Abstract
Background
Arterial stiffness and homocysteine are both powerful predictors of cardiovascular disease, especially in older populations. Previous studies have investigated the association of homocysteine with arterial stiffness in human subjects, while the relationship between homocysteine and arterial stiffness in the elderly is still indefinite. The current study examined the association of homocysteine with arterial stiffness in Chinese community-based elderly persons.
Methods
We related serum levels of homocysteine to two measures of arterial stiffness (carotid-femoral pulse wave velocity (PWV) and carotid-radial PWV) in 780 participants (46.3% men, mean age 71.9 years (ranging 65–96 years old)) from two communities of Beijing, China. Arterial stiffness was measured within two days of the time of biomarker measurement.
Results
In multiple-adjusted models, homocysteine levels were strongly associated with the carotid-femoral PWV (standardized β = 0.13, P < 0.001), even after adjustment for classical risk factors of cardiovascular disease. The association is also stronger when the carotid-femoral PWV is elevated above normal, whereas no significant association with homocysteine was observed for carotid-radial PWV.
Conclusions
In Chinese elderly persons, serum homocysteine levels are associated with alterations of aortic stiffness.
Keywords: The elderly, Homocysteine, Arterial stiffness, Pulse wave velocity
1. Introduction
Arterial stiffness gradually occurs with aging and has been increasingly recognized as a strong predictor of future fatal and nonfatal cardiovascular disease, especially in older individuals.[1]–[4] Structural and functional abnormalities of the arterial wall can be assessed by noninvasive, reproducible, and relatively inexpensive techniques, one of which is pulse wave velocity (PWV) measurement.[5] Aortic stiffness expressed as aortic PWV offers an extremely powerful and independent predictor of cardiovascular mortality in elderly populations.[4] The alteration of arterial elasticity is proposed to be a function of a complex interaction with age, hypertension and multiple conventional risk factors of cardiovascular disease.[6]
Elevated serum homocysteine has been considered as another independent risk factor among elderly persons for cardiovascular diseases, as well as subsequent mortality.[7]–[10] The relationship of homocysteine with these diseases may be mediated by its adverse effects on vascular endothelium and smooth muscle with resultant alterations in subclinical arterial structure and function. Among the putative mechanisms that these effects exert on are increased vascular smooth muscle cell proliferation, oxidative damage, endothelial dysfunction, increased collagen synthesis and deterioration of elastic material of the arterial wall.[11]–[13]
Investigators have reported a significant association of serum homocysteine concentration with different indices of arterial stiffness, including pulse pressure and aortic stiffness as assessed by carotid-femoral PWV in the general population.[14]–[16] While in older subjects who have multiple cardiovascular risk factors, findings are still inconsistent.[17],[18]
The aim of our study was to determine whether there exists an association between homocysteine concentration and arterial stiffness in the elderly. We related serum levels of homocysteine to two vascular functional measurements (carotid-femoral PWV and carotid-radial PWV) in a community-based sample from Beijing, China.
2. Methods
2.1. Study population and design
This was a community-based cross-sectional study of people living in the Pingguoyuan area of the Shijingshan district, a metropolitan area of Beijing, China. Originally, a total of 1,148 permanent residents aged 65 years, or older, were eligible to enroll as members of the study after a routine health check-up between September 2007 and January 2009. Thirty-one subjects with bedridden status, mental illness, and severe systemic diseases were excluded from the analysis.
Arterial stiffness assessments were performed in 1,107 subjects. Adequate measurements were either not attempted, or not obtained, in 83 participants. Excluding them, the homocysteine results were available from 904 participants. After excluding 124 participants for overt CVD, 780 subjects (mean age 71.9 years; range 65–96 years) comprised the present study.
The study was approved by the ethics committee of the Chinese People's Liberation Army (PLA) General Hospital, and each participant provided written informed consent.
