Abstract
Ethanol causes pathological changes in GABAA receptor trafficking and function. These changes are mediated in part by ethanol activation of protein kinase A (PKA). The current study investigated the expression of the GABAA α1 and α4 subunits and the kinase anchoring protein AKAP150, as well as bicuculline-induced seizure threshold, at baseline and following acute injection of ethanol (3.5 g/kg IP) in a mouse line lacking the regulatory RIIβ subunit of PKA. Whole cerebral cortices were harvested at baseline, 1 h, or 46 h following injection of ethanol or saline and subjected to fractionation and western blot analysis. Knockout (RIIβ−/−) mice had similar baseline levels of PKA RIIα and GABAA α1 and α4 subunits compared to wild type (RIIβ+/+) littermates, but had deficits in AKAP150. GABAA α1 subunit levels were decreased in the P2 fraction of RIIβ−/−, but not RIIβ+/+, mice following 1 h ethanol, an effect that was driven by decreased α1 expression in the synaptic fraction. GABAA α4 subunits in the P2 fraction were not affected by 1 h ethanol; however, synaptic α4 subunit expression was increased in RIIβ+/+, but not RIIβ−/− mice, while extrasynaptic α4 expression was decreased in RIIβ−/−, but not RIIβ+/+ mice. Finally, RIIβ knockout was protective against bicuculline-induced seizure susceptibility. Overall, the results suggest that PKA has differential roles in regulating GABAA receptor subunits. PKA may protect against ethanol-induced deficits in synaptic α1 and extrasynaptic α4 receptors, but may facilitate the increase of synaptic α4 receptors.
Keywords: Alcohol, GABA, PKA, knockout, seizure, AKAP79/150, kinase anchoring protein
Introduction
Ethanol exposure produces a number of maladaptive GABAergic adaptations in the CNS [1]. Among these changes are a decrease in synaptic GABAA α1 and extrasynaptic α4 receptors, and an increase in synaptic α4 receptors [2, 3], resulting in deficits in GABAergic inhibition and overall CNS hyperexcitability. Studies using knockout mouse lines suggest that these changes may underlie some of the pathologies associated with chronic alcohol misuse, including increased seizure susceptibility, increased anxiety, and benzodiazepine tolerance [4, 5]. Interestingly, these GABAergic effects can be recapitulated following a single binge episode of ethanol [6].
Most GABAA receptors are composed of 2α(1-6), 2β(1-3), and either a γ subunit for synaptic receptors or δ subunit for extrasynaptic receptors [7]. GABAA synaptic and extrasynaptic receptors are thought to be responsible for conduction of phasic and tonic inhibition, respectively, within the CNS [8, 9]. Though α1 receptors are the most abundant synaptic GABAA receptor, there is evidence of a tonic conductance carried by α1 receptors [10, 11]. Conversely, extrasynaptic receptors are often composed of α4 receptors [9, 12], though there is also a detectable phasic current conducted by α4 receptors [6, 13, 14]. The function of synaptic 1 and 4 receptors, as well as extrasynaptic 4 receptors has been shown to be rapidly regulated by ethanol [1, 6, 15]. However, previous studies have not separated these synaptic and extrasynaptic receptor subtypes by subcellular fractionation to investigate ethanol adaptations in membrane expression.
Evidence has increasingly pointed to a role for the cAMP-dependent protein kinase (PKA) in mediating the physiological effects of ethanol. Recently, we found that PKA modulates the ethanol-induced trafficking of GABAA α1 subunits both in vivo [16], and in cultured cerebral cortical neurons [17]. PKA activation reversed the effects of ethanol on the synaptic and evoked electrophysiological signatures of GABAA 1 receptors as well as their surface expression. These studies suggest that activation of PKA by ethanol leads to increased membrane levels of synaptic GABAA α1 receptors and may oppose some of the pathological effects produced by ethanol activation of PKC [18]. It has not been established, however, whether these effects require both PKA RII and activation, whether PKA modulates the actions of ethanol in mouse lines or the physiological significance with respect to ethanol-mediated behaviors. Additionally, while the PKA scaffolding protein AKAP150 appears to play an important role in mediating PKA regulation of synapses [19, 20], it is unclear what role this protein may play modulation of GABAergic signaling by ethanol.
Studies using knockout mouse lines have suggested a key role for the PKA pathway in mediating the behavioral effects of ethanol. Mice with a null mutation for the RIIβ subunit of PKA drink more ethanol relative to wild type littermates and are resistant to the sedative effects of ethanol [21]. Interestingly, increased drinking is not associated with altered basal levels of anxiety [22] or increased operant self-administration [23]. It is unknown, however, whether global knockout of PKA regulatory subunits alters GABAergic trafficking either constitutively or following ethanol exposure, and whether this might relate to some of the observed behavioral phenotypes. The present study investigated the potential for altered trafficking of GABAA receptors in RIIβ−/− mice at baseline and following acute ethanol challenge. Additionally, we determined the bicuculline-induced seizure threshold in these mice as a potential behavioral correlate of altered GABAergic signaling.
Materials and Methods
Animals
All experiments were conducted in accordance with guidelines from the National Institutes of Health and Institutional Animal Care and Use Committee. RIIβ−/− mice were generated through targeted disruption by homologous recombination in 129/SvJ mice. Chimeras were crossbred with C57BL/6J mice to obtain heterozygotes. These heterozygotes were then backcrossed with C57BL/6J mice over eight generations to obtain RIIβ+/− mice on an ~100% C57BL/6J genetic background. Non-littermate RIIβ+/− mice were then bred to yield RIIβ+/+ and RIIβ−/− F2 littermates used in these experiments. Mice were ~3 months of age and ~15–25 g at the time of the experiments. Mice were on a reverse 12 h light cycle and were injected with ethanol at the beginning of the last hour of lights on.
