Summary
A Yersinia pseudotuberculosis (Yptb) murine model of lung infection was previously developed using the serotype III IP2666NdeI strain, which robustly colonized lungs but only sporadically disseminated to the spleen and liver. We demonstrate here that a serotype Ib Yptb strain, IP32953, colonizes the lungs at higher levels and disseminates more efficiently to the spleen and liver compared to IP2666NdeI. The role of adhesins was investigated during IP32953 lung infection by constructing isogenic Δail, Δinv, ΔpsaE and ΔyadA mutants. An IP32953ΔailΔyadA mutant initially colonized but failed to persist in the lungs and failed to disseminate to the spleen and liver. Yptb expressing these adhesins selectively bound to and targeted neutrophils for translocation of Yops. This selective targeting was critical for virulence because persistence of the ΔailΔyadA mutant was restored following intranasal infection of neutropenic mice. Furthermore, Ail and YadA prevented killing by complement-mediated mechanisms during dissemination to and/or growth in the spleen and liver, but not in the lungs. Combined, these results demonstrate that Ail and YadA are critical, redundant virulence factors during lung infection, because they thwart neutrophils by directing Yop-translocation specifically into these cells.
Introduction
Yersinia pseudotuberculosis (Yptb) is a human pathogen primarily implicated in cases of gastroenteritis. It is also the direct ancestor of Y. pestis (Achtman, et al., 1999, Chain, et al., 2004), an extremely virulent mammalian pneumonic pathogen (Perry, et al., 1997, Lathem, et al., 2005). Because of its close genetic similarity, Yptb virulence during intranasal infection has been compared with that of Y. pestis (Price, et al., 2012, Worsham, et al., 2012) and has been used to study therapeutics that target virulence features shared by both Yptb and Y. pestis (Balada-Llasat, et al., 2007, Garrity-Ryan, et al., 2010). Previously, a pneumonic mouse model of Yersinia infection was characterized using Yptb strain IP2666NdeI to study Yersinia infection of the lung, wherein the mice developed a fulminant pneumonia (Fisher, et al., 2007). However, this bacterial infection failed to mimic the quick systemic spread of Y. pestis (Lathem, et al., 2005, Fisher, et al., 2007, Price, et al., 2012). Rather, IP2666 NdeI spread to distal sites later in infection (Fisher, et al., 2007). Thus, use of IP2666 NdeI can model infection with gram-negative lung pathogens, but it does not recapitulate the infectious course of pathogens that rapidly seed other tissues from the lungs.
For successful colonization and dissemination to occur during bacterial lung infections, host defenses must be evaded or suppressed. Complement components are one of the initial innate immune barriers encountered by bacteria in the lungs (Watford, et al., 2000). Complement is present at high levels in the lungs (Watford, et al., 2000) and plays multiple immunological roles, including as mediators of inflammation, components of the membrane attack complex (MAC), which directly lyses bacteria, and opsonins (Watford, et al., 2000, Daha, 2010, Ricklin, et al., 2010). A first wave of cellular responders that is often predominated by neutrophils is also encountered during early bacterial infection (Lathem, et al., 2005, Fisher, et al., 2007, Crimmins, et al., 2012, Kolaczkowska, et al., 2013). Neutrophils eliminate bacteria through functions such as opsonophagocytosis, reactive oxygen species (ROS) production and neutrophil extracellular trap (NET) formation, and often work in concert with complement components (Ricklin, et al., 2010, Lu, et al., 2012, Kolaczkowska, et al., 2013).
Yersinia employs multiple methods to evade or suppress innate immune responses during infection (Matsumoto, et al., 2009, Bliska, et al., 2013). A major virulence factor that is critical for infection by pathogenic Yersinia is the Type 3 secretion system (T3SS) (Cornelis, 2002, Bliska, et al., 2013). The T3SS delivers effector proteins, called Yops, from within the bacteria into a host cell (Cornelis, 2002, Matsumoto, et al., 2009, Dewoody, et al., 2013) in a process termed translocation. Once inside the host cell, Yops disable normal cellular functions, often resulting in an inhibition of the immune response (Bliska, et al., 2013). Mechanisms of immune suppression by Yops include interfering with phagocytic uptake, inducing apoptosis in phagocytes, altering cytokine production, and preventing chemotaxis of responding immune cells (Viboud, et al., 2005, Matsumoto, et al., 2009, Bliska, et al., 2013). Translocation of Yops requires tight binding to cells (Boyd, et al., 2000, Grosdent, et al., 2002, Mejía, et al., 2008). Outer membrane proteins known as adhesins can mediate this tight binding (Mikula, et al., 2012). Of the known Yersinia adhesins, Invasin, Ail, pH 6 Antigen and YadA have all been demonstrated to support translocation (Bliska, et al., 1993, Mota, et al., 2005, Mejía, et al., 2008, Felek, et al., 2009, Felek, et al., 2010, Maldonado-Arocho, et al., 2013). Specifically, Y. pestis has been shown to mediate translocation into HEp2 and THP-1 cells using adhesins Ail and pH 6 antigen (Felek, et al., 2009, Felek, et al., 2010, Tsang, et al., 2010), Yptb uses Ail, YadA and Invasin (Bliska, et al., 1993, Mejía, et al., 2008, Maldonado-Arocho, et al., 2013) and Y. enterocolitica uses Invasin and YadA (Heesemann, et al., 1987, Kapperud, et al., 1987, Visser, et al., 1995, Uliczka, et al., 2011).
Invasin, Ail, YadA and pH 6 Antigen also all have functions independent of their role in delivery of T3SS effectors that could contribute to successful Yersinia infection (Pepe, et al., 1995, Marra, et al., 1997, Heise, et al., 2006, Mikula, et al., 2012). Based on the strain of Yptb, resistance to complement-mediated killing is conferred by Ail, Ail or YadA, or factors independent of these adhesins (Yang, et al., 1996, Ho, et al., 2012a). Y. enterocolitica is protected by YadA, Ail and LPS (Wachter, et al., 1989, Pierson, et al., 1993, Biedzka-Sarek, et al., 2005, Kolodziejek, et al., 2012). In Y. pestis, where a functional YadA is not expressed due to a frameshift mutation (Skurnik, et al., 1989), Ail is the only adhesin shown to be crucial in this capacity (Kolodziejek, et al., 2007, Bartra, et al., 2008). Furthermore, in Y. pestis, Ail prevents neutrophil influx during bubonic infection (Hinnebusch, et al., 2011), possibly through a lack of production of C5a chemokine which is normally generated after initiation of the complement pathway. In addition to facilitating adherence to cells, pH 6 antigen binds to low density lipoprotein, which may mask Yersinia from innate immune effectors (Makoveichuk, et al., 2003, Cathelyn, et al., 2006). Both YadA and Invasin are critical for colonization of the Peyer’s patches after oral inoculation for both enteric Yersinia species (Pepe, et al., 1995, Marra, et al., 1997, Heise, et al., 2006, Durand, et al., 2010), however, the LD50 for Yptb is unchanged in the absence of YadA whereas a Y. enterocolitica ΔyadA mutant is attenuated (Tamm, et al., 1993, Roggenkamp, et al., 1996, Han, et al., 1997, Mikula, et al., 2012).
The importance of these four adhesins during primary Yptb pneumonia for the translocation of Yops and for dissemination of Yptb has not been previously studied. Work by others suggests a role for YadA in Yptb systemic spread to and/or colonization of the lung that is independent of the T3SS (Hudson, et al., 2006). While in Y. pestis, Ail is critical for virulence during primary pneumonia (Kolodziejek, et al., 2010). Its specific function in the lungs has not been delineated in any pneumonic model of Yersinia infection to date. In Y. pestis, pH 6 antigen plays only a modest role during colonization of lungs (Cathelyn, et al., 2006). Our previous work has investigated the role of adhesins in the spleen using a serotype III strain of Yptb. We found that a ΔailΔinvΔyadA mutant in strain IP2666 was attenuated for infection and was significantly less efficient at delivering Yops into splenocytes when it is present at levels comparable to a WT strain (Maldonado-Arocho, et al., 2013). This indicates that one or more of these adhesins plays a role facilitating injection of Yops into these cells. In addition, in the absence of complement, the virulence of this strain was restored indicating that one or more of these adhesins protect against bactericidal effects of complement in the spleen (Maldonado-Arocho, et al., 2013). In this work, we characterized the role of these adhesins for Yptb growth in the lung and dissemination to other tissues. Using IP32953, a serotype Ib strain of Yptb that is a closer evolutionary relative to Y. pestis than IP2666NdeI (Achtman, et al., 1999, Skurnik, et al., 2000), we demonstrate that intranasal inoculation with IP32953 results in an extremely virulent infection of the lung that quickly disseminates to the liver and spleen. Ail and YadA played redundant roles in persistence in lungs and dissemination to distal tissues. In the lungs, Ail and YadA circumvented neutrophilic attack by mediating preferential translocation into these cells. By contrast, inhibition of complement by Ail and YadA was critical for dissemination of Yptb to distal sites. Together, these data suggest a role for Ail and YadA in warding off attacks by neutrophils in lungs and complement during dissemination and/or growth in spleens, allowing Yptb to mount a virulent pneumonic and systemic infection.
Results
Yptb IP32953 is a virulent lung pathogen that efficiently colonizes the lung and rapidly disseminates
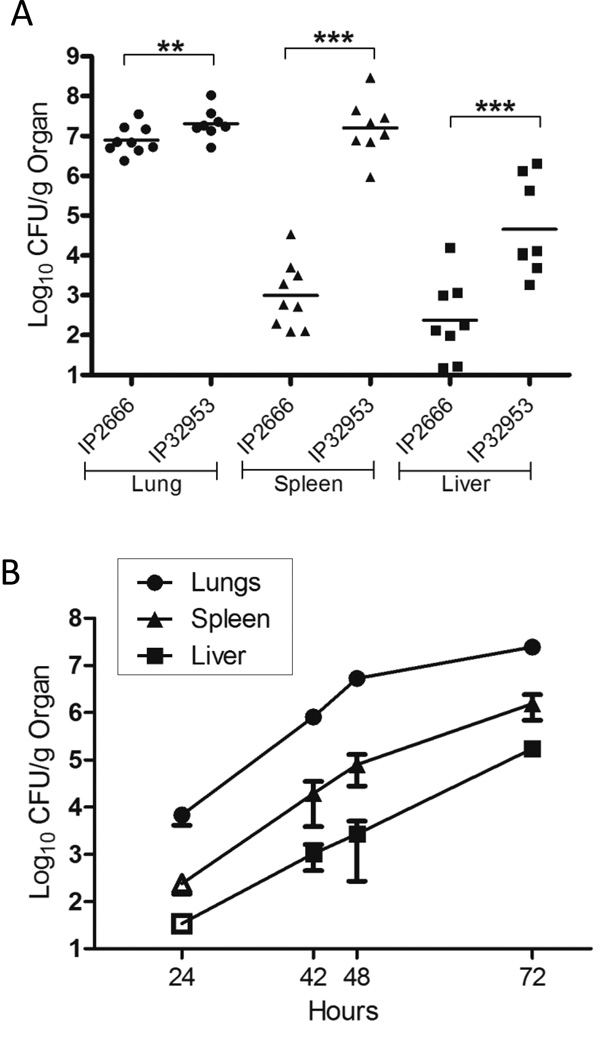
A lung infection model using the Yptb serotype III strain IP2666 pIB1NdeI (IP2666NdeI) has been previously established (Fisher, et al., 2007). In this model, IP2666NdeI robustly colonized the lungs, but only sporadically colonized the spleen and liver when compared to Y. pestis (Lathem, et al., 2005, Agar, et al., 2009, Price, et al., 2012). Serotype Ib strains of Yptb are more closely related to Y. pestis than serotype III strains (Achtman, et al., 1999, Skurnik, et al., 2000), and therefore we hypothesized that the serotype Ib IP32953 strain would be a more virulent lung pathogen than IP2666NdeI. To determine if IP32953 could better colonize the lungs and systemic organs, BALB/c mice were infected intranasally with 500 CFU of either WT IP32953 or WT IP2666NdeI, and the number of bacteria in the lung, spleen and liver was enumerated at 96 hours post-infection (h.p.i.) (Fig. 1A). In the lungs, WT IP32953 had a ∼6 fold greater bacterial load than WT IP2666NdeI. Furthermore, significantly higher numbers of IP32953 were recovered in the spleen and liver (10,000-fold and 1,000-fold, respectively), indicating that IP32953 was more efficient at systemic spread than IP2666NdeI (Fig. 1A).
Figure 1. IP32953 disseminates more rapidly than IP2666 following intranasal infection.
(A) BALB/c mice were intranasally infected with (A) 500 CFU of the WT IP2666NdeI or WT IP32953 strains, or (B) 250 CFU of WT IP32953. (A) 96 h.p.i. lungs (circles), spleens (triangles), and livers (squares) were harvested and plated for CFU. Data is representative of at least two independent experiments with 2–4 mice per experiment, Each dot represents an individual mouse. The bars represent the geometric mean and the asterisks represent significance as analyzed by Student’s T-test (**p<0.01, ***p<0.001). (B) At time points indicated, lungs (circles), spleens (triangles) and livers (squares) were harvested and plated for CFU. Open symbol indicates the limit of detection and that no bacteria were recovered at that time-point in any mice. The error bars are the standard error of the mean (SEM). Each point is the mean from 3 independent experiments consisting of 3 mice per time point per experiment.