2.2. Clinical data collection
Trained physicians and nurses provided health assessments using standardized structured interviews and comprehensive clinical examinations. The health assessment covered medical history, family history of CVD, and lifestyle. Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters. Two blood pressure recordings were obtained from the right arm of participants; measurements were taken at 5-min intervals and the mean values were calculated. The categories for smoking were never smoking, previously smoking, and currently smoking. Hypertension was defined as a mean of three independent measures of blood pressure ≥ 140/90 mmHg, or current use of antihypertensive drugs. Diabetes mellitus (DM) was defined as a fasting glucose ≥ 7.0 mmol/L, nonfasting glucose ≥ 11.1 mmol/L, or current use of anti-diabetic agents. The estimated glomerular filtration rate (eGFR) was estimated with the re-expressed four-variable Modification of Diet in Renal Disease (MDRD) equation: eGFR (mL/min per 1.73 m2) = 175 × standardized Scr − 1.154 × age − 0.203 (if female, × 0.742), where Scr is serum creatinine concentration (mg/dL).[19]
2.3. Biomarker variable determination
Blood samples were obtained between 8 a.m. and 10 a.m. after participants fasted overnight. The samples were centrifuged within 2 h, and stored at −80°C until assays were performed. Concentrations of fasting glucose, total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C) were determined on a Roche autoanalyser (Roche Diagnostics, Indianapolis, IN). Concentrations of homocysteine were determined by high-performance chromatography with fluorometric detection using a Dimension RxL Max analyzer (Siemens Healthcare Diagnostics Inc., Germany). High sensitive C reactive protein (hsCRP) was measured using immunoturbidometry (Siemens Healthcare Diagnostics Inc., Germany). All biochemical evaluations were performed by well-trained personnel blinded to clinical data in the Department of Biochemistry of Chinese PLA General Hospital.
2.4. Assessment of arterial stiffness
Arterial stiffness was assessed within two days after the collection of blood samples, within the morning, in a quiet environment, and at stable temperature. Subjects were requested to abstain from caffeine, smoking and alcohol for at least 12 h before the assessment was performed. Regional arterial stiffness was assessed by automatic carotid-femoral and carotid-radial PWV measurements using the Complior Colson device (Createch Industrie, France); the technical characteristics of this device have been described.[20] PWV along the artery was measured by using two strain-gauge transducers. The procedure is noninvasive and uses a TY-306 Fukuda pressure-sensitive transducer (Fukuda Denshi Co, Japan) that is fixed transcutaneously over the course of a pair of arteries separated by a known distance; the carotid, femoral, and radial arteries (all on the right side) were used. During preprocessing analysis, the gain of each waveform was adjusted to obtain an equal signal for the two waveforms. During PWV measurements, after pulse waveforms of sufficient quality were recorded, the digitization process was initiated by the operator, and automatic calculation of the time delay between two upstrokes was started. Measurements were repeated over 10 different cardiac cycles, and the mean value was used for the final analysis. PWV was calculated from the measurement of the pulse transit time and the distance traveled by the pulse between the two recording sites (measured on the surface of the body in meters), according to the following formula: PWV (m/s) = distance (m)/transit time (s).
2.5. Statistical analyses
Continuous variables are presented as mean ± SD or median (with inter-quartile range); dichotomous variables are presented as numbers and percentages. The continuous variables were tested for normality before being tested. Homocysteine was natural logarithmically transformed to normalize its distribution. Differences in baseline levels of risk factors, clinical characteristics and arterial stiffness measures (carotid-femoral PWV and carotid-radial PWV) between participants with high homocysteine level (≥ 15 µmol/L) and normal homocysteine level (< 15 µmol/L)[21] were analyzed with chi-square or t tests; the Wilcoxon two-sample test was used for continuous variables that were not normally distributed.
Associations between arterial stiffness measures (carotid-femoral PWV and carotid-radial PWV) and levels of homocysteine (natural logarithm transformed) were investigated by stepwise multiple linear regression analysis. In multiple linear models, the dependent variable is continuous (fitting normal distribution) and the independent variables are either continuous, or categorical variables. Regression models were adjusted for age and sex (Model 1) and additionally for hypertension, DM, current smoking, BMI, eGFR, and levels of serum TG, TC, HDL-C, LDL-C and hsCRP (Model 2).
