Abstract
Rationale
Fluid shear stress (FSS) differentially regulates endothelial cell (EC) stress fiber formation with decreased stress fibers in areas of disturbed-flow (d-flow) compared to steady-flow (s-flow) areas. Importantly, stress fibers are critical for several EC functions including cell shape, mechano-signal transduction, and EC cell-cell junction integrity. A key mediator of s-flow induced stress fiber formation is Src, which regulates downstream signaling mediators such as phosphorylation of cortactin, activity of focal adhesion kinase and small GTPases.
Objective
Previously we showed that thioredoxin-interacting protein (TXNIP, also VDUP1 and TBP-2) was regulated by FSS; TXNIP expression was increased in d-flow compared to s-flow areas. While TXNIP was originally characterized for its role in redox and metabolic cellular functions, recent reports show important scaffold functions related to its α-arrestin structure. Based on these findings, we hypothesized that TXNIP acts as a biomechanical sensor that regulates Src kinase activity and stress fiber formation.
Methods and Results
Using en face immunohistochemistry of the aorta and cultured EC, we show inverse relationship between TXNIP expression and Src activity. Specifically, s-flow increased Src activity and stress fiber formation, while it decreased TXNIP expression. In contrast, d-flow had opposite effects. We studied the role of TXNIP in regulating SHP2 plasma membrane localization and VE-cadherin binding, because SHP2 indirectly regulates dephosphorylation of Src tyrosine 527 that inhibits Src activity. Using immunohistochemistry and immunoprecipitation we found that TXNIP prevented SHP2-VE-cadherin interaction.
Conclusion
In summary, these data characterize a FSS mediated mechanism for stress fiber formation that involves a TXNIP-dependent VE-cadherin-SHP2-Src pathway.
Keywords: Fluid shear stress, TXNIP, endothelial cell, Src, stress fibers, protein
INTRODUCTION
Although not fully understood, the mechanism by which blood flow transduces a signal into endothelial cells (EC) is dependent on the frictional force generated by blood flow, known as fluid shear stress (FSS). Changes in blood flow patterns are a well-established regulator of EC morphology, function and homeostasis. Specifically, regions that are exposed to steady blood flow (s-flow; e.g. the greater curvature of the aortic arch), are known to be atherosclerosis-protected while disturbed blood flow regions (d-flow; e.g. the lesser curvature of the aortic arch or flow divider regions) are atherosclerosis-prone 1–3.
Physiologically, s-flow regions are characterized by unidirectional high FSS force (>10 dyn/cm2) while d-flow regions are characterized with non-unidirectional, non-steady flow patterns and low shear stress force (<5 dyn/cm2) 4–6. Mechanistically, s-flow regions are atherosclerosis-protected mainly due to increases in anti-inflammatory and anti-oxidative stress protein expression, including Kruppel-like factor 2, endothelial nitric oxide synthase, thioredoxin and unidirectional stress fiber formation 7–9. In contrast, d-flow regions are atherosclerosis-prone due to endothelial dysfunction that is characterized by an inhibition of anti-inflammatory factors, increased expression of adhesion molecules, increase in reactive oxygen species generation and reduced stress fibers number 10–12. As a result of EC dysfunction, circulating free leukocytes are recruited to and bind at the site of injury, transmigrate through the endothelium and induce chronic inflammation to promote atherogenesis.
Importantly, FSS regulates the activation of multiple plasma membrane (PM) tyrosine kinase receptors, including vascular endothelial growth factor receptor 2 (VEGFR2), Tie1 and platelet-derived growth factor receptor. Consequently, Src family kinases are activated, promoting phosphorylation of PM scaffolds such as platelet endothelial cell adhesion molecule 1 and vascular endothelial cadherin (VE-cadherin) 13–17. These pathways are important for multiple endothelial functions, such as vasodilation, inflammation, angiogenesis and migration. Many of these events require formation and remodeling of stress fibers, which are specialized actin structures in EC 18–20.
In particular, VE-cadherin is a trans-membrane receptor that acts as a FSS-sensing molecule to regulate endothelial stress fiber formation by two mechanisms: first, by increased phosphorylation and transduction of extracellular signals into the cytoplasm; and second, by acting as a docking site for adapter proteins and phosphatases 21,22. VE-cadherin interacts with Src homology phosphatase-2 (SHP2) and Src to regulate EC stress fiber formation 23,24. For example, in response to vascular endothelial growth factor, the endothelial receptor VEGFR2 is activated, leading to three consequent steps: recruitment of SHP2 to the VE-cadherin complex followed by activation of Src kinase protein, and changes in stress fiber dynamics 25,26.
We recently showed that thioredoxin-interacting protein (TXNIP) is highly regulated by changes in blood flow. In d-flow regions TXNIP is highly induced and promotes endothelial inflammation and apoptosis, while in s-flow regions TXNIP expression is suppressed 27. Furthermore, TXNIP is an α-arrestin protein, which has an important function in cellular signaling, acting as a scaffold protein, to regulate formation of signaling complexes and subcellular specific signaling events 28–31. Specifically, TXNIP is important for activation of VEGFR2 at the PM and downstream signaling, which is important for Src activation 13. Based on our recent TXNIP structure-function analyses 32, we propose that in response to FSS, TXNIP regulates Src activation via regulation of VE-cadherin-SHP2-Src complex, resulting in altered EC stress fibers.
METHODS
Animals
All animal experiments were conducted in accordance with experimental protocols that were approved by the University Committee on Animal Resources of the University of Rochester. Animals were maintained under pathogen-free conditions at the Aab Cardiovascular Research Institute of the University of Rochester.
En face staining
Immunofluorescence staining of mouse aortic EC was performed using 24-week-old control or TXNIP knock-out (KO) mice. Mice were anesthetized with ketamine/xylazine cocktail (0.13/0.0088 mg/g body weight), the jugular vein was cut and the arterial tree was perfused with saline containing 40 USPU/ml heparin from left ventricle for 5 min, followed by perfusion of pre-chilled 4% paraformaldehyde in PBS (pH 7.4) for 10 min. Subsequently, the whole aorta was dissected from iliac bifurcation to the heart, cut open longitudinally, permeabilized with 0.1% Triton X-100 in PBS for 10 min and blocked with 10% normal goat serum in Tris-buffered saline (TBS) containing 2.5% Tween-20 for 1 hour at room temperature. Next, aortas were incubated with primary antibody in blocking buffer overnight at 4°c. After rinsing with washing solution (TBS containing 2.5% Tween-20) 3 times, fluorescence-conjugated secondary antibodies were applied for 1 hour at room temperature. Finally, after another 3 rinses in the washing solution, aortas were mounted in the ProLong antifade reagent (Invitrogen, Eugene, OR). Aortas were examined by a laser-scanning confocal microscope (FV-1000 mounted on IX81, Olympus) with UPlanSApo 20× or UPlanFL N 40× lens.
Cell culture, western blot and siRNA transfection
Human umbilical vein endothelial cell (HUVEC) or bovine aortic endothelial cell (BAEC) were grown and transfected with plasmid/siRNA as detailed in the online-only data and previously described 33.
In vitro flow experiments
To perform biochemical studies, we used a cone-and-plate flow apparatus and HUVEC cultured in 60-mm dishes were exposed to s-flow at 12 dyn/cm2 by using smooth cones (120 rpm), or exposed to disturbed flow at 2 dyn/cm2 by using grooved cones (5 rpm) for 24 hours. The shear stress imposed on the surface of the plate was calculated by the formula ωμ/θ where ω is the rotation speed, μ is the fluid viscosity, and θ is the angle of the cone.
Immunofluorescence
Treated HUVEC in 35mm-dishes were fixed in 4% paraformaldehyde in PBS (pH 7.4) for 15 min, permeabilized with 0.1% Triton X-100 in PBS for 5 min, and blocked with 10% normal goat serum in PBS containing 0.5% Tween-20 for 1 hour at room temperature. Then cells were stained with primary antibodies overnight at 4°c in blocking solution. Cells were then rinsed with 0.5% Tween-20 in PBS 3 times and incubated with the fluorescence-conjugated secondary antibodies for 1 hour at room temperature. After another 3 rinses with the washing solution, images were acquired using an inverted epi-fluorescence microscope (IX50, Olympus) equipped with a charge-coupled device camera (Spot; Diagnostic Instruments, Inc.) with Cropland water 40× (N.A. 0.8) or 60× (N.A. 0.9) objective lens or laser-scanning confocal microscope (FV-1000 mounted on IX81, Olympus) with UPlanSApo 20× or UPlanFL N 40× lens.