Next, we studied the kinetics of IP32953 colonization of the lungs and spread to systemic organs. Mice were infected intranasally with 250 CFU of WT IP32953, and the bacteria in the lungs, spleens and liver were quantified at 24, 42, 48 and 72 h.p.i. (Fig. 1B). Within the lungs, IP32953 rapidly established an infection and grew to about 104 CFU/g of lung at 24 h.p.i. and 107 CFU/g at 48 h.p.i. Between 48 and 96 h.p.i., bacterial growth plateaued at levels between 1×107–1×108/g of lung (Fig. 1A–1B, see below). Detectable levels of IP32953 first appeared in the spleen and liver at 42 h.p.i. and increased through 96 h.p.i. (Fig. 1A–B). Curiously, IP32953 was better at disseminating to and/or colonizing the spleen than the liver, as the bacterial load was at least 10-fold higher at each time point in the spleen than in the liver. This phenomenon has also been observed during lung infection with Y. pestis (Agar, et al., 2009), but did not occur with Yptb IP2666NdeI (Fisher, et al., 2007). Together, these data demonstrate that IP32953 is a more aggressive lung pathogen than IP2666NdeI as measured by increased lung colonization and systemic spread.
Adhesin expression and activity differ in IP2666NdeI as compared to IP32953
In the Yersinia species, adhesins serve as virulence factors during infection with important roles in colonization and dissemination (Roggenkamp, et al., 1996, Heise, et al., 2006, Hudson, et al., 2006, Lathem, et al., 2007, Felek, et al., 2009, Durand, et al., 2010, Hinnebusch, et al., 2011, Mikula, et al., 2012). However, several adhesins exhibit differences in expression between Yptb strains, which may impact the disease outcome by the specific Yptb strain (Simonet, et al., 1992, Maldonado-Arocho, et al., 2013). For instance, the gene encoding Invasin is poorly expressed in IP2666 as compared to IP32953 (Simonet, et al., 1992, Maldonado-Arocho, et al., 2013). Sequence analysis of ail, psaEFABC and yadA revealed differences between yadA and psaC in IP32953 versus IP2666NdeI. YadAIP32953 contained a two amino acid deletion, A95G96, and four predicted amino acid changes, N99D, D104G, P105I, and Y106H, as compared to YadAIP2666 (Fig. S1A). Interestingly, these changes clustered immediately downstream of the amino acids 53–83, which are necessary for autoagglutination, fibronectin binding and invasion of HEp-2 cells (Heise, et al., 2006). A functional comparison revealed that both strains exhibited YadA-dependent autoagglutination, but IP32953 autoagglutinated faster than IP2666NdeI. These results, combined with previous work demonstrating that IP32953 has lower expression of YadA than IP2666 (Maldonado-Arocho, et al., 2013), suggests that YadAIP32953 has increased functional efficiency as compared toYadAIP2666 (Fig. S1B). This could be due to differences between these two forms of YadA or in other factors on the surface of these two serotypes.
PsaC, which encodes the usher protein, PsaC, for the pH 6 antigen system (Zavialov, et al., 2007), had an adenosine insertion at position 153. This results in a frameshift that should generate a truncated version of PsaC due to an early stop codon (Fig. S2A). Therefore the IP2666NdeI strain should be deficient for formation of pH 6 antigen since it lacks a functional PsaC. To confirm that pH 6 antigen was not functional in the IP2666NdeI strain, the ability of IP2666NdeI to hemagglutinate sheep red blood cells (SRBC) was compared to that of IP32953 (Fig. S2B). As expected, the IP2666NdeI strain could not hemagglutinate SRBC at a 1:16 dilution, indicating that this strain is defective for pH 6 antigen expression on the bacterial cell surface (Fig. S2B). (Note added in Proof: Not all IP2666 strains have this insertion). By contrast, the IP32953 strain showed no diminishment in hemagglutination until a 1:128 dilution, whereas an IP32953 ΔpsaE mutant, which does not express pH 6 antigen (Yang, et al., 1997), failed to hemagglutinate SRBC at a dilution of 1:8 (Fig. 2B). In summary, three adhesins, Invasin, pH 6 antigen and YadA, display differences in activities between IP2666NdeI and IP32953, while a fourth, Ail, contributes to Yop translocation in IP2666 (Maldonado-Arocho, et al., 2013). Therefore, these four adhesins were assessed for their contribution to colonization and dissemination by IP32953 during infection.
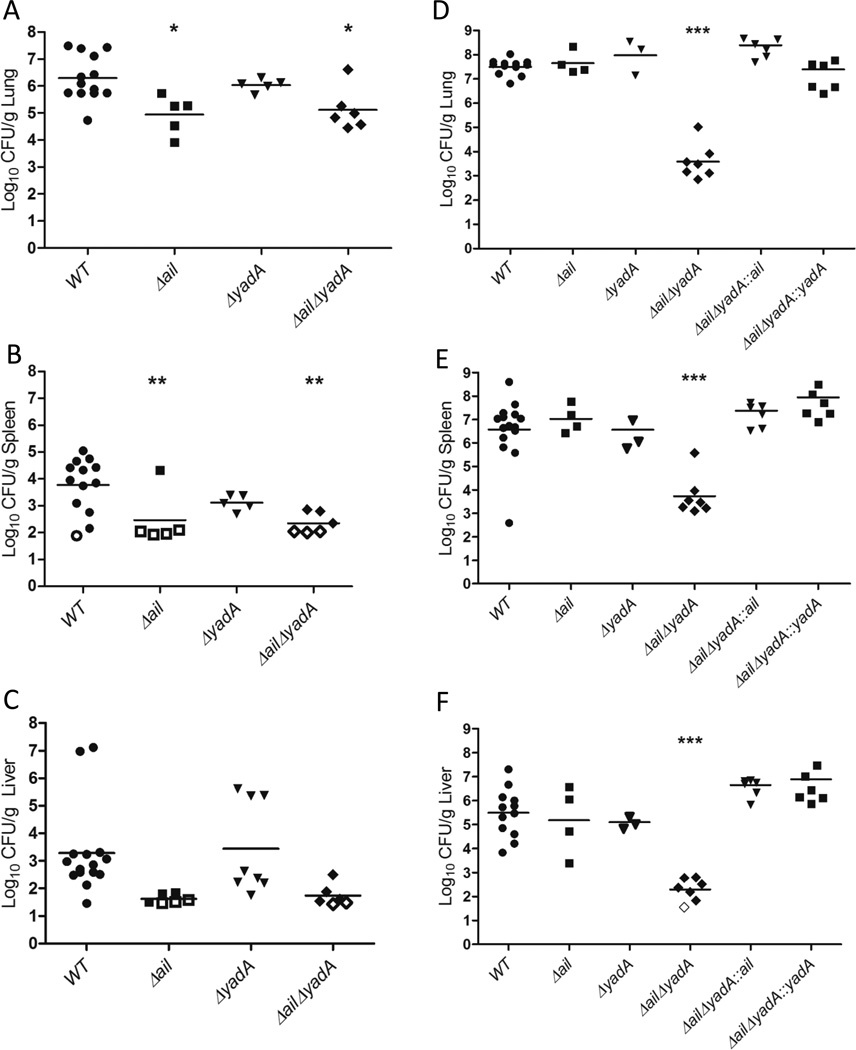
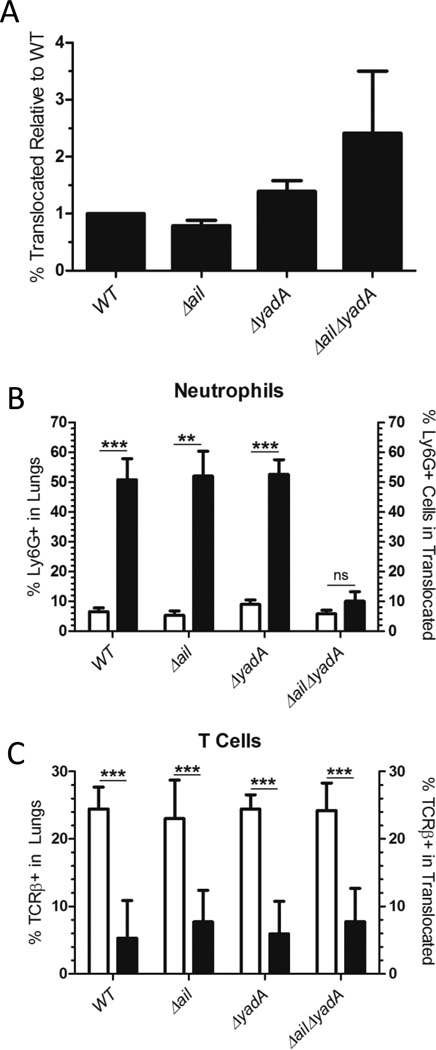
Figure 2. Ail is critical for early lung colonization and dissemination to the spleen and liver, while Ail and YadA are redundant and critical for IP32953 persistence in the lungs.
BALB/c mice were infected intranasally with 250 CFU of the indicated IP32953 strain. At (A–C) 44 h.p.i. or (D–F) 96 h.p.i., the (A, D) lungs, (B, E) spleens, and (C, F) livers were harvested and plated for CFU. Each dot represents the CFU recovered from an individual mouse, and open symbols indicate that no bacteria were recovered at that limit of detection. The bars represent the geometric mean. (A-C) 2–4 independent experiments of 2 or 3 mice per strain were performed. (D–F) 1–4 independent experiments/strain of 2–3 mice per experiment were performed. Significance was determined by One-Way ANOVA with Tukey’s post-test. Asterisks indicate significance of mutants as compared to WT values (*p<0.05, **p<0.01, ***p<0.001).
Ail and YadA are necessary for persistence of IP32953 in the lungs
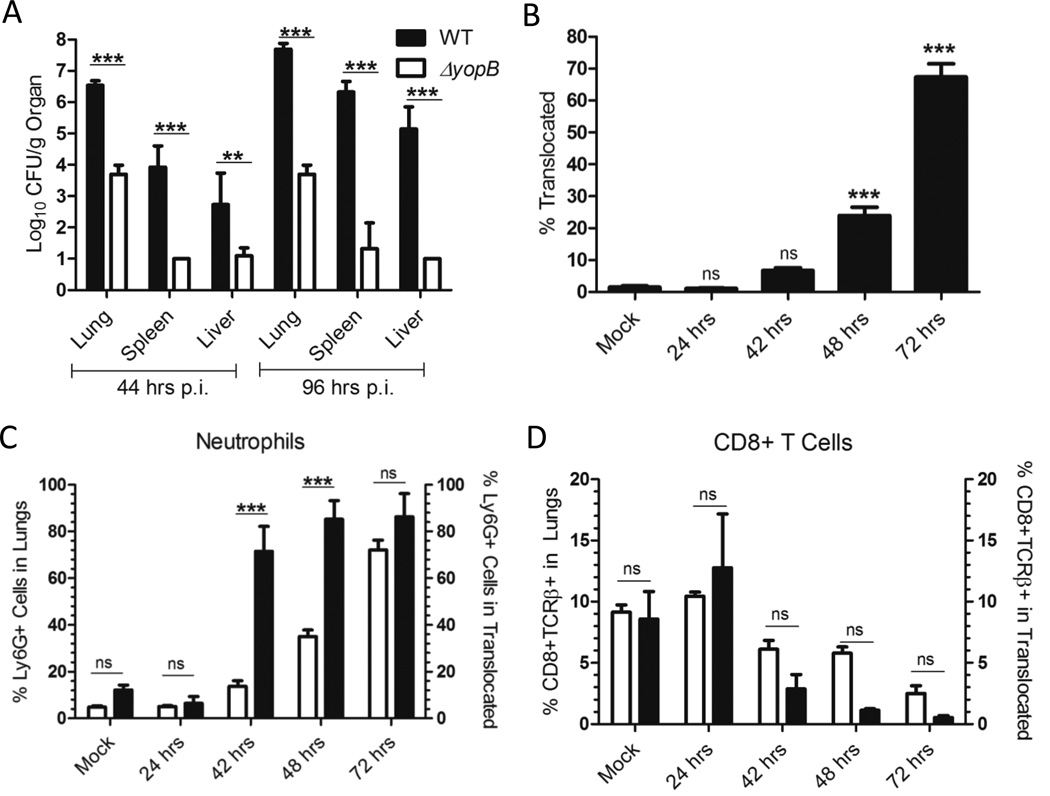
To determine if adhesins participate in colonization of and/or dissemination from lungs, isogenic strains of IP32953 with deletions of yadA, ail, psaE, invasin or with deletions in two or three of these genes were inoculated intranasally into BALB/c mice (Fig. 2A–F, S3A–F). One cohort of mice was sacrificed at 44 h.p.i. to assess initial bacterial colonization and growth in the lung, as well as early dissemination to the spleen and liver (Fig. 2A–C, S3A–C). A second cohort of mice was sacrificed at 96 h.p.i. to compare the ability of these adhesin mutants to persist in tissues (Fig. 2D–F, S3D–F). At 44 h.p.i. the Δail, ΔailΔyadA, and ΔailΔinvΔyadA mutants colonized the lungs and disseminated to the spleen and liver less efficiently than WT as evidenced by a significant decrease in the number of CFU as compared to WT or no recovery of the mutants (Fig. 2A–C, S3A–C). This suggests that early in infection, Ail enhances events required for lung colonization and possibly for dissemination. At 96 h.p.i., fewer CFU of the ΔailΔyadA and ΔailΔinvΔyadA mutants were recovered in the lungs than at 44 h.p.i., indicating that these mutants cannot persist in the lungs (Fig. 2A and D, S3A and D). By contrast, neither the Δail nor ΔyadA single mutants had defects in colonizing the lungs, spleens or livers at 96 h.p.i. (Fig. 2D–F). These results indicate that the Δail mutant can recover from its initial defect in lung colonization. They also indicate that Ail and YadA play redundant roles in promoting lung infection, as the presence of either protein is sufficient for Yptb to persist in the lungs. The ΔailΔyadA and ΔailΔinvΔyadA mutants also failed to colonize the spleen and liver to high levels at 96 h.p.i., which could reflect their low levels in the lungs or indicate an additional growth defect in these tissues (Fig. 2E–F, S3E–F). Complementation of either ail or yadA in the ΔailΔyadA mutant restored growth and dissemination to WT levels (Fig. 2D–F), further demonstrating that either Ail or YadA is sufficient for persistence in the lung and dissemination to the liver and spleen by IP32953. Taken together, Ail plays a non-redundant role in early colonization during lung infection, while Ail and YadA play necessary, yet redundant, roles in IP32953 growth and persistence in the lungs and subsequent spread to distal sites.