In addition, we assessed the association of homocysteine level with carotid-femoral PWV (elevated (≥ 12 m/s) versus normal level (< 12 m/s))[22] by means of logistic regression models. Forward stepwise multiple logistic regression analysis was performed to obtain the odds ratios (OR) and 95% confidence intervals (CI). In multiple logistic models, the dependent variable is carotid-femoral PWV (elevated (≥ 12 m/s) versus normal level (< 12 m/s)) and the independent variables are either continuous, or categorical variables (the same as variables in linear models).
Data were analyzed using Stata software (version 9.0; Stata Corporation, College Station, TX). A two-sided value of P < 0.05 was considered significant.
3. Results
3.1. Study sample characteristics
Altogether, we included 780 participants in the present study. The mean age of participants was 71.9 ± 7.8 years and 46.3% were men. Clinical characteristics of the study sample, values of biomarkers and mean arterial stiffness values are presented in Table 1.
Table 1. Characteristics of the study sample.
| Characteristics | All subjects (n = 780) | Homocysteine groups |
P value | |
| Low (< 15µmol/L, n = 138) | High (≥ 15 µmol/L, n = 642) | |||
| Age, yrs | 71.7 ± 4.7 | 70.7 ± 4.4 | 71.9 ± 4.7 | 0.009 |
| Men, % | 47.3 | 33.3 | 50.3 | < 0.001 |
| BMI, kg/m2 | 22.4 ± 9.0 | 16.9 ± 11.9 | 23.6 ± 7.7 | < 0.001 |
| SBP, mm Hg | 122.1 ± 24.4 | 120.6 ± 28.2 | 122.4 ± 23.5 | 0.438 |
| DBP, mm Hg | 72.3 ± 14.8 | 71.6 ± 15.8 | 72.4 ± 14.5 | 0.551 |
| TC, mmol/L | 5.04 ± 1.00 | 5.18 ± 1.10 | 5.01 ± 0.98 | 0.068 |
| TG, mmol/L | 1.47 (1.09, 2.05) | 1.48 (1.14, 2.01) | 1.46 (1.08, 2.06) | 0.499 |
| HDL-C, mmol/L | 1.37 ± 0.38 | 1.40 ± 0.44 | 1.36 ± 0.36 | 0.307 |
| LDL-C, mmol/L | 3.03 ± 0.76 | 3.11 ± 0.82 | 3.01 ± 0.76 | 0.136 |
| Fasting glucose, mmol/L | 5.37 ± 1.61 | 5.34 ± 1.21 | 5.38 ± 1.68 | 0.819 |
| Homocysteine, µmol/L | 19.1 (16.1, 23.5) | 13.5 (11.5, 14.4) | 20.4 (17.8, 24.8) | < 0.001 |
| hsCRP, mg/L | 0.25 (0.15, 0.38) | 0.26 (0.18, 0.38) | 0.24 (0.14, 0.38) | 0.077 |
| eGFR, mL/min per 1.73 m2 | 84.4 ± 14.2 | 87.2 ± 12.8 | 83.8 ± 14.4 | 0.011 |
| Carotid-femoral PWV, m/s | 12.9 ± 3.8 | 12.2 ± 4.2 | 13.1 ± 3.7 | 0.012 |
| Carotid-radial PWV, m/s | 9.18 ± 2.0 | 9.00 ± 2.42 | 9.21 ± 1.90 | 0.25 |
| Current smoking, % | 27.8 | 19.5 | 29.6 | 0.017 |
| Diabetes, % | 17.7 | 21.1 | 16.9 | 0.116 |
| Hypertension, % | 50.1 | 45.7 | 51.1 | 0.305 |
Data are presented as mean ± SD, percent or median (quartile 1, quartile 3) as indicated. BMI: body mass index; DBP: diastolic blood pressure; eGFR: estimated glomerular filtration rate; HDL-C: high-density lipoprotein cholesterol; hsCRP: homocysteine and high sensitive C reactive protein; LDL-C: low-density lipoprotein cholesterol; PWV: pulse wave velocity; SBP: systolic blood pressure; TC: total plasma cholesterol; TG: triglycerides.