Statistical analysis
Group differences were analyzed using the standard Student t test. All values are expressed as mean ± SE. P<0.05 was considered statistically significant.
RESULTS
TXNIP regulates src phosphorylation in vivo
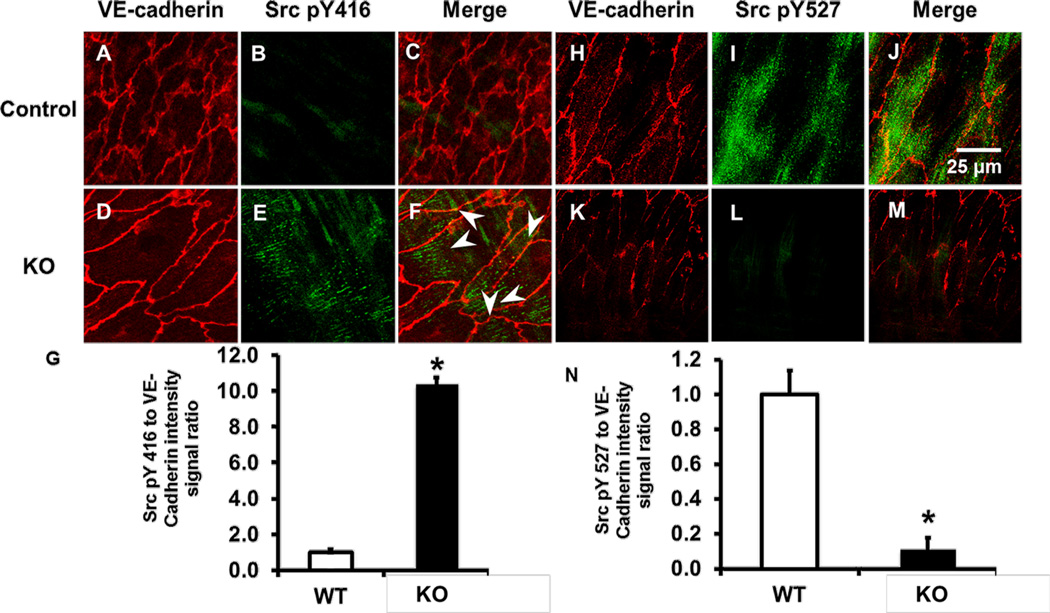
To evaluate the role of TXNIP in Src phosphorylation in vivo, we measured the phosphorylation state of tyrosine 416 (Y416) and tyrosine 527 (Y527) using en face staining of EC lining the aorta. Under basal conditions Src Y527 is phosphorylated (pY527), which maintains low kinase activity. In response to many stimuli, Src pY527 is dephosphorylated, which induces a conformational change in the activation loop. Full kinase activation than occurs by phosphorylation of Src Y416 (pY416) 34. To demonstrate an endothelial role of TXNIP in differential phosphorylation states of Y416 and Y527 of Src, we utilized TXNIP-KO mice. In the aorta exposed to s-flow, control animals exhibited low levels of phospho-Y416 (pY416) and high levels of pY527 (Figure 1A–C; H–J). In contrast, the aorta of KO animals showed a dramatic increase in Src pY416 and decrease in pY527 (Figure 1D–G & K–N). To confirm these findings we also scanned sites where the spinal arteries branch from the aorta; these sites are characterized by both s- and d-flow regions. There was a clear increase in Src pY416 and decrease in pY527, as shown in Supplemental Figure I. Total Src expression was not significantly different between these animals (Sup. Figure II & III), suggesting that the effect observed was due to TXNIP’s effect on a Src regulatory mechanism.
Figure 1. En face staining demonstrates differential regulation of Src pY416 and pY527 in KO animals compared to control.
Aortas of control (A–C; H–J) or KO (D–F; K–M) were immuno-stained for VE-cadherin (A, D, H, K), Src pY416 (B, E), Src pY527 (I, L) or presented as merged images (C, F, J, M). (G, N) Quantification of the data using Image J show Src pY416 and pY527 intensity (* P<0.05 vs. control; n=4). White arrowheads denote formation of linear active Src patterns.
Src phosphorylation state of tyrosine 416 and 527 is differentially regulated by TXNIP in vitro
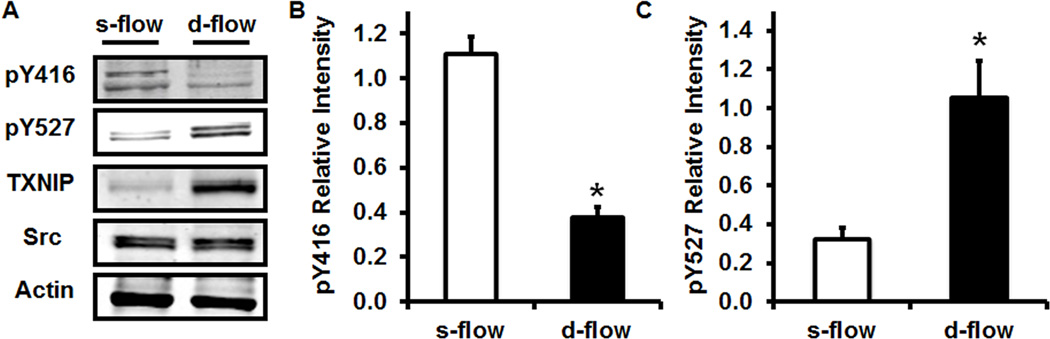
To confirm this observation in vitro, we measured Src pY416 and pY527 in cultured HUVEC exposed to s-flow or d-flow. Shown in Figure 2, TXNIP expression is reduced under s-flow conditions while dramatically increased under d-flow conditions. In addition, Y416 is highly phosphorylated under s-flow conditions and dephosphorylated under d-flow conditions (Figure 2). Finally, Y527 phosphorylation level was low under s-flow conditions but highly phosphorylated under d-flow conditions (Figure 2). This observation was also confirmed by an immunofluorescence experiment using HUVEC exposed to s-flow or d-flow (Sup. Figure IV).
Figure 2. Western blot analysis demonstrates TXNIP expression.
Src pY416 and pY527 are regulated by flow. (A) Total cell lysates of HUVEC exposed to s-flow or d-flow were immuno-blotted for pY416, pY527, Src and TXNIP. (B) Quantification of the data using Image J (* P<0.05 vs. s-flow; n=4).
In addition, we also measured pY416 and pY527 in HUVEC in which TXNIP was depleted using siRNA. HUVEC transfected with control siRNA, had low levels of pY416 and high levels of pY527, while TXNIP siRNA transfected cells had a 2.5 fold increase in pY416 and 0.7-fold decrease in pY527 were observed (Sup. Figure V). Furthermore, we confirmed this differential phosphorylation state of Y416 and Y527, using an immunofluorescence approach to measure pY416 and pY527 in cultured HUVEC in which TXNIP was depleted using siRNA. In HUVEC transfected with control siRNA, low levels of pY416 were observed, while in TXNIP siRNA transfected cells a 2.5-fold increase in pY416 was observed (Sup. Figure VI A–F& M). Increased pY416 was at the PM and diffusely in the cytosol. The results obtained for pY527 were opposite for those observed with pY416, as expected. In control siRNA transfected cells, high levels of pY527 were observed, while in TXNIP siRNA transfected cells there was a significant 60% decrease in pY527 (Sup. Figure VI G-L& N). To confirm these results we also utilized confocal microscopy as shown in Supplemental Figure VII. All together our in vivo and in vitro data strongly suggest that TXNIP acts as an endogenous inhibitor of Src activation in EC.