Yop translocation by IP32953 is critical for lung, spleen and liver colonization, and is predominately targeted into neutrophils
The observation that the ΔailΔyadA mutant population in the lung decreases between 44 and 96 h.p.i. (Fig. 2A and D) suggests that this mutant is subjected to clearance by the innate immune system. Adhesins, such as Ail and YadA, are thought to play vital roles in preventing innate immune clearance by several mechanisms, including binding to host cells to facilitate Yop injection into cells (Bliska, et al., 1993, Visser, et al., 1995, Mota, et al., 2005, Felek, et al., 2009, Felek, et al., 2010, Tsang, et al., 2010, Maldonado-Arocho, et al., 2013). We hypothesized that these adhesins may be involved in preferential binding to phagocytes, expediting Yop delivery to these cells and thereby inactivating this aspect immune system in the lungs and permitting growth of Yptb. The inability to translocate properly would hinder bacterial squelching of phagocytes, and thus, hinder the survival of the ΔailΔyadA mutant.
First, to establish that translocation is critical for Yptb colonization and persistence in the lung and dissemination to the spleen and liver, the virulence of an isogenic ΔyopB strain was compared to WT IP32953 following intranasal infection. ΔyopB mutants express the T3SS needle complex and secrete Yops, but fail to assemble a translocon and deliver Yops into host cells (Harmon, et al., 2013). At 44 h.p.i., the bacterial burden of WT was 1,000-fold higher than ΔyopB in the lung and spleen and 100-fold higher in the liver, indicating that translocation of Yops is critical for early colonization and dissemination (Fig. 3A). At 96 h.p.i., colonization of WT had increased by at least 10-fold in the lung, 100-fold in the spleen and 1000-fold in the liver, but ΔyopB had failed to grow in any of these tissues. In fact, in many mice, the ΔyopB mutant was not recovered in the spleen or liver at either 44 or 96 h.p.i. Therefore, translocation is necessary for colonization, persistence and dissemination of Yptb IP32953 during pneumonic infection.
Figure 3. Yop translocation by IP32953 is critical for lung colonization and dissemination to the spleen and liver, and is targeted preferentially into neutrophils during lung infection.
(A) BALB/c mice were infected intranasally with 250 CFU of IP32953 WT (black) or ΔyopB (grey). At 44 and 96 h.p.i., the lungs, spleens and livers were harvested and plated for CFU. Data is from 2 independent experiments with 3 mice per strain per experiment. The log10 mean of the CFU and SEM are shown. Statistical significance was determined by One-Way ANOVA with Tukey’s post-test. (B–D) BALB/c mice were infected intranasally with 250 CFU of ETEM expressing WT IP32953. At the indicated time-points, the lungs were harvested, plated for CFU and single cell suspensions were generated in the presence of gentamicin. The lung cells were stained with CCF4-AM, and for (C) Ly6G or (D) CD8 and TCRβ. Data is a compilation of 2–3 independent experiments of 3 mice each per time point per experiment. (B) Total percentage of ETEM+ cells in the lungs for each time-point was compared to PBS-treated mice (mock). Statistical significance determined by One-Way ANOVA followed by Dunnett’s post-test compared to the mock controls. (C, D) White bars represent mean percentage of the (C) Ly6G+ or (D) CD8+TCRβ+ population in the lung and black bars represent the mean percentage of (C) Ly6G+ or (D) CD8+TCRβ+ cells within the ETEM+ population. Error bars represent the SEM. Statistical significance was determined by One-Way ANOVA with Bonferroni’s posttest. (ns=not significant, *p<0.05, **p<0.01, ***p<0.001)
Next, the number of lung cells that contained Yops during the course of infection was determined using the CCF4-AM/β-lactamase system (Charpentier, et al., 2004, Durand, et al., 2010, Harmon, et al., 2010, Maldonado-Arocho, et al., 2013). The fusion protein, ETEM (Harmon, et al., 2010)), which contains the YopE secretion and translocation signal sequence fused to a β-lactamase (TEM), was introduced into WT IP32953 (Maldonado-Arocho, et al., 2013). This strain colonized lungs and disseminated to spleen and liver at levels comparable to the WT IP32953 strain (data not shown). Translocation of ETEM into host cells depends on the T3SS. In a cell loaded with CCF4-AM, ETEM cleaves the CCF4-AM resulting in a shift from green (ETEM−) to blue (ETEM+) fluorescence. Therefore, differences in the fluorescence of lung cells indicate the translocation status of these cells (Fig. S4B). BALB/c mice were infected intranasally with WT IP32953 expressing ETEM (WT-ETEM), and the number of total lung cells containing ETEM was measured at 24, 42, 48 and 72 h.p.i. (Fig. 3B). By 48 h.p.i., the number of ETEM+ cells in the lung was significantly above background and by 72 h.p.i. this percentage reached ∼75% (Fig. 3B). Translocation levels in the lung closely mirrored the bacterial load in the lung with a correlation coefficient of 0.921 (Fig. 1B, 3B), indicating that as the bacterial population increased in the lung, the number of cells translocated with Yops increased proportionally.
Previous work has demonstrated that Yersinia selectively targets phagocytic cells for translocation with Yops compared to T and B cells in the Peyer’s patches and the spleen during oral and intravenous infection (Marketon, et al., 2005, Köberle, et al., 2009, Durand, et al., 2010, Maldonado-Arocho, et al., 2013), but whether this preferential targeting is also observed in the lungs has not been addressed. To determine if IP32953 selectively directs Yops into neutrophils in the lungs, the proportion of each cell type in the lung was compared to the proportion of each cell type in the translocated (ETEM+) population within the lung as measured by FACS. The immune cells present in the lung over the time course of infection were defined and analyzed as follows: Ly6G+ are neutrophils; CD11bint CD11chi are alveolar macrophages; CD11bhiCD11chi are dendritic cells; GR1loCD11b+ are resident monocytes; CD4+TCRβ+ are CD4 T cells; CD8+TCRβ+ are CD8 T cells; and B220+CD19+ are B cells (Fig. S4C). Cell type analysis revealed an early response to Yptb in the lung predominated by an influx of neutrophils (Fig. 3C–D, S5, white bars). Notably, neutrophils were infiltrating the lungs at such a rate that their population doubled between 42 and 48h.p.i. from 17% to 38%. By 72 h.p.i., the population of neutrophils reached 70% of the total cells within the lung (Fig. 3C, white bars). The steady decrease in the relative population of B cells, T cells and alveolar macrophages could be attributed to a shift in the overall population size due to the large increase in neutrophils (Fig. 3C–D, S5).
The proportion of each cell type in the lung was compared to the proportion of each cell type within the translocated population of the lung to determine whether specific cell types were over- or under-represented in the translocated cell population. If the two populations were equal, then that specific cell type was not over- nor under-represented in the translocation population. Only neutrophils were significantly over-represented in the ETEM+ cell population compared to the total cell population in the lung when levels of translocation were higher than that seen in the mock infected (Fig. 3C–D, S5). In fact, neutrophils comprised greater than 75% of the Yop-injected population by 42 h.p.i. (Fig. 3C). Due to fact that the neutrophil population in the lung at 72 h.p.i. was over 70% of the total cells, selective translocation could not be discerned. By contrast, while resident macrophages (GR1loCD11b+) and alveolar macrophages (CD11bint CD11chi) appeared to be over-represented in the translocated population 24 h.p.i., these cell types inherently have a high degree of autofluorescence as seen by the higher percentage of cells in the mock infected control (Fig. S5A and C). Therefore, we could not accurately assess whether these cell types were targeted early in infection. None of the other cell types were significantly over-represented nor under-represented in the ETEM+ population (Fig. 3D, S5). In summary, translocation of Yops was essential for colonization, persistence and dissemination of IP32953, and neutrophils were both the predominant immune cell responder to the infection and the preferential target for Yop translocation.
Ail and YadA play redundant roles for targeting neutrophils for translocation in vivo
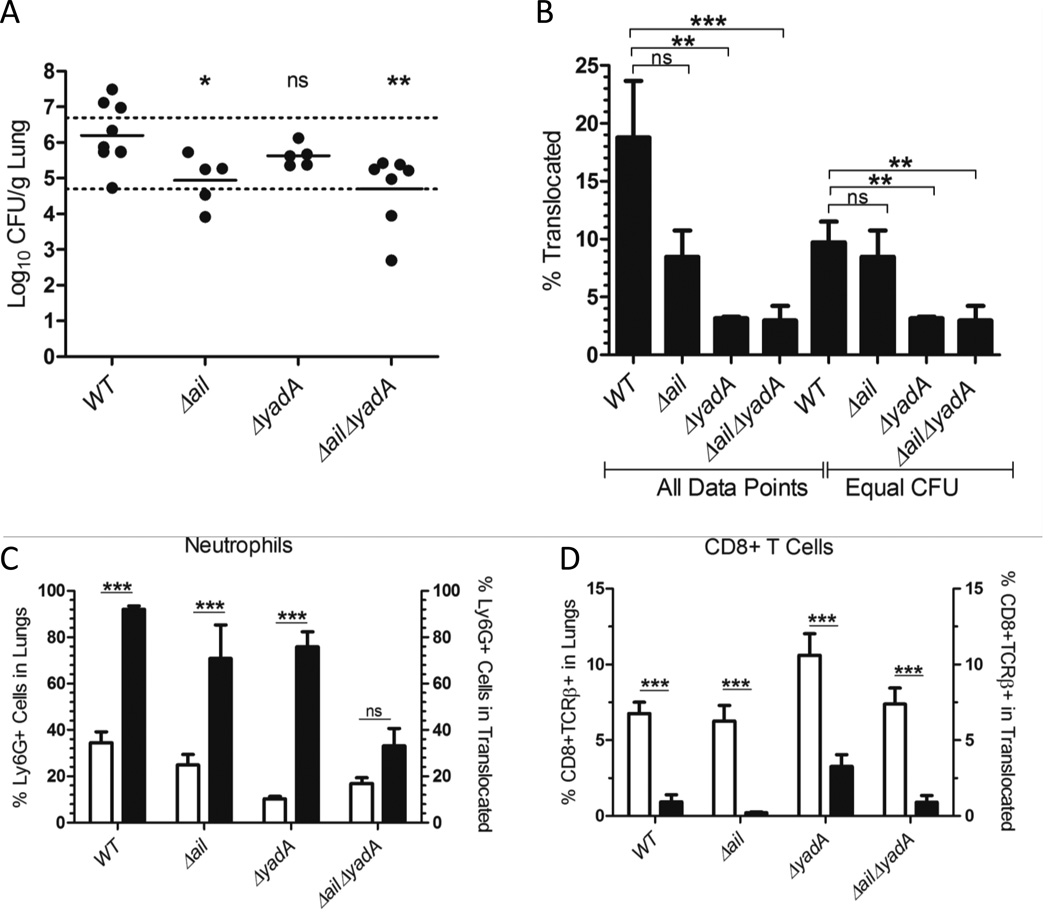
Next, we investigated whether Ail and/or YadA promote bacterial survival and persistence in the lung by directing Yop translocation into neutrophils. BALB/c mice were infected with ETEM-expressing WT, Δail, ΔyadA or ΔailΔyadA strains. The importance of these adhesins in directing translocation into neutrophils was analyzed at 44 h.p.i. At this time-point there was a moderate bacterial load, a distinct population of ETEM+ cells, and a clearly discernible preference for translocation into neutrophils compared to other cell types (Fig. 1B, 3B–C). Because the ΔailΔyadA mutant had a survival defect in the lungs compared to the other strains (Fig. 2D), mice were infected with 10,000 CFU of ΔailΔyadA with the goal of recovering comparable CFU in the lungs of all four strains. In order to determine whether translocation levels are affected by an adhesin, equivalent numbers of bacteria should be recovered from an organ as the number of cells injected with Yops increases as the bacterial burden increases (Fig. 1B, 3B). Nonetheless, despite the differences in initial input doses, the mean bacterial load recovered after infection among these four strains was not equivalent (Fig. 4A). This inability to achieve comparable colonization levels between WT and the ΔailΔyadA mutant may reflect a bottleneck for any Δail mutant in initial seeding and colonization of the lung that is overcome by later time points (Fig. 2A and D, 4A). Therefore, we established a range of CFU in which there was no statistically significant difference among the strains (Fig. 4A, black dotted lines) at which to analyze translocation levels (Fig. 4B). With equivalent bacterial burdens in the lung, Δail had no translocation defect compared to WT (Fig. 4B). However, infection with either the ΔyadA or ΔailΔyadA mutants resulted in a significant reduction in the number of Yop-translocated cells with ETEM+ cells comprising only 4% of the lung population, compared to 10% in lungs infected with WT (Fig. 4B). These results indicate that YadA is necessary for promoting high levels of translocation in the lung. However, as the ΔyadA mutant showed no significant growth defect at either 44 or 96 h.p.i. (Fig. 2A and D), this decrease in the overall number of Yop-translocated cells may not be detrimental to bacterial survival. This suggests that the specific cell types that are targeted for translocation is more critical for successful infection rather than the overall number of cells.