Among participants with high homocysteine levels, numbers with mildly elevated homocysteine levels (≥ 15 µmol/L, < 25 µmol/L), moderately elevated levels (≥ 25 µmol/L, <100 µmol/L) were 487 and 155, respectively. None of the participants had a higher level than 100 µmol/L homocysteine.
3.2. Difference of arterial stiffness measures in subjects with different homocysteine levels
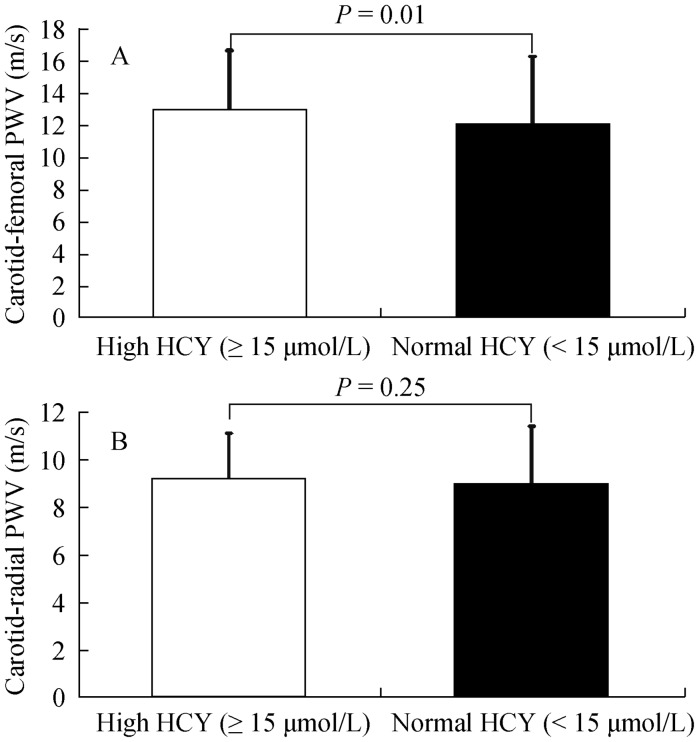
The carotid-femoral PWV was significantly higher in the high homocyteine group than in the normal homocysteine group (P = 0.01, Figure 1A), however, there was no differences in carotid-radial PWV between the high homocyteine group and the normal homocysteine group (Figure 1B).
Figure 1. Carotid-femoral (A) and carotid-radial (B) pulse wave velocity (PWV) in high and normal homocysteine (HCY) group.
3.3. The relationship between homocysteine and arterial stiffness measurements
Homocysteine levels were significantly related to carotid-femoral PWV (P < 0.001) by linear regression analysis, whereas no association was found with carotid-radial PWV (Table 2). In the adjusted models (1 and 2), the relationship between homocysteine and carotid-femoral PWV remained statistically significant (P < 0.003). According to demographic and clinical indices, age, gender and mean arterial pressure were the most important influencing factors for the arterial stiffness measurement (Table 2).
