Abstract
Objectives
Determine whether adaptation to a swing phase perturbation during gait transferred from treadmill to overground walking, the rate of overground deadaptation, and whether overground aftereffects improved step length asymmetry in persons with hemiparetic stroke and gait asymmetry.
Methods
Ten participants with stroke and hemiparesis and 10 controls walked overground on an instrumented gait mat, adapted gait to a swing phase perturbation on a treadmill, then walked overground on the gait mat again. Outcome measures, primary: overground step length symmetry, rates of treadmill step length symmetry adaptation and overground step length symmetry deadaptation; secondary: overground gait velocity, stride length, and stride cycle duration.
Results
Step length symmetry aftereffects generalized to overground walking and adapted at a similar rate on the treadmill in both groups. Aftereffects decayed at a slower rate overground in participants with stroke and temporarily improved overground step length asymmetry. Both groups’ overground gait velocity increased post adaptation due to increased stride length and decreased stride duration.
Conclusions
Stroke and hemiparesis do not impair generalization of step length symmetry changes from adapted treadmill to overground walking, but prolong overground aftereffects.
Significance
Motor adaptation during treadmill walking may be an effective treatment for improving overground gait asymmetries post-stroke.
Keywords: Gait, motor adaptation, CVA, motor learning
1. Introduction
Alterations in the normal pattern of walking often occur after stroke and addressing these walking deficits is a major focus of neurological rehabilitation. In individuals with post-stroke hemiparesis, gait is characterized by decreased speed and cadence along with other spatiotemporal changes that frequently lead to asymmetries of step length (Brandstater et al., 1983; von Schroeder et al., 1995; Hesse et al., 1999; De Bujanda et al., 2003). These deficits can result in an inefficient (e.g. requires increased energy to walk a given distance compared to non-disabled) and functionally less effective (e.g. unable to cross a street before a traffic light changes) gait pattern (Wall and Turnbull, 1986; Hsu et al., 2003; Chen et al., 2005; Balasubramanian et al., 2007; Oken and Yavuzer, 2008). Many interventions have been shown to improve some features of walking in persons with hemiparetic gait (Silver et al., 2000; Teixeira-Salmela et al., 2001; Peurala et al., 2005; Dunsky et al., 2008; Patterson et al., 2008; Regnaux et al., 2008), but they have generally shown little ability to alter gait asymmetries. Furthermore, although gait speed is an important rehabilitation goal (Schmid et al., 2007; Tilson et al., 2010), the relationship between gait speed and asymmetry is unclear.
Recently, we have shown that motor adaptation to a swing phase perturbation during treadmill walking can temporarily alter gait symmetry in nondisabled individuals (Savin et al., 2010) and those with stroke and hemiparesis (Savin et al., 2013). Motor adaptation is a practice-dependent alteration of an established movement pattern caused by a sensorimotor perturbation (Martin et al., 1996). It requires the cerebellum (Morton and Bastian, 2006) and produces aftereffects, i.e. movement errors that are opposite those seen during the initial adaptation. Aftereffects indicate that feedforward motor commands are updated and stored by the central nervous system (Weiner et al., 1983; Shadmehr and Mussa-Ivaldi, 1994) and can be thought of as evidence for short-term motor learning (Shadmehr and Wise, 2005). Evidence suggests that by perturbing hemiparetic gait so that baseline (pre-perturbed) asymmetry is initially increased, the resulting aftereffects can temporarily improve symmetry (Reisman et al., 2007, 2009, 2013; Savin et al., 2013). As such, locomotor adaptation (motor adaptation of gait) has been suggested as a potential treatment for the asymmetries of hemiparetic gait (Reisman et al., 2009).
To be an effective treatment for gait asymmetries, aftereffects resulting from locomotor adaptation during treadmill walking must generalize to overground walking. However, locomotor adaptation in animal models has been suggested to be context specific. For example, when cats adapt their gait on a treadmill, aftereffects are present during subsequent post-adaptation treadmill walking but not during overground walking (McVea and Pearson, 2007). However, in humans, evidence suggests that locomotor adaptation can generalize to a different context (Anstis, 1995; Weber et al., 1998; Earhart et al., 2002; Reisman et al., 2009, 2013; Torres-Oviedo and Bastian, 2010, 2012). Yet to our knowledge, only two studies have shown that locomotor adaptation generalizes from treadmill to overground walking in participants with stroke (Reisman et al., 2009, 2013) and none have investigated rates of overground deadaptation.
The rate at which motor adaptation occurs has been frequently studied (Martin et al., 1996; Smith et al., 2006; Wei and Körding, 2010; Savin et al., 2013) while the rate of deadaptation has not. We previously showed that the rate of initial fast (i.e. the first 10 – 30 strides) locomotor adaptation did not differ between controls and participants with stroke during treadmill walking (Savin et al., 2013). Therefore it would be reasonable to expect that the initial deadaptation rates in persons with stroke would be similar compared to nondisabled individuals. It is also unknown whether our specific adaptation paradigm, utilizing a swing phase resistance, can produce symmetric step lengths that will generalize to overground walking in persons with stroke and hemiparesis.
The primary purpose of this study was to test the extent to which locomotor adaptation to a swing phase perturbation during treadmill walking generalized to overground walking in participants with post-stroke hemiparesis and controls. We hypothesized that all participants would show a generalization of step length symmetry adaptation and that participants with stroke and controls would have similar rates of deadaptation overground. We also hypothesized that by perturbing the leg with the shorter overground step length in persons post-stroke, the resulting aftereffects would decrease overground step length asymmetry. The secondary purpose of this study was to investigate the effects of aftereffect-induced changes in step length symmetry on overground gait parameters (e.g., speed) in participants with stroke.
2. Methods
2.1. Participants
Ten participants with stroke and hemiparesis (7 female, aged 62.8 ± 9.4 years) and 10 age- (± 5 years) and gender matched nondisabled controls (aged 61.8 ± 9.3 years) were recruited to participate in the study. All participants gave informed consent and the study protocol was approved by the joint Baltimore Veterans Administration and University of Maryland Baltimore Institutional Review Board. Participants with stroke were included if they had a history of unilateral ischemic stroke occurring >9 months earlier and were able to walk ≥0.4 m/s on a treadmill. Lesion location was determined by CT or MRI and classified by a neurologist as cortical, subcortical, and/or brainstem. Participants with stroke were excluded if they had a history of stroke affecting both hemispheres, cerebellar damage, other neurological or orthopedic conditions affecting the legs or a Mini Mental State Exam (Folstein et al., 1975) score <22. All participants with stroke walked without the use of an ankle-foot orthosis or assistive device. See Table 1 for further details.