Figure 4. Ail and YadA drive translocation into neutrophils during animal infection.
BALB/c mice were infected intranasally with 250 CFU of ETEM expressing IP32953 WT, Δail, or ΔyadA, or 10,000 CFU of ΔailΔyadA. At 44 h.p.i., the lungs were harvested, plated for CFU and homogenized in the presence of gentamicin. Lung cells were stained with CCF4-AM and the indicated cell surface markers; the percentage of ETEM+ cells was measured with FACS. (A) Total CFU/g of lung following infection. Statistical significance determined by One-Way ANOVA with Tukey’s post-test for mutant strains as compared to WT. There was no statistically significant difference between mice within the dotted lines and these mice were used for analysis of “equal CFU” in (B). (B) The mean percentage of ETEM+ cells in the lungs of mice infected with adhesin mutant strains was compared to WT IP32953 infection. Either all data points were used or points that lie within the dotted lines in (A) (Equal CFU) as indicated. (C, D) White bars represent population of (C) Ly6G+ or (D) CD8+TCRβ+ cells in the lung and black bars represent population of indicated cell type within the ETEM+ population. Data is a compilation of 2–3 independent experiments of 2–3 mice per strain. Error bars indicate the SEM. Statistical significance determined by One-Way ANOVA with (A-B) Tukey’s or (C-D) Bonferroni’s post-test. (ns = not significant, *p<0.05, **p<0.01, ***p<0.001)
To further explore this possibility, the types of cells that comprise the Yop-containing cell population were compared between the WT and the adhesin mutant strains (Fig. 4C–D, S6). Strikingly, neutrophils were not over-represented in the translocated population of the ΔailΔyadA infected lungs (Fig. 4C). By contrast, neutrophils were still overrepresented in the ETEM+ population for all the other strains (Fig. 4C). In addition, upon infection with the ΔailΔyadA mutant, the alveolar macrophages became over-represented in the ETEM+ population, while CD4+ T cells were no longer under-represented (Fig. S6). Finally, we noted a trend of an increase in both the total numbers of CD8+ T cells and the number of injected CD8+ T cells during ΔyadA mutant infection; however, these were not significant (Fig. 4D). Combined these data indicate that Ail and YadA play redundant roles in selectively targeting neutrophils for Yop translocation in lung infection. Furthermore, the colonization defect of the ΔailΔyadA mutant is likely explained by its inability to translocate Yops efficiently into neutrophils and its aberrant interactions with other cell types in the lung.
Ail and YadA mediate translocation into neutrophils in a lung cell suspension
We next sought to determine whether the specific targeting of neutrophils by Ail and YadA we observed during Yptb infection was due to the lung tissue architecture positively influencing bacteria co-localization with neutrophils or if, alternatively, targeting of cells was independent of lung architecture. Therefore, an ‘ex vivo’ translocation assay was performed on cell suspensions isolated from the lungs of naive BALB/c mice that were infected with ETEM expressing IP32953 strains. Then, the number and cell types targeted for Yop translocation were determined by FACS. Interestingly, the overall levels of Yop translocation did not differ between the adhesin mutants and WT (Fig. 5A). However, analysis of the cell types present in the ETEM+ population demonstrated that the pattern of selective translocation ex vivo mimicked that seen in vivo (Fig. 4C–D, 5B–C, S6, S7). Neutrophils were over-represented in the translocation population during infection with the WT, Δail, and ΔyadA strains while B and T cells were under-represented (Fig. 5B–C, S7). Likewise as compared to infection of mice, the ΔailΔyadA strain failed to selectively target neutrophils (Fig. 5B) and this strain exhibited an increase in the percentage of B cells (B220+) present in the ETEM+ population (Fig. S7D). These results recapitulate our findings in mouse infections that Ail and YadA function redundantly in their ability to selectively direct translocation into neutrophils. Furthermore, these data demonstrate that this targeting of translocation is independent of lung tissue architecture.
Figure 5. Ail and YadA drive translocation by IP32953 into neutrophils in lung single cell suspensions.
Naive BALB/c mouse lungs were harvested, homogenized and infected at an MOI of 1:1 for 1h at 37°C with ETEM expressing IP32953 strains WT, Δail ΔyadA, ΔailΔyadA or ΔyopB. Lung cells were then incubated with gentamicin, CCF4-AM and the indicated cell surface markers. (A) The percentage of ETEM+ cells from lung cell suspensions infected with WT was set to 1 and the mean relative percentage of ETEM+ cells from lung suspensions infected with various adhesins mutants was normalized to WT. ΔyopB infected cells were used as a gating control for ETEM. The absolute percentage of ETEM+ cells infected with WT ranged from 1.33–3.65%. The mean and SEM is from 41.33–3.65%. The mean and SEM is from 4–6 independent 6 independent experiments per strain. Statistical significance was determined by One-Way ANOVA with Tukey’s post-test. No statistical significance was observed. (B-C) White bars represent the percentage of total (B) Ly6G+ or (C) TCRβ+ cells in the lung cell suspensions and black bars represent percentage of ETEM+ cells that are (B) Ly6G+ or (C) TCRβ+. Data is from 41.33–3.65%. The mean and SEM is from 4–6 independent 6 independent experiments per strain. Statistical significance determined by One-Way ANOVA with (A) Dunnett’s or (B–C) Bonferroni’s post-test. (ns=not significant, *p<0.05, **p<0.01, ***p<0.001)
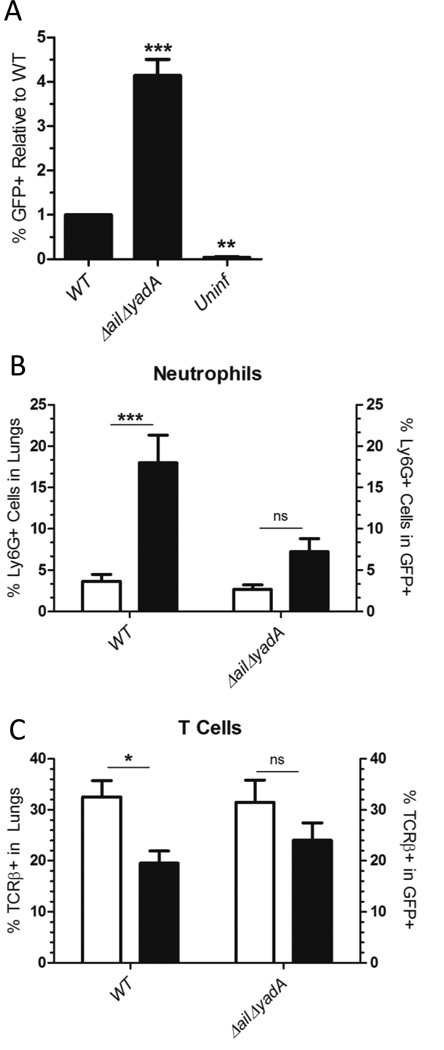
Ail and YadA promote binding to neutrophils over other immune cell types in the lung
Adhesins are critical for binding by Yersinia to cells and proper binding is necessary for translocation (Bliska, et al., 1993, Grosdent, et al., 2002, Mejía, et al., 2008, Tsang, et al., 2010, Mikula, et al., 2012). Therefore, the adhesin mutant strains were assessed for their ability to bind to cells from lung homogenates to determine if the binding phenotypes correlated with the translocation phenotypes. In an ex vivo binding assay, lung cell suspensions from naive BALB/c mice were infected with GFP-expressing WT IP32953 or ΔailΔyadA, and both the total GFP+ lung cell population and the cells present in the GFP+ population were analyzed by FACS (Fig. S5A, S5C, S8A). When the overall levels of binding were examined, the ΔailΔyadA mutant showed a ∼3-fold increased level of binding compared to WT (Fig. 6A). WT bound selectively to neutrophils, resident monocytes and dendritic cells, while B cells and T cells were under-represented in the bound (GFP+) population (Fig. 6B–C, Fig. S8B–D). By contrast, the ΔailΔyadA mutant exhibited no significant enrichment of neutrophils, dendritic cells or resident macrophages as compared to WT, while at the same time B and T cells were no longer under-represented in the GFP+ population (Fig. 6B–C, S8B–D). These data suggest that Ail and/or YadA are not necessary for binding to the total population of cells within the lungs. However, they impart an important specificity for binding neutrophils, which likely accounts for the differences in translocation observed (Fig. 4, 5, S6, S7).
Figure 6. Ail and YadA mediate binding to neutrophils in lung single cell suspensions.
Naive BALB/c mouse lungs were harvested, homogenized and infected at an MOI of 1:1 for 20 min at 37°C with GFP expressing WT IP32953 or ΔailΔyadA. Lung cell suspensions were stained with Ly6G or TCRβ antibodies, fixed in 4% formamide and analyzed by FACS. (A) The total percentage of GFP+ (bacteria bound) cells from lung cell suspensions infected with WT was set to 1 and the mean relative percentage of GFP+ cells from lung suspensions infected with ΔailΔyadA was compared for significance against WT. The absolute percentage of GFP+ cells infected with WT ranged from 2.241.33–3.65%. The mean and SEM is from 4–6 independent 13.6%. (B–C) White bars represent the mean percentage of (B) Ly6G+ or (C) TCRβ+ cells in the lung cell suspensions. Black bars represent mean percentage of (B) Ly6G+ or (C) TCRβ+ cells within the GFP+ population. The mean and SEM from 41.33–3.65%. The mean and SEM is from 4–6 independent 8 independent experiments per strain is plotted. Statistical significance determined by One-Way ANOVA with (A) Dunnett’s as compared to WT or (B–C) Bonferroni’s posttest. (ns=not significant, *p<0.05, **p<0.01, ***p<0.001)
Depletion of neutrophils restores persistence of IP32953 ΔailΔyadA in the lung while depletion of complement permits dissemination to spleen and liver
The observation that levels of the ΔailΔyadA mutant in the lung decrease between 44–96 h.p.i. (Fig. 2A and D) suggests that this mutant is subjected to clearance by the immune system. As highlighted above, the lung experiences a massive neutrophil influx following infection with Yptb intranasally. Moreover, the inability of the ΔailΔyadA strain to colonize the lung and deliver Yops into neutrophils suggests the importance of counteracting these cells for successful infection. Therefore, we hypothesized that infection in the absence of neutrophils may allow the ΔailΔyadA mutant to persist. To test this, BALB/c mice were depleted of neutrophils with α-Ly6G (Daley, et al., 2008) or mock depleted with an isotype control, and then infected with WT IP32953 or ΔailΔyadA (Fig. 7). By 96 h.p.i., the WT population had grown to the same extent in the presence or absence of neutrophils in the lung, suggesting that this strain is proficient at evading neutrophils at this site (Fig. 7B). Additionally, this suggests that other immune effectors are sufficient for limiting rampant growth of Yptb in the lungs in the absence of neutrophils, or that Yptb WT growth is restricted only by availability of resources by this time-point. By contrast, growth of the ΔailΔyadA mutant was restored to WT levels in the lung in the absence of neutrophils and was ∼10,000-fold higher than growth of ΔailΔyadA in the presence of neutrophils (Fig. 7B). This indicates that neutrophils effectively eliminate ΔailΔyadA and that this mutant can thrive in the absence of neutrophils. Combined our results indicate that specific targeting of neutrophils by Ail and YadA contributes to the virulence of IP32953 in the lungs.
Figure 7. Ail and YadA are critical for circumventing neutrophils in the lung.