TXNIP prevents SHP2 phosphatase interaction with VE-cadherin
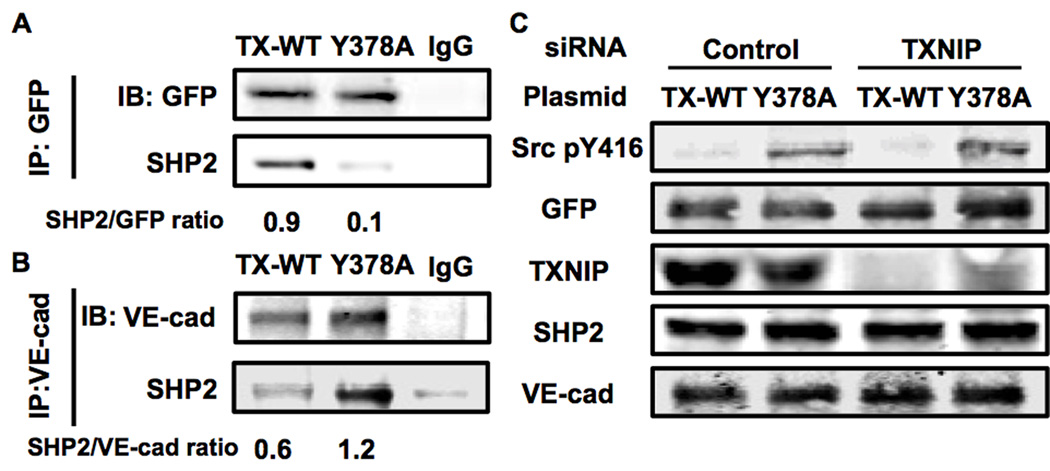
Next we hypothesized that TXNIP prevents SHP2 to be recruited to VE-cadherin. Previously it was shown that SHP2 dephosphorylates C-terminal Src Kinase (CSK), which is important inhibitor of Src via phosphorylation of the inhibitory Y527 25. Inhibition of CSK allows relief of the inhibitory effect of pY527 and activation of Src. SHP2 activity is regulated in EC through an interaction with VE-cadherin that acts as a docking site for a complex of proteins including Src, CSK and SHP2 25. Because TXNIP is an α-arrestin protein, and contains several protein-protein binding motifs 29 (e.g. PPxY motif or ITIM motif) we hypothesized that TXNIP prevents SHP2 from interacting with VE-cadherin. To test this hypothesis we measured VE-cadherin-SHP2 interaction (Figure 3) in cells transfected with TXNIP or control siRNA. In addition, we co-transfected these cells with two different plasmids: TXNIP wild type (TX-WT) or TXNIP mutant Y378A (Y378A). Tyrosine 378 (Y378) is part of an identified PPxY motif within the arrestin domain of TXNIP. Based on an extensive mutagenesis analysis, we discovered that Y378A fails to interact with SHP2 (Figure 3A). To test the hypothesis that TXNIP regulates SHP2 ability to interact with VE-cadherin, we introduced two green fluorescent protein (GFP) tagged plasmids TX-WT-GFP or Y378A-GFP and measured VE-cadherin-SHP2 interaction (Figure 3B). In control TX-WT-GFP transfected cells, low levels of VE-cadherin-SHP2 interaction were observed, as expected. In contrast, Y378A-GFP transfected cells showed a 2-fold increased interaction between VE-cadherin and SHP2. These data demonstrate that TXNIP binding to SHP2 is regulated by the PPxY domain (AA375-378).
Figure 3. VE-cadherin-SHP2 interaction is regulated by TXNIP.
(A, B) Western blot analysis of immunoprecipitation samples from BAEC transfected with TX-WT-GFP or Y378A-GFP plasmids. (C) Western blot analysis of total cell lysates samples from BAEC transfected with control or TXNIP siRNA and TX-WT-GFP or Y378A-GFP mutant TXNIP. Quantification of the data using Image J (n=3).
To demonstrate the functional mechanism by which TXNIP-SHP2 binding regulates Src activation, we performed a rescue experiment. We co-transfected cells with control or TXNIP siRNA, and than expressed TX-WT-GFP or Y378A-GFP plasmids, to rescue the activation of Src. When TX-WT-GFP was expressed, Src pY416 was low but when Y378A-GFP was expressed, a 3.5-fold increase of Src pY416was observed. When Y378A-GFP was expressed, Src pY416 was significantly increased similar to transfection with control siRNA, as shown in Figure 3C. These data suggest that Y378A outcompetes endogenous TX-WT.
The in vivo data (Figures 1 and Sup. Figure I) suggest that s-flow promotes the VE-cadherin-SHP2 interaction, based on the high levels of Src pY416. To show further the role of TXNIP in regulating VE-cadherin-SHP2 interaction we exposed HUVEC to s- or d-flow conditions. There was a significant 3.5-fold increase in VE-cad-SHP2 interaction when s-flow was applied to HUVEC, compared to d-flow samples (Sup. Figure VIII; P<0.05). To provide further evidence that TXNIP regulates SHP2 function, we measured SHP2 localization in control or TXNIP siRNA transfected cells by confocal microscopy (Sup. Figure VIII). In control siRNA cells, SHP2 was found mainly in perinuclear and nuclear regions (Sup. Figure VIII). In contrast, in TXNIP siRNA transfected cells, SHP2 was excluded from the nucleus, and was more evenly distributed throughout the cell (Sup. Figure VIII).
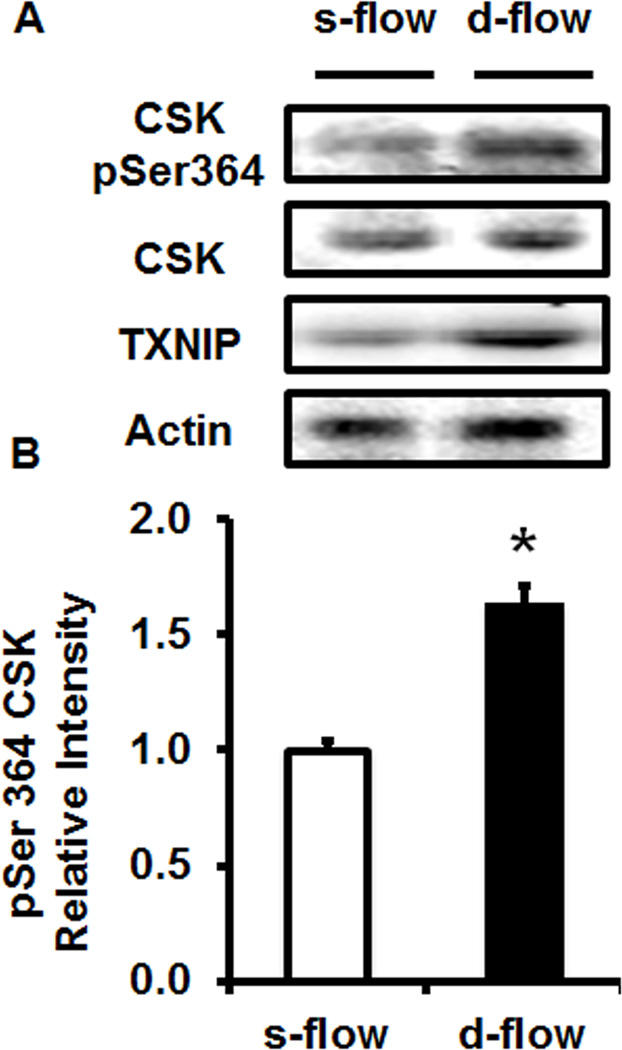
Finally, to prove this mechanism we measured CSK activation (serine 364 phosphorylation; pSer364) under s-flow or d-flow conditions. Under s-flow conditions CSK activation was low, consistent with low TXNIP expression and high Src activity. In contrast, under d-flow conditions CSK activation was high, consistent with high TXNIP levels and low Src activity (Figure 4). To confirm this observation we also transfected HUVEC with control or TXNIP siRNA. In control siRNA transfected cells a basal level of active CSK was observed, while in TXNIP siRNA transfected cells, a 60% reduction in active CSK was observed (Sup. Figure IX).
Figure 4. CSK activity is regulated by flow.
(A) Western blot analysis of HUVEC exposed to s-flow or d-flow and immuno-stained for active CSK, TXNIP and Actin. (B) Quantification of CSK activation in response to flow (* p<0.05 vs. s-flow; n=3).
These data confirm our hypothesis that TXNIP regulates Src activation through indirect regulation of CSK inhibition by SHP2. Specifically, TXNIP regulates SHP2 subcellular recruitment to VE-cadherin to dephosphorylate and inhibit CSK.