Table 1.
Demographics of participants with stroke.
| Participant | Age | Gender | Time Since Onset | Lesion Information |
|---|---|---|---|---|
| S1 | 63 | F | 7.5 y | Subcortical, internal capsule |
| S2 | 67 | F | 48.8 y | Cortical |
| S3 | 69 | M | 9.8 y | MCA infarct, cortex + subcortical |
| S4 | 60 | F | 2.3 y | Cortical |
| S5 | 54 | M | 7.4 y | Cortical |
| S6 | 76 | F | 9.5 y | Brainstem |
| S7 | 56 | F | 6.8 y | Subcortical |
| S8 | 77 | M | 13.8 y | Subcortical, internal capsule |
| S9 | 57 | F | 4.8 y | Cortical |
| S10 | 49 | F | 4.8 y | MCA infarct, cortical |
|
| ||||
| Mean | 62.8(9.4) | 11.6(13.5) y | ||
Abbreviations: MCA = middle cerebral artery; LE = lower extremity. Means ± 1SD.
2.2. Testing paradigm
Participants undertook four consecutive testing conditions: Overground Baseline, Treadmill Baseline, Treadmill Adaptation and Overground Generalization. During overground conditions participants walked on a 7.9 meter-long GAITRite mat (CIR Systems, Inc., Sparta, NJ). They were instructed to walk at their preferred speed with their arms free to swing. During treadmill conditions, participants walked on a motorized treadmill (Woodway, Inc., Waukesha, WI). While walking on the treadmill, participants were instructed to hold onto the front hand rail, look straight ahead, avoid looking at their feet, and not think about their walking. For safety, all participants wore a harness to prevent falling. In all conditions, participants wore custom-made padded cuffs around each of their lower legs to which the perturbation device could be attached. See Figure 1A.
Figure 1.

A. Illustration of the treadmill setup. The small circles represent position marker placement. The weight and pulley system was positioned directly behind the perturbed leg. B. Time course of the experiment showing conditions and key testing periods.
During Overground Baseline participants walked the length of the GAITRite mat three times. During treadmill walking, the treadmill’s speed was set to 80% of a participant’s overground gait speed to minimize any confounding effect on gait due to the perception that they were walking faster on the treadmill compared to overground (Dal et al., 2010). Details of the treadmill paradigm have been previously published (Savin et al., 2010). Briefly, participants walked on the treadmill during Treadmill Baseline and Adaptation conditions, lasting five and 10 minutes respectively. During the Treadmill Adaptation condition, a rope was attached to the cuff on the leg having the shorter overground step length as determined by the GAITRite mat. The other end of the rope passed through a set of pulleys and was connected to a weight equal to 1.25% of the participant’s body weight, rounded to the nearest 0.11 Kg, which resisted forward movement of that leg during its swing phase. See Figure 1A. Following Treadmill Adaptation, participants were instructed to remain on the treadmill belt while a wheelchair was brought to them. The harness and weight were unhooked and participants sat in the wheelchair. They were then wheeled off the treadmill and positioned at the end of the GAITRite mat. During the Overground Generalization condition, participants walked the length of the mat five times, again at their preferred speed. See Figure 1B. When participants reached the end of the mat, they were instructed to walk off and stop without turning around. They were then seated in the wheelchair and turned around. In this manner, all strides during this condition occurred on the GAITRite mat.
2.3. Data Collection
Spatial and temporal gait parameters during overground walking were collected at 120 samples/s with the GAITRite system. Position data during treadmill walking were collected with two Optotrak Certus position sensors (NDI, Waterloo, Ontario, Canada), one on each side of the treadmill. We placed infrared emitting diodes over the head of the fifth metatarsal and the lateral malleolus bilaterally to define the foot and ankle positions. Position data were collected continuously at 100 samples/s during treadmill walking.
2.4. Data Analysis
Overground gait parameters were analyzed with GAITRite software. Treadmill position data were analyzed with custom written MATLAB (MathWorks, Natick, MA) software. Position data were low-pass filtered at 6 Hz. During treadmill walking, we identified each stride as the time from initial contact on one foot to the next initial contact on the same foot. Initial contact was identified as the time when the ankle marker reached its maximum forward position. Lift off was the time when the foot marker reached its maximum backward position (Noble and Prentice, 2006). Step length was defined as the forward distance between the two ankle markers at initial contact. Single limb support (SLS) time was defined as the time from lift off on one foot to the next initial contact on the same foot.
Primary outcome variables were step length symmetry, rate of step length symmetry adaptation on the treadmill, and rate of step length symmetry deadaptation overground. Secondary outcome variables were overground gait velocity, overground SLS time symmetry (measured as a percentage of the stride cycle), variability of SLS time and step length symmetry (standard deviation), and stride length and stride cycle duration changes. Symmetry was quantified with a symmetry index: SI = (Xu − Xp)/(Xu + Xp), where u is the unperturbed leg (e.g. the leg with the longer overground baseline step length, irrespective of side of paresis in the stroke group), p is the perturbed leg (Noble and Prentice, 2006), and X represents the variable of interest (i.e., step length or SLS time). Perfect symmetry will result in a symmetry index value of zero. Rates of step length symmetry adaptation and deadaptation were calculated by first smoothing each participant’s data using a moving average with a window width of 2 data points. To determine adaptation and deadaptation rates, an exponential decay function was then fit to each participant’s data (Lang and Bastian, 1999) with the form of y = a + (b * e−t/c) where a is the final value that the exponential decay function approaches, i.e. the plateau reached at the end of adaptation/deadaptation, b is the magnitude of adaptation or deadaptation required from the first trial value to the value a, t is the stride number and c is the decay constant or the rate at which adaptation or deadaptation occurs. In our paradigm, c is the number of strides it will take to obtain (1 − e−1) or approximately two thirds of the adaptation or deadaptation (Lang and Bastian, 1999). In order to ensure a logical and reasonable fit, we broadly constrained a to vary between −0.25 and +0.25, b was set to equal the difference between the first and final adaptation/deadaptation symmetry values on a participant by participant basis, and c to vary between 1 and 40.
