Abstract
Background
Brain natriuretic peptide (BNP) is normally present in low levels in the circulation, but it is elevated in parallel with the degree of congestion in heart failure subjects (CHF). BNP has natriuretic effects and is a potent vasodilator. It is suggested that BNP could be a therapeutic alternative in CHF. However, we postulated that the high levels of circulating BNP in CHF may downregulate the response of microvascular natriuretic receptors. This was tested by comparing 15 CHF patients (BNP > 3000 ng/L) with 10 matched, healthy controls.
Methods
Cutaneous microvascular blood flow in the forearm was measured by laser Doppler Flowmetry. Local heating (+44°C, 10 min) was used to evoke a maximum local dilator response.
Results
Non-invasive iontophoretic administration of either BNP or acetylcholine (ACh), a known endothelium-dependent dilator, elicited an increase in local flow. The nitric oxide synthase inhibitor, l-N-Arginine- methyl-ester (L-NAME), blocked the BNP response (in controls). Interestingly, responses to BNP in CHF patients were reduced to about one third of those seen in healthy controls (increase in flow: 251% in CHF vs. 908% in controls; P < 0.001). In contrast, the vasodilator responses to ACh and to local heating were only somewhat attenuated in CHF patients. Thus, dilator capacity and nitric oxide signalling were not affected to the same extent as BNP-mediated dilation, indicating a specific downregulation of the latter response.
Conclusions
The findings show for the first time that microvascular responses to BNP are markedly reduced in CHF patients. This is consistent with the hypothesis of BNP receptor function is downregulated in CHF.
Keywords: Heart failure; Cutaneous microcirculation; Endothelial responses; Acetylcholine, Brain natriuretic peptide; Nitric oxide
1. Introduction
Patients with congestive heart failure exhibit a number of cardiac conditions both structural and functional such as left-ventricular systolic and diastolic dysfunction, abnormal right-ventricular size and function, valvular heart disease, pulmonary artery hypertension and arterial fibrillations.[1] The outcome of acute heart failure is still dismal with high rates of early death and rehospitalisation and there is no major improvement over the past decades.[2] The myocyte stress from volume overload and myocardium wall stretch triggers natriuretic peptide gene expression and the release of atrial and brain natriuretic peptides (ANP/BNP) and pro-brain natriuretic peptide (NT-proBNP).[3] The biology of ANP/BNP and NT-proBNP strongly indicate that they are suitable as objective tools for monitoring and managing patients with chronic HF.[1]
We have shown in previous publications that a single measurement of plasma NT-proBNP correlates well with the prognosis and survival outcome in congestive heart failure (CHF) patients.[4] In agreement Januzzi and colleagues consider BNP and NT-proBNP measurements to be the current gold-standard biomarkers for prognosis in chronic CHF.[3] Recent clinical practice guidelines also imply that serial measurements of BNP and NT-proBNP provide valuable information regarding the progression of CHF disease, need for hospitalization and mortality.[1]
We have previously studied the peripheral microcirculation as a surrogate for microvascular changes in subjects with different degrees of CHF and of different ages.[5],[6] The cutaneous microvascular responses to iontophoretic administration of the endothelium-dependent dilatators acetylcholine (ACh) and the endothelium-independent vasodilator sodium nitroprusside (SNP) were reduced in CHF.[5] These responses were also attenuated by the age of the subjects; hence it is important to perform experiments in well-matched clinical groups.
Because of the need of novel strategies it has been suggested that BNP could serve as a “novel” type of pharmacological treatment to reduce overload in CHF patients.[3],[7] Thus, recombinant BNP (Nesiritide) has shown vasodilator properties and is approved for use in acute CHF.[8],[9] However, a recent large randomized study showed in patients with acute decompensated heart failure no change in rate of death and hospitalization, and had no significant effect on dyspnoea.[7] Because of highly elevated levels of endogenous BNP in severe CHF, we hypothesize that administration of exogenous BNP would be unsuitable for treatment of severe heart failure patients probably due to downregulation of natriuretic receptor function. It is known that BNP receptors can downregulate following constant exposure to BNP,[10] and this may occur in CHF patients.
The aim of this study was to investigate if responses to BNP in the peripheral microcirculation are altered in CHF patients. We also compared vasodilatory blood flow responses of BNP with that of ACh and local heat.