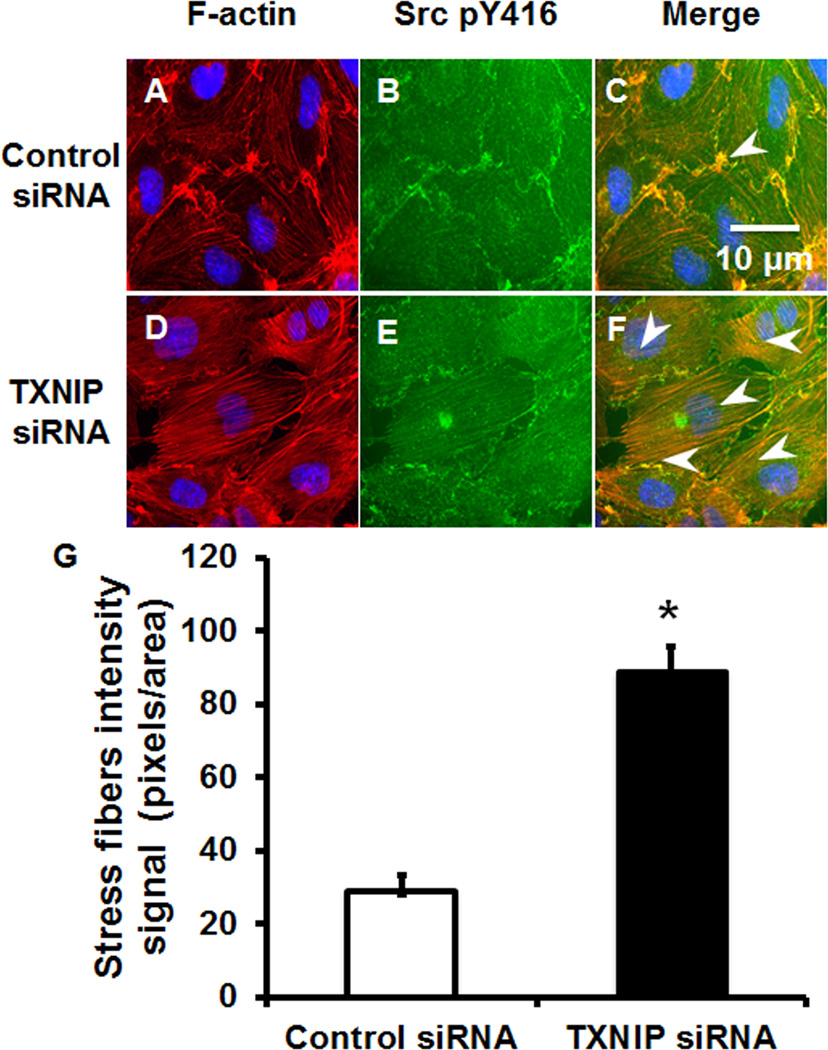
TXNIP acts as a negative regulator of Src and stress fibers formation in vitro
As Src activity is important for F-actin stress fiber homeostasis 35, we determined the role of TXNIP in stress fiber formation. We transfected HUVEC with control or TXNIP siRNA to evaluate Src activation state (pY416) and F-actin fibers (rhodamine-phalloidin fluorescence). Cells transfected with control siRNA had low amount of stress fibers under baseline conditions that was consistent with low Src activation (Figure 5A–C). In contrast, after transfection of TXNIP siRNA, stress fibers formed spontaneously that correlated with increased Src activation at the periphery of the cells and in cytosolic structures (Figure 5D–F). Active Src localized with stress fibers, suggesting a link between reduced TXNIP expression and Src regulation of F-actin stress fibers (Figure 5F).
Figure 5. TXNIP depletion results in spontaneous stress fiber formation.
HUVEC transfected with control siRNA (A–C) or TXNIP siRNA (D–F) were immuno-stained for F-actin (A, D), Src pY416 (B, E) or presented as merged image (C, F). White arrows demonstrate sites of F-actin and Src pY416 colocalization. (G) Stress fiber area was measured and analyzed for signal intensity using Image J (* P<0.05 vs. control siRNA, n=4).
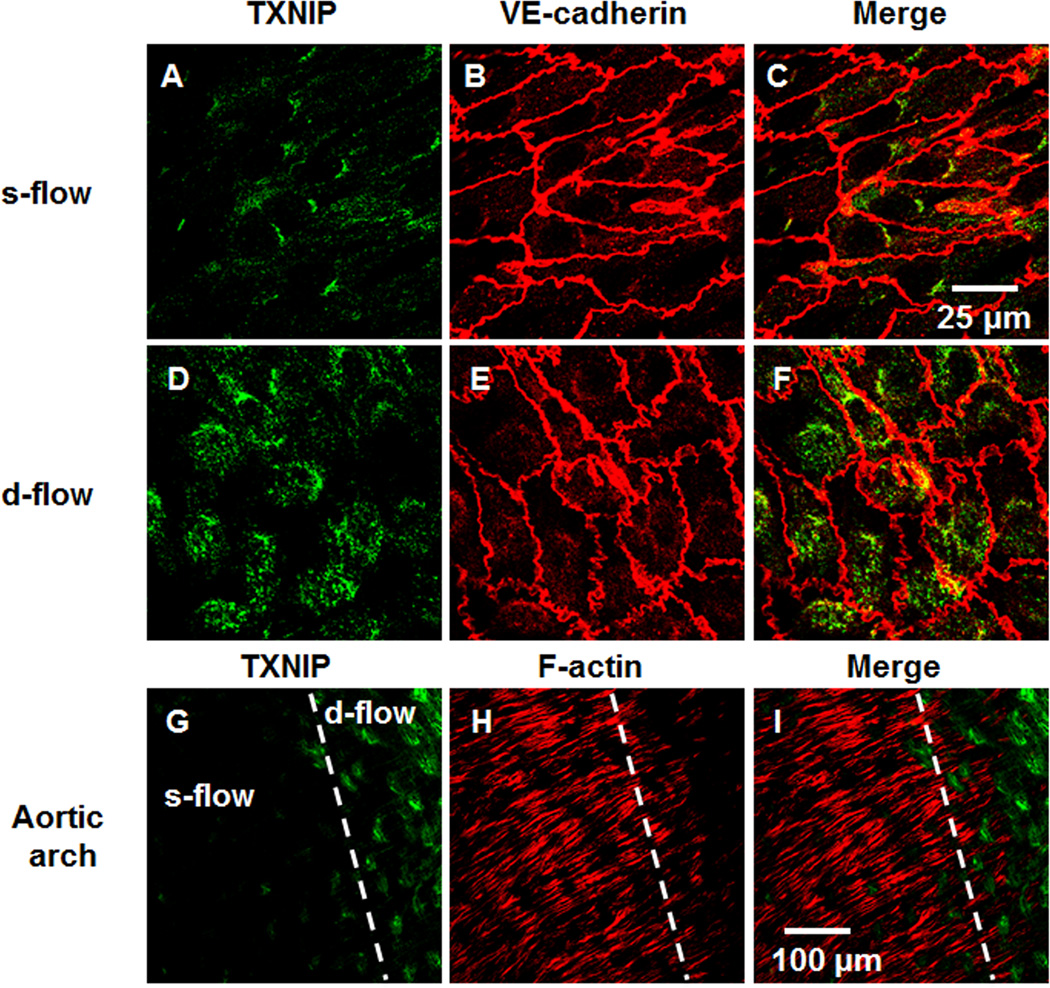
Flow regulates TXNIP expression and F-actin stress fiber in vitro and in vivo
To study the physiologic relevance of these observations, we analyzed the relationship between s-flow or d-flow, TXNIP expression and F-actin stress fiber formation. First, we performed en face staining for TXNIP in s-flow and d-flow regions of the aortic arch of WT animals (Figure 6A–F). Consistent with previous studies 9, TXNIP expression was significantly suppressed in regions of s-flow (greater curvature; Figure 6A–C), compared to d-flow regions (lesser curvature; Figure 6D–F). Interestingly, TXNIP expression in s-flow and d-flow regions inversely correlated with formation of F-actin stress fibers. These data suggest an important inhibitory effect of TXNIP on EC stress fiber formation (Figure 6G–I). Measuring the correlation between TXNIP and F-actin fibers showed a clear inverse correlation between TXNIP expression and F-actin formation (Sup. Figure X).