2.5. Statistical Analysis
We compared step length symmetry across key testing periods by averaging the last 5 strides of baseline conditions (Overground and Treadmill Late Baseline) and the first and last 5 strides of Treadmill Adaptation and Overground Generalization conditions (Early and Late Treadmill Adaptation, and Early and Late Overground Generalization respectively). Secondarily, we compared overground SLS time symmetry, variability of SLS time and step length symmetry, gait velocity, stride length and stride cycle duration by averaging the last 5 strides of Overground Late Baseline, Overground Early and Late Adaptation conditions. See Figure 1B. Statistical comparisons were completed using Statistica software (StatSoft, Tulsa, OK). Step length symmetry indices and all secondary variables were compared with a factorial ANOVA with factors group (control and participants with stroke) and testing period, with repeated measures on testing period. When an ANOVA yielded a significant result, post hoc analyses were performed using Tukey’s honest significant difference test. Adaptation and deadaptation rates were compared with independent samples t-tests. The level of statistical significance for these variables was set at p <0.05. Data are presented as mean ± 1 SD.
3. Results
One participant with stroke (S2) was removed from all analyses because she complained of vertigo-like symptoms after the treadmill adaptation and required assistance to walk during the first overground generalization trial. There was no difference between subject groups with respect to age (p =0.81). Average overground baseline and treadmill gait speeds for participants with stroke was 0.76 ± 0.3 and 0.61 ± 0.2 m/s, and for controls 1.24 ± 0.2 and 0.99 ± 0.2 m/s respectively. Average overground baseline step length asymmetry was greater for participants with stroke compared to controls (0.087 ± 0.062 versus 0.014 ± 0.013, p =0.002).
3.1. Perturbation-induced step length symmetry changes
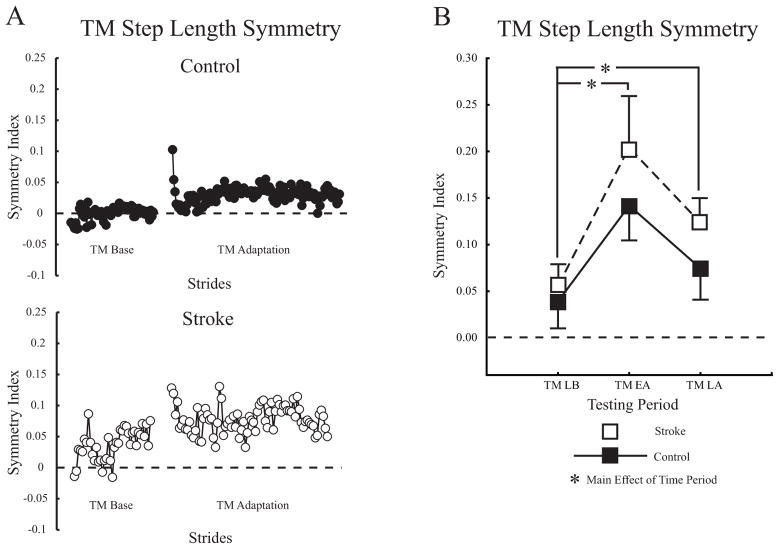
Figure 2A shows individual step length symmetry changes during treadmill walking for a typical control and participant with stroke. When first perturbed in Treadmill Adaptation, symmetry index values showed an immediate increase (i.e. became more positive), indicating that the unperturbed leg had a longer step length than the perturbed. As walking continued, both participants’ step symmetry index values were adjusted back toward baseline. Figure 2B shows group average step length symmetry changes during treadmill walking for controls and participants with stroke. The ANOVA had a main effect of testing period (p <0.0001) but no group or interaction effects. Post hoc analysis indicated the perturbation caused step length symmetry indices to immediately increase in Treadmill Early Adaptation compared to Late Baseline (0.163 ± 0.15 vs. 0.046 ± 0.08, p <0.001). By Treadmill Late Adaptation, step length symmetry indices adjusted toward, but remained above baseline values (0.101 ± 0.09 vs. 0.046 ± 0.08, p <0.05).
Figure 2.
A. Step length symmetry during treadmill walking plotted on a stride-by-stride basis for a typical control and participant with stroke. Each circle represents the average of three consecutive strides. B. Group average step length symmetry changes across treadmill conditions for all participants. Treadmill Late Baseline (TM LB), Treadmill Early Adaptation (TM EA), Treadmill Late Adaptation (TM LA). The dashed horizontal line in all plots indicates perfect symmetry. Asterisks indicate significance level of p <0.05 from post hoc analyses of each condition vs. late baseline for the main effect of testing period. Error bars ± 1 SEM.