Drug Exposure
For acute ethanol exposure mice were injected intraperitoneally (IP) with 3.5 g/kg ethanol (20% v/v in isotonic saline) or isotonic saline. Mice were then sacrificed 1 h or 46 h post-injection, whole brains were removed and the cortices were isolated.
For seizure threshold determination, mice were restrained in a plexiglass plunger-style mouse restraint (Braintree Scientific, Braintree, MA). Bicuculline (Sigma-Aldrich, St. Louis, MO) was dissolved in 0.1 N HCl, and diluted with isotonic saline to a final concentration of 0.05 mg/ml, pH 7. Bicuculline was administered by lateral tail vein infusion at a constant rate of 0.5 ml/min; the endpoint was taken as the first myoclonic jerk of the head and neck as determined by experienced observers who were blind to the experimental conditions. Seizure thresholds were calculated from the time of infusion X dose of bicuculline per body weight and presented as milligrams per kilogram of bicuculline.
Fractionation and Western Blot Analysis
Tissues were weighed, homogenized in 0.32M sucrose and centrifuged at 1000 g for 10 min. The supernatant was then centrifuged twice for 30 min at 12,000 g. The final pellet was resuspended in PBS to yield the P2 fraction. Synaptic and extrasynaptic fractions of the P2 fraction were prepared according to the methods of Goebel-Goody and colleagues [24]. The fractions were separated by 30 min incubation in 0.5% Triton-X, followed by two centrifugations at 32,000 g for 30 min. The resulting pellet was resuspended for the synaptic fraction. The supernatant was incubated overnight at −20°C in acetone (1:8 ratio supernatant:acetone). The resulting solution was spun twice for 30 min at 12,000 g to produce the extrasynaptic fraction. Protein concentrations for fractions were determined using the bicinchoninic acid protein assay kit (Thermo Fisher Scientific, Waltham, MA).
Protein samples from all fractions were subjected to SDS-PAGE using BioRad TGX (4–15%) gels and transferred to PVDF membranes (Life Technologies, Carlsbad, CA). Membranes were probed with GABAA receptor α1 (Novus, Littleton, CO), α4 (Abcam, Cambridge, MA), γ2 (Novus), δ (Novus), AKAP150 (Santa Cruz, Dallas, TX), and gephyrin (BD Biosciences, San Jose, CA) antibodies. Blots were then exposed to an antibody for -actin (Millipore, Billerica, MA) for normalization. Proteins were detected with enhanced chemiluminescence (GE Healthcare, Amersham, UK). Blots were visualized digitally using GE LAS-4000 and analyzed using Image Quant software. Comparisons were made within blots. Data were analyzed using Student’s t-test or 2-way ANOVA.
Results
RIIβ(−/−) mice show unaltered basal cortical levels of PKA RIIα and GABAA subunits, but deficits in AKAP150
Brains were collected from untreated animals to determine if there is altered constitutive regulation of membrane levels of GABAA subunits and proteins regulating GABAA receptors. As expected, RIIβ−/− mice completely lacked membrane PKA RIIβ (Fig. 1A). Further, membrane levels of PKA RIIα were unaltered relative to RIIβ+/+ littermates (Fig. 1B), suggesting that there is not a compensatory increase in RIIα. Interestingly, RIIβ−/− mice showed consistently lower membrane levels of the kinase anchoring protein AKAP150 compared to RIIβ+/+ littermates (Fig. 1C, p<0.01, Student’s t test). Finally, basal cortical P2 fraction levels of the GABAA α1 and α4 subunits were similar in knockout animals and wild type littermates (Fig. 2).
Figure 1.
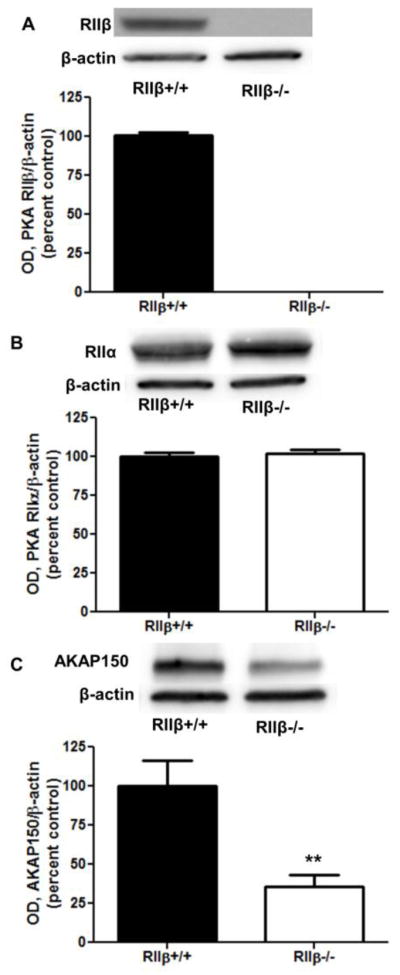
Comparison of baseline membrane levels of PKA-associated regulatory proteins. P2 fraction levels of PKA proteins were measured in RIIβ+/+ and RIIβ−/− mice. (A) RIIβ was absent in the knockout mice, and (B) there was no compensatory increase in RIIα. (C) Analysis of AKAP150 revealed membrane deficits in RIIβ−/− mice. ** p<0.01, Student’s t test, n=8 per group.
Figure 2.