Figure 6. En face staining demonstrates flow regulation of TXNIP and stress fiber formation.
s-flow (A–C) and d-flow (D–F) regions of animals demonstrate differential TXNIP expression (A, D), VE-cadherin (B, E) or presented as merged images (C, F). Aortic arch immuno-staining for TXNIP (G), F-actin (H) or presented as merged image (I) demonstrating both s-flow and d-flow regions as indicated by the broken white line.
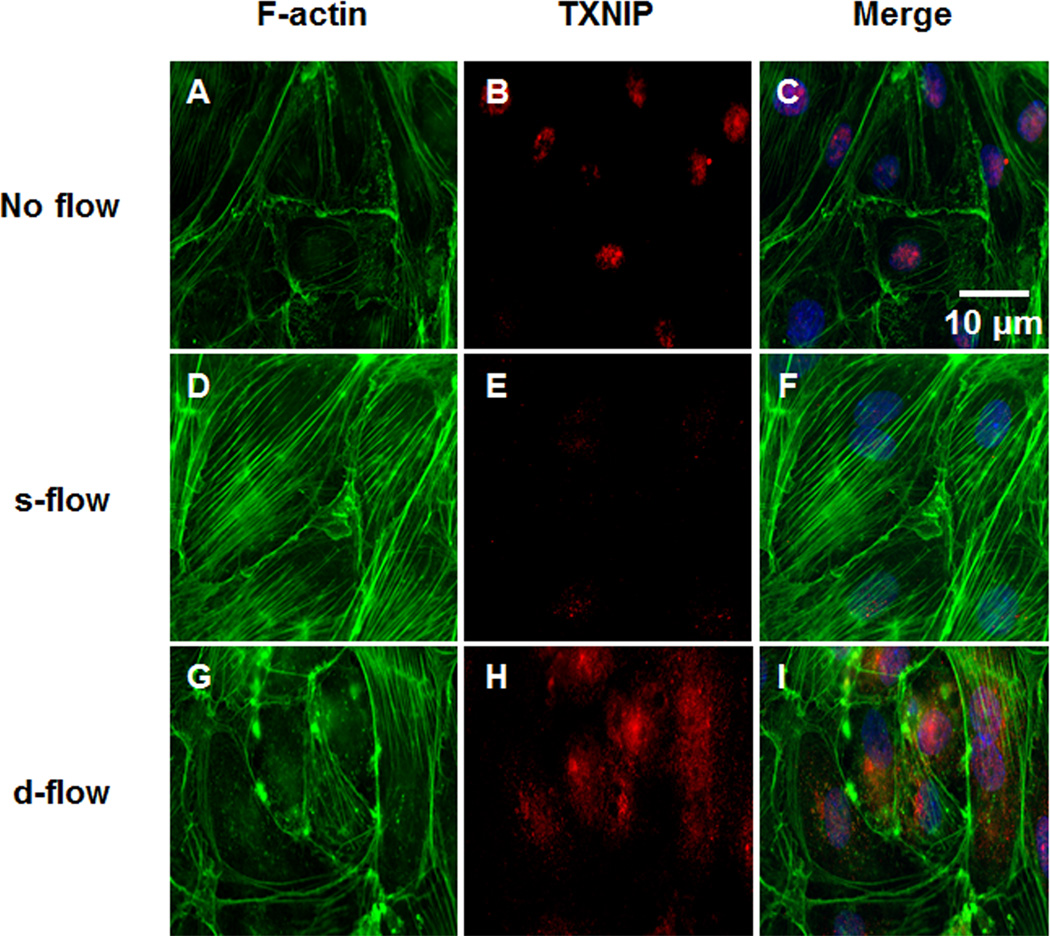
To confirm a role for TXNIP in the regulation of stress fibers, HUVEC were exposed to no flow, s-flow or d-flow conditions and TXNIP and F-actin levels were measured using immunofluorescence. Under no flow conditions, there were few stress fibers, and TXNIP was localized to the nucleus, as indicated by colocalization of TXNIP and DAPI staining (Figure 7A–C). After s-flow stimulation (12 dyn/cm2; 24-hours), EC aligned in the direction of flow and were characterized by increased stress fibers, and TXNIP expression was decreased (Figure 7D–F). In contrast, EC that were exposed to d-flow (2 dyn/cm2; 24-hour), demonstrated no cell alignment, low stress fibers, and significantly increase TXNIP expression in both nucleus and cytosol (Figure 7G–I). (Quantification of these data presented in Sup. Figure XI). These data confirm the inhibitory role TXNIP plays in stress fiber formation under different flow patterns.
Figure 7. Flow-mediated regulation of TXNIP expression and stress fiber formation.
HUVEC were exposed to no flow (A–C), s-flow (D–F) or d-flow (G–I) conditions. Cells were fluorescently labeled for F-actin (A, D, G), TXNIP (B, E, H) or presented as merged (C, F, I).
DISCUSSION
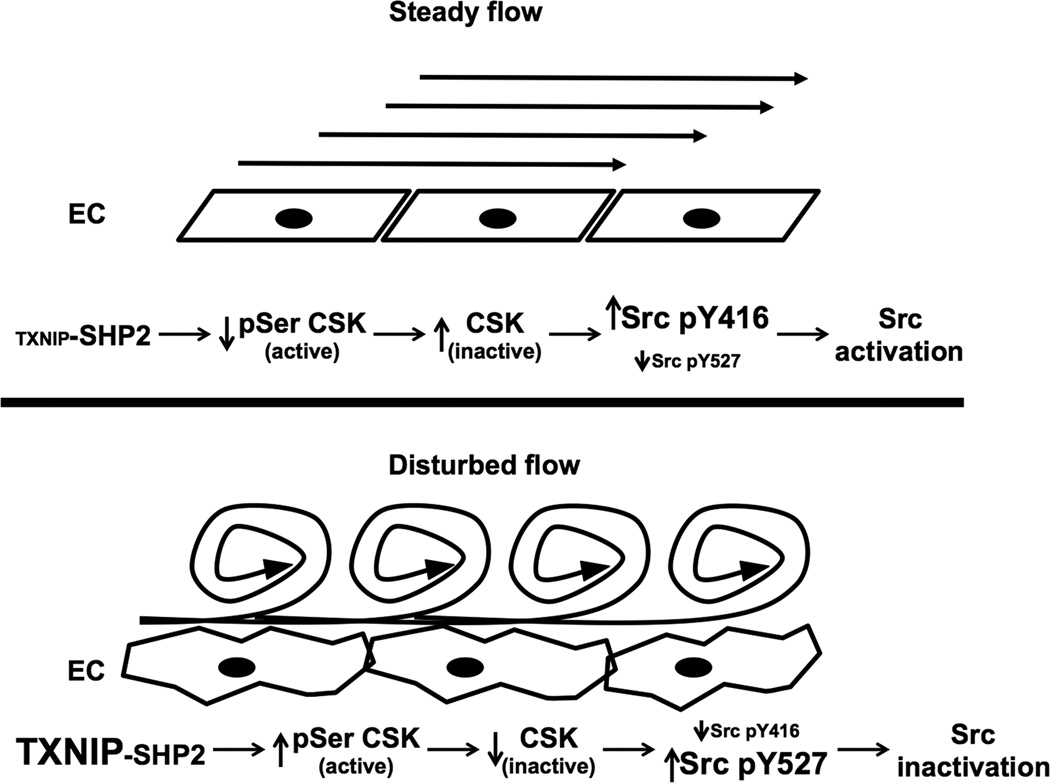
The major findings of this study are that TXNIP is a biomechanical regulator of Src activity that controls EC stress fibers formation. Based on both in vitro and in vivo studies we propose that TXNIP regulates a SHP2-CSK-Src signaling cascade (Figure 8). Under d-flow conditions TXNIP expression is high and CSK is active leading to increased Src Y527 phosphorylation and low Src activity. In contrast, s-flow reduces TXNIP-SHP2 interaction, which leads to dephosphorylation of CSK, decreased Src Y527 phosphorylation, and activation of Src. Importantly, active Src was observed mainly in linear structures that appeared similar to stress fibers, as indicated with white arrows in Figure 1. In EC, Src activation correlated with increased F-actin stress fibers formation, both in vivo and in vitro. Physiologically, this model explains the presence of stress fibers in regions of s-flow and absence of stress fibers in regions of d-flow, as originally described by Gotleib et al. 36. Furthermore, stress fiber formation is likely to be important in diverse endothelial cell functions, such as migration, permeability and apoptosis, cell shape change and alignment, mechano-signal transduction, EC cell-cell junction and EC structural and functional integrity 37–44.