BALB/c mice treated with an isotype antibody control or depleted of neutrophils with αLy6G (IA8) antibody were intranasally infected with 250 CFU of IP32953 WT or ΔailΔyadA. At 96 h.p.i, lungs were harvested and homogenized. (A) Neutrophil depletion was confirmed by staining lung homogenate with αLy6G (IA8) and analyzing with FACS. (B) Bacterial levels were determined by plating homogenate for CFU. Data is representative of two independent experiments of 3 mice per experiment, each dot representing an individual mouse. Statistical significance determined by One-Way ANOVA with Tukey’s post test. (ns=not significant, *p<0.05, **p<0.01, ***p<0.001)
We next examined the contribution of complement to the elimination of the ΔailΔyadA mutant. Previously, we have shown that the combined absence of Ail, Invasin and YadA renders IP2666 susceptible to complement-mediated killing in the spleen after intravenous infection (Maldonado-Arocho, et al., 2013). While both Ail and YadA have been implicated in providing resistance to killing by the complement-mediated membrane attack complex (MAC) (Ho, et al., 2012a, Mikula, et al., 2012), Yersinia is not susceptible to MAC-based lysis in mice (Bartra, et al., 2008) and a serotype Ib strain, 2812/79, has been previously found to use factors aside from ail and those encoded by pYV for resistance to killing by human serum (Ho, et al., 2012a). Therefore, we reasoned that if complement participates in killing of WT or ΔailΔyadA, it does so as an opsonin during phagocytosis by neutrophils or by serving as a pro-inflammatory signal. However, we first determined if Ail and/or YadA mediate resistance of IP32953 to the membrane attack complex (MAC) and subsequent bacterial lysis when IP32953 is exposed to sera from other animal sources. WT IP32953 or isogenic ΔyadA, Δail, or ΔailΔyadA strains were incubated in either fetal bovine serum (FBS) or heat-inactivated fetal bovine serum (HIS) for 2 hours, and then bacterial survival was assessed (Fig. 8A). In addition, IP2666 and IP2666 Δail were included as controls, as IP2666 Δail is susceptible to serum-mediated killing (Fig. 8A). In contrast to the IP2666 Δail strain, none of the IP32953 adhesin mutants were susceptible to serum-mediated killing. These results suggest that other factors, such as LPS or other adhesins, are responsible for MAC resistance by IP32953, and not the adhesins Ail or YadA. This result further strengthens the idea that if complement plays a role in controlling IP32953 and IP32953ΔailΔyadA, it is through serving as an opsonin for neutrophil phagocytosis or as a proinflammatory signal.
Figure 8. Complement inhibits IP32953 ΔailΔyadA dissemination to the spleen, but not persistence in the lungs.
(A) The indicated IP32953 and IP2666 strains were diluted in a 1:1 volume in either FBS or HIS. Following a 2h incubation at 37°C, the incubated cultures were plated for CFU. Killing by serum was determined by comparing the CFU recovered in FBS to that recovered after incubation in HIS. Values were normalized to WT IP32953, which was set at 1. Statistical significance was determined by a One-Way ANOVA followed by Dunnett’s post-test. Asterisk(s) indicate statistical significance as compared to WT IP32953 values. (**p<0.01). Data is a compilation of 3 independent experiments done in triplicate. Bars indicate mean and error bars indicate SEM. (B–D) BALB/c mice were intraperitoneally injected with CVF to deplete complement or mock treated with PBS. At 24 hours post treatment, the mice were intranasally infected with 250 CFU of WT IP32953 or ΔailΔyadA. At 96 h.p.i., the (B) lungs, (C) spleens and (D) livers were harvested and plated for CFU. Data from 2 independent experiments of 21.33–3.65%. The mean and SEM is from 4–6 independent 3 mice each is shown, where each dot represents an individual mouse. Open symbols represent no bacteria recovered and limit of detection. Bars represent the geometric mean. Statistical significance was determined by One-Way ANOVA with Tukey’s post-test (ns=not significant, *p<0.05, **p<0.01, ***p<0.001)
To determine the role of complement in containing IP32953 during mouse pneumonia, cobra venom factor (CVF) was used to deplete complement from mice (Shapiro, et al., 2002) and, following depletion, mice were infected with WT or ΔailΔyadA IP32953. Colonization and dissemination of the WT IP32953 and ΔailΔyadA strains was then assessed in the lungs, spleen and liver of complement-depleted mice or mock-depleted mice. In the lungs, no significant difference in WT or ΔailΔyadA bacterial levels was found in the presence or absence of complement (Fig. 8B), suggesting that either Ail and YadA are not necessary for protecting against complement-mediated functions in the lung or that complement does not play a major role in preventing IP32953 colonization of the lung. However, it should be noted that in the absence of infection, there was an increase from ∼3% neutrophils in PBS treated mouse lungs to ∼11% neutrophils 72h post CVF treatment (Vogel, et al., 2010)and data not shown). Therefore, it is possible that this increase in neutrophils following CVF treatment could compensate for the lack of complement.
Despite the observation that Ail and YadA do not prevent killing by complement-dependent immune mechanisms in the lung, complement appeared to play a key role in preventing dissemination of the ΔailΔyadA mutant to the spleen and liver (Fig. 8C–D). Notably there was an ∼15-fold restoration in the spleen and ∼100-fold restoration in the liver of the CFU recovered from ΔailΔyadA infected mice in the absence of complement as compared to in the presence of complement (Fig. 8C–D). Combined, these results suggest that Ail and/or YadA are not important for preventing complement-mediated inhibition of Yptb colonization in the lung, but do facilitate dissemination to and/or growth in the spleen and liver via resistance to complement.
Discussion
Growth and dissemination of bacteria in host tissues is a multifaceted process, requiring a number of bacterial factors to overcome innate immune responses that occur during infection. Members of the Yersinia genus that are pathogenic to humans are all capable of growing and spreading from their initial sites of colonization to other tissues. A number of studies have demonstrated that both adhesins and the T3SS, in addition to other factors, are required to colonize and spread from various infection sites (Matsumoto, et al., 2009, Mikula, et al., 2012, Bliska, et al., 2013). However, whether and how adhesins and the T3SS collaborate in the process of persistence in and dissemination from the lungs in vivo has not been investigated in any Yersinia species to date. Here we show that in Yptb, the adhesins Ail and YadA functioned redundantly to promote growth in the lungs and dissemination to the spleen and liver. In the lungs, these adhesins function to direct Yop injection specifically into neutrophils. In their absence Yop injection still occurred, however, there was no enhancement of injection into neutrophils and other cell types were still found to contain Yops. Interestingly, a ΔyadA strain also had translocated into fewer total cells, but colonized the lungs to the same level as WT and still directed injection of Yops into neutrophils. In addition, WT Yptb, but not the ΔailΔyadA mutant, selectively bound to neutrophils. Combined, these observations suggest that it is the specificity of bacterial binding to and Yop injection into neutrophils rather than the overall number of cells translocated into that is critical for virulence in lungs. Furthermore, Ail and YadA are required for this specificity. Additional support for this idea is the observation that growth of the ΔailΔyadA mutant in the lungs was restored in the absence of neutrophils. Thus, these two adhesins allow Yptb to specifically target a subset of host cells for translocation, which is critical for growth and persistence of Yptb in lungs. In the absence of these adhesins, Yop injection occurs, but into cell types that are less toxic to Yptb. Thus Yptb fails to efficiently disrupt normal functions of neutrophils with the T3SS and is cleared from the lungs.
To our knowledge, this is the first study that demonstrates a non-redundant role for Ail for Yptb during in vivo infection, that is, that Ail enhances early colonization and growth in lungs. We also uncovered a redundant need for Ail and YadA later in infection during Yptb persistence in and dissemination from the lungs. This redundancy is particularly interesting when viewed from an evolutionary perspective. While Yptb is a close evolutionary relative of Y. pestis (Achtman, et al., 1999, Skurnik, et al., 2000), these two pathogens have two very different infectious cycles (Perry, et al., 1997, Wren, 2003). Yptb is an enteric pathogen primarily spread by the fecal-oral route of infection, while Y. pestis is a pneumonic or bubonic pathogen acquired either intradermally through the bite of an infected flea or by inhalation of bacteria containing aerosols (Perry, et al., 1997, Wren, 2003). During the evolution of Y. pestis, yadA became a pseudogene due to an inactivating frameshift mutation (Skurnik, et al., 1989). Both species retain an intact ail gene (Kolodziejek, et al., 2007, Bartra, et al., 2008, Maldonado-Arocho, et al., 2013). If spread from one host’s lungs to the next host was one selective pressure that Y. pestis underwent, the functional redundancy of Ail and YadA in vivo suggests one reason why yadA was not under selective pressure to be retained in the Y. pestis genome. However, conservation of ail would have been beneficial because it is required early during pneumonic infection. This redundancy of Ail with YadA, along with the fact that mouse strains lack the ability to mount an effective MAC against Yersinia (Bartra, et al., 2008), may also explain why it has been difficult to detect roles for Ail in mouse infection for the enteric species of Yersinia. This study, therefore, clarifies a role for Ail during infection, as well as highlights some of the functional redundancy that occurs between adhesins during Yptb infection.
In cultured cells, Yop translocation requires tight binding of Yptb to host cells. A variety of adhesins and receptors are sufficient for this binding to promote Yop delivery (Bliska, et al., 1993, Grosdent, et al., 2002, Mota, et al., 2005, Felek, et al., 2009, Felek, et al., 2010). For instance, in the absence of three major adhesins, complement or Fc receptor suffices to promote interactions between Y. enterocolitica and neutrophils or macrophages (Grosdent, et al., 2002). Nonetheless, Ail and/or YadA were required to direct IP32953 Yop translocation specifically into neutrophils. Ail and YadA may interact with receptors found exclusively or primarily on neutrophils and/or may impede interactions with receptors that are on other cells. The receptors used by Ail and YadA to bind to host cells and deliver Yops have been an area of intensive investigation. Both adhesins may bind to β1 integrins via either direct binding by YadA or indirect binding to extracellular matrix proteins by Ail and YadA (Emody, et al., 1989, Tertti, et al., 1992, Eitel, et al., 2002, Heise, et al., 2006, Tsang, et al., 2010, Yamashita, et al., 2011, Tsang, et al., 2012). Alternatively, because both adhesins have also been shown to bind to components of complement (Biedzka-Sarek, et al., 2008, Kirjavainen, et al., 2008, Ho, et al., 2012b, Schindler, et al., 2012), these components could serve as bridges between adhesins and host cell receptors that are highly expressed on neutrophils. However, the virulence of the WT strain and attenuation of the ΔailΔyadA strain in the lung was unaffected by the absence of complement. This suggests that these adhesins do not use complement components as a bridge to facilitate translocation into neutrophils. Therefore, we favor the idea that these adhesins either directly bind neutrophils or exploit extracellular matrix proteins to selectively bind to neutrophils.
Complement controls bacterial growth by a number of mechanisms including MAC lysis, as an opsonin for phagocytosis and as a mediator of inflammation. Generally, pathogenic Yersinia species rely predominantly on Ail and/or YadA for resistance to the MAC when immersed in fetal bovine serum (Kolodziejek, et al., 2007, Bartra, et al., 2008, Kolodziejek, et al., 2010, Leo, et al., 2011, Kolodziejek, et al., 2012, Mikula, et al., 2012). However, a serotype Ib strain, 2812/79, has been previously shown to need neither Ail nor pYV for resistance to killing by human serum (Ho, et al., 2012a). Likewise our work showed that IP32953 was resistant to serum even in the absence of both Ail and YadA (a chromosomal and pYV encoded factor, respectively), indicating that other factors provide protection against lysis by the MAC for IP32953. These factors could include LPS, adhesins or other outer membrane proteins that may be expressed on the surface of IP32953. Furthermore, our work taken with previous findings, suggests Yptb factors used for serum resistance may be serotype specific (Ho, et al., 2012a). In addition, our work in mice suggests that coating of Yptb by complement components did not enhance opsonophagocytosis and that Ail and YadA are not required for blocking these components in the lungs. However, our work does suggest a role for complement in restricting systemic spread of Yptb in the absence of Ail and YadA to the spleen and liver. The mechanism by which complement restricts this systemic spread is unknown. One possibility is that the bacterial factor(s) that mediate complement resistance in the lungs are not expressed in the blood, spleen and liver, rendering the bacteria more reliant on YadA and Ail. The role of complement in dissemination compared to colonization of the lung may also reflect that these adhesins have different functions in the lungs as compared to the blood, spleen, and liver. Alternatively, since cobra venom factor treatment increases neutrophil migration to the lungs, there may be an increase in the leakiness of the lungs, which could increase speed of dissemination by allowing bacteria through the epithelial barrier (Tamang, et al., 2012). The enhanced growth of IP32953ΔailΔyadA in the spleen and liver in the absence of complement was similar to that observed for an IP2666ΔailΔinvΔyadA mutant in the spleen (Maldonado-Arocho, et al., 2013), suggesting that these adhesins function to counteract complement regardless of strain background. How these adhesins interface with complement in dissemination to or growth in the liver and spleen still remains to be unraveled.
A second striking finding from our studies is that IP32953 disseminates much more rapidly than IP2666NdeI. The genetic basis for the differences here is not known. One or more of the known differences in these strains, such as YadA, pH 6 antigen, Invasin, YopT or LPS (Simonet, et al., 1992, Achtman, et al., 1999, Skurnik, et al., 2000, Viboud, et al., 2006), may account for the variances in dissemination. Alternatively, unknown factor(s) may enhance growth and dissemination of IP32953. Nonetheless, the pathogenesis of Yptb in the lungs remains a robust model for the study of gram-negative bacterial pathogens as it seed lungs at high efficiency and grows over the period of 96–144 hours. IP2666 provides a model for infections that remain primarily in the lungs for the first 96–120 hours (Fisher, et al., 2007), while IP32953 allows for the study of virulence factors required for faster dissemination from the lung to other tissues.