Table 2. Linear regression analysis for the association between arterial stiffness measures and levels of homocysteine in the elderly.
| Measures of arterial stiffness |
|||||||
| Carotid-femoral PWV |
Carotid-radial PWV |
||||||
| β (95% CI) | Standard β | P Value | β (95% CI) | Standard β | P Value | ||
| Unadjusted | 1.36 (0.61–2.12) | 0.13 | < 0.001 | 0.39 (–0.01–0.79) | 0.07 | 0.053 | |
| Model 1 | 1.19 (0.41–1.97) | 0.11 | 0.003 | 0.24 (–0.17–0.65) | 0.04 | 0.25 | |
| Model 2 | 1.52 (0.69–2.35) | 0.14 | < 0.001 | 0.32 (–0.11–0.76) | 0.06 | 0.114 | |
Carotid-femoral PWV, carotid-radial PWV and levels of homocysteine were considered as continuous variable; levels of homocysteine were natural logarithm transformed. Model 1 is adjusted for age and sex; Model 2 is additionally adjusted for hypertension, diabetes mellitus, current smoking, body mass index, the estimated glomerular filtration rate and levels of serum triglycerides, total plasma cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol and homocysteine and high sensitive C reactive protein. PWV: pulse wave velocity.
In addition, the association of homocysteine levels with elevated carotid–femoral PWV was stronger than that observed with normal carotid-femoral PWV (P < 0.01). In the adjusted models (1 and 2), the relationship remained statistically significant (Table 3).
Table 3. Logistic regression analysis for the association between carotid-femoral PWV and levels of homocysteine in the elderly.
| Carotid-femoral PWV |
||
| Odds Ratio (95% CI) | P Value | |
| Unadjusted | 1.79 (1.17–2.74) | 0.008 |
| Model 1 | 1.66 (1.07–2.58) | 0.025 |
| Model 2 | 1.71 (1.06–2.77) | 0.028 |
Odds ratios for Carotid-femoral PWV are elevated (≥ 12 meters/s) versus normal levels (< 12 meters/s). Model 1 is adjusted for age and sex. Model 2 is additionally adjusted for hypertension, diabetes mellitus, current smoking, body mass index, the estimated glomerular filtration rate, levels of serum triglycerides, total plasma cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, homocysteine and high sensitive C reactive protein. PWV: pulse wave velocity.
4. Discussion
This is the first study to investigate the relationship between homocysteine and arterial stiffness in a community- dwelling, elderly sample. From the present study, we found that serum homocysteine concentration was positively associated with central artery stiffness (carotid-femoral PWV), while levels of homocysteine were not related with peripheral artery stiffness (carotid-radial PWV) in the elderly subjects. Importantly, homocysteine was an independent predictor of central arterial stiffness in the older population after controlling for age, gender as well as other conventional cardiovascular risk factors. Furthermore, elevated carotid-femoral PWV showed a stronger association with homocysteine levels than normal carotid-femoral PWV.
Few studies have investigated the association of homocysteine with arterial stiffness in the elderly. In a population based sample of 376 middle-aged and elderly men, Nakhai-Pour, et al.[17] found that homocysteine concentration was associated with large artery stiffness and thickness in crude analysis but not after adjustment for classical risk factors of cardiovascular disease. Potential explanations for the findings of Nakhai-Pour could be that the study had modest sample size and was exclusively performed in men. Recently, van Dijk, et al.[18] concluded that the homocysteine level was independently and significantly associated with aortic stiffening in the elderly and particularly in the oldest old subjects from the cross-sectional B-PROOF (B-Vitamins for the PRevention Of Osteoporotic Fractures) trial. However, the B-PROOF trial included only participants with hyperhomocysteinemia. In the present study we observed a consistent and strongly positive association of homocysteine with aortic (carotid-femoral) PWV based on a large community-based cohort of older subjects who were not selected on gender difference, or the basis of biomarker levels.
The possible mechanisms which may explain the relationship between hyperhomocysteinemia and aortic stiffness are not yet fully well established. Main hypotheses are that homocysteine plays a potential role in remodeling of the arterial wall leading to vascular damage. Elevated homocysteine levels enhance oxidative stress and inflammation of vascular endothelial cells, reduce the production and bioavailability of nitric oxide, a strong relaxing factor by the endothelium.[23] Hyperhomocysteinemia induced endothelial dysfunction may further stimulate vascular smooth muscle cell proliferation and matrix deposition of sulfated glycosaminoglycans, which impair endothelium-dependent vasodilatation.[16] Increased homocysteine levels also promote platelet adhesion to endothelial cells and are associated with higher levels of prothrombotic factors, such as β-thromboglobulin, tissue plasminogen activator and factor VIIc, which lead to the augmentation of thrombus formation.[24] In addition, Yun, et al.[25] showed that enhanced arterial stiffness in hyperhomocysteinemia might be attributed, in part, to homocysteine-related LDL atherogenicity, such as small LDL particle size and oxidative modification of LDL.