2. Methods
2.1. Study population
Group 1 consisted of 15 patients with CHF, 9 men and 6 women, mean age of 77.8 years. They were diagnosed earlier with chronic CHF. Due to worsening of the condition, they were admitted to the emergency ward clinic at Lund University hospital, Lund University, Sweden, with New York Heart Association (NYHA) class III/IV symptoms and NT-pro-BNP levels ≥ 3000 ng/L.
Group 2 consisted of 10 healthy elderly age- and gender-matched subjects recruited from the community registry. These subjects had a mean age of 78.8 years of age. Their NT-pro-BNP levels were in the normal range; varying between 50 ng/L and 450 ng/L. They did not take any medication for cardiovascular disease. For demographic details of the two groups of subjects see Table 1. We found no difference between them in general parameters.
Table 1. The demographics of congestive heart failure patients vs. healthy subjects.
| Heart failure n = 15 | Healthy n = 10 | |
| Sex (F/M) | 6/9 | 5/5 |
| Age | 77.8 ± 1.5 (77–89) | 78.8 ± 1.2 (72–85) |
| BMI (kg/m2) | 26.5 ± 1.47 (18–40) | 23.2 ± 1.0 (20–29) |
| Systolic BP (mmHg) | 125.0 ± 4.8 | 131.8 ± 5.1 |
| Diastolic BP (mmHg) | 72.1 ± 4.0 | 73.3 ± 2.6 |
| Pulse/min | 79 (67–92) | 67 (55–72) |
Data given as mean ± SE, and/or range in parenthesis. No statistical differences with Mann-Whitney's nonparametric test were found between Heart Failure and Healthy subjects. BMI: Body mass index; BP: blood pressure.
The chronic congestive heart failure patients had reduced left ventricular function as assessed by echocardiography and were all non-current smokers when entering the clinical study to avoid any effects on flow measurements.[5] All patients were kept on their prescribed medication but refrained from long-lasting nitrates 6 h before the Laser Doppler blood flow measurement. No other co-morbidity resulted in exclusion of participation in the study; only tremor was considered not suitable for the laser Doppler blood flow method. For demographic details on the subjects, see Table 2.
Table 2. Medical history and treatment of chronic congestive heart failure patients.
| Heart failure n = 15 | Healthy n = 10 | |
| NYHA III | 5 | N/A |
| NYHA IV | 10 | N/A |
| Pharmacological treatment | ||
| Beta-adrenoreceptor antagonists | 13/15 | N/A |
| ACE-inhibitors | 11/15 | N/A |
| ARB | 3/15 | N/A |
| Diuretics | 14/15 | N/A |
| Digoxin | 1/15 | N/A |
| Spironolactone | 0/15 | N/A |
| ASA | 8/15 | N/A |
| Warfarin | 9/15 | N/A |
| Chest X-ray | ||
| Pulmonary oedema | 11/15 | N/A |
| Electrocardiogram | ||
| Atrial fibrillation | 12/15 | N/A |
| Pacemaker | 4/15 | N/A |
| QRS complex width (ms) | 115.5 ± 8.6 | N/A |
| Ejection fraction (%) | 37.7 ± 1.9 | N/A |
ACE: angiotensin converting enzyme; ARB: angiotensin receptor blockers; ASA: acetylsalicylic acid; N/A: not applicable; NYHA: New York Heart Association classification.
2.2. Ethics
The investigation conformed to the principles outlined in the Declaration of Helsinki, Seoul 2008. The Ethics Committee of Lund University approved of the protocol (No: 2012/224). Written informed consent was obtained from all patients and healthy controls by the investigators before they were entered into the study and this was verified in the electronic medical charts.
2.3. Clinical parameters
Hemodynamic measurements consisted of arterial blood pressure and heart rate. Blood pressure was measured non- invasively in the supine position from the upper left arm with the cuff inflated at heart level. Blood pressure was taken after the blood flow measurement when the patients had been resting for about 1 h. The diastolic value was accepted as Korotkoff`s phase V. All blood pressure measurements were taken by the same investigator. Heart rate was counted for 1min (Tables 1 & 2).