Our findings also highlight tissue specific differences in the function of adhesins. For example, in ex vivo studies, the IP32953 ΔyadA mutant demonstrated increased translocation into splenocytes as compared to WT (Maldonado-Arocho, et al., 2013), while for lung cells, these strains had equivalent translocation levels. Furthermore, in mouse infections, the IP2666 ΔailΔinvΔyadA mutant did not display any differences in cell type specificity for translocation after intravenous infection (Maldonado-Arocho, et al., 2013). By contrast, we found here that IP32953 ΔailΔyadA had a marked defect in translocation into neutrophils following intranasal infection. This could reflect several variations between these studies, including the mouse strains used, the organs studied, and the temperature at which the bacterial cultures were grown.
In conclusion, we have found that Ail and YadA act redundantly in lungs to direct injection of Yops into lung neutrophils, and therefore prevent killing and clearing by neutrophils in the lungs. It is still unclear, however, how neutrophils are killing the ΔailΔyadA mutant in the lungs. While our work suggests that complement mediated opsonophagocytosis is not the predominant method of bacterial killing, there are still other neutrophil effector functions to explore, such as ROS production and NETs (Kolaczkowska, et al., 2013). Ail and YadA also function redundantly in enhancing dissemination and/or growth in the spleen by warding off complement-mediated killing mechanisms. Future work will focus on understanding on how these adhesins interact with neutrophils, the function of the Yops within neutrophils following translocation, and the role of adhesins and complement during Yersinia systemic spread.
Experimental Procedures
Bacterial Strain Construction
Strains used in this study are listed in Table 1, and primers are listed in Table 2 and Supplemental Table 1. Yptb strains were grown for conjugation and mouse infections as previously described (Logsdon, et al., 2003)).
Table 1.
Bacterial Strains
| Genotype | Strain Name | Reference |
|---|---|---|
| Y. pseudotuberculosis | ||
| IP2666 pIB1NdeI WT | MKP001 | (Ivanov, et al., 2005) |
| IP2666 pIB1NdeI ÄyadA | MKP031 | This study |
| IP2666 WT | FM025 | (Simonet, et al., 1992) |
| IP2666 Äail | FM141 | (Maldonado-Arocho, et al., 2013) |
| IP32953 WT | MLF049 | (Chain, et al., 2004) |
| IP32953 Äail | FM347 | (Maldonado-Arocho, et al., 2013) |
| IP32953 Äinv | FM496 | (Maldonado-Arocho, et al., 2013) |
| IP32953 ÄpsaE | MLF086 | This study |
| IP32953 ÄyadA | MLF70 | This study |
| IP32953 ÄailÄinv | FM486 | (Maldonado-Arocho, et al., 2013) |
| IP32953 ÄailÄyadA | FM352 | (Maldonado-Arocho, et al., 2013) |
| IP32953 ÄinvÄyadA | FM121 | (Maldonado-Arocho, et al., 2013) |
| IP32953 ÄpsaEÄyadA | MLF076 | This study |
| IP32953 ÄailÄinvÄyadA | FM376 | (Maldonado-Arocho, et al., 2013) |
| IP32953 ÄinvÄpsaEÄyadA | FM119 | This study |
| IP32953 ÄailÄyadA∷ail | MKP086 | This study |
| IP32953 ÄailÄyadA∷yadA | MKP126 | This study |
| IP32953 ÄyopB | MLF073 | This study |
| IP32953 WT-ETEM | MKP032 | This study |
| IP32953 Äail-ETEM | MKP038 | This study |
| IP32953 ÄyadA-ETEM | MLF090 | This study |
| IP32953 ÄailÄyadA-ETEM | MKP040 | This study |
| IP32953 ÄyopB-ETEM | MKP074 | This study |
| IP32953 WT-GFP | MKP054 | This study |
| IP32953 Äail-GFP | MKP060 | This study |
| IP32953 ÄyadA-GFP | MKP106 | This study |
| IP32953 ÄailÄyadA-GFP | MKP062 | This study |
| Escherichia coli | ||
| DH5áëpir+pCVD442-ail | FM425 | (Maldonado-Arocho, et al., 2013) |
| SM10λpir+pCVD442-yadA | MKP049 | This study |
| SY327λpir+pSR47s–YopE100-TEM | CC99 (pSR47S–E-TEM) |
(Harmon, et al., 2010) |
| DH125+pACYC184-GFP | JM582 | (Durand, et al., 2010) |
| SM10ëpir+pCVD442-ÄyopB | LL19 | (Logsdon, et al., 2003) |
| SY327λpir+pCVD442-ÄyadA | MM83 | This study |
| DH5αλpir + pRK600 | JM549 | (Kessler, et al., 1992) |
| DH5αλpir+pRS47-ÄpsaE | MLF083 | This study |
Table 2.
Primers
| Primer Name | Primer Sequence |
|---|---|
| AD59 | CTATCTCGCGTGACAGAGGTGC |
| AD60 | GGCACCCCTGATGCGTTCGCGG |
| FM025 | GAATTCGTCGACCATCCGGTTTGAGGTGAGG |
| FM026 | GAATTCGAGCTCGTAGCAAATATCGGAGAGATTG |
| FM029 | GATCCGCATGCCGTGTGAAACAAGTTCTG |
| FM030 | GATCCTCTAGATTTTCTAGCTGAGCGATAGCCA |
| FM033 | GATCCGCATGCCATCCGGTTTGAGGTGAGG |
| FM034 | GATCCTCTAGAGTAGCAAATATCGGAGAGATTG |
| P18 | GTACCGTCGACCATCCGGTTTGAGGTGAGG |
| P19 | TTTGTGCATGCGAACTGCTGACAAACGAGGA |
| P20 | AGTTCGCATGCACAAAGGTTTAGCCAGTTCAGC |
| P21 | GATCCGAGCTCCGTAGCAAATATCGGAGAGATTG |
| TEM1F | GAGACAGCGGCCGCCACCCAGAAACGCTGGTG |
| TEM1R | GAGACAGAGCTCGCATGCTGAGTAAACTTGGTCTGACAGT |
The yadA gene was deleted by allelic exchange as previously described (Logsdon, et al., 2003). Briefly, overlapping PCR was used to generate fragments flanking the upstream and downstream regions of YadA. The primer pairs P18/P19 and P20/P21 were used to amplify the upstream and downstream regions of yadA from IP32953 pYV. A second PCR was carried out to stitch together the overlapping PCR fragments using primers P18 and P21. The overlapping PCR product was cloned into pCVD442 using Sac I and Sal I restriction sites. The resulting plasmid, pCVD442-ΔyadA, was then introduced into E. coli SM10λpir to generate strain MM83. Allelic exchange through mating between donor MM83 and recipient Yptb strain IP32953 was used to remove the coding region of yadA. Deletion of yadA was confirmed by PCR using primers FM025 and FM026, western blot using polyclonal antibody against YadA (bA-17, Santa Cruz) and for lack of autoagglutination (see Supplemental Experimental Procedures).
To complement the IP32953 ΔailΔyadA strain with Ail or YadA, the ail and yadA genes were amplified from IP32953 using primer pairs FM011/FM014 (Maldonado-Arocho, et al., 2013)and FM033/FM034, respectively. The PCR products were cloned into pCVD442 using Sac I and Sal I restriction sites for ail or Sph I and Xba I sites for yadA, and the resulting plasmids, FM425 (ail) and MKP049 (yadA), were then introduced into E. coli Sy327λpir and E. coli SM10λpir respectively. Allelic exchange was used as described above to introduce ail and yadA genes into IP32953 ΔailΔyadA strain. Restoration of Ail was confirmed by PCR using primers FM029 and FM030. Restoration of YadA was confirmed by PCR using primers FM025 and FM026 and for the ability to autoagglutinate (see Supplemental Experimental Procedures).
The IP32953 ΔpsaE strain was generated by tri-parental mating between helper (JM549), donor (MLF083) and recipient IP32953 (MLF049). Transconjugants were selected as described (Logsdon, et al., 2003). The IP32953 ΔyopB mutant was generated as described (Logsdon, et al., 2003) by mating LL-19 with MLF049. IP32953 ΔyopB clones were verified by PCR with primers AD59 and AD60, the inability to cause cell rounding following infection of HEp-2 cells (Harmon, et al., 2010)) and western blot with anti-serum against YopB (gift from J. Bliska, Stony Brook).
All YopE-TEM (ETEM) expressing strains were generated as described previously by triparental mating E. coli SY327λpir carrying pSR47S–E-TEM and JM549 with the indicated Yptb strains (Harmon, et al., 2010). The presence of ETEM in all strains was confirmed by PCR using primers TEM1F and TEM1R. In addition, secretion of ETEM and other Yops was verified by TCA precipitation of cultured supernatants followed by SDS-PAGE analysis and western blot analysis of supernatants with antibody against TEM (QED Bioscience Inc) (Davis, et al., 2007, Harmon, et al., 2010). All GFP-expressing strains were constructed by transforming the pACYC184-GFP plasmid (Durand, et al., 2010) into the indicated Yptb strains (Table 1).
Mouse Infections
Yptb strains were cultured and mouse infections were carried out as described (Fisher, et al., 2007). Briefly, 6–8 week old female BALB/c mice (NCI) were anesthetized with isoflurane and inoculated intranasally with a 40µl bolus of ∼250 CFU Yptb in PBS, unless otherwise noted in figure legends. At the indicated times after infection, mice were sacrificed by CO2 asphyxiation followed by cervical dislocation. Tissues were harvested into RPMI supplemented with 5% HIS, weighed, homogenized using a Biospec miniBeadbeater, diluted 1:1 in 50% glycerol, and serial dilutions were plated to determine the CFU. Data are reported as the CFU/g organ for lung, liver or spleen.
In vivo Neutrophil depletion assay
6–8 week old BALB/c females were depleted of neutrophils as previously described (Daley, et al., 2008). Briefly, mice were injected intraperitoneally with 100µL of either αLy6G depletion antibody (0.5mg/mL, IA8, Fisher Scientific) or an isotype control (IgG2a κ isotype, 0.5mg/mL, Biolegend) one day prior to infection and two days post-infection. Mice were infected as described above with either WT IP32953 or ΔailΔyadA, and sacrificed 4 days post infection. Neutrophil depletion was confirmed by cell staining of lung homogenate with Ly6G PE-Cy7 (IA8, Fisher Scientific) and FACS analysis using the LSRII (BD Bioscience).
Complement depletion
6–8 week old BALB/c females were depleted of complement using CVF (Quidel Corporation) as previously described (Shapiro, et al., 2002, Maldonado-Arocho, et al., 2013). Briefly, one day prior to infection, mice were injected intraperitoneally twice at 4h apart with PBS or two 5 Unit doses of CVF diluted in PBS (50U/mL). 24 hours later, mice were infected intranasally with Yptb.
In vitro serum resistance assay
Overnight cultures of Yptb were diluted 1:40 in 2XYT+5mM CaCl2 and incubated 2h at 26°C with aeration followed by a 2h incubation at 37°C with aeration. Bacteria were diluted to 1×104 CFU/mL and then 50µL of each dilution was transferred to a 96 well polypropylene plate. Input numbers of bacteria were confirmed via serial dilutions on L plates. 50µL fetal bovine serum (FBS) or heat-inactivated fetal bovine serum (HIS) was added to each well, each condition was performed in triplicate. The bacteria were incubated for 2h at 37°C with aeration, and serial dilutions of each well were plated on L plates containing 0.5µg/mL Irgasan to determine number of Yptb that survived treatment. Percent survival was calculated as CFU recovered after incubation in FBS divided by CFU recovered after incubation with HIS and then that value was normalized to WT IP32953, which was set to 1.
In vivo CCF4 conversion assays
7–8 week old BALB/c female mice were infected intranasally with the indicated Yptb strain and dose indicated in the figure legends. At the indicated time post infection, lungs, spleens and livers were collected in RPMI supplemented with 5% HIS. Spleens and livers were processed as described above for quantifying CFU. Lung tissues were homogenized by passing through a 70µM cell filter and each sample was diluted and plated for CFU. All subsequent steps were done in the presence of gentamicin (100µg/mL). Lung cell suspension was treated with Collagenase D (Fisher Scientific, 1mg/mL in RPMI+5% HIS) for 60 min at 37°C. Cells were then pelleted by centrifuging at 1300RPM for 5 min, resuspended in 1X Pharmlyse (BD Biosciences, diluted in sterile H2O) for 1 min, centrifuged, resuspended in RPMI+5% HIS, and passed again through a 70µM cell strainer to remove any clumped cellular debris. Cells were loaded with CCF4-AM as previously described (Durand, et al., 2010) by incubating single cell suspensions for 20 min in the dark in media containing 1µg/mL CCF4-AM (Invitrogen), 1.5mM probenecid (Sigma) and 100µg/mL gentamicin. For cell type staining, Fc receptors were first blocked by a 10 min incubation at 4°C with Fc Block (BD Pharmingen), and then stained for cell surface markers for 30 min at 4°C with the following fluorescent antibodies: Ly6G PE-Cy7 (IA8, Fisher Scientific), GR1 PE-Cy5 (eBioscience,) CD11b PE-Cy7 (eBioscience,) CD11c PE-Cy5 (eBioscience,) TCRβ PE-Cy7 (Fisher Scientific,) CD4 PE-Cy5 (BD Pharmingen,) CD8 PE-Cy5 (BD Pharmingen,) B220 PE-Cy5 (eBioscience,) and CD19 PE-Cy7 (eBioscience). Following staining, cells were resuspended in PBS, sampled on a LSRII (BD) and analyzed using FlowJo 7.6.4 software. Negative controls included uninfected cells that were not incubated with CCF4-AM and/or antibodies.