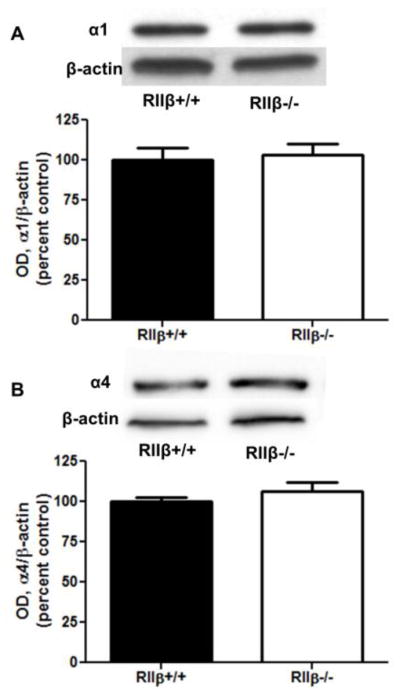
Baseline membrane levels of GABAA subunits are unaltered in RIIβ−/− mice. P2 fraction levels of GABAA (A) α1 and (B) α4 were not different in untreated RIIβ+/+ and RIIβ−/− mice. n=8 per group.
RIIβ(−/−) mice exhibit ethanol-induced deficits GABAA α1 subunit levels in the P2 fraction
Cortical levels of GABAA subunits were analyzed 1 h after IP injection of 3.5 g/kg ethanol or saline. P2 fraction levels of GABAA α1 subunits were decreased in ethanol-treated mice [two-way ANOVA main effect of treatment, F(1,30)=7.164, p<0.05], which was driven by decreases in RIIβ−/− mice (Fig. 3A, Bonferroni post test, p<0.01). GABAA α4 subunit and AKAP150, however, were unaltered in the P2 fraction following ethanol injection in both RIIβ−/− and RIIβ+/+ mice (Fig. 3B and 3C). Consistent with baseline data, AKAP150 levels were lower in RIIβ−/− mice [main effect of genotype, F(1,28)=15.77, p<0.001].
Figure 3.
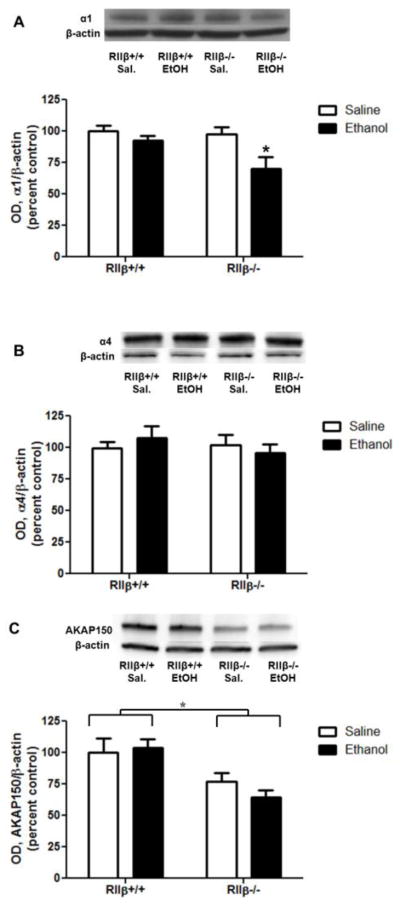
RIIβ−/− mice exhibit ethanol-induced decreases in GABAA α1 subunits. Mice were injected with 3.5 g/kg ethanol or saline for 1 h. P2 fraction levels of (A) GABAA α1 subunits were decreased by ethanol, due to significant differences in RIIβ−/− mice [two-way ANOVA main effect of treatment, F(1,30)=7.164, p<0.05]. (B) GABAA α4 subunits were unaffected by acute ethanol exposure in both RIIβ+/+ and RIIβ−/− mice. *p<0.05, Bonferroni post test, n=7–9 per group.
Synaptic and extrasynaptic GABAA receptors are differentially regulated by PKA following ethanol injection
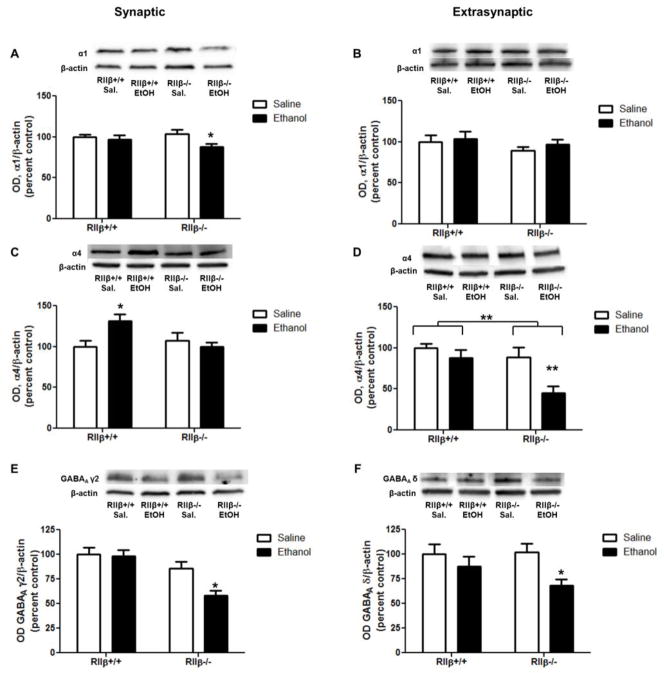
We further purified the P2 fraction into synaptic and extrasynaptic fractions to assess the potential for site-specific changes in GABAA receptor subunits. We assessed markers of synaptic and extrasynaptic GABAA receptors to confirm enrichment of these proteins in the refined fractions. The synaptic proteins gephyrin and GABAA γ2 were enriched in the synaptic fraction relative to the extrasynaptic and P2 fractions, while the extrasynaptic GABAA δ was enriched in extrasynaptic fractions relative to synaptic and P2 (Fig. 4). Similar to our findings in the P2 fraction, overall synaptic GABAA α1 subunit levels were decreased by ethanol, which was driven by decreases in RIIβ−/− mice [Fig. 5A, two-way ANOVA main effect of treatment, F(1,30)=4.601, p<0.05, Bonferroni post test, p<0.05 RIIβ−/− saline vs ethanol]. GABAA α1 subunits in the extrasynaptic fraction, however, were unaffected in both genotypes (Fig. 5B).