There are several potential factors which may explain the relation between homocysteine and central artery stiffness in elderly. Gori, et al.[26] observed that in subjects aged ≥ 65 years, interleukin 6 (IL-6) and interleukin 1 receptor antagonist were independent predictors of homocysteine concentration. This mild proinflammatory state may induce the structural and functional alteration of the arterial wall. In addition, an experimental study had demonstrated that low folate concentrations resulted in elevation of homocysteine, which may combine with the elevated homocysteine and contributed to arterial permeability and stiffness.[27] In elderly individuals, low to low-normal concentrations or deficiencies of folate resulting from a number of factors (eg., reduced intake, impaired absorption, interactions with medications) are not uncommon. Moreover, age itself, as the most important cardiovascular risk factor, strengthened the ill effects of homocysteine on central artery stiffness.
The effects of homocysteine on central, predominantly elastic artery, and peripheral, predominantly muscular artery are different. It may be attributed to the different structural and functional properties of the arterial wall within the arterial tree. In a experimental study of minipigs, mild hyperhomocysteinemia was found to cause an arterial, site-dependent deterioration of the elastic structure involving metalloproteinase-related elastolysis.[28] Nevertheless, future research will be necessary to investigate the underlying mechanisms.
In this study, we observed that the associations between homocysteine and aortic stiffness were stronger in subjects with elevated carotid-femoral PWV than with normal carotid-femoral PWV. This result might be explained by the fact that elevated central artery PWV is related to more cardiovascular risk factors, and all together, increase the susceptibility of the arterial wall to the influence of elevated homocysteine.
There are several strengths to our study. First, to the best of our knowledge, the present investigation is the first large community-based study evaluating the association of homocysteine with alterations in arterial stiffness in a relatively large sample of elderly subjects. Second, unlike the previous studies that selected subjects on either gender or homocysteine level differences, the present study was based on a sample in which selection bias was inherently low. Third, well-characterized cardiovascular risk factors enable adjustment for a wide range of other covariates that may have confounded the association of biomarkers with vascular data.
However, the present study had several potential limitations. First, the present study was performed in Chinese residents from two communities in Beijing, thus the results may not represent Chinese from other areas of China, and might not be applicable to other ethnic groups who may have a different pattern in biomarkers, or in vascular stiffness. Second, this cross-sectional study was not designed to establish causal relationship, which should be confirmed by longitudinal and interventional studies. Finally, whereas the results were adjusted for multiple covariates that may be associated with circulating homocysteine levels, or with altered vascular properties, the possibility of the existence of residual confounding factors remains, such as the profile of vitamin B12 and folic acid, hereditary factors, and several negative lifestyle factors (e.g., alcohol intake and low physical activity).
In conclusion, this study demonstrated a clear association between homocysteine level and aortic stiffness in elderly, indicating that homocysteine may be involved in artery stiffening in older population. Considering the cross-sectional design of the present study, the contributions of serum homocysteine measurement in risk assessment and risk reduction strategies in older population requires further investigation by prospective, interventional studies.
Acknowledgments
The authors have no conflicts of interest to declare. This work was supported by grants from the Key National Basic Research Program of China (2013CB530804) and Nature Science Foundation of China (81270941) to Ye P, and the Nature Science Foundation of China (81100878) and the Beijing Nova Program (Z121107002513124) to Bai Y.