Ex vivo CCF4 conversion assay
Cultures of WT IP32953 or adhesin mutants expressing ETEM were grown in 2XYT+5mM CaCl2 supplemented with 50µg/mL kanamycin and incubated with aeration at 37°C overnight. The cultures were diluted 1:40 into 2mL of 2XYT supplemented with 5mM CaCl2 and 50µg/mL kanamycin, and then incubated for 3–4 h at 37°C with aeration. Cultures were centrifuged at 14,000RPM for 2 min, washed 3 times and resuspended in RPMI+5% HIS. Lungs from naive BALB/c female mice were aseptically collected in RPMI+5% HIS and processed as described above for cell staining except that no gentamicin was used. After the second passage through the 70uM filter, lung cells were resuspended in RPMI+5% HIS to ∼1–3.5 ×105 lung cells in a final volume of 200µL per strain and transferred to a 24 well plate. Bacteria were added at an MOI 1:1 and incubated with the lung cells at 37°C for 1h. CCF4-AM loading, cell surface marker staining and FACS analysis was performed in the presence of gentamicin as described above.
Assay of Yptb adherence to lung cell suspension
Overnight cultures of GFP-expressing Yptb strains were diluted 1:40 into 2XYT supplemented with 5mM CaCl2 and 10µg/mL chloramphenicol, and then incubated for 3–4h at 37°C with aeration. Cultures were centrifuged, washed 3 times with RPMI+5% HIS, and resuspended in RPMI+5% HIS. Lungs from naive BALB/c female mice were collected in RPMI+5% HIS and processed as described for ex vivo cell staining. After the second passage through the 70uM filter, lung cells were resuspended in RPMI+5% HIS to 5–18 ×105 cell/mL lung cells and 200µL were transferred to a 24 well plate. Bacteria were added at an MOI 1:1 and plate was incubated at 37°C for 30 min. Cells were labeled with antibodies as described above, the plate was spun at 300 RCF for 5 min, and cells were fixed in 4% formamide prior to analysis on the LSRII (BD).
Statistics
Statistical analyses for each experiment are described in figure legends. Statistics for CFU was done on the Log10 transformed values. All statistical analysis was performed using GraphPad Prism 5 software. For data analyzed using One-Way ANOVA, the post-test was applied as follows: Tukey’s for comparing all cohorts within the data set, Dunnett’s for comparing cohorts against a control cohort, and Bonferroni’s for comparing specific pairs of cohorts.
Supplementary Material
Acknowledgements
We thank Melissa McCoy for MM83. We thank members of the Mecsas laboratory for critical reading of the manuscript and useful suggestions.
Michelle Paczosa is a Howard Hughes Medical Institute (HHMI) Med into Grad Scholar supported in part by a grant to the Sackler School of Biomedical Sciences, Tufts University School of Medicine from HHMI through the Med into Grad Initiative. MKP was supported by 2T32AI007077-31 and T32AI007422, MLF was supported by T32AI007422, FJMA was supported by 5K12GM074869, and JM was supported by AI056068 and AI073759.
References
- Achtman M, Zurth K, Morelli G, Torrea G, Guiyoule A, Carniel E. Yersinia pestis, the cause of plague, is a recently emerged clone of Yersinia pseudotuberculosis. Proc Natl Acad Sci U S A. 1999;96:14043–14048. doi: 10.1073/pnas.96.24.14043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agar SL, Sha J, Baze WB, Erova TE, Foltz SM, Suarez G, et al. Deletion of Braun lipoprotein gene (lpp) and curing of plasmid pPCP1 dramatically alter the virulence of Yersinia pestis CO92 in a mouse model of pneumonic plague. Microbiology. 2009;155:3247–3259. doi: 10.1099/mic.0.029124-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balada-Llasat JM, Panilaitis B, Kaplan D, Mecsas J. Oral inoculation with Type III secretion mutants of Yersinia pseudotuberculosis provides protection from oral, intraperitoneal, or intranasal challenge with virulent Yersinia. Vaccine. 2007;25:1526–1533. doi: 10.1016/j.vaccine.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Bartra SS, Styer KL, O'Bryant DM, Nilles ML, Hinnebusch BJ, Aballay A, Plano GV. Resistance of Yersinia pestis to complement-dependent killing is mediated by the Ail outer membrane protein. Infect Immun. 2008;76:612–622. doi: 10.1128/IAI.01125-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biedzka-Sarek M, Salmenlinna S, Gruber M, Lupas AN, Meri S, Skurnik M. Functional mapping of YadA- and Ail-mediated binding of human factor H to Yersinia enterocolitica serotype O:3. Infect Immun. 2008;76:5016–5027. doi: 10.1128/IAI.00314-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biedzka-Sarek M, Venho R, Skurnik M. Role of YadA, Ail, and Lipopolysaccharide in Serum Resistance of Yersinia enterocolitica Serotype O:3. Infect Immun. 2005;73:2232–2244. doi: 10.1128/IAI.73.4.2232-2244.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliska JB, Copass MC, Falkow S. The Yersinia pseudotuberculosis adhesin YadA mediates intimate bacterial attachment to and entry into HEp-2 cells. Infect Immun. 1993;61:3914–3921. doi: 10.1128/iai.61.9.3914-3921.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliska JB, Wang X, Viboud GI, Brodsky IE. Modulation of innate immune responses by Yersinia type III secretion system translocators and effectors. Cell Microbiol. 2013 doi: 10.1111/cmi.12164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd AP, Grosdent N, Totemeyer S, Geuijen C, Bleves S, Iriarte M, et al. Yersinia enterocolitica can deliver Yop proteins into a wide range of cell types: development of a delivery system for heterologous proteins. European journal of cell biology. 2000;79:659–671. doi: 10.1078/0171-9335-00098. [DOI] [PubMed] [Google Scholar]
- Cathelyn JS, Crosby SD, Lathem WW, Goldman WE, Miller VL. RovA, a global regulator of Yersinia pestis, specifically required for bubonic plague. Proc Natl Acad Sci U S A. 2006;103:13514–13519. doi: 10.1073/pnas.0603456103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chain PS, Carniel E, Larimer FW, Lamerdin J, Stoutland PO, Regala WM, et al. Insights into the evolution of Yersinia pestis through whole-genome comparison with Yersinia pseudotuberculosis. Proc Natl Acad Sci U S A. 2004;101:13826–13831. doi: 10.1073/pnas.0404012101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charpentier X, Oswald E. Identification of the secretion and translocation domain of the enteropathogenic and enterohemorrhagic Escherichia coli effector Cif, using TEM-1 beta-lactamase as a new fluorescence-based reporter. J Bacteriol. 2004;186:5486–5495. doi: 10.1128/JB.186.16.5486-5495.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelis GR. The Yersinia Ysc-Yop 'type III' weaponry. Nat Rev Mol Cell Biol. 2002;3:742–752. doi: 10.1038/nrm932. [DOI] [PubMed] [Google Scholar]
- Crimmins GT, Mohammadi S, Green ER, Bergman MA, Isberg RR, Mecsas J. Identification of MrtAB, an ABC transporter specifically required for Yersinia pseudotuberculosis to colonize the mesenteric lymph nodes. PLoS Pathog. 2012;8:e1002828. doi: 10.1371/journal.ppat.1002828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daha MR. Role of complement in innate immunity and infections. Critical reviews in immunology. 2010;30:47–52. doi: 10.1615/critrevimmunol.v30.i1.30. [DOI] [PubMed] [Google Scholar]
- Daley JM, Thomay AA, Connolly MD, Reichner JS, Albina JE. Use of Ly6G–specific monoclonal antibody to deplete neutrophils in mice. Journal of leukocyte biology. 2008;83:64–70. doi: 10.1189/jlb.0407247. [DOI] [PubMed] [Google Scholar]
- Davis AJ, Mecsas J. Mutations in the Yersinia pseudotuberculosis type III secretion system needle protein, YscF, that specifically abrogate effector translocation into host cells. J Bacteriol. 2007;189:83–97. doi: 10.1128/JB.01396-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewoody RS, Merritt PM, Marketon MM. Regulation of the Yersinia type III secretion system: traffic control. Frontiers in cellular and infection microbiology. 2013;3:4. doi: 10.3389/fcimb.2013.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand EA, Maldonado-Arocho FJ, Castillo C, Walsh RL, Mecsas J. The presence of professional phagocytes dictates the number of host cells targeted for Yop translocation during infection. Cell Microbiol. 2010;12:1064–1082. doi: 10.1111/j.1462-5822.2010.01451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eitel J, Dersch P. The YadA protein of Yersinia pseudotuberculosis mediates high-efficiency uptake into human cells under environmental conditions in which invasin is repressed. Infect Immun. 2002;70:4880–4891. doi: 10.1128/IAI.70.9.4880-4891.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emody L, Heesemann J, Wolf-Watz H, Skurnik M, Kapperud G, O'Toole P, Wadstrom T. Binding to collagen by Yersinia enterocolitica and Yersinia pseudotuberculosis: evidence for yopA-mediated and chromosomally encoded mechanisms. J Bacteriol. 1989;171:6674–6679. doi: 10.1128/jb.171.12.6674-6679.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felek S, Krukonis ES. The Yersinia pestis Ail protein mediates binding and Yop delivery to host cells required for plague virulence. Infect Immun. 2009;77:825–836. doi: 10.1128/IAI.00913-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felek S, Tsang TM, Krukonis ES. Three Yersinia pestis adhesins facilitate Yop delivery to eukaryotic cells and contribute to plague virulence. Infect Immun. 2010;78:4134–4150. doi: 10.1128/IAI.00167-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher ML, Castillo C, Mecsas J. Intranasal inoculation of mice with Yersinia pseudotuberculosis causes a lethal lung infection that is dependent on Yersinia outer proteins and PhoP. Infect Immun. 2007;75:429–442. doi: 10.1128/IAI.01287-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrity-Ryan LK, Kim OK, Balada-Llasat JM, Bartlett VJ, Verma AK, Fisher ML, et al. Small molecule inhibitors of LcrF, a Yersinia pseudotuberculosis transcription factor, attenuate virulence and limit infection in a murine pneumonia model. Infect Immun. 2010;78:4683–4690. doi: 10.1128/IAI.01305-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosdent N, Maridonneau-Parini I, Sory MP, Cornelis GR. Role of Yops and adhesins in resistance of Yersinia enterocolitica to phagocytosis. Infect Immun. 2002;70:4165–4176. doi: 10.1128/IAI.70.8.4165-4176.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han YW, Miller VL. Reevaluation of the virulence phenotype of the inv yadA double mutants of Yersinia pseudotuberculosis. Infect Immun. 1997;65:327–330. doi: 10.1128/iai.65.1.327-330.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon DE, Davis AJ, Castillo C, Mecsas J. Identification and characterization of small-molecule inhibitors of Yop translocation in Yersinia pseudotuberculosis. Antimicrob Agents Chemother. 2010;54:3241–3254. doi: 10.1128/AAC.00364-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon DE, Murphy JL, Davis AJ, Mecsas J. A mutant with aberrant extracellular LcrV-YscF interactions fails to form pores and translocate Yop effector proteins but retains the ability to trigger Yop secretion in response to host cell contact. J Bacteriol. 2013;195:2244–2254. doi: 10.1128/JB.02011-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heesemann J, Gross U, Gruter L. Genetic manipulation of virulence of Yersinia enterocolitica. Contributions to microbiology and immunology. 1987;9:312–316. [PubMed] [Google Scholar]
- Heise T, Dersch P. Identification of a domain in Yersinia virulence factor YadA that is crucial for extracellular matrix-specific cell adhesion and uptake. Proc Natl Acad Sci U S A. 2006;103:3375–3380. doi: 10.1073/pnas.0507749103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinnebusch BJ, Jarrett CO, Callison JA, Gardner D, Buchanan SK, Plano GV. Role of the Yersinia pestis Ail protein in preventing a protective polymorphonuclear leukocyte response during bubonic plague. Infect Immun. 2011;79:4984–4989. doi: 10.1128/IAI.05307-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho DK, Riva R, Kirjavainen V, Jarva H, Ginstrom E, Blom AM, et al. Functional recruitment of the human complement inhibitor C4BP to Yersinia pseudotuberculosis outer membrane protein Ail. Journal of immunology. 2012a;188:4450–4459. doi: 10.4049/jimmunol.1103149. [DOI] [PubMed] [Google Scholar]
- Ho DK, Riva R, Skurnik M, Meri S. The Yersinia pseudotuberculosis outer membrane protein Ail recruits the human complement regulatory protein factor H. Journal of immunology. 2012b;189:3593–3599. doi: 10.4049/jimmunol.1201145. [DOI] [PubMed] [Google Scholar]
- Hudson KJ, Bouton AH. Yersinia pseudotuberculosis adhesins regulate tissue-specific colonization and immune cell localization in a mouse model of systemic infection. Infect Immun. 2006;74:6487–6490. doi: 10.1128/IAI.00718-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov MI, Stuckey JA, Schubert HL, Saper MA, Bliska JB. Two substrate-targeting sites in the Yersinia protein tyrosine phosphatase co-operate to promote bacterial virulence. Molecular microbiology. 2005;55:1346–1356. doi: 10.1111/j.1365-2958.2005.04477.x. [DOI] [PubMed] [Google Scholar]