Figure 8. Schematic model for TXNIP regulation of Src signaling and stress fiber formation.
Under basal conditions, TXNIP prevents SHP2 to interact with VE-cadherin resulting in pY527 Src by CSK. Upon decreased TXNIP expression, SHP2 can be recruited to VE-cadherin, where it dephosphorylates CSK, relieving the inhibitory pY527 and allowing activation of Src via increased pY416. As a consequent of active Src, stress fibers formation is observed.
The present study further supports the role of TXNIP as an arrestin-like scaffold protein that coordinates cell-signaling mechanisms that occur in specific sub-cellular locations. Recently we performed a sequence analysis of TXNIP and described several binding motifs that can facilitate TXNIP interaction with multiple proteins under different biological conditions 32. Particularly important are the four SH3 and two PPxY motifs. The current paper demonstrating the critical role for Y378 in the C-terminal PPxY is the first description of a functional TXNIP motif that interacts with SHP2. Future studies will describe the binding partners that mediate TXNIP localization to specific sub-cellular locations, such as nucleus, cytosol and PM 45–47. For example, we and others showed previously that PARP1 and thioredoxin regulate TXNIP localization 48. Recently, we also showed that in response to VEGFR2 activation, TXNIP translocates from the nucleus and interacts with Rab5 to regulate endocytosis and angiogenesis49.
Our data show that TXNIP is a key regulator of Src activity, which is important in physiologic and pathologic cell functions. For example, under physiologic conditions when EC are exposed to s-flow, TXNIP expression is low. Therefore, Src activity is high since Src pY527 is low and pY416 is high, resulting in EC stress fiber formation. Other examples that the relationship between TXNIP expression and Src activity include both tumorogenesis and metabolism. Decreased expression of TXNIP was shown to result in spontaneous tumorigenesis in multiple tissue types, which is related to its role as growth arrest protein 50. This is consistent with data that Src is a well-known oncogene, which is highly active in tumor cells 51. In addition, tumor metastasis requires stress fiber rearrangement and loss of cell interaction with matrix; processes facilitated by decreased TXNIP expression 52. In terms of metabolism, Src and TXNIP have been shown to play important roles in glucose metabolism, mitochondrial function and cell death 53- 59. In summary, this is the first demonstration that a specific TXNIP sub-cellular motif (C-terminal PPxY) mediates a critical cell function; specifically the interaction with SHP2 and regulation of Src activity. Furthermore, these findings suggest that TXNIP may play a broader role in regulating Src activity in multiple cell types, particularly in response to oncogenic and inflammatory stimuli.
Supplementary Material
Novelty and Significance.
What Is Known?
Thioredoxin-interacting protein (TXNIP), a scaffold protein of the α-arrestin family, regulates target protein activity through multiple protein-protein interacting domains.
TXNIP is highly regulated by multiple mechanisms, such as glucose, redox state and biomechanical forces, including shear stress and stretch.
Src kinase activity regulates stress fibers formation via multiple targets, such as paxillin or FAK.
What is New Information Does This Article Contribute?
Steady flow (s-flow) increases Src-activity and stress fiber formation, while decreasing TXNIP expression. While disturbed flow (d-flow) has the opposite effect.
Our observation that TXNIP plays a role in stress fibers formation expands the role of TXNIP as a biomechanical sensor.
Stress fiber formation under conditions of fluid shear stress is mediated by the TXNIP-SHP2-CSK-VE-cadherin-Src signaling pathway
Fluid shear stress differentially regulates endothelial cell (EC) function; most notably atherosclerosis is greater in areas of d-flow compared with s-flow). Conversely, stress fiber formation is greater in s-flow then d-flow areas. Importantly, stress fibers are critical for several EC functions including cell shape, mechano-signal transduction, and cell-cell junction integrity. A key mediator of s-flow induced stress fiber formation is Src, although the exact mechanisms that control Src remain unclear. A new signal mediator that links biomechanical forces with redox state in EC has been identified as the Thioredoxin-interacting protein (TXNIP), which is highly induced by d-flow. Using en face immunohistochemistry of the aorta and cultured EC, we show that s-flow increased Src activity and stress fiber formation, while it decreased TXNIP expression. In contrast, d-flow had opposite effects. We found that TXNIP regulated Src by binding to SHP2 and inhibiting the Src negative regulator CSK. These data show that TXNIP plays a role in stress fibers formation and expands its role as a biomechanical mediator. Furthermore, these findings suggest that TXNIP is a novel regulator of Src. Because TXNIP is highly regulated by multiple factors (e.g., glucose, circadian-rhythm, redox), these results provide new insights into Src regulation in many pathophysiologic conditions.
Acknowledgments
SOURCES OF FUNDING
This work was supported by NIH HL 106158 to B.Berk.
Nonstandard Abbreviations and Acronyms
- BAEC
Bovine aortic endothelial cell
- CSK
C-terminal Src Kinase
- d-flow
Disturbed flow
- EC
Endothelial cell
- KO
Knockout
- FSS
Fluid shear stress
- GFP
Green fluorescent protein
- HUVEC
Human umbilical vein endothelial cell
- PM
Plasma membrane
- pSer364
phospho-serine 364
- pY416
phospho-tyrosine 416
- pY527
phospho-tyrosine 527
- s-flow
Steady flow
- SHP2
Src homology phosphatase-2
- TXNIP
Thioredoxin-interacting protein
- VE-cadherin
Vascular endothelial cadherin
- VEGFR2
Vascular endothelial growth factor receptor 2
- WT
Wild type
Footnotes
DISCLOSURES
None.