Figure 4.
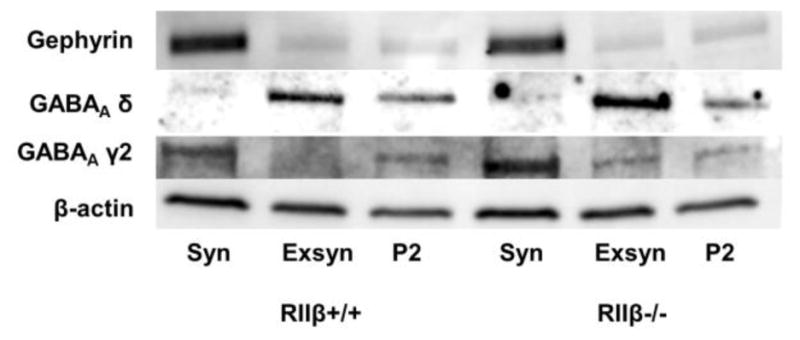
Characterization of synaptic and extrasynaptic markers of GABAergic synapses. Representative blots for samples with equal total protein (20 μg) for the synaptic (Syn), extrasynaptic (Exsyn), and P2 fractions for both RIIβ+/+ and RIIβ−/− genotypes are shown. Blots were probed with antibodies targeted to the synaptic proteins gephyrin and GABAA γ2 and extrasynaptic protein GABAA δ.
Figure 5.
Synaptic and extrasynaptic GABAA subunits are differentially regulated by ethanol and PKA. (A) Synaptic GABAA α1 subunits were decreased by ethanol in RIIβ−/− mice, while (B) extrasynaptic GABAA α1 subunits were unaffected in both genotypes.(C) Synaptic GABAA α4 subunits increased after ethanol in RIIβ+/+ mice, but did not change in RIIβ−/− animals. (D) Extrasynaptic GABAA α4 subunits decreased following ethanol in RIIβ−/− mice, but not wild type littermates. (E) Synaptic GABAA γ2 and (F) extrasynaptic GABAA δ decreased in RIIβ−/− mice, but not wild type littermates. *p<0.05, **p<0.01, n=6–9 per group.
Synaptic α4 subunits increased following ethanol injection in RIIβ+/+ mice, but were unaffected in the RIIβ−/− mice [Fig. 5C, two-way ANOVA interaction, F(1,31)=5.739, p<0.05, Bonferroni post test, p<0.05 RIIβ+/+ saline vs ethanol]. Conversely, extrasynaptic α4 subunit levels were unaffected in RIIβ+/+ mice, but decreased in RIIβ−/− mice after acute ethanol [Fig. 5D, two-way ANOVA, main effect of treatment, F(1,27)=8.938, p<0.01, main effect of treatment, F(1,27)=8.697, p<0.01, Bonferroni post test, p<0.01 RIIβ−/− saline vs ethanol].
There was a significant effect of ethanol treatment [two-way ANOVA, F(1,19)=5.415, p<0.05] and genotype [two-way ANOVA, F(1,19)=18.33, p<0.001] on the synaptic GABAA γ2 subunit, that was driven by decreases in the RIIβ−/− mice (Bonferroni posttest, p<0.05). There was also an effect of ethanol treatment [two-way ANOVA, F(1,26)=6.796, p<0.05] on the extrasynaptic GABAA δ subunit, that was similarly driven by decreases in RIIβ−/− mice (Bonferroni posttest, p<0.05).
Synaptic AKAP150 increased in response to ethanol in RIIβ+/+, but not RIIβ−/−, mice [Fig. 6A, two-way ANOVA interaction, F(1,27)=14.75, p<0.001, main effect of genotype, F(1,27)=164.3, p<0.0001, Bonferroni post test, p<0.05 RIIβ−/− saline vs ethanol]. Extrasynaptic AKAP150 was unaffected by ethanol in both genotypes; however, there was significantly less in the RIIβ−/− mice [Fig. 6B, main effect of genotype, F(1,29)=16.03, p<0.001].
Figure 6.
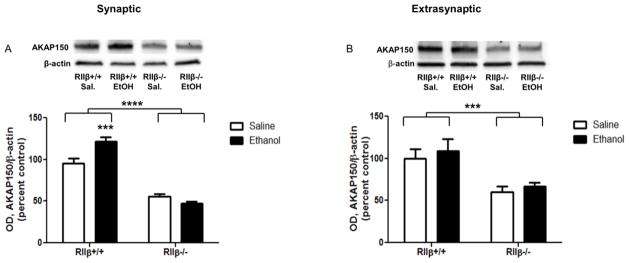
Synaptic and extrasynaptic AKAP150 are differentially regulated by ethanol and PKA. (A) Synaptic AKAP150 increased following ethanol in RIIβ+/+ mice, and (B) extrasynaptic AKAP150 was unaffected by ethanol in both genotypes. ***p<0.001, ****p<0.00001, n=7–9 per group.