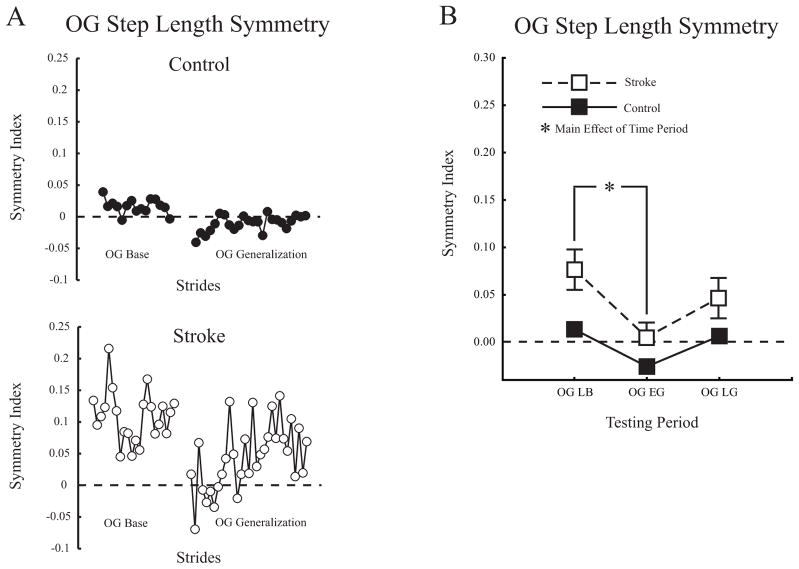
Figure 3A shows stride-by-stride changes in overground step length symmetry for a typical control and participant with stroke. Note that aftereffects, present initially during Generalization, resulted in step length symmetry for the participant with stroke and asymmetry for the control participant. Figure 3B shows average overground step length symmetry changes for both groups. The ANOVA had main effects of group and testing period (p <0.01 for both) but no interaction. Post hoc analysis on group showed symmetry indices for participants with stroke were more positive and therefore more asymmetrical than controls (0.042 ± 0.07 vs. −0.002 ± 0.02, p <0.01). Post hoc analysis on testing period showed the perturbation resulted in a significant step length symmetry aftereffect (Overground Late Baseline to Overground Early Generalization, 0.044 ± 0.06 vs. −0.012 ± 0.04, p <0.001). Aftereffects resulted in participants with stroke as a group temporarily walking with step length symmetry overground. By Overground Late Generalization, step length symmetry indices returned to Baseline (0.025 ± 0.05 vs. 0.044 ± 0.05, p =0.22). See Table 2 for group average step length values for the perturbed and unperturbed legs across all experimental conditions.
Figure 3.
A. Step length symmetry during overground walking plotted on a stride-by-stride basis (not averaged) for a typical control and participant with stroke. B. Group average step length symmetry changes in overground walking. Overground Late Baseline (OG LB), Overground Early Generalization (OG EG), Overground Late Generalization (OG LG). The dashed horizontal lines indicate perfect symmetry. Asterisk indicates significance level of p <0.05 from post hoc analyses of each condition vs. late baseline for the main effect of testing period. Error bars ± 1 SEM.
Table 2.
Group average step length for perturbed and unperturbed legs across all experimental conditions.
| Leg | OG LB | TM LB | TM EA | TM LA | OG EG | |
|---|---|---|---|---|---|---|
| Step Length (cm) Stroke | Perturbed | 45.9 (15.1) | 38.6 (9.1) | 32.8 (12.6) | 36.3 (10.1) | 48.2 (13.6) |
| Unperturbed | 53.0 (11.4) | 42.2 (7.6) | 45.9 (7.3) | 45.1 (8.0) | 48.1 (14.9) | |
| Step Length (cm) Control | Perturbed | 67.8 (9.4) | 50.5 (6.6) | 44.6 (9.0) | 49.0 (8.1) | 68.9 (10.0) |
| Unperturbed | 69.9 (8.4) | 54.5 (5.9) | 58.6 (5.3) | 56.5 (5.4) | 66.5 (9.3) |
Abbreviations: OG LB = Overground Late Baseline, TM LB = Treadmill Late Baseline, TM EA = Treadmill Early Adaptation, TM LA = Treadmill Late Adaptation, OG EG = Overground Early Generalization, OG LG = Overground Late Generalization. Data presented as means ± 1SD.
3.2. Adaptation and deadaptation rates
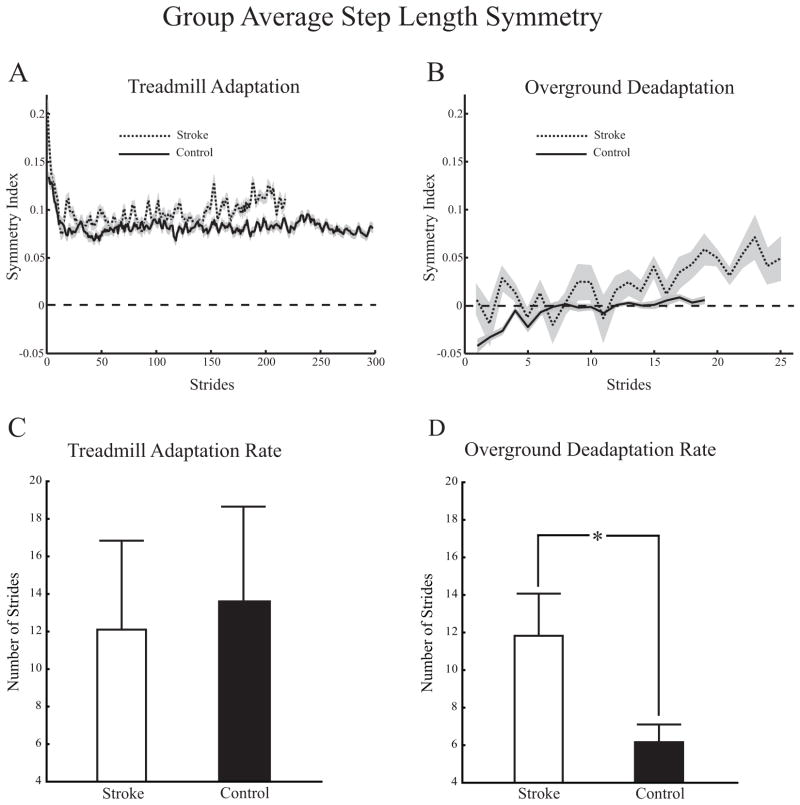
Figures 4A and 4B show group averaged step length symmetry treadmill adaptation and overground deadaptation curves for controls and participants with stroke. Overground aftereffects for participants with stroke resulted in them initially walking with decreased step length asymmetry whereas controls walked with increased step length asymmetry. Note that while both groups appear to reach a plateau during treadmill adaptation, only the control group appears to reach a similar plateau during overground deadaptation. Figures 4C and 4D show treadmill step length symmetry adaptation rates and overground step length symmetry deadaptation rates for controls and participants with stroke respectively (goodness of fit for deadaptation rates, average adjusted r2 = 0.25 for participants with stroke and 0.59 for controls). There was no difference in adaptation rates during treadmill walking between participants with stroke and controls (12.1 ± 15.0 vs. 13.6 ± 15.2 strides). However there was a difference in overground deadaptation rates. Participants with stroke required more strides to deadapt overground than controls (11.8 ± 6.7 vs. 6.2 ± 3.0, p <0.05). While controls took more steps during treadmill adaptation than participants with stroke (787 ± 131 versus 604 ± 128, p = 0.007) and fewer steps during Overground Generalization (48 ± 7 versus 73 ± 21, p = 0.003 independent samples t-test for both), we found no significant correlations between the number of steps taken during treadmill adaptation and overground deadaptation rate (R = −0.46, p = 0.21 for controls and R = −0.14, p = 0.71 for participants with stroke, Spearman Rank Order correlation).