2.4. Blood analysis
Plasma levels of inflammatory markers, C-reactive protein (CRP), cytokines; interleukin-6 (IL-6) and soluble IL-2 receptor (s-IL2r) were measured as well as NT-proBNP (P-NT-proBNP on Cobas, NPU21571, Roche Diagnostics, Switzerland), and blood glucose levels. In addition, plasma levels of haemoglobin (Hb), sodium, potassium, creatinine and cystatin-C, and uric acid were analyzed at the Department of Clinical Chemistry and Pharmacology, Lund University Hospital. IL was measured at the Clinical Immunology laboratory at Lund University Hospital. All blood samples were obtained from peripheral venous access in heart failure patients and in the controls and measured by validated techniques. For details see Table 3.
Table 3. Laboratory blood analysis (mean ± SE).
| Heart failure n = 15 | Healthy n = 10 | |
| NT-proBNP, ng/L | 5286 ± 893* | 251 ± 85 |
| Hemoglobin, g/L | 121 ± 3.6 | 130 ± 1.5 |
| Sodium, mmol/L | 141 ± 1.1 | 141 ± 0.6 |
| Potassium, mmol/L | 4.0 ± 0.1 | 4.2 ± 0.1 |
| Creatinine, μmol/L | 132.2 ± 12.1# | 78.8 ± 4.1 |
| Uric acid, μmol/L | 579 ± 40# | 281 ± 21 |
| CRP, mg/L | 15.0 ± 3.7# | 1.4 ± 0.3 |
| IL-6, ng/L | 28.2 ±10.6# | 3.6 ± 0.5 |
| IL-2r, kU/L | 967 ± 131# | 406 ± 39 |
| eGFR, mL/min | 42.1 ± 4.6* | 64.8 ± 4.2 |
CRP: sensitive C reactive protein; eGFR: estimated glomeruli filtration rate (Cockcroft-Gaults adults); IL: interleukin; IL-2r: soluble IL 2 receptor; NT-proBNP: nerve terminal-pro-brain natriuretic peptide. Statistical analysis was performed using the non-parametric Mann-Whitney's test. *P < 0.05, #P < 0.01 compared to healthy subjects.
2.5. Blood flow measurements
Cutaneous blood flow was measured using the PeriFlux system 5000 (Perimed, Järfälla, Sweden). This method is non-invasive and gives minimal discomfort to the patients which make it suitable for severely ill patients at bedside.[11] Laser-generated light at a wavelength of 780 nm is directed to the skin using a fibre optic probe. The light reflected from moving blood cells in the superficial skin microvessels undergoes a shift in frequency (Doppler Effect) that is proportional to the number and velocity of moving blood cells. The laser-Doppler output is semi-quantitative, and we have presented all data as the percentage change compared with the baseline perfusion value. Temperature of the skin was recorded continuously.
2.6. Laser Doppler calculation
Light is transmitted to the tissue via a fibre-optic probe. When the light hits moving blood cells, it undergoes a change in wavelength (Doppler shift). The magnitude and frequency distribution of these changes are directly related to the number and velocity of blood cells, i.e., the blood perfusion. Measurements are expressed in arbitrary Perfusion Units (PU). Full linear correlation to absolute perfusion value is achieved using Perimed's analysis technology (including a linearization function to avoid underestimation in highly perfused tissues) and calibration using automatic instrument zeroing and Perimed's Motility Standard. The responses are expressed as the maximum percent change in PU from baseline flow to the iontophoretic administration of ACh and BNP. The perfusion change after local heating (e.g., +44 °C) is a measure of the tissue reserve capacity.
2.7. Iontophoresis
Constant current iontophoresis was used to enhance the perfusion of charged molecules into the skin of the dorsal side of the lower arm. The PeriIont System (Perimed) used in this study consists of an applicator with a small recess in the centre and a circular temperature probe surrounding the application site. The recess in the centre allows the insertion of a fibre optic probe to measure the blood flow in the stimulated area. An additional temperature probe containing a fibre optic probe was placed at a distance of 10–15 cm and to avoid large veins. This was used as a reference during the iontophoresis and was subsequently used to determine the response to local warming.
Endothelium-dependent vasodilatation was evoked by iontophoresis of ACh (2% dissolved in MilliQ water; Sigma, St. Louis, MO, USA.) using anodal current to deliver the positively charged molecule. BNP-32 human (Batch No.1A, TOCRIS bioscience, UK, 0.05% dissolved in MilliQ water) is also a positively charged molecule and was delivered using the anodal current.