Membrane proteins return to baseline 46 h after ethanol injection
As Liang et al. (2007) found contrasting effects 1 and 48 h after ethanol exposure in rats, we assessed membrane proteins 46 h after IP injection. The earlier time point was chosen to account for the faster metabolism of mice [25]. All membrane proteins in the ethanol-treated animals had returned to saline-treated levels by 46 h post-injection (Table 1).
Table 1.
Summary of GABAA receptor subunit and AKAP150 levels 46 h after ethanol injection.
| Subunit | RIIβ+/+ Saline | RIIβ+/+ Ethanol | RIIβ−/− Saline | RIIβ−/− Ethanol |
|---|---|---|---|---|
| P2 Fraction | ||||
| GABAA α1 | 100 ± 3.7 | 104.8 ± 4.8 | 106.9 ± 3.9 | 104.3 ± 5.6 |
| GABAA α4 | 100 ± 4.6 | 93.6 ± 6.4 | 109.7 ± 2.5 | 111.9 ± 6.1 |
| AKAP150 | 100 ± 8.4 | 93.8 ± 4.0 | ***60.8 ± 6.1 | ***52.9 ± 4.6 |
| Synaptic Fraction | ||||
| GABAA α1 | 100 ± 6.8 | 110.9 ± 5.2 | 109.3 ± 5.6 | 109.9 ± 7.9 |
| GABAA α4 | 100 ± 8.5 | 95.3 ± 10.3 | 106.4 ± 11.9 | 110.7 ± 17.5 |
| AKAP150 | 100 ± 9.6 | 100.6 ± 8.3 | ***66.2 ± 9.2 | ***59.4 ± 7.2 |
| Extrasynaptic Fraction | ||||
| GABAA α1 | 100 ± 6.6 | 96.1 ± 3.0 | 87.9 ± 5.0 | 100.4 ± 8.5 |
| GABAA α4 | 100 ± 7.3 | 98.2 ± 7.9 | 102.5 ± 9.1 | 107.3 ± 6.2 |
| AKAP150 | 100 ± 9.6 | 85.2 ± 8.0 | **64.4 ± 5.7 | **65.0 ± 5.9 |
p<0.01,
p<0.001, two-way ANOVA main effect of genotype
Knockout of PKA RIIβ is protective against bicuculline-induced seizure
Mice were tested for bicuculline-induced seizure threshold at baseline and 1 h after 3.5 g/kg ethanol injection. There was an overall effect of ethanol administration, indicating that ethanol was protective against seizure [Fig. 7; two-way ANOVA main effect of treatment, F(1,31)=5.512, p<0.05], as determined by an increase in the seizure threshold in mice injected with ethanol. Bonferroni post test revealed a significant effect of ethanol in RIIβ−/− mice (p<0.05), but not in RIIβ+/+, indicating a greater protective effect of ethanol in these mice. Additionally, there was an effect of genotype, with the RIIβ−/− having a higher overall seizure threshold compared to RIIβ+/+ littermates [two-way ANOVA main effect of genotype, F(1,31)=6.155, p<0.05].
Figure 7.
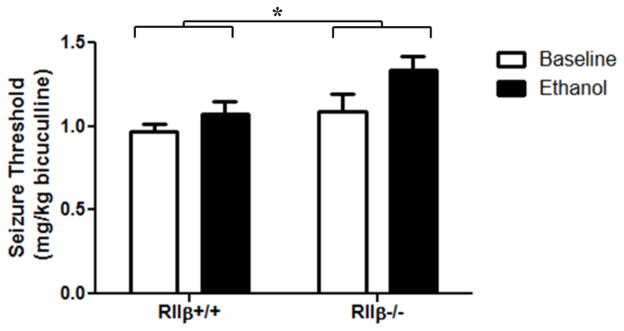
RIIβ knockout is protective against bicuculline-induced seizure. Bicuculline seizure threshold was measured at baseline and at 1 h following 3.5 g/kg ethanol. Two-way ANOVA revealed a main effect of both treatment [F(1,31)=5.512, p<0.05] and genotype [F(1,31)=6.155, p<0.05]. Ethanol was more protective against seizure in RIIβ−/− mice (Bonferroni post test, p<0.05). n= 6–14 per group.
Discussion
Our results further establish a role for PKA in modulating the effects of ethanol on GABAA receptors. Null mutation of RIIβ produced vulnerability to ethanol-induced decreases in synaptic GABAA α1 and extrasynaptic α4 subunits, but prevented increases in synaptic α4 subunits by ethanol. Surprisingly, RIIβ knockout was protective against bicuculline-induced seizure and enhanced the seizure-protective effects of ethanol. The vulnerability to deficits in synaptic GABAA α1 subunits in RIIβ−/− mice is consistent with our previous findings in vitro [17]. Pharmacological inhibition of PKA during ethanol exposure in cerebral cortical neurons produced decreases in biochemical and electrophysiological indices of synaptic α1 receptors. Our similar findings in null mutation mice reported here further supports the hypothesis that PKA may act as an endogenous protective mechanism against pathological adaptations. The observation that PKA activation is protective in two different model species would suggest that this likely represents a conserved mechanism. Further, whereas our in vitro studies using pharmacological inhibitors of PKA were not specific to different regulatory subunits, the present study clearly establishes a role for the RIIβ subunit in particular in regulating GABAergic adaptations.
The effects on synaptic α1 and extrasynaptic α4 subunits are corroborated by the changes in the synaptic γ2 and extrasynaptic δ subunits, respectively. The data suggest that it is likely synaptic α1βγ2 and extrasynaptic α4βδ receptors are both downregulated in the RIIβ−/− mice. Interestingly, effects on the synaptic α1 subunit were apparent in both the P2 and synaptic fraction, while effects on α4 subunits were apparently absent in the P2 and only uncovered in the more refined subcellular fractions. This would suggest that the synaptic α1 subunit makes up a greater overall fraction of total protein in the P2 fraction, whereas α4 subunits may be more evenly divided between the synaptic and extrasynaptic fractions.