Figure 4.
A and B. Group average treadmill and overground step length symmetry adaptation and deadaptation plots for controls and participants with stroke. Dashed lines indicate perfect symmetry. Solid lines represent the control group; dotted lines represent the stroke group. Gray shading indicates ± 1 SD. C. Treadmill adaptation rates for control participants and those with stroke. D. Overground deadaptation rates for control participants and those with stroke. Asterisk indicates significance level of p < 0.05. Error bars ± 1 SEM.
3.3. Secondary measures
Figure 5A shows group average overground gait speed changes. The ANOVA had main effects of group and testing period (p <0.01 for both) but no interaction effect. Post hoc analysis on group indicated that participants with stroke walked slower overground than controls (0.74 ± 0.3m/s vs. 1.27 ± 0.20m/s, p <0.001). Post hoc analysis on testing period indicated overground gait speed was slower during Overground Early Generalization and faster during Overground Late Generalization compared to Overground Late Baseline (0.96 ± 0.4m/s vs. 1.02 ± 0.4m/s, p <0.01 and 1.08 ± 0.4m/s vs. 1.02 ± 0.4m/s, p <0.02 respectively). Figures 5B and 5C show group average overground stride length and stride cycle duration changes, respectively. Similar to overground gait speed, there were significant main effects of group and testing period for both (p <0.01 for all), but no group by testing period interactions. For the group effects, as expected, the stroke group had decreased stride lengths and increased stride cycle durations compared to controls (post hocs, p <0.01 for both). For the testing period effects, stride lengths were reduced during Overground Early Generalization compared to Overground Late Baseline (post hoc, p <0.05) and increased during Overground Late Generalization compared to Overground Late Baseline (post hoc, p <0.01). Stride cycle durations were reduced during Overground Late Generalization compared to Overground Early Generalization (p <0.01). Overall, the secondary analyses of overground gait speed, stride length, and stride cycle duration all showed the typical changes expected post-stroke (slower speed, reduced stride length, and increased stride duration) as well as a relative slowing in Overground Early Generalization with restoration of levels toward Overground Late Baseline by Overground Late Generalization.
Figure 5.
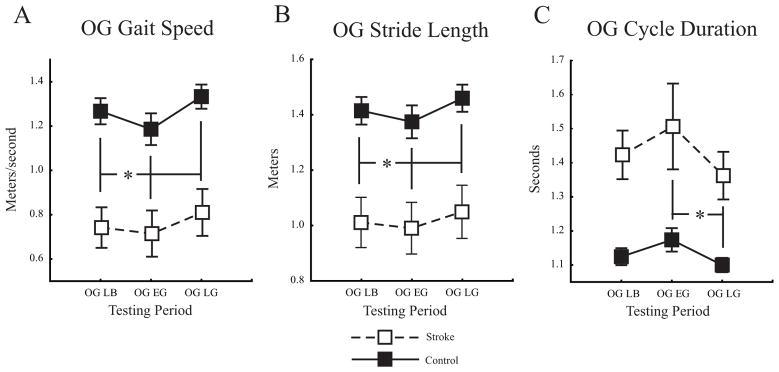
A. Group average overground gait velocity. B. Group average overground stride length. C. Group average overground stride cycle duration. Overground Late Baseline (OG LB), Overground Early Generalization (OG EG), Overground Late Generalization (OG LG). Asterisks for A and B indicate significance level of p < 0.05 from post hoc analyses of each condition vs. Overground Late Baseline for the main effect of testing period. Asterisk in C indicates significance level of p < 0.05 from post hoc analysis of Early vs. Late Overground Generalization for the main effect of testing period. Error bars ± 1 SEM.
There were no interactions or main effects for group or testing period for SLS time symmetry across overground conditions (p = 0.26, 0.08 and 0.18 respectively). (See Table 3 for group average SLS time values for each leg across overground conditions.) There were main effects for group for SLS time and step length symmetry variability, with stroke participants having greater variability than controls (p = 0.02 and 0.01 respectively). However there were no interactions or main effects for testing period for variability of SLS time symmetry (p = 0.18 and 0.10 respectively) or step length symmetry (p = 0.75 and 0.36 respectively) across overground testing conditions. Thus it did not appear that our swing phase perturbation altered overground SLS time or SLS time and step length variability.
Table 3.
Group average single limb support (SLS) time as a percent of the stride cycle across overground conditions.
| Leg | OG LB | OG EG | OG LG | |
|---|---|---|---|---|
| SLS Time Stroke | Perturbed | 33.9 (4.8) | 33.7 (4.9) | 33.8 (4.9) |
| Unperturbed | 29.1 (6.2) | 27.8 (8.0) | 29.9 (6.2) | |
| SLS Time Control | Perturbed | 37.0 (1.8) | 36.9 (3.0) | 37.4 (1.5) |
| Unperturbed | 36.0 (2.6) | 35.7 (2.5) | 36.7 (2.1) |
Abbreviations: OG LB = Overground Late Baseline, OG EG = Overground Early Generalization, OG LG = Overground Late Generalization. Data presented as means ± 1SD.
4. Discussion
Our data supported our hypotheses that all participants would show a generalization of step length adaptation to overground walking and that overground aftereffects would temporarily decrease step length asymmetry in participants with stroke. Our data did not support our hypothesis that deadaptation rates would be similar between participants with stroke and controls. Step length symmetry aftereffects in participants with stroke generalized to overground walking, demonstrating that despite stroke and hemiparesis, the motor system retained the capability to significantly alter its output through error-based learning mechanisms. This resulted in participants with stroke walking with step length symmetry, albeit temporarily, during the real world task of overground walking. This is significant for gait rehabilitation post-stroke given that these results occurred after only one 10-minute bout of treadmill walking, similar to those of Kahn and Hornby (2009) and Reisman et al., (2007, 2013), whereas traditional interventions lasting longer have not been able to alter gait symmetry (Silver et al., 2000; Peurala et al., 2005; Regnaux et al., 2008).