2.8. Protocol
All studies were performed at room temperature (+22– 24 °C). For the severely ill CHF patients, the measurements were obtained at bedside at the hospital internal medicine ward. For the healthy subjects, blood flow measurements were carried out at the emergency medicine ward, MAVA, Lund University Hospital, Lund, Sweden. All subjects were resting in a supine position. Blood pressure and heart rate were measured before and after stimulation and the lowest value is given. The skin of the dorsal lower arm was gently cleansed and the iontophoretic applicators/fibre optic probes were applied to the forearm resting on a pillow to give comfort and provide stabilization. The basal blood flow was studied for 2 min after which ACh was transferred by iontophoresis (anodal current, 0.2 mA for 20 s). The current alone did not affect the blood flow (results not shown). The protocol was based on our previous studieswhen we determined that successive iontophoretic stimuli at 60 s intervals, produces a cumulative stimuli-response curve.[6] We measured the maximum response after 5 stimuli. The vasodilatory effect was studied by iontophoresis of BNP as above (anodal current, 0.2 mA for 60 s). The stimulation was repeated 4 times at 60 s intervals. Finally, the response to heat was measured following local warming to +44 °C for 10 min. This response was considered as maximum vasodilatation in the microvessels of the skin using this technique.
L-N-Arginine-methyl-ester (L-NAME, 2%, Sigma, USA) was administered by iontophoresis to 3 healthy persons by a separate protocol to test for effects on the BNP response. First, BNP 0.05% was given with 0.2 mA current for 1 min and repeated for 4 stimulations. After that, L-NAME was given with 0.1 mA current, for 1 min and repeated 4 times on the dorsal side of the lower arm skin area. Then BNP was administered once more and with the same procedure as above and on the same probe site of skin area where L-NAME was given.
2.9. Statistical analysis
Statistical analysis was performed by Mann-Whiney U test. Statistical differences with a P value < 0.05 or less were considered significant. Calculations were performed using GraphPad Prism 5 (GraphPad Software, La Jolla, CA).
3. Results
3.1. Basic characteristics
There was no significant difference between the two groups, the healthy aged and the heart failure patients regarding gender, age, body mass index (BMI), pulse rate and blood pressure (Table 1).
The heart failure patients consisted of 5 subjects with NYHA III and 10 subjects with NYHA IV. The healthy group did not have any cardiovascular diagnosis. The diagnosis of the heart failure subjects were based on symptoms but also in 11/15 patients, a chest X-ray showed significant pulmonary oedema. ECG showed atrial fibrillation in 12/15 of the subjects. The ejection fraction was reduced and showed a mean of 37.7%. Treatment in this group of CHF patients was dominated by ACE inhibitors, beta-adrenoceptor blockers, diuretics, acetylsalicylic acid (ASA) or warfarin. None of the healthy aged controls had these medications (Table 2).
As expected, blood samples showed much higher levels of NT-proBNP in the CHF group (5286 ± 893 ng/L) as compared to the healthy controls (251 ± 85 ng/L; P < 0.05). The CHF group also showed significantly higher levels of CRP (P < 0 .001), creatinine (P < 0.01), uric acid (P < 0.001), IL-6 (P < 0.001) and s-IL2r (P < 0.001). These represent indications of a pro-inflammatory state in CHF subjects (Table 3). The calculated absolute glomerular filtration rate[12] was 42.1 ± 4.6 mL/min in NYHA III/IV and 64.8 ± 4.2 mL/min in the aged healthy controls (P < 0.05).