REFERENCES
- 1.Nakashima Y, Plump AS, Raines EW, Breslow JL, Ross R. ApoE-deficient mice develop lesions of all phases of atherosclerosis throughout the arterial tree. Arterioscler Thromb. 1994;14:133–140. doi: 10.1161/01.atv.14.1.133. [DOI] [PubMed] [Google Scholar]
- 2.Caro CG, Fitz-Gerald JM, Schroter RC. Arterial wall shear and distribution of early atheroma in man. Nature. 1969;223:1159–1160. doi: 10.1038/2231159a0. [DOI] [PubMed] [Google Scholar]
- 3.VanderLaan PA, Reardon CA, Getz GS. Site Specificity of Atherosclerosis. Site-Selective Responses to Atherosclerotic Modulators. Arterioscler Thromb Vasc Biol. 2003 Nov 6; doi: 10.1161/01.ATV.0000105054.43931.f0. [DOI] [PubMed] [Google Scholar]
- 4.Malek AM, Alper SL, Izumo S. Hemodynamic shear stress and its role in atherosclerosis. Jama. 1999;282:2035–2042. doi: 10.1001/jama.282.21.2035. [DOI] [PubMed] [Google Scholar]
- 5.Cooke JP. Flow, NO, and atherogenesis. Proc Natl Acad Sci U S A. 2003 Feb 4;100:768–770. doi: 10.1073/pnas.0430082100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cunningham KS, Gotlieb AI. The role of shear stress in the pathogenesis of atherosclerosis. Lab Invest. 2005 Jan;85:9–23. doi: 10.1038/labinvest.3700215. [DOI] [PubMed] [Google Scholar]
- 7.Wang W, Ha CH, Jhun BS, Wong C, Jain MK, Jin ZG. Fluid shear stress stimulates phosphorylation-dependent nuclear export of HDAC5 and mediates expression of KLF2 and eNOS. Blood. 2010 Apr 8;115:2971–2979. doi: 10.1182/blood-2009-05-224824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ishibazawa A, Nagaoka T, Takahashi T, Yamamoto K, Kamiya A, Ando J, Yoshida A. Effects of shear stress on the gene expressions of endothelial nitric oxide synthase, endothelin-1, and thrombomodulin in human retinal microvascular endothelial cells. Investigative ophthalmology & visual science. 2011 Oct;52:8496–8504. doi: 10.1167/iovs.11-7686. [DOI] [PubMed] [Google Scholar]
- 9.Yamawaki H, Pan S, Lee RT, Berk BC. Fluid shear stress inhibits vascular inflammation by decreasing thioredoxin-interacting protein in endothelial cells. J Clin Invest. 2005 Mar;115:733–738. doi: 10.1172/JCI200523001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Keulenaer GW, Chappell DC, Ishizaka N, Nerem RM, Alexander RW, Griendling KK. Oscillatory and steady laminar shear stress differentially affect human endothelial redox-state: Role of a superoxide-producing NADH oxidase. Circ Res. 1998;82:1094–1101. doi: 10.1161/01.res.82.10.1094. [DOI] [PubMed] [Google Scholar]
- 11.Himburg HA, Grzybowski DM, Hazel AL, LaMack JA, Li XM, Friedman MH. Spatial comparison between wall shear stress measures and porcine arterial endothelial permeability. Am J Physiol Heart Circ Physiol. 2004 May;286:H1916–H1922. doi: 10.1152/ajpheart.00897.2003. [DOI] [PubMed] [Google Scholar]
- 12.Chappell DC, Varner SE, Nerem RM, Medford RM, Alexander RW. Oscillatory shear stress stimulates adhesion molecule expression in cultured human endothelium. Circulation research. 1998 Mar 23;82:532–539. doi: 10.1161/01.res.82.5.532. [DOI] [PubMed] [Google Scholar]
- 13.Jin ZG, Ueba H, Tanimoto T, Lungu AO, Frame MD, Berk BC. Ligand-independent activation of vascular endothelial growth factor receptor 2 by fluid shear stress regulates activation of endothelial nitric oxide synthase. Circ Res. 2003 Aug 22;93:354–363. doi: 10.1161/01.RES.0000089257.94002.96. [DOI] [PubMed] [Google Scholar]
- 14.Chen-Konak L, Guetta-Shubin Y, Yahav H, Shay-Salit A, Zilberman M, Binah O, Resnick N. Transcriptional and post-translation regulation of the Tie1 receptor by fluid shear stress changes in vascular endothelial cells. FASEB J. 2003 Nov;17:2121–2123. doi: 10.1096/fj.02-1151fje. [DOI] [PubMed] [Google Scholar]
- 15.Woodfin A, Voisin MB, Nourshargh S. PECAM-1: a multi-functional molecule in inflammation and vascular biology. Arterioscler Thromb Vasc Biol. 2007 Dec;27:2514–2523. doi: 10.1161/ATVBAHA.107.151456. [DOI] [PubMed] [Google Scholar]
- 16.Liu SQ, Tang D, Tieche C, Alkema PK. Pattern formation of vascular smooth muscle cells subject to nonuniform fluid shear stress: mediation by gradient of cell density. Am J Physiol Heart Circ Physiol. 2003 Sep;285:H1072–H1080. doi: 10.1152/ajpheart.01009.2002. [DOI] [PubMed] [Google Scholar]
- 17.Liu Y, Sweet DT, Irani-Tehrani M, Maeda N, Tzima E. Shc coordinates signals from intercellular junctions and integrins to regulate flow-induced inflammation. J Cell Biol. 2008 Jul 14;182:185–196. doi: 10.1083/jcb.200709176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sipkema P, van der Linden PJ, Westerhof N, Yin FC. Effect of cyclic axial stretch of rat arteries on endothelial cytoskeletal morphology and vascular reactivity. J Biomech. 2003 May;36:653–659. doi: 10.1016/s0021-9290(02)00443-8. [DOI] [PubMed] [Google Scholar]
- 19.Chava KR, Tauseef M, Sharma T, Mehta D. Cyclic AMP response element-binding protein prevents endothelial permeability increase through transcriptional controlling p190RhoGAP expression. Blood. 2012 Jan 5;119:308–319. doi: 10.1182/blood-2011-02-339473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu X, Zhao D, James L, Li J, Zeng H. Requirement of the nuclear localization of transcription enhancer factor 3 for proliferation, migration, tube formation, and angiogenesis induced by vascular endothelial growth factor. FASEB J. 2011 Apr;25:1188–1197. doi: 10.1096/fj.10-167619. [DOI] [PubMed] [Google Scholar]
- 21.Dejana E, Tournier-Lasserve E, Weinstein BM. The control of vascular integrity by endothelial cell junctions: molecular basis and pathological implications. Dev Cell. 2009 Feb;16:209–221. doi: 10.1016/j.devcel.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 22.Harris ES, Nelson WJ. VE-cadherin: at the front, center, and sides of endothelial cell organization and function. Curr Opin Cell Biol. 2010 Oct;22:651–658. doi: 10.1016/j.ceb.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baum B, Georgiou M. Dynamics of adherens junctions in epithelial establishment, maintenance, and remodeling. The Journal of cell biology. 2011 Mar 21;192:907–917. doi: 10.1083/jcb.201009141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dejana E, Orsenigo F, Lampugnani MG. The role of adherens junctions and VE-cadherin in the control of vascular permeability. Journal of cell science. 2008 Jul 1;121:2115–2122. doi: 10.1242/jcs.017897. [DOI] [PubMed] [Google Scholar]
- 25.Ha CH, Bennett AM, Jin ZG. A novel role of vascular endothelial cadherin in modulating c-Src activation and downstream signaling of vascular endothelial growth factor. J Biol Chem. 2008 Mar 14;283:7261–7270. doi: 10.1074/jbc.M702881200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Usatyuk PV, Parinandi NL, Natarajan V. Redox regulation of 4-hydroxy-2-nonenal-mediated endothelial barrier dysfunction by focal adhesion, adherens, and tight junction proteins. J BIol Chem. 2006 Nov 17;281:35554–35566. doi: 10.1074/jbc.M607305200. [DOI] [PubMed] [Google Scholar]
- 27.Wang XQ, Nigro P, World C, Fujiwara K, Yan C, Berk BC. Thioredoxin interacting protein promotes endothelial cell inflammation in response to disturbed flow by increasing leukocyte adhesion and repressing Kruppel-like factor 2. Circ Res. 2012 Feb 17;110:560–568. doi: 10.1161/CIRCRESAHA.111.256362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.World C, Spindel ON, Berk BC. Thioredoxin-interacting protein mediates TRX1 translocation to the plasma membrane in response to tumor necrosis factor-alpha: a key mechanism for vascular endothelial growth factor receptor-2 transactivation by reactive oxygen species. Arteriosclerosis, thrombosis, and vascular biology. 2011 Aug;31:1890–1897. doi: 10.1161/ATVBAHA.111.226340. [DOI] [PubMed] [Google Scholar]