Overground step length symmetry deadaptation rate differences could be due to several factors. The slower rate in persons with stroke could be reflective of their walking overground with greater step length symmetry immediately after treadmill adaptation, possibly representing a cost savings to the motor system since a symmetrical gait pattern could be more efficient and functional, e.g. allowing effective community ambulation, than an asymmetrical one (Wall and Turnbull, 1986; Hsu et al., 2003; Chen et al., 2005; Oken and Yavuzer, 2008; Hall et al., 2012; Finley et al., 2013). It is also possible that walking overground has a higher attentional demand for persons with stroke and this distraction interferes with deadaptation. A previous study on split-belt treadmill adaptation in healthy adults found that distraction slows adaptation and subsequent deadaptation rates but the effect of distraction during deadaptation was not tested (Malone and Bastian, 2010). Evidence also suggests persons with stroke may have impairments integrating sensory information (Marigold et al., 2004; Lamontagne et al., 2007; Lamontagne and Fung, 2009; Lamontagne et al., 2010), which may increase sensory feedback variability, potentially decreasing deadaptation rates (Wei and Körding, 2010). However, post hoc we found no correlation between step length symmetry variability during treadmill adaptation and deadaptation rates, (Spearman Rank Order correlations, R = −0.42, p = 0.21 for controls, R = 0.08, p = 0.83 for participants with stroke). This finding agrees with the results of Torres-Oviedo and Bastian (2012) who found that variability during adaptation was positively related to the magnitude, but not the deadaptation of the aftereffect.
Reisman et al., (2009) reported findings consistent with ours when they found no differences in adaptation but a greater transfer of split-belt treadmill adaptation to overground walking in persons with stroke compared to controls. They speculated that deficits in the ability of participants with stroke to modify their motor plan in response to a contextual change (e.g. from walking on a split-belt treadmill to walking overground) could explain the greater transfer magnitude. Anecdotally, we used the methods described in this study to re-analyze data from a previous study (Savin et al., 2013) involving controls and persons with stroke adapting and de-adapting their gait to our swing phase perturbation during treadmill walking. We found no group differences in deadaptation rates on the treadmill. Additionally, when comparing treadmill and overground deadaptation rates within groups, controls deadapted significantly faster overground than on the treadmill while there were no differences in rates for participants with stroke. While somewhat speculative, taken together these further suggest that persons with stroke have impairments in modifying their gait pattern when the walking context changes (e.g. from treadmill to overground). Regardless of the mechanisms, this impairment in locomotor deadaptation rate overground may have the potential to be beneficial in gait rehabilitation, since it allowed participants with stroke to walk with step length symmetry changes for a longer time than controls. Further study will be needed to determine whether this can be used advantageously in the rehabilitation of asymmetrical gait.
We found no significant group by testing period interaction effects in our secondary outcome measures. Thus for these measures, participants with stroke behaved like nondisabled controls. It is interesting to note that while all participants walked slower in the Overground Early Generalization condition compared to Overground Late Baseline, they walked faster in Overground Late Generalization. It is perhaps not surprising that gait speed was slower in the Overground Early Generalization condition given that we set the treadmill speed to 80% of baseline overground gait speed.
While asymmetry often results in decreased gait function and efficiency (Wall and Turnbull 1986; Chen et al. 2005; Hsu et al. 2003; Oken and Yavuzer 2008), step length symmetry may not be as important to gait speed as is sometimes thought (c.f. Latash and Anson, 1996). Indeed, the rehabilitation goal of a symmetrical gait pattern is somewhat controversial since with asymmetrical constraints an asymmetrical gait may be more functionally effective. For our participants with stroke, the return to asymmetry with accompanying speed increases from early to late generalization could be an indication of gait becoming more comfortable. For nondisabled it may also be an indication of gait becoming more comfortable as they return to symmetry. In other words, both groups return to their respective, well-practiced baseline gait patterns while increasing their preferred overground speed. In sum, improving asymmetry was not actually detrimental to gait speed for individuals with stroke and returning to asymmetry was beneficial.
5. Study Limitations
One study limitation was that participants with stroke were mildly impaired based on their lower extremity Fugl-Myer assessment scores, possibly decreasing our ability to generalize our findings to stroke survivors as a whole. Another limitation is that differences in gait speed between controls and participants with stroke resulted in controls taking more strides during adaptation than participants with stroke. Thus controls were exposed to the perturbation for a greater number of stride cycles. While this could have resulted in differences in aftereffects and deadaptation rates between groups, we do not believe this to be the case. First there was no group by testing period interaction in treadmill step length symmetry, indicating both groups responded similarly. Second, both groups had significant aftereffects overground. If anything, we would have expected participants with stroke, who on the whole took fewer steps during adaptation, to have had less robust aftereffects, which our data do not support.
6. Conclusions
In summary, we have shown that adaptation to our swing phase perturbation during treadmill walking generalizes to overground walking in controls and persons with stroke and individuals with post-stroke hemiparesis retain the ability to alter gait symmetry in the real-world task of overground walking. Locomotor adaptation to a swing phase perturbation during treadmill walking appears to have the potential to be an effective and low-cost means of bringing about short-term improvements in overground step length symmetry. Decreased rates of deadaptation in persons post-stroke allowed them to walk with gait symmetry for a longer time. This may prove beneficial if this paradigm is used to improve hemiparetic gait asymmetry since it allows more time on the task of walking with gait symmetry. Further study is needed to determine whether aftereffect-driven symmetry can be made more durable and whether improved asymmetry also results in improvements in functional gait.
Highlights.
Locomotor adaptation to a unilateral swing phase resistance during treadmill walking generalized to overground walking in all study participants.
Overground aftereffects resulted in a temporary reduction of step length asymmetry in participants with stroke who had baseline step length asymmetry.
After effects in participants with stroke decayed at a slower rate overground compared to controls, despite no difference in the rate of treadmill adaptation between the two groups.