References
- 1.Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. 2010;55:1318–1327. doi: 10.1016/j.jacc.2009.10.061. [DOI] [PubMed] [Google Scholar]
- 2.Mattace-Raso FU, van der Cammen TJ, Hofman A, et al. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam Study. Circulation. 2006;113:657–663. doi: 10.1161/CIRCULATIONAHA.105.555235. [DOI] [PubMed] [Google Scholar]
- 3.Matsuoka O, Otsuka K, Murakami S, et al. Arterial stiffness independently predicts cardiovascular events in an elderly community—Longitudinal Investigation for the Longevity and Aging in Hokkaido County (LILAC) study. Biomed Pharmacother. 2005;59:40–44. doi: 10.1016/s0753-3322(05)80008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meaume S, Benetos A, Henry OF, et al. Aortic pulse wave velocity predicts cardiovascular mortality in subjects > 70 years of age. Arterioscler Thromb Vasc Biol. 2001;21:2046–2050. doi: 10.1161/hq1201.100226. [DOI] [PubMed] [Google Scholar]
- 5.Asmar R, Benetos A, Topouchian J, et al. Assessment of arterial distensibility by automatic pulse wave velocity measurement. Validation and clinical application studies. Hypertension. 1995;26:485–490. doi: 10.1161/01.hyp.26.3.485. [DOI] [PubMed] [Google Scholar]
- 6.Benetos A, Waeber B, Izzo J, et al. Influence of age, risk factors, and cardiovascular and renal disease on arterial stiffness: clinical applications. Am J Hypertens. 2002;15:1101–1108. doi: 10.1016/s0895-7061(02)03029-7. [DOI] [PubMed] [Google Scholar]
- 7.Aronow WS, Ahn C. Increased plasma homocysteine is an independent predictor of new coronary events in older persons. Am J Cardiol. 2000;86:346–347. doi: 10.1016/s0002-9149(00)00931-0. [DOI] [PubMed] [Google Scholar]
- 8.Kuo HK. Relationship between homocysteine and cardiovascular diseases in older adults. J Am Geriatr Soc. 2004;52:1955–1956. doi: 10.1111/j.1532-5415.2004.52526.x. [DOI] [PubMed] [Google Scholar]
- 9.Wong YY, Golledge J, Flicker L, et al. Plasma total homocysteine is associated with abdominal aortic aneurysm and aortic diameter in older men. J Vasc Surg. 2013;58:364–370. doi: 10.1016/j.jvs.2013.01.046. [DOI] [PubMed] [Google Scholar]
- 10.Bates CJ, Mansoor MA, Pentieva KD, et al. Biochemical risk indices, including plasma homocysteine, that prospectively predict mortality in older British people: the National Diet and Nutrition Survey of People Aged 65 Years and Over. Br J Nutr. 2010;104:893. doi: 10.1017/S0007114510001236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Welch GN, Loscalzo J. Homocysteine and atherotrombosis. N Engl J Med. 1998;338:1042–1050. doi: 10.1056/NEJM199804093381507. [DOI] [PubMed] [Google Scholar]
- 12.Tawakol A, Omland T, Gerhard M, et al. Hyperhomocysteinemia is associated with impaired endothelium-dependant vasodilatation in humans. Circulation. 1997;95:1119–1121. doi: 10.1161/01.cir.95.5.1119. [DOI] [PubMed] [Google Scholar]
- 13.Charpiot P, Bescond A, Augier T, et al. Hyperhomocysteinemia induces elastolysis in minipig arteries: structural consequences, arterial site specificity and effect of captoril–hydroclorothiaszide. Matrix Biol. 1998;17:559–574. doi: 10.1016/s0945-053x(98)90108-1. [DOI] [PubMed] [Google Scholar]
- 14.Feng SQ, Ye P, Luo LM, et al. Associations of plasma homocysteine and high-sensitivity C-reactive protein levels with arterial stiffness in Chinese population: a community-based study. Chin Med J (Engl) 2012;125:44–49. [PubMed] [Google Scholar]