The RIIβ subunit appears to have differential effects in regulating trafficking of synaptic vs. extrasynaptic GABAA α4 receptors. Genetic knockout of RIIβ produced vulnerability to ethanol-induced deficits in extrasynaptic α4 and subunit expression, believed to be responsible for conducting tonic currents in the presence of relatively low concentrations of GABA. Conversely, the absence of RIIβ prevented ethanol-induced increases in synaptic α4 subunits. Together these results indicate an overall deficit in GABAergic inhibition in the RIIβ−/− mice following acute ethanol. This would suggest that PKA is protective against reduced GABAA signaling, one of the major pathologies associated with chronic alcohol exposure [1],. The observations of decreased extrasynaptic α4 receptors and increased synaptic α4 subunits following ethanol exposure have been well-established in the hippocampus [2, 3, 26]. Our results suggest that these same processes likely occur in the cortex and are largely mediated by activation of PKA. Further, we previously found opposing effects of activation of PKA and PKC by ethanol in regulating GABAA α1 subunit trafficking [17]. Activation of PKC by ethanol leads to increases in synaptic α4 and a trend towards decreases in extrasynaptic α4 receptors in rat cerebral cortical neurons [13]. The decreased extrasynaptic α4 and subunits observed in knockout animals in the current study suggests that PKA activation similarly may oppose PKC-induced decreases in mice. Conversely, the absence of increased synaptic α4 subunits in knockout animals, which occurred in wild-type animals, would indicate that PKA actually facilitates this adaptation in mice. While these data would suggest a role for the RIIβ subunit in particular in mediating the actions of ethanol, as there was no compensatory increase in RIIα expression, we cannot discount potential altered expression of other regulatory subunits, such as RIα [27], or altered activity of the catalytic subunits Cα and β.
Interestingly, whereas GABAA receptor adaptations had returned to baseline levels 46 h after ethanol injection, previous studies have found further alterations up to 48 h following ethanol exposure [6]. It is possible that the different findings are due to differences in dose and administration methods (3.5 g/kg IP in the present study versus 5.0 g/kg gavage in Liang et al., 2007). Previous studies, however, have found relatively similar times to ethanol clearance (~450 min) following mouse IP and rat IG administration [25]. Thus, this discrepancy may represent a fundamental difference in ethanol-induced GABA regulation either between brain regions of interest (hippocampus versus cortex) or between model systems (Sprague-Dawley rats versus C57BL/6J mice).
The observed deficit in AKAP150 levels in RIIβ−/− was surprising given that previous reports did not find altered levels of this anchoring protein in RIIβ null mutation animals (“unpublished data” cited in [28]) and that there is not a difference in basal kinase activity in RIIβ−/− mice [21]. This result was extremely consistent, however, across samples and experiments (Figs. 1C, 3C, 4E and F, Table 1). The discrepancy between our results and previous studies may be due to different genetic backgrounds (~100% C57BL/6J in the present study versus 50% C57BL/6 and 50% 129SvJ in Brandon et al.) or to different brain regions of study (cortex in the present study versus striatum in Brandon et al.). Regardless, this finding is likely functionally relevant in interpreting our results. A kinase anchoring proteins are believed to act as important regulators of PKA signaling via subcellular localization [19]. The data suggest, however, that the reverse may be true in that PKA may play an active role in the regulation of AKAP79/150 or in directing AKAP79/150 to the plasma membrane. Previous studies have established that AKAP150 is critical in mediating PKA interactions with GABA receptors [29]. Further, the observation that ethanol increased expression of AKAP150 in wild type mice is consistent with the ability of ethanol to increase PKA expression [17]. Thus, it is unclear if the alterations in GABA receptor trafficking during ethanol exposure were due to knockout of RIIβ, to deficits in membrane levels of AKAP150, or some combination thereof. Studies utilizing animals with selective knockout of AKAP150 could resolve this question.
The finding that knockout of the RIIβ subunits is protective against bicuculline-induced seizures was unexpected. Particularly, it was surprising that ethanol had a greater protective effect in the RIIβ−/− mice as deletion of RIIβ reduces the sedative effect of ethanol [21], and given that we only found deficits in GABAA subunits following ethanol exposure in these mice (Figs. 2A, 3A and D). It is possible that other GABAA receptor subunits that we did not study may be upregulated while synaptic α1 and extrasynaptic α4 are downregulated. PKA is also known to regulate other receptors that modulate seizure susceptibility, including AMPA and NMDA receptors [30–32]. Further, it may be that other factors, such as differential neurosteroid regulation, are altering the sensitivity of these animals to bicuculline [33] as kinase phosphorylation is known to alter GABAA receptor sensitivity to neurosteroid modulation [34, 35]. Analysis of baseline and ethanol-induced neurosteroid levels and neurosteroid modulation of GABAA receptors in these mice could be informative.
Together the results further extend our understanding of the functional relevance of the PKA pathway in regulating GABA receptor trafficking following ethanol exposure. Overall, RIIβ was found to be protective against reductions in membrane levels of GABAA subunits produced by ethanol; however, RIIβ appears to increase susceptibility to bicuculline-induced seizure through an as yet unknown mechanism. The data underscore the mechanistic potential for the PKA pathway in the treatment of pathological GABAergic adaptations associated with alcohol use disorders.