Acknowledgments
The authors wish to thank M. Falbo for assistance with data collection and R. Roche for helpful discussions regarding our findings and thoughtful comments and suggestions regarding previous versions of this manuscript. This work was supported by grants NIH T32 HD041899, NIH K01 HD050369, NIH R21 NS067189, NIA P30 AG028747, and a VA predoctoral fellowship award.
Footnotes
This material was presented as a poster for the American Physical Therapy Association’s Combined Sections Meeting, February 2012, Chicago, IL, USA.
The authors declare that there is no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anstis S. Aftereffects from jogging. Exp Brain Res. 1995;103:476–478. doi: 10.1007/BF00241507. [DOI] [PubMed] [Google Scholar]
- Balasubramanian CK, Bowden MG, Neptune RR, Kautz SA. Relationship between step length asymmetry and walking performance in subjects with chronic hemiparesis. Arch Phys Med Rehabil. 2007;88:43–49. doi: 10.1016/j.apmr.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Brandstater ME, de Bruin H, Gowland C, Clark BM. Hemiplegic gait: analysis of temporal variables. Arch Phys Med Rehabil. 1983;64:583–587. [PubMed] [Google Scholar]
- De Bujanda E, Nadeau S, Bourbonnais D, Dickstein R. Associations between lower limb impairments, locomotor capacities and kinematic variables in the frontal plane during walking in adults with chronic stroke. J Rehabil Med. 2003;35:259–264. doi: 10.1080/16501970310012428. [DOI] [PubMed] [Google Scholar]
- Chen G, Patten C, Kothari DH, Zajac FE. Gait differences between individuals with post-stroke hemiparesis and non-disabled controls at matched speeds. Gait Posture. 2005;22:51–56. doi: 10.1016/j.gaitpost.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Dal U, Erdogan T, Resitoglu B, Beydagi Determination of preferred walking speed on treadmill may lead to high oxygen cost on treadmill walking. Gait Posture. 2010;31:366–369. doi: 10.1016/j.gaitpost.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Dunsky A, Dickstein R, Marcovitz E, Levy S, Deutsch J. Home-based motor imagery training for gait rehabilitation of people with chronic poststroke hemiparesis. Arch Phys Med Rehabil. 2008;89:1580–1588. doi: 10.1016/j.apmr.2007.12.039. [DOI] [PubMed] [Google Scholar]
- Earhart GM, Jones GM, Horak FB, Block EW, Weber KD, Fletcher WA. Transfer of podokinetic adaptation from stepping to hopping. J Neurophysiol. 2002;87:1142–1144. doi: 10.1152/jn.00588.2001. [DOI] [PubMed] [Google Scholar]
- Finley JM, Bastian AJ, Gottschall JS. Learning to be economical: the energy cost of walking tracks motor adaptation. J Physiol. 2013;591.4:1081–1095. doi: 10.1113/jphysiol.2012.245506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Hall AL, Bowden MG, Kautz SA, Neptune RR. Biomechanical variables related to walking performance 6-months following post-stroke rehabilitation. Clin Biomech. 2012;27:1017–1022. doi: 10.1016/j.clinbiomech.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesse S, Konrad M, Uhlenbrock D. Treadmill walking with partial body weight support versus floor walking in hemiparetic participants. Arch Phys Med Rehabil. 1999;80:421–427. doi: 10.1016/s0003-9993(99)90279-4. [DOI] [PubMed] [Google Scholar]
- Hsu AL, Tang PF, Jan MH. Analysis of impairments influencing gait velocity and asymmetry of hemiplegic patients after mild to moderate stroke. Arch Phys Med Rehabil. 2003;84:1185–1193. doi: 10.1016/s0003-9993(03)00030-3. [DOI] [PubMed] [Google Scholar]
- Kahn JH, Hornby TG. Rapid and long-term adaptations in gait symmetry following unilateral step training in people with hemiparesis. Phys Ther. 2009;89:474–483. doi: 10.2522/ptj.20080237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamontagne A, Fung J. Gaze and postural reorientation in the control of locomotor steering after stroke. Neurorehabil Neural Repair. 2009;23:356–366. doi: 10.1177/1545968308324549. [DOI] [PubMed] [Google Scholar]
- Lamontagne A, Fung J, McFadyen BJ, Faubert J. Modulation of walking speed by changing optic flow in persons with stroke. J Neuroeng Rehabil. 2007;4:22. doi: 10.1186/1743-0003-4-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamontagne A, Fung J, McFadyen B, Faubert J, Paquette C. Stroke affects locomotor steering responses to changing optic flow directions. Neurorehabil Neural Repair. 2010;24:457–468. doi: 10.1177/1545968309355985. [DOI] [PubMed] [Google Scholar]
- Lang CE, Bastian AJ. Cerebellar participants show impaired adaptation of anticipatory EMG during catching. J Neurophysiol. 1999;82:2108–2119. doi: 10.1152/jn.1999.82.5.2108. [DOI] [PubMed] [Google Scholar]
- Latash ML, Anson JG. What are “normal movements” in atypical populations? Behav Brain Sci. 1996;19:55–106. [Google Scholar]
- Malone LA, Bastian AJ. Thinking about walking: effects of conscious correction versus distraction on locomotor adaptation. J Neurophysiol. 2010;103:1954–1962. doi: 10.1152/jn.00832.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marigold DS, Eng JJ, Tokuno CD, Donnelly CA. Contribution of muscle strength and integration of afferent input to postural instability in persons with stroke. Neurorehabil Neural Repair. 2004;18:222–229. doi: 10.1177/1545968304271171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin TA, Keating JG, Goodkin HP, Bastian AJ, Thach WT. Throwing while looking through prisms I. Focal olivocerebellar lesions impair adaptation. Brain. 1996;119:1183–1198. doi: 10.1093/brain/119.4.1183. [DOI] [PubMed] [Google Scholar]
- McVea DA, Pearson KG. Long-lasting, context-dependent modification of stepping in the cat after repeated stumbling-corrective responses. J Neurophysiol. 2007;97:659–669. doi: 10.1152/jn.00921.2006. [DOI] [PubMed] [Google Scholar]