3.2. Microvascular responses
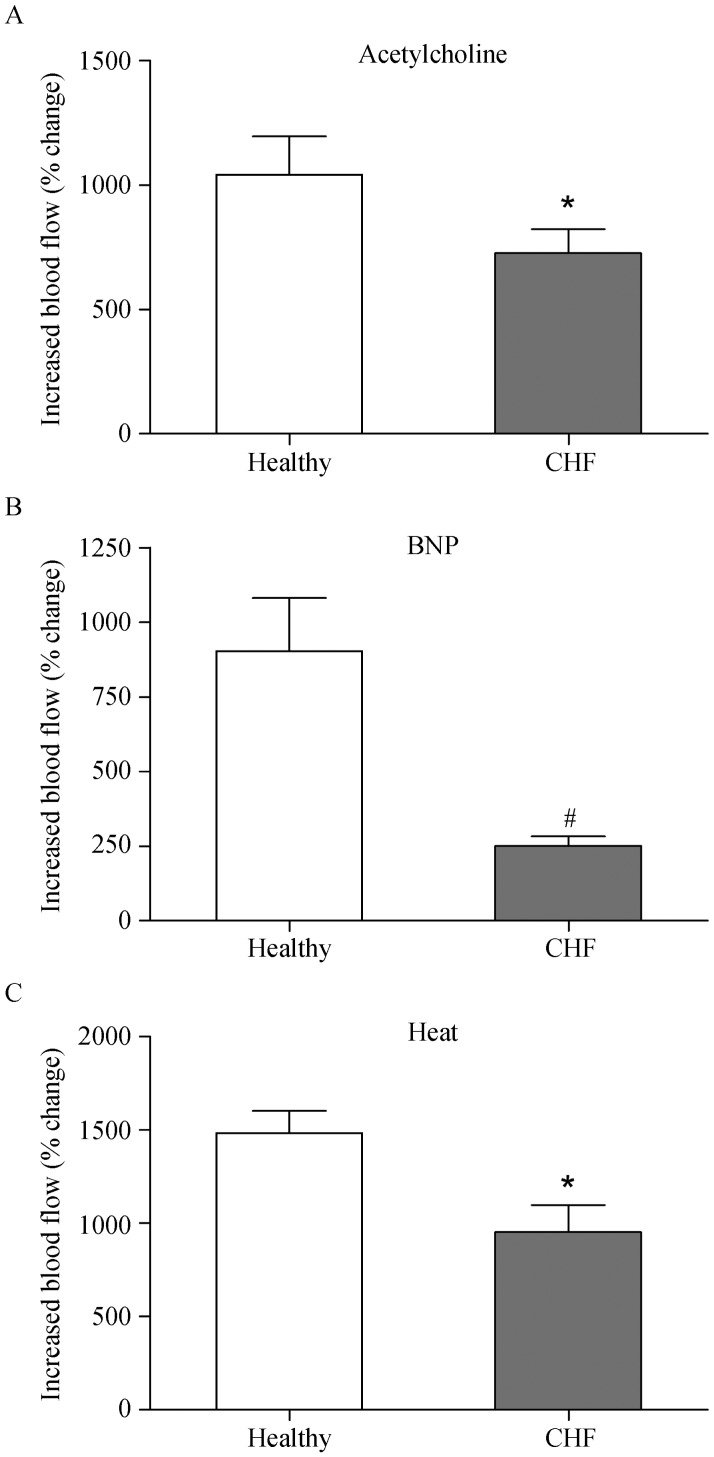
The basal laser Doppler flow values at rest did not differ between the two groups (P > 0.05). The increase in local skin temperature (heat) caused a maximum dilatation and this was used as a way to cause near maximum dilation of the local cutaneous vascular bed.[6] We found that the response to heat was an increase in blood flow of 1484% ± 118% relative to baseline in the healthy controls. In the CHF group, the response to heat was reduced (959% ± 114%; P < 0.05) as compared to healthy controls.
ACh stimulates the release of nitric oxide (NO) and results in subsequent dilatation of cutaneous blood vessels. Following iontophoretic application of ACh, the control subjects showed a mean increase in blood flow of 1041% ± 154%; the CHF patients showed a lower mean increase of 727% ± 96% (P < 0.05).
The responses to BNP in the controls were more variable than responses to ACh (Figure 1). Flow was increased by 908% ± 178% in the healthy controls. There was a markedly lower response to BNP in the CHF patients (251% ± 32%; P < 0.001) as compared to controls.
Figure 1. Microvascular relaxant responses in healthy individuals (n = 10) and of patients with congestive heart failure of NYHA class III-IV (n = 15).
(A): Endothelium-dependent responses to acetylcholine; (B): Relaxation response to BNP; (C): general vasodilator response to local heating to +44 °C. Values represent mean ± SE; *P < 0.05, #P< 0.001 relative to healthy age-matched controls. BNP: brain natriuretic peptide; CHF: congestive heart failure.
