect baculovirus vector for high-level expression and secretion of biologically active mouse interleukin-3. Gene. 1987;58(2-3):273–281. doi: 10.1016/0378-1119(87)90382-9. [DOI] [PubMed] [Google Scholar]
- Nabel G., Galli S. J., Dvorak A. M., Dvorak H. F., Cantor H. Inducer T lymphocytes synthesize a factor that stimulates proliferation of cloned mast cells. Nature. 1981 May 28;291(5813):332–334. doi: 10.1038/291332a0. [DOI] [PubMed] [Google Scholar]
- Nicholson S. E., Oates A. C., Harpur A. G., Ziemiecki A., Wilks A. F., Layton J. E. Tyrosine kinase JAK1 is associated with the granulocyte-colony-stimulating factor receptor and both become tyrosine-phosphorylated after receptor activation. Proc Natl Acad Sci U S A. 1994 Apr 12;91(8):2985–2988. doi: 10.1073/pnas.91.8.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quelle F. W., Sato N., Witthuhn B. A., Inhorn R. C., Eder M., Miyajima A., Griffin J. D., Ihle J. N. JAK2 associates with the beta c chain of the receptor for granulocyte-macrophage colony-stimulating factor, and its activation requires the membrane-proximal region. Mol Cell Biol. 1994 Jul;14(7):4335–4341. doi: 10.1128/mcb.14.7.4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman P., Kreider B., Azam M., Levy D., Wegenka U., Eilers A., Decker T., Horn F., Kashleva H., Ihle J. Cytokines and growth factors signal through tyrosine phosphorylation of a family of related transcription factors. Immunity. 1994 Sep;1(6):457–468. doi: 10.1016/1074-7613(94)90088-4. [DOI] [PubMed] [Google Scholar]
- Sakamaki K., Miyajima I., Kitamura T., Miyajima A. Critical cytoplasmic domains of the common beta subunit of the human GM-CSF, IL-3 and IL-5 receptors for growth signal transduction and tyrosine phosphorylation. EMBO J. 1992 Oct;11(10):3541–3549. doi: 10.1002/j.1460-2075.1992.tb05437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato N., Sakamaki K., Terada N., Arai K., Miyajima A. Signal transduction by the high-affinity GM-CSF receptor: two distinct cytoplasmic regions of the common beta subunit responsible for different signaling. EMBO J. 1993 Nov;12(11):4181–4189. doi: 10.1002/j.1460-2075.1993.tb06102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler C., Fu X. Y., Improta T., Aebersold R., Darnell J. E., Jr Proteins of transcription factor ISGF-3: one gene encodes the 91-and 84-kDa ISGF-3 proteins that are activated by interferon alpha. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7836–7839. doi: 10.1073/pnas.89.16.7836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler C., Shuai K., Prezioso V. R., Darnell J. E., Jr Interferon-dependent tyrosine phosphorylation of a latent cytoplasmic transcription factor. Science. 1992 Aug 7;257(5071):809–813. doi: 10.1126/science.1496401. [DOI] [PubMed] [Google Scholar]
- Schrader J. W. The panspecific hemopoietin of activated T lymphocytes (interleukin-3). Annu Rev Immunol. 1986;4:205–230. doi: 10.1146/annurev.iy.04.040186.001225. [DOI] [PubMed] [Google Scholar]
- Shuai K., Horvath C. M., Huang L. H., Qureshi S. A., Cowburn D., Darnell J. E., Jr Interferon activation of the transcription factor Stat91 involves dimerization through SH2-phosphotyrosyl peptide interactions. Cell. 1994 Mar 11;76(5):821–828. doi: 10.1016/0092-8674(94)90357-3. [DOI] [PubMed] [Google Scholar]
- Shuai K., Schindler C., Prezioso V. R., Darnell J. E., Jr Activation of transcription by IFN-gamma: tyrosine phosphorylation of a 91-kD DNA binding protein. Science. 1992 Dec 11;258(5089):1808–1812. doi: 10.1126/science.1281555. [DOI] [PubMed] [Google Scholar]
- Shuai K., Stark G. R., Kerr I. M., Darnell J. E., Jr A single phosphotyrosine residue of Stat91 required for gene activation by interferon-gamma. Science. 1993 Sep 24;261(5129):1744–1746. doi: 10.1126/science.7690989. [DOI] [PubMed] [Google Scholar]
- Silvennoinen O., Witthuhn B. A., Quelle F. W., Cleveland J. L., Yi T., Ihle J. N. Structure of the murine Jak2 protein-tyrosine kinase and its role in interleukin 3 signal transduction. Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8429–8433. doi: 10.1073/pnas.90.18.8429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen P. H., Mui A. L., Murthy S. C., Krystal G. Interleukin-3, GM-CSF, and TPA induce distinct phosphorylation events in an interleukin 3-dependent multipotential cell line. Blood. 1989 Feb;73(2):406–418. [PubMed] [Google Scholar]
- Sorensen P., Mui A. L., Krystal G. Interleukin-3 stimulates the tyrosine phosphorylation of the 140-kilodalton interleukin-3 receptor. J Biol Chem. 1989 Nov 15;264(32):19253–19258. [PubMed] [Google Scholar]
- Stahl N., Boulton T. G., Farruggella T., Ip N. Y., Davis S., Witthuhn B. A., Quelle F. W., Silvennoinen O., Barbieri G., Pellegrini S. Association and activation of Jak-Tyk kinases by CNTF-LIF-OSM-IL-6 beta receptor components. Science. 1994 Jan 7;263(5143):92–95. doi: 10.1126/science.8272873. [DOI] [PubMed] [Google Scholar]
- Torigoe T., O'Connor R., Santoli D., Reed J. C. Interleukin-3 regulates the activity of the LYN protein-tyrosine kinase in myeloid-committed leukemic cell lines. Blood. 1992 Aug 1;80(3):617–624. [PubMed] [Google Scholar]
- Wakao H., Gouilleux F., Groner B. Mammary gland factor (MGF) is a novel member of the cytokine regulated transcription factor gene family and confers the prolactin response. EMBO J. 1994 May 1;13(9):2182–2191. doi: 10.1002/j.1460-2075.1994.tb06495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakao H., Schmitt-Ney M., Groner B. Mammary gland-specific nuclear factor is present in lactating rodent and bovine mammary tissue and composed of a single polypeptide of 89 kDa. J Biol Chem. 1992 Aug 15;267(23):16365–16370. [PubMed] [Google Scholar]
- Witthuhn B. A., Quelle F. W., Silvennoinen O., Yi T., Tang B., Miura O., Ihle J. N. JAK2 associates with the erythropoietin receptor and is tyrosine phosphorylated and activated following stimulation with erythropoietin. Cell. 1993 Jul 30;74(2):227–236. doi: 10.1016/0092-8674(93)90414-l. [DOI] [PubMed] [Google Scholar]
- Zhong Z., Wen Z., Darnell J. E., Jr Stat3 and Stat4: members of the family of signal transducers and activators of transcription. Proc Natl Acad Sci U S A. 1994 May 24;91(11):4806–4810. doi: 10.1073/pnas.91.11.4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Z., Wen Z., Darnell J. E., Jr Stat3: a STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science. 1994 Apr 1;264(5155):95–98. doi: 10.1126/science.8140422. [DOI] [PubMed] [Google Scholar]