Acknowledgments
We would like to thank J. Peyton Bohnsack for helpful suggestions. This work was supported by National Institute of Health grants AA011605, AA013573, AA015148, and AA007573.
References
- 1.Kumar S, Porcu P, Werner DF, Matthews DB, Diaz-Granados JL, Helfand RS, Morrow AL. The role of GABAA receptors in the acute and chronic effects of ethanol: a decade of progress. Psychopharmacology (Berl) 2009;205:529–564. doi: 10.1007/s00213-009-1562-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cagetti E, Liang J, Spigelman I, Olsen RW. Withdrawal from chronic intermittent ethanol treatment changes subunit composition, reduces synaptic function, and decreases behavioral responses to positive allosteric modulators of GABAA receptors. Mol Pharmacol. 2003;63:53–64. doi: 10.1124/mol.63.1.53. [DOI] [PubMed] [Google Scholar]
- 3.Liang J, Cagetti E, Olsen RW, Spigelman I. Altered pharmacology of synaptic and extrasynaptic GABAA receptors on CA1 hippocampal neurons is consistent with subunit changes in a model of alcohol withdrawal and dependence. J Pharmacol Exp Ther. 2004;310:1234–1245. doi: 10.1124/jpet.104.067983. [DOI] [PubMed] [Google Scholar]
- 4.Kralic JE, Korpi ER, O’Buckley TK, Homanics GE, Morrow AL. Molecular and pharmacological characterization of GABAA receptor α1 subunit knockout mice. J Pharmacol Exp Ther. 2002;302:1037–1045. doi: 10.1124/jpet.102.036665. [DOI] [PubMed] [Google Scholar]
- 5.Kralic JE, O’Buckley TK, Khisti RT, Hodge CW, Homanics GE, Morrow AL. GABAA receptor α1 subunit deletion alters receptor subtype assembly, pharmacological and behavioral responses to benzodiazepines and zolpidem. Neuropharmacology. 2002;43:685–694. doi: 10.1016/s0028-3908(02)00174-0. [DOI] [PubMed] [Google Scholar]
- 6.Liang J, Suryanarayanan A, Abriam A, Snyder B, Olsen RW, Spigelman I. Mechanisms of reversible GABAA receptor plasticity after ethanol intoxication. J Neurosci. 2007;27:12367–12377. doi: 10.1523/JNEUROSCI.2786-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olsen RW, Sieghart W. GABAA receptors: Subtypes provide diversity of function and pharmacology. Neuropharmacology. 2009;56:141–148. doi: 10.1016/j.neuropharm.2008.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABAA receptors. Nat Rev Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- 9.Belelli D, Harrison NL, Maguire J, Macdonald RL, Walker MC, Cope DW. Extrasynaptic GABAA receptors: form, pharmacology, and function. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009;29:12757–12763. doi: 10.1523/JNEUROSCI.3340-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herman MA, Contet C, Justice NJ, Vale W, Roberto M. Novel subunit-specific tonic GABA currents and differential effects of ethanol in the central amygdala of CRF receptor-1 reporter mice. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2013;33:3284–3298. doi: 10.1523/JNEUROSCI.2490-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glykys J, Peng Z, Chandra D, Homanics GE, Houser CR, Mody I. A new naturally occurring GABAA receptor subunit partnership with high sensitivity to ethanol. Nat Neurosci. 2007;10:40–48. doi: 10.1038/nn1813. [DOI] [PubMed] [Google Scholar]
- 12.Jia F, Pignataro L, Harrison NL. GABAA receptors in the thalamus: alpha4 subunit expression and alcohol sensitivity. Alcohol. 2007;41:177–185. doi: 10.1016/j.alcohol.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 13.Werner DF, Kumar S, Criswell HE, Suryanarayanan A, Fetzer JA, Comerford CE, Morrow AL. PKCgamma is required for ethanol-induced increases in GABA(A) receptor alpha4 subunit expression in cultured cerebral cortical neurons. J Neurochem. 2011;116:554–563. doi: 10.1111/j.1471-4159.2010.07140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liang J, Suryanarayanan A, Chandra D, Homanics GE, Olsen RW, Spigelman I. Functional consequences of GABAA receptor α4 subunit deletion on synaptic and extrasynaptic currents in mouse dentate granule cells. Alcohol Clin Exp Res. 2008;32:19–26. doi: 10.1111/j.1530-0277.2007.00564.x. [DOI] [PubMed] [Google Scholar]
- 15.Pignataro L, Varodayan FP, Tannenholz LE, Harrison NL. The regulation of neuronal gene expression by alcohol. Pharmacology & Therapeutics. 2009;124:324–335. doi: 10.1016/j.pharmthera.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumar S, Ren Q, Beckley JH, O’Buckley TK, Gigante ED, Santerre JL, Werner DF, Morrow AL. Ethanol activation of protein kinase A regulates GABA(A) receptor subunit expression in the cerebral cortex and contributes to ethanol-induced hypnosis. Front Neurosci. 2012;6:44. doi: 10.3389/fnins.2012.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carlson SL, Kumar S, Werner DF, Comerford CE, Morrow AL. Ethanol activation of PKA regulates GABAA alpha1 receptor function and trafficking in cultured cerebral cortical neurons. J Pharmacol Exp Ther. 2013 doi: 10.1124/jpet.112.201954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumar S, Suryanarayanan A, Boyd KN, Comerford CE, Lai MA, Ren Q, Morrow AL. Ethanol reduces GABAA alpha1 subunit receptor surface expression by a protein kinase Cgamma-dependent mechanism in cultured cerebral cortical neurons. Mol Pharmacol. 2010;77:793–803. doi: 10.1124/mol.109.063016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dell’Acqua ML, Smith KE, Gorski JA, Horne EA, Gibson ES, Gomez LL. Regulation of neuronal PKA signaling through AKAP targeting dynamics. Eur J Cell Biol. 2006;85:627–633. doi: 10.1016/j.ejcb.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 20.Wong W, Scott JD. AKAP signalling complexes: focal points in space and time. Nat Rev Mol Cell Biol. 2004;5:959–970. doi: 10.1038/nrm1527. [DOI] [PubMed] [Google Scholar]