- 29.Spindel ON, World C, Berk BC. Thioredoxin interacting protein: redox dependent and independent regulatory mechanisms. Antioxid Redox Signal. 2012 Mar 15;16:587–596. doi: 10.1089/ars.2011.4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alvarez CE. On the origins of arrestin and rhodopsin. BMC Evol Biol. 2008;8:222. doi: 10.1186/1471-2148-8-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chutkow WA, Lee RT. Thioredoxin Regulates Adipogenesis through Thioredoxin-interacting Protein (Txnip) Protein Stability. J Biol Chem. 2011 Aug 19;286:29139–29145. doi: 10.1074/jbc.M111.267666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spindel ON, World C, Berk B. Thioredoxin Interacting Protein (TXNIP): Redox dependent and independent regulatory mechanisms. Antioxid Redox Signal. 2011 Sep 19; doi: 10.1089/ars.2011.4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamawaki H, Haendeler J, Berk BC. Thioredoxin: a key regulator of cardiovascular homeostasis. Circ Res. 2003 Nov 28;93:1029–1033. doi: 10.1161/01.RES.0000102869.39150.23. [DOI] [PubMed] [Google Scholar]
- 34.Roskoski R., Jr Src protein-tyrosine kinase structure and regulation. Biochemical and biophysical research communications. 2004 Nov 26;324:1155–1164. doi: 10.1016/j.bbrc.2004.09.171. [DOI] [PubMed] [Google Scholar]
- 35.Ishida T, Takahashi M, Corson MA, Berk BC. Fluid shear stress-mediated signal transduction: how do endothelial cells transduce mechanical force into biological responses? Ann N Y Acad Sci. 1997;811:12–23. doi: 10.1111/j.1749-6632.1997.tb51984.x. discussion 23-14. [DOI] [PubMed] [Google Scholar]
- 36.Kim DW, Langille BL, Wong MK, Gotlieb AI. Patterns of endothelial microfilament distribution in the rabbit aorta in situ. Circ Res. 1989;64:21–31. doi: 10.1161/01.res.64.1.21. [DOI] [PubMed] [Google Scholar]
- 37.Tricot O, Mallat Z, Heymes C, Belmin J, Leseche G, Tedgui A. Relation between endothelial cell apoptosis and blood flow direction in human atherosclerotic plaques. Circulation. 2000;101:2450–2453. doi: 10.1161/01.cir.101.21.2450. [DOI] [PubMed] [Google Scholar]
- 38.Hahn C, Wang C, Orr AW, Coon BG, Schwartz MA. JNK2 Promotes Endothelial Cell Alignment under Flow. PloS one. 2011;6:e24338. doi: 10.1371/journal.pone.0024338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stockton RA, Schaefer E, Schwartz MA. p21-activated kinase regulates endothelial permeability through modulation of contractility. J Biol Chem. 2004 Nov 5;279:46621–46630. doi: 10.1074/jbc.M408877200. [DOI] [PubMed] [Google Scholar]
- 40.Wu CC, Li YS, Haga JH, Kaunas R, Chiu JJ, Su FC, Usami S, Chien S. Directional shear flow and Rho activation prevent the endothelial cell apoptosis induced by micropatterned anisotropic geometry. Proc Natl Acad Sci U S A. 2007 Jan 23;104:1254–1259. doi: 10.1073/pnas.0609806104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang N, Miao H, Li YS, Zhang P, Haga JH, Hu Y, Young A, Yuan S, Nguyen P, Wu CC, Chien S. Shear stress regulation of Kruppel-like factor 2 expression is flow pattern-specific. Biochem Biophys Res Commun. 2006 Mar 24;341:1244–1251. doi: 10.1016/j.bbrc.2006.01.089. [DOI] [PubMed] [Google Scholar]
- 42.Malek AM, Izumo S. Mechanism of endothelial cell shape change and cytoskeletal remodeling in response to fluid shear stress. J Cell Sci. 1996 Apr;109:713–726. doi: 10.1242/jcs.109.4.713. [DOI] [PubMed] [Google Scholar]
- 43.Birukov KG, Birukova AA, Dudek SM, Verin AD, Crow MT, Zhan X, DePaola N, Garcia JG. Shear Stress-Mediated Cytoskeletal Remodeling and Cortactin Translocation in Pulmonary Endothelial Cells. Am J Respir Cell Mol Biol. 2002;26:453–464. doi: 10.1165/ajrcmb.26.4.4725. [DOI] [PubMed] [Google Scholar]
- 44.Katoh K, Kano Y, Ookawara S. Role of stress fibers and focal adhesions as a mediator for mechano-signal transduction in endothelial cells in situ. Vasc Health Risk Manag. 2008;4:1273–1282. doi: 10.2147/vhrm.s3933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.World C, Spindel ON, Berk BC. Thioredoxin-Interacting Protein Mediates TRX1 Translocation to the Plasma Membrane in Response to Tumor Necrosis Factor-{alpha}: A Key Mechanism for Vascular Endothelial Growth Factor Receptor-2 Transactivation by Reactive Oxygen Species. Arterioscler Thromb Vasc Biol. 2011 Jun 2; doi: 10.1161/ATVBAHA.111.226340. [DOI] [PubMed] [Google Scholar]
- 46.Shin D, Jeon JH, Jeong M, Suh HW, Kim S, Kim HC, Moon OS, Kim YS, Chung JW, Yoon SR, Kim WH, Choi I. VDUP1 mediates nuclear export of HIF1alpha via CRM1-dependent pathway. Biochim Biophys Acta. 2008 May;1783:838–848. doi: 10.1016/j.bbamcr.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 47.Ago T. A Redox-Dependent Pathway for Regulating Class II HDACs and Cardiac Hypertrophy. Cell. 2008;133:978–993. doi: 10.1016/j.cell.2008.04.041. [DOI] [PubMed] [Google Scholar]
- 48.Spindel ON, Yan C, Berk BC. Thioredoxin-interacting protein mediates nuclear-to-plasma membrane communication: role in vascular endothelial growth factor 2 signaling. Arterioscler Thromb Vasc Biol. 2012 May;32:1264–1270. doi: 10.1161/ATVBAHA.111.244681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Park Y, Park SY, Shi X, Pang J, Yan C, Berk BC. Thioredoxin-Interacting Protein Mediates Sustained VEGFR2 Signaling in Endothelial Cells Required for Angiogenesis. Arterioscler Thromb Vasc Biol. 2013 Feb 7; doi: 10.1161/ATVBAHA.112.300386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yamaguchi F, Takata M, Kamitori K, Nonaka M, Dong Y, Sui L, Tokuda M. Rare sugar Dallose induces specific up-regulation of TXNIP and subsequent G1 cell cycle arrest in hepatocellular carcinoma cells by stabilization of p27kip1. Int J Oncol. 2008 Feb;32:377–385. [PubMed] [Google Scholar]
- 51.Kim LC, Song L, Haura EB. Src kinases as therapeutic targets for cancer. Nature reviews. Clinical oncology. 2009 Oct;6:587–595. doi: 10.1038/nrclinonc.2009.129. [DOI] [PubMed] [Google Scholar]
- 52.Ridley AJ. Life at the leading edge. Cell. 2011 Jun 24;145:1012–1022. doi: 10.1016/j.cell.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 53.Cvrljevic AN, Akhavan D, Wu M, Martinello P, Furnari FB, Johnston AJ, Guo D, Pike L, Cavenee WK, Scott AM, Mischel PS, Hoogenraad NJ, Johns TG. Activation of Src induces mitochondrial localisation of de2-7EGFR (EGFRvIII) in glioma cells: implications for glucose metabolism. Journal of cell science. 2011 Sep 1;124:2938–2950. doi: 10.1242/jcs.083295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Livigni A, Scorziello A, Agnese S, Adornetto A, Carlucci A, Garbi C, Castaldo I, Annunziato L, Avvedimento EV, Feliciello A. Mitochondrial AKAP121 links cAMP and src signaling to oxidative metabolism. Molecular biology of the cell. 2006 Jan;17:263–271. doi: 10.1091/mbc.E05-09-0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lim SK, Choi YW, Lim IK, Park TJ. BTG2 suppresses cancer cell migration through inhibition of Src-FAK signaling by downregulation of reactive oxygen species generation in mitochondria. Clinical & experimental metastasis. 2012 May 6; doi: 10.1007/s10585-012-9479-z. [DOI] [PubMed] [Google Scholar]
- 56.Indovina P, Giorgi F, Rizzo V, Khadang B, Schenone S, Di Marzo D, Forte IM, Tomei V, Mattioli E, D'Urso V, Grilli B, Botta M, Giordano A, Pentimalli F. New pyrazolo[3,4-d]pyrimidine SRC inhibitors induce apoptosis in mesothelioma cell lines through p27 nuclear stabilization. Oncogene. 2012 Feb 16;31:929–938. doi: 10.1038/onc.2011.286. [DOI] [PubMed] [Google Scholar]
- 57.Hui ST, Andres AM, Miller AK, Spann NJ, Potter DW, Post NM, Chen AZ, Sachithanantham S, Jung DY, Kim JK, Davis RA. Txnip balances metabolic and growth signaling via PTEN disulfide reduction. Proc Natl Acad Sci U S A. 2008 Mar 11;105:3921–3926. doi: 10.1073/pnas.0800293105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Saxena G, Chen J, Shalev A. Intracellular shuttling and mitochondrial function of thioredoxininteracting protein. J BIol Chem. 2010 Feb 5;285:3997–4005. doi: 10.1074/jbc.M109.034421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Spindel ON, Yan C, Berk BC. Thioredoxin-Interacting Protein (TXNIP) Mediates Nuclear-to-Plasma Membrane Communication: Role in Vascular Endothelial Growth Factor 2 (VEGFR2) Signaling. Arteriosclerosis, thrombosis, and vascular biology. 2012 Feb 16; doi: 10.1161/ATVBAHA.111.244681. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.