- 15.Mayer O, Filipovský J, Dolejsová M, et al. Mild hyperhomocysteinaemia is associated with increased aortic stiffness in general population. J Hum Hypertens. 2006;20:267–271. doi: 10.1038/sj.jhh.1001983. [DOI] [PubMed] [Google Scholar]
- 16.Vyssoulis G, Karpanou E, Kyvelou SM, et al. Associations between plasma homocysteine levels, aortic stiffness and wave reflection in patients with arterial hypertension, isolated office hypertension and normotensive controls. J Hum Hypertens. 2009;24:183–189. doi: 10.1038/jhh.2009.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakhai-Pour HR, Grobbee DE, Bots ML, et al. Circulating homocysteine and large arterial stiffness and thickness in a population-based sample of middle-aged and elderly men. J Hum Hypertens. 2007;21:942–948. doi: 10.1038/sj.jhh.1002247. [DOI] [PubMed] [Google Scholar]
- 18.van Dijk SC, Smulders YM, Enneman AW, et al. Homocysteine level is associated with aortic stiffness in elderly: cross-sectional results from the B-PROOF study. J Hypertens. 2013;31:952–959. doi: 10.1097/HJH.0b013e32835eb6b9. [DOI] [PubMed] [Google Scholar]
- 19.Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 20.Asmar R, Benetos A, Topouchian J, et al. Assessment of arterial distensibility by automatic pulse wave velocity measurement. Validation and clinical application studies. Hypertension. 1995;26:485–490. doi: 10.1161/01.hyp.26.3.485. [DOI] [PubMed] [Google Scholar]
- 21.Ueland PM, Refsum H, Stabler SP, et al. Total homocysteine in plasma or serum: methods and clinical applications. Clin Chem. 1993;39:1764–1779. [PubMed] [Google Scholar]
- 22.Blacher J, Guérin AP, Pannier B, et al. Impact of aortic stiffness on survival in end-stage renal disease. Circulation. 1999;99:2434–2439. doi: 10.1161/01.cir.99.18.2434. [DOI] [PubMed] [Google Scholar]
- 23.Spoelstra-De Man AM, Smulders YM, Dekker JM, et al. Homocysteine levels are not associated with cardiovascular autonomic function in elderly Caucasian subjects without and with type 2 diabetes mellitus: Hoom study. J Intern Med. 2005;258:536–543. doi: 10.1111/j.1365-2796.2005.01578.x. [DOI] [PubMed] [Google Scholar]
- 24.Weir DG, Scott JM. The biochemical basis of neuropathy in cobalamin deficiency. Bailliere's Clin Heamatol. 1995;8:479–497. doi: 10.1016/s0950-3536(05)80217-3. [DOI] [PubMed] [Google Scholar]
- 25.Yun J, Kim JY, Kim OY, et al. Associations of plasma homocysteine level with brachial-ankle pulse wave velocity, LDL atherogenicity, and inflammation profile in healthy men. Nutr Metab Cardiovasc Dis. 2011;21:136–143. doi: 10.1016/j.numecd.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 26.Gori AM, Corsi AM, Fedi S, et al. A proinflammatory state is associated with hyperhomocysteinemia in the elderly. Am J Clin Nutr. 2005;82:335–341. doi: 10.1093/ajcn.82.2.335. [DOI] [PubMed] [Google Scholar]
- 27.Symons JD, Zaid UB, Athanassious CN, et al. Influence of folate on arterial permeability and stiffness in the absence or presence of hyperhomocysteinemia. Arterioscler Thromb Vasc Biol. 2006;26:814–818. doi: 10.1161/01.ATV.0000204408.01416.16. [DOI] [PubMed] [Google Scholar]
- 28.Bortolotto LA, Safar ME, Billaud E, et al. Plasma homocysteine, aortic stiffness, and renal function in hypertensive patients. Hypertension. 1999;34:837–842. doi: 10.1161/01.hyp.34.4.837. [DOI] [PubMed] [Google Scholar]