In order to examine if BNP mediates its effects via an endothelial mechanism, the NO synthase inhibitor L-NAME was tested. The relaxant response to iontophoretically administered BNP was tested in the microcirculation before and after L-NAME treatment. We found that BNP (studied in 3 healthy subjects) elicited an increase of blood flow of 1280% ± 127%. L-NAME alone had almost no effect (25% ± 5% change in PU). When BNP was given in the presence of L-NAME, its effect was markedly reduced (109% ± 9% increase in flow; P < 0.05 compared to BNP alone). These data show that the vasorelaxation of BNP is dependent on production of NO.
3.3. Gender aspects
There were no significant differences in the relaxant responses to ACh, BNP or heat between males and females in either the healthy controls or in the CHF subjects. For example ACh, increased flow by 751% in male patients and 692% in females. For BNP in CHF subjects, the flow response was 356% for men and 303% for women.
4. Discussion
The present study shows for the first time that patients with severe CHF have reduced microvascular reactivity to BNP. To study this, we used a non-invasive method combining iontophoresis drug administration with laser Doppler flow measurements to assess the vasomotor reactivity of cutaneous blood vessels in ill and healthy elderly subjects.[11] The CHF patients showed highly elevated blood levels of NT-proBNP, in agreement with what we and others have found previously, originating from their enhanced formation and release from stressed myocytes in the hearts of CHF patients. The findings are consistent with the hypothesis that chronic exposure to circulating BNP downregulates the vasodilator response to BNP in the peripheral vasculature of CHF patients. Kuhn, et al.[13] observed in myocardial biopsies from CHF patients that ANP and BNP were markedly elevated (30-fold) while the natriuretic peptide receptor type C was only 4-fold increased. This may seem in opposition to our observation but the function of the regulatory element guanylyl cyclase-A (GC-A) was abolished in severe CHF.[13] The findings suggest that therapeutic use of BNP or natriuretic peptide analogues may be limited due to decreased effectiveness in patients with advanced heart failure.
Early studies on BNP showed that it was a dilator of different vessel types in the circulation.[3] Our study is the first to show that BNP also acts as a vasodilator in the cutaneous microcirculation of humans. Under normal conditions or in early stages of CHF, peripheral vasodilatation by BNP is likely beneficial to reduce overload when the heart experiences stress. However, our studies indicate that the effectiveness of this mechanism may decline with advanced heart failure. In situation of left ventricular assisted device use, some reversal may occur which could indicate a way to counterbalance the refractoriness of systemic BNP.[13]
The reduction in BNP-mediated vasodilatation may be due to several interacting mechanisms: (1) BNP may act on all three subtypes of natriuretic peptide receptors (NP-A, -B and -C) so alterations in or more of these may exist. The present study did not quantify their protein expression which is a future avenue. (2) The hypothesis that the vascular natriuretic receptors have been desensitized by the chronic exposure to high levels of circulating hormone in CHF patients is supported by the literature.[13],[14] This may occur at different sites such as at the receptor or on the function of the receptor. BNP acts on NPR-A, NPR-B, NPR-C, guanylate cyclase-linked receptors, and a Gi-protein-linked receptor.[9] The latter receptor is likely responsible for the NO mediated dilation observed in the present study.[1] (3) Although it has been found that CHF induces increase in NPR-C mRNA analysis,[13],[14] this is not equivalent to demonstration of actual receptor reduction because this must be shown by protein or functional quantification. Clearly this fact needs demonstration.
An alternative explanation for the reduced BNP response is that, in elderly subjects, there is a general decline in vascular responsiveness with aging. In a previous study, we showed microvascular relaxant capacity in skin vessels is reduced in healthy elderly people and attenuated further by the presence of heart failure.[6] Nevertheless, the BNP response measured here in the healthy elderly controls was reasonably robust. Moreover, the cutaneous microvasculature of controls, as well as the CHF patients, dilated appropriately to a local heat stimulus. While the heat response was somewhat diminished in the CHF patients relative to controls, it was less affected than the BNP response. This suggests other mechanisms underlie the larger reduction in BNP-mediated vasodilatation about72%, as compared to about 30% in heat or ACh responses.