- Morton SM, Bastian AJ. Cerebellar contributions to locomotor adaptations during splitbelt treadmill walking. J Neurosci. 2006;26:9107–9116. doi: 10.1523/JNEUROSCI.2622-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble JW, Prentice SD. Adaptation to unilateral change in lower limb mechanical properties during human walking. Exp Brain Res. 2006;169:482–495. doi: 10.1007/s00221-005-0162-3. [DOI] [PubMed] [Google Scholar]
- Oken O, Yavuzer G. Spatio-temporal and kinematic asymmetry ratio in subgroups of patients with stroke. Eur J Phys Rehabil Med. 2008;44:127–132. [PubMed] [Google Scholar]
- Patterson SL, Rodgers MM, Macko RF, Forrester LW. Effect of treadmill exercise training on spatial and temporal gait parameters in participants with chronic stroke: a preliminary report. J Rehabil Res Dev. 2008;45:221–228. doi: 10.1682/jrrd.2007.02.0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peurala SH, Titianova EB, Mateev P, Pitkanen K, Sivenius J, Tarkka IM. Gait characteristics after gait-oriented rehabilitation in chronic stroke. Restor Neurol Neurosci. 2005;23:57–65. [PubMed] [Google Scholar]
- Regnaux JP, Pradon D, Roche N, Robertson J, Bussel B, Dobkin B. Effects of loading the unaffected limb for one session of locomotor training on laboratory measures of gait in stroke. Clin Biomech. 2008;23:762–768. doi: 10.1016/j.clinbiomech.2008.01.011. [DOI] [PubMed] [Google Scholar]
- Reisman DS, McLean H, Keller J, Danks KA, Bastian AJ. Repeated split-belt treadmill training improves poststroke step length asymmetry. Neurorehabil Neural Repair. 2013;27:460–468. doi: 10.1177/1545968312474118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisman DS, Wityk R, Silver K, Bastian AJ. Locomotor adaptation on a split-belt treadmill can improve walking symmetry post-stroke. Brain. 2007;130:1861–1872. doi: 10.1093/brain/awm035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisman DS, Wityk R, Silver K, Bastian AJ. Split-belt treadmill adaptation transfers to overground walking in persons poststroke. Neurorehabil Neural Repair. 2009;23:735–744. doi: 10.1177/1545968309332880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savin DN, Tseng SC, Morton SM. A bilateral adaptation during locomotion following a unilaterally-applied resistance to swing in non-disabled adults. J Neurophysiol. 2010;104:3600–3611. doi: 10.1152/jn.00633.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savin DN, Tseng SC, Whitall J, Morton SM. Spatial and temporal gait parameters adapt similarly to a unilateral swing-phase perturbation in nondisabled and persons post stroke. Neurorehabil Neural Repair. 2013;27:24–34. doi: 10.1177/1545968311434552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid A, Duncan PW, Studenski S, Lai SM, Richards L, Perera S, et al. Improvements in speed-based gait classifications are meaningful. Stroke. 2007;38:2096–2100. doi: 10.1161/STROKEAHA.106.475921. [DOI] [PubMed] [Google Scholar]
- von Schroeder HP, Coutts RD, Lyden PD, Billings E, Nickel VL. Gait parameters following stroke: a practical assessment. J Rehabil Res Dev. 1995;32:25–31. [PubMed] [Google Scholar]
- Shadmehr R, Mussa-Ivaldi FA. Adaptive representation of dynamics during learning of a motor task. J Neurosci. 1994;14:3208–3224. doi: 10.1523/JNEUROSCI.14-05-03208.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadmehr R, Wise SP. The computational neurobiology of reaching and pointing: A foundation for motor learning. Cambridge, MA: The MIT Press; 2005. [Google Scholar]
- Silver KHC, Macko RF, Forrester LW, Goldberg AP, Smith GV. Effects of aerobic treadmill training on gait velocity, cadence, and gait symmetry in chronic hemiparetic stroke: A preliminary report. Neurorehabil Neural Repair. 2000;14:65–71. doi: 10.1177/154596830001400108. [DOI] [PubMed] [Google Scholar]
- Smith MA, Ghazizadeh A, Shadmehr R. Interacting adaptive processes with different timescales underlie short-term motor learning. PLoS Biol. 2006;4:e179. doi: 10.1371/journal.pbio.0040179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira-Salmela LF, Nadeau S, Mcbride I, Olney SJ. Effects of muscle strengthening and physical conditioning training on temporal, kinematic and kinetic variables during gait in chronic stroke survivors. J Rehabil Med. 2001;33:53–66. doi: 10.1080/165019701750098867. [DOI] [PubMed] [Google Scholar]
- Tilson JK, Sullivan KJ, Cen SY, Rose DK, Koradia CH, Azen SP, et al. Meaningful gait speed improvement during the first 60 days poststroke: minimal clinically important difference. Phys Ther. 2010;90:196–208. doi: 10.2522/ptj.20090079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Oviedo G, Bastian AJ. Seeing is believing: effects of visual contextual cues on learning and transfer of locomotor adaptation. J Neurosci. 2010;30:17015–17022. doi: 10.1523/JNEUROSCI.4205-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Oviedo G, Bastian AJ. Natural error patterns enable transfer of motor learning to novel contexts. J Neurophysiol. 2012;107:346–356. doi: 10.1152/jn.00570.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall JC, Turnbull GI. Gait asymmetries in residual hemiplegia. Arch Phys Med Rehabil. 1986;67:550–553. [PubMed] [Google Scholar]
- Weber KD, Fletcher WA, Gordon CR, Jones GM, Block EW. Motor learning in the “podokinetic” system and its role in spatial orientation during locomotion. Exp Brain Res. 1998;120:377–385. doi: 10.1007/s002210050411. [DOI] [PubMed] [Google Scholar]
- Wei K, Körding K. Uncertainty of feedback and state estimation determines the speed of motor adaptation. Front Comput Neurosci. 2010;11:4–11. doi: 10.3389/fncom.2010.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner MJ, Hallett M, Funkenstein HH. Adaptation to lateral displacement of vision in patients with lesions of the central nervous system. Neurology. 1983;33:766–772. doi: 10.1212/wnl.33.6.766. [DOI] [PubMed] [Google Scholar]