- 21.Thiele TE, Willis B, Stadler J, Reynolds JG, Bernstein IL, McKnight GS. High ethanol consumption and low sensitivity to ethanol-induced sedation in protein kinase A-mutant mice. J Neurosci. 2000;20:RC75. doi: 10.1523/JNEUROSCI.20-10-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fee JR, Sparta DR, Knapp DJ, Breese GR, Picker MJ, Thiele TE. Predictors of high ethanol consumption in RIIbeta knock-out mice: assessment of anxiety and ethanol-induced sedation. Alcohol Clin Exp Res. 2004;28:1459–1468. doi: 10.1097/01.ALC.0000141809.53115.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferraro FM, 3rd, Sparta DR, Knapp DJ, Breese GR, Thiele TE. Increased consumption but not operant self-administration of ethanol in mice lacking the RIIbeta subunit of protein kinase A. Alcohol Clin Exp Res. 2006;30:825–835. doi: 10.1111/j.1530-0277.2006.00096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goebel-Goody SM, Davies KD, Alvestad Linger RM, Freund RK, Browning MD. Phospho-regulation of synaptic and extrasynaptic N-methyl-d-aspartate receptors in adult hippocampal slices. Neuroscience. 2009;158:1446–1459. doi: 10.1016/j.neuroscience.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 25.Livy DJ, Parnell SE, West JR. Blood ethanol concentration profiles: a comparison between rats and mice. Alcohol. 2003;29:165–171. doi: 10.1016/s0741-8329(03)00025-9. [DOI] [PubMed] [Google Scholar]
- 26.Liang J, Zhang N, Cagetti E, Houser CR, Olsen RW, Spigelman I. Chronic intermittent ethanol-induced switch of ethanol actions from extrasynaptic to synaptic hippocampal GABAA receptors. J Neurosci. 2006;26:1749–1758. doi: 10.1523/JNEUROSCI.4702-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amieux PS, McKnight GS. The essential role of RI alpha in the maintenance of regulated PKA activity. Annals of the New York Academy of Sciences. 2002;968:75–95. doi: 10.1111/j.1749-6632.2002.tb04328.x. [DOI] [PubMed] [Google Scholar]
- 28.Brandon EP, Logue SF, Adams MR, Qi M, Sullivan SP, Matsumoto AM, Dorsa DM, Wehner JM, McKnight GS, Idzerda RL. Defective motor behavior and neural gene expression in RIIbeta-protein kinase A mutant mice. J Neurosci. 1998;18:3639–3649. doi: 10.1523/JNEUROSCI.18-10-03639.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brandon NJ, Jovanovic JN, Colledge M, Kittler JT, Brandon JM, Scott JD, Moss SJ. A-kinase anchoring protein 79/150 facilitates the phosphorylation of GABA(A) receptors by cAMP-dependent protein kinase via selective interaction with receptor beta subunits. Mol Cell Neurosci. 2003;22:87–97. doi: 10.1016/s1044-7431(02)00017-9. [DOI] [PubMed] [Google Scholar]
- 30.Skeberdis VA, Chevaleyre V, Lau CG, Goldberg JH, Pettit DL, Suadicani SO, Lin Y, Bennett MV, Yuste R, Castillo PE, Zukin RS. Protein kinase A regulates calcium permeability of NMDA receptors. Nature Neuroscience. 2006;9:501–510. doi: 10.1038/nn1664. [DOI] [PubMed] [Google Scholar]
- 31.Man HY, Sekine-Aizawa Y, Huganir RL. Regulation of {alpha}-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor trafficking through PKA phosphorylation of the Glu receptor 1 subunit. Proc Natl Acad Sci U S A. 2007;104:3579–3584. doi: 10.1073/pnas.0611698104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang Y, Takeuchi K, Rodenas-Ruano A, Takayasu Y, Bennett MV, Zukin RS. Developmental switch in requirement for PKA RIIbeta in NMDA-receptor-dependent synaptic plasticity at Schaffer collateral to CA1 pyramidal cell synapses. Neuropharmacology. 2009;56:56–65. doi: 10.1016/j.neuropharm.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Devaud LL, Purdy RH, Morrow AL. The neurosteroid, 3 alpha-hydroxy-5 alpha-pregnan-20-one, protects against bicuculline-induced seizures during ethanol withdrawal in rats. Alcohol Clin Exp Res. 1995;19:350–355. doi: 10.1111/j.1530-0277.1995.tb01514.x. [DOI] [PubMed] [Google Scholar]
- 34.Vergnano AM, Schlichter R, Poisbeau P. PKC activation sets an upper limit to the functional plasticity of GABAergic transmission induced by endogenous neurosteroids. The European journal of neuroscience. 2007;26:1173–1182. doi: 10.1111/j.1460-9568.2007.05746.x. [DOI] [PubMed] [Google Scholar]
- 35.Harney SC, Frenguelli BG, Lambert JJ. Phosphorylation influences neurosteroid modulation of synaptic GABAA receptors in rat CA1 and dentate gyrus neurones. Neuropharmacology. 2003;45:873–883. doi: 10.1016/s0028-3908(03)00251-x. [DOI] [PubMed] [Google Scholar]