Another possible reason for the diminished BNP response in CHF patients could be that specific dilator signalling mechanisms are blunted in heart failure. Using the NO synthase inhibitor, L-NAME, we showed here that dilation to BNP was dependent on NO formation in human cutaneous microvessels. L-NAME iontophoresis has recently been validated as a tool to assess NO-mediated dilatation in humans.[15] We have recently performed a study of isolated human subcutaneous arteries where we also find that BNP acts via an endothelium-dependent dilator mechanism (unpublished data). To further assess the NO pathway for vasodilatation, we measured blood flow in response to ACh, a well-established dilator that activates endothelial NO synthase in a manner similar to BNP. There was some reduction in ACh dilation in CHF patients; however, this response was not affected to the same degree as that of BNP in the present study. In a comparative trial of nesiritide and nitroglycerine on the effectiveness of vasodilatation in the management of acute CHF the outcome showed weaker effect of BNP than of nitroglycerine.[9]
CHF subjects are typically of advanced age; in agreement the mean age of the present CHF patients was 77.8 years and for controls 78.8 years, hence well matched. This was important because we have found previously that cutaneous vascular reactivity is reduced by age and is even further attenuated by the presence of heart failure.[6] Gender differences were another consideration; little is known regarding possible sex differences in the peripheral microvasculature of CHF patients. In the present study, there were 9 male and 6 female patients, which is reflective of the gender distribution observed generally in CHF.[16],[17] In the Swedish Heart Failure registry, a large CHF cohort of 50,827 patients, the age group 75–84 years contains 59% males and 41% females.[16],[17] We found no sex differences in cutaneous microvascular Laser Doppler flow in response to BNP, ACh or heat in CHF and control subjects of similar age, hence it is justified to group them together.
The reason for the reduction in responses in the subcutaneous microcirculation could in part be associated with a low degree of inflammation. In our CHF patients the inflammatory cytokines IL-6 and IL-2 receptor, CRP and uric acid were all highly elevated compared to controls. Increases in these circulating markers of inflammation have been reported in earlier studies of CHF.[6],[18] The IL-2 receptor is of particular interest as blood levels of this marker seem to be linked specifically to CHF and may be useful as a diagnostic measure for following the progression of CHF. At this point, we do not know how inflammation may influence the vasodilatory capacity of the microcirculation in CHF, but this point needs further investigation.
4.1. Limitations
Although the number of patients in this study was limited, the values obtained were highly significant. Hence more patients would not change the general concept or conclusions.
Medication is another consideration in evaluating responses of CHF patients. The drug profiles for the CHF patients in this study were in accord with the standard recommendations for this disease. However, because of the severity of their condition, they were not on the optimum doses recommended for CHF treatment. Particularly in elderly subjects, they cannot tolerate the high levels of β-adrenoceptor blockade and other vasodilators due to reductions in many compensatory systems with age. Clearly, many of these drugs may affect the vascular system in general, and thus might have the potential to influence the measurements of microcirculatory blood flow. However, it is unlikely that the specific CHF drugs might influence the differences between reactivity between BNP and ACh/heat.
In evaluating patient responses, it is also important to keep in mind that CHF patients often have co-morbid conditions that could influence the results. Arterial fibrillation is a common co-morbidity in CHF.[17] In CHF group, 12 of the 15 patients had atrial fibrillation, and 4 of these had a pacemaker. One might argue that the controls should have included subjects with atrial fibrillation. However, it is known that such patients may have some degree of elevated circulating natriuretic peptides which does not speak in favour of such a suggestion. Clinical parameters of importance such as the QRS complexes with a width > 120 ms have now been shown to be a marker for severity of heart failure with poor outcome and higher mortality rate in CHF.
4.2. Conclusions
We showed for the first time that the cardiac hormone BNP acts on human cutaneous microvessels to increase blood flow via a NO dependent mechanism. A major finding was that BNP has poor relaxant effects in patients with severe CHF. The mechanism behind this is not known, but may involve receptor downregulation or reduction in GC-A activity with reduced formation of cGMP in response to the elevated circulating levels of BNP in advanced stages of CHF.
Acknowledgments
This studies were supported by the Lisa & Johan Grönbergs Stiftelse, SEB Enskilda Banken, Stockholm, Sweden.