- Kapperud G, Namork E, Skurnik M, Nesbakken T. Plasmid-mediated surface fibrillae of Yersinia pseudotuberculosis and Yersinia enterocolitica: relationship to the outer membrane protein YOP1 and possible importance for pathogenesis. Infect Immun. 1987;55:2247–2254. doi: 10.1128/iai.55.9.2247-2254.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler B, de Lorenzo V, Timmis KN. A general system to integrate lacZ fusions into the chromosomes of gram-negative eubacteria: regulation of the Pm promoter of the TOL plasmid studied with all controlling elements in monocopy. Molecular & general genetics : MGG. 1992;233:293–301. doi: 10.1007/BF00587591. [DOI] [PubMed] [Google Scholar]
- Kirjavainen V, Jarva H, Biedzka-Sarek M, Blom AM, Skurnik M, Meri S. Yersinia enterocolitica serum resistance proteins YadA and ail bind the complement regulator C4b–binding protein. PLoS Pathog. 2008;4:e1000140. doi: 10.1371/journal.ppat.1000140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köberle M, Klein-Günther A, Schütz M, Fritz M, Berchtold S, Tolosa E, et al. Yersinia enterocolitica targets cells of the innate and adaptive immune system by injection of Yops in a mouse infection model. PLoS Pathog. 2009;5:e1000551. doi: 10.1371/journal.ppat.1000551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nature reviews. Immunology. 2013;13:159–175. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- Kolodziejek AM, Hovde CJ, Minnich SA. Yersinia pestis Ail: multiple roles of a single protein. Frontiers in cellular and infection microbiology. 2012;2:103. doi: 10.3389/fcimb.2012.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodziejek AM, Schnider DR, Rohde HN, Wojtowicz AJ, Bohach GA, Minnich SA, Hovde CJ. Outer membrane protein X (Ail) contributes to Yersinia pestis virulence in pneumonic plague and its activity is dependent on the lipopolysaccharide core length. Infect Immun. 2010;78:5233–5243. doi: 10.1128/IAI.00783-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodziejek AM, Sinclair DJ, Seo KS, Schnider DR, Deobald CF, Rohde HN, et al. Phenotypic characterization of OmpX, an Ail homologue of Yersinia pestis KIM. Microbiology. 2007;153:2941–2951. doi: 10.1099/mic.0.2006/005694-0. [DOI] [PubMed] [Google Scholar]
- Lathem WW, Crosby SD, Miller VL, Goldman WE. Progression of primary pneumonic plague: a mouse model of infection, pathology, and bacterial transcriptional activity. Proc Natl Acad Sci U S A. 2005;102:17786–17791. doi: 10.1073/pnas.0506840102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lathem WW, Price PA, Miller VL, Goldman WE. A plasminogen-activating protease specifically controls the development of primary pneumonic plague. Science. 2007;315:509–513. doi: 10.1126/science.1137195. [DOI] [PubMed] [Google Scholar]
- Leo JC, Skurnik M. Adhesins of human pathogens from the genus Yersinia. Advances in experimental medicine and biology. 2011;715:1–15. doi: 10.1007/978-94-007-0940-9_1. [DOI] [PubMed] [Google Scholar]
- Logsdon LK, Mecsas J. Requirement of the Yersinia pseudotuberculosis effectors YopH and YopE in colonization and persistence in intestinal and lymph tissues. Infect Immun. 2003;71:4595–4607. doi: 10.1128/IAI.71.8.4595-4607.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu T, Kobayashi SD, Quinn MT, Deleo FR. A NET Outcome. Frontiers in immunology. 2012;3:365. doi: 10.3389/fimmu.2012.00365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makoveichuk E, Cherepanov P, Lundberg S, Forsberg A, Olivecrona G. pH6 antigen of Yersinia pestis interacts with plasma lipoproteins and cell membranes. Journal of lipid research. 2003;44:320–330. doi: 10.1194/jlr.M200182-JLR200. [DOI] [PubMed] [Google Scholar]
- Maldonado-Arocho FJ, Green C, Fisher ML, Paczosa MK, Mecsas J. Adhesins and host serum factors drive Yop translocation by yersinia into professional phagocytes during animal infection. PLoS Pathog. 2013;9:e1003415. doi: 10.1371/journal.ppat.1003415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marketon MM, DePaolo RW, DeBord KL, Jabri B, Schneewind O. Plague bacteria target immune cells during infection. Science. 2005;309:1739–1741. doi: 10.1126/science.1114580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marra A, Isberg RR. Invasin-dependent and invasin-independent pathways for translocation of Yersinia pseudotuberculosis across the Peyer's patch intestinal epithelium. Infect Immun. 1997;65:3412–3421. doi: 10.1128/iai.65.8.3412-3421.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto H, Young GM. Translocated effectors of Yersinia. Current opinion in microbiology. 2009;12:94–100. doi: 10.1016/j.mib.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mejía E, Bliska JB, Viboud GI. Yersinia controls type III effector delivery into host cells by modulating Rho activity. PLoS Pathog. 2008;4:e3. doi: 10.1371/journal.ppat.0040003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikula KM, Kolodziejczyk R, Goldman A. Yersinia infection tools-characterization of structure and function of adhesins. Frontiers in cellular and infection microbiology. 2012;2:169. doi: 10.3389/fcimb.2012.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mota LJ, Cornelis GR. The bacterial injection kit: type III secretion systems. Ann Med. 2005;37:234–249. doi: 10.1080/07853890510037329. [DOI] [PubMed] [Google Scholar]
- Pepe JC, Wachtel MR, Wagar E, Miller VL. Pathogenesis of defined invasion mutants of Yersinia enterocolitica in a BALB/c mouse model of infection. Infect Immun. 1995;63:4837–4848. doi: 10.1128/iai.63.12.4837-4848.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry RD, Fetherston JD. Yersinia pestis--etiologic agent of plague. Clinical microbiology reviews. 1997;10:35–66. doi: 10.1128/cmr.10.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierson DE, Falkow S. The ail gene of Yersinia enterocolitica has a role in the ability of the organism to survive serum killing. Infect Immun. 1993;61:1846–1852. doi: 10.1128/iai.61.5.1846-1852.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price PA, Jin J, Goldman WE. Pulmonary infection by Yersinia pestis rapidly establishes a permissive environment for microbial proliferation. Proc Natl Acad Sci U S A. 2012;109:3083–3088. doi: 10.1073/pnas.1112729109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 2010;11:785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roggenkamp A, Ruckdeschel K, Leitritz L, Schmitt R, Heesemann J. Deletion of amino acids 29 to 81 in adhesion protein YadA of Yersinia enterocolitica serotype O:8 results in selective abrogation of adherence to neutrophils. Infect Immun. 1996;64:2506–2514. doi: 10.1128/iai.64.7.2506-2514.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler MK, Schutz MS, Muhlenkamp MC, Rooijakkers SH, Hallstrom T, Zipfel PF, Autenrieth IB. Yersinia enterocolitica YadA mediates complement evasion by recruitment and inactivation of C3 products. Journal of immunology. 2012;189:4900–4908. doi: 10.4049/jimmunol.1201383. [DOI] [PubMed] [Google Scholar]
- Shapiro S, Beenhouwer DO, Feldmesser M, Taborda C, Carroll MC, Casadevall A, Scharff MD. Immunoglobulin G monoclonal antibodies to Cryptococcus neoformans protect mice deficient in complement component C3. Infect Immun. 2002;70:2598–2604. doi: 10.1128/IAI.70.5.2598-2604.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonet M, Falkow S. Invasin expression in Yersinia pseudotuberculosis. Infect Immun. 1992;60:4414–4417. doi: 10.1128/iai.60.10.4414-4417.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skurnik M, Peippo A, Ervela E. Characterization of the O-antigen gene clusters of Yersinia pseudotuberculosis and the cryptic O-antigen gene cluster of Yersinia pestis shows that the plague bacillus is most closely related to and has evolved from Y. pseudotuberculosis serotype O:1b. Molecular microbiology. 2000;37:316–330. doi: 10.1046/j.1365-2958.2000.01993.x. [DOI] [PubMed] [Google Scholar]
- Skurnik M, Wolf-Watz H. Analysis of the yopA gene encoding the Yop1 virulence determinants of Yersinia spp. Molecular microbiology. 1989;3:517–529. doi: 10.1111/j.1365-2958.1989.tb00198.x. [DOI] [PubMed] [Google Scholar]
- Tamang DL, Pirzai W, Priebe GP, Traficante DC, Pier GB, Falck JR, et al. Hepoxilin A(3) facilitates neutrophilic breach of lipoxygenase-expressing airway epithelial barriers. Journal of immunology. 2012;189:4960–4969. doi: 10.4049/jimmunol.1201922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamm A, Tarkkanen AM, Korhonen TK, Kuusela P, Toivanen P, Skurnik M. Hydrophobic domains affect the collagen-binding specificity and surface polymerization as well as the virulence potential of the YadA protein of Yersinia enterocolitica. Molecular microbiology. 1993;10:995–1011. doi: 10.1111/j.1365-2958.1993.tb00971.x. [DOI] [PubMed] [Google Scholar]
- Tertti R, Skurnik M, Vartio T, Kuusela P. Adhesion protein YadA of Yersinia species mediates binding of bacteria to fibronectin. Infect Immun. 1992;60:3021–3024. doi: 10.1128/iai.60.7.3021-3024.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang TM, Annis DS, Kronshage M, Fenno JT, Usselman LD, Mosher DF, Krukonis ES. Ail protein binds ninth type III fibronectin repeat (9FNIII) within central 120-kDa region of fibronectin to facilitate cell binding by Yersinia pestis. J Biol Chem. 2012;287:16759–16767. doi: 10.1074/jbc.M112.358978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang TM, Felek S, Krukonis ES. Ail binding to fibronectin facilitates Yersinia pestis binding to host cells and Yop delivery. Infect Immun. 2010;78:3358–3368. doi: 10.1128/IAI.00238-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uliczka F, Pisano F, Schaake J, Stolz T, Rohde M, Fruth A, et al. Unique cell adhesion and invasion properties of Yersinia enterocolitica O:3, the most frequent cause of human Yersiniosis. PLoS Pathog. 2011;7:e1002117. doi: 10.1371/journal.ppat.1002117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viboud GI, Bliska JB. Yersinia outer proteins: role in modulation of host cell signaling responses and pathogenesis. Annu Rev Microbiol. 2005;59:69–89. doi: 10.1146/annurev.micro.59.030804.121320. [DOI] [PubMed] [Google Scholar]
- Viboud GI, Mejia E, Bliska JB. Comparison of YopE and YopT activities in counteracting host signalling responses to Yersinia pseudotuberculosis infection. Cell Microbiol. 2006;8:1504–1515. doi: 10.1111/j.1462-5822.2006.00729.x. [DOI] [PubMed] [Google Scholar]
- Visser LG, Annema A, van Furth R. Role of Yops in inhibition of phagocytosis and killing of opsonized Yersinia enterocolitica by human granulocytes. Infect Immun. 1995;63:2570–2575. doi: 10.1128/iai.63.7.2570-2575.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel CW, Fritzinger DC. Cobra venom factor: Structure, function, and humanization for therapeutic complement depletion. Toxicon. 2010;56:1198–1222. doi: 10.1016/j.toxicon.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Wachter E, Brade V. Influence of surface modulations by enzymes and monoclonal antibodies on alternative complement pathway activation by Yersinia enterocolitica. Infect Immun. 1989;57:1984–1989. doi: 10.1128/iai.57.7.1984-1989.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watford WT, Ghio AJ, Wright JR. Complement-mediated host defense in the lung. American journal of physiology. Lung cellular and molecular physiology. 2000;279:L790–L798. doi: 10.1152/ajplung.2000.279.5.L790. [DOI] [PubMed] [Google Scholar]
- Worsham PL, Mou S, Cote CK, Fritz D. Virulence of Yersinia pseudotuberculosis in aerosol models. Advances in experimental medicine and biology. 2012;954:217–222. doi: 10.1007/978-1-4614-3561-7_27. [DOI] [PubMed] [Google Scholar]
- Wren BW. The yersiniae--a model genus to study the rapid evolution of bacterial pathogens. Nature reviews. Microbiology. 2003;1:55–64. doi: 10.1038/nrmicro730. [DOI] [PubMed] [Google Scholar]
- Yamashita S, Lukacik P, Barnard TJ, Noinaj N, Felek S, Tsang TM, et al. Structural insights into Ail-mediated adhesion in Yersinia pestis. Structure. 2011;19:1672–1682. doi: 10.1016/j.str.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Isberg RR. Transcriptional regulation of the Yersinia pseudotuberculosis pH6 antigen adhesin by two envelope-associated components. Molecular microbiology. 1997;24:499–510. doi: 10.1046/j.1365-2958.1997.3511719.x. [DOI] [PubMed] [Google Scholar]
- Yang Y, Merriam JJ, Mueller JP, Isberg RR. The psa locus is responsible for thermoinducible binding of Yersinia pseudotuberculosis to cultured cells. Infect Immun. 1996;64:2483–2489. doi: 10.1128/iai.64.7.2483-2489.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavialov A, Zav'yalova G, Korpela T, Zav'yalov V. FGL chaperone-assembled fimbrial polyadhesins: anti-immune armament of Gram-negative bacterial pathogens. FEMS microbiology reviews. 2007;31:478–514. doi: 10.1111/j.1574-6976.2007.00075.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.