References
- 1.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;128:e240–e319. doi: 10.1161/CIR.0b013e31829e8776. [DOI] [PubMed] [Google Scholar]
- 2.Bueno H, Ross JS, Wang Y, et al. Trends in length of stay and short-term outcomes among Medicare patients hospitalized for heart failure, 1993–2006. JAMA. 2010;303:2141–2147. doi: 10.1001/jama.2010.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Motiwala SR, Januzzi JL., Jr The role of natriuretic peptides as biomarkers for guiding the management of chronic heart failure. Clin Pharmaco Ther. 2013;93:57–67. doi: 10.1038/clpt.2012.187. [DOI] [PubMed] [Google Scholar]
- 4.Andersson SE, Edvinsson ML, Björk J, Edvinsson L. High NT-proBNP is a strong predictor of outcome in elderly heart failure patients. Am J Geriatr Cardiol. 2008;17:13–20. doi: 10.1111/j.1076-7460.2007.06674.x. [DOI] [PubMed] [Google Scholar]
- 5.Edvinsson ML, Uddman E, Andersson SE. Deteriorated function of cutaneous microcirculation in chronic congestive heart failure. J Geriatr Cardiol. 2011;8:82–87. doi: 10.3724/SP.J.1263.2011.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andersson SE, Edvinsson ML, Edvinsson L. Cutaneous vascular reactivity is reduced in aging and in heart failure: association with inflammation. Clin Sci (Lond) 2003;105:699–707. doi: 10.1042/CS20030037. [DOI] [PubMed] [Google Scholar]
- 7.O'Connor CM, Starling RC, Hernandez AF, et al. Effect of nesiritide in patients with acute decompensated heart failure. N Engl J Med. 2011;365:32–43. doi: 10.1056/NEJMoa1100171. [DOI] [PubMed] [Google Scholar]
- 8.Colucci WS, Elkayam U, Horton DP, et al. Intravenous nesiritide, a natriuretic peptide, in the treatment of decompensated congestive heart failure. N Engl J Med. 2000;343:246–253. doi: 10.1056/NEJM200007273430403. [DOI] [PubMed] [Google Scholar]
- 9.Committee for the VMAC investigators Intravenous nesiritide verses nitroglycerine for treatment of decompensated congestive heart failure: a randomized controlled trial. JAMA. 2002;287:1531–1540. doi: 10.1001/jama.287.12.1531. [DOI] [PubMed] [Google Scholar]
- 10.Potter LR, Abbey-Hosch S, Dickey DM. Natriuretic peptides, their receptors, and cyclic guanosine monophospate-dependent signaling functions. Endocrine Rev. 2006;27:47–72. doi: 10.1210/er.2005-0014. [DOI] [PubMed] [Google Scholar]
- 11.Edvinsson L, Andersson SE. Commentary on viewpoint: the human cutaneous circulation as a model of generalized microvascular function. J Appl Physiol. 2008;105:381; author reply: 389. doi: 10.1152/japplphysiol.90301.2008. [DOI] [PubMed] [Google Scholar]
- 12.Grubb A, Nyman U, Björk J, et al. Simple cystatin C-based prediction equations for glomerular filtration rate compared with the modification of diet in renal disease prediction equation for adults and the Schwartz and the Counahan- Barratt prediction equations for children. Clin Chem. 2005;51:1420–1431. doi: 10.1373/clinchem.2005.051557. [DOI] [PubMed] [Google Scholar]
- 13.Kuhn M, Voss M, Mitko D, et al. Left ventricular assist device support reverses altered cardiac expression and function of natriuretic peptides and receptors in end-stage heart failure. Cardiovasc Res. 2004;64:308–314. doi: 10.1016/j.cardiores.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 14.Houben AJHM, van der Zander K, de Leeuw PW. Vascular and renal actions of brain natriuretic peptide in man: physiology and pharmacology. Fund Clin Pharmacol. 2005;19:411–419. doi: 10.1111/j.1472-8206.2005.00336.x. [DOI] [PubMed] [Google Scholar]
- 15.Dreyfuss C, Wauters A, Adamopoulos D, et al. L-NAME iontophoresis: A tool to assess NO-mediated vasoreactivity during thermal hyperaemic vasodilation in humans. J Cardiovas Pharmacol. 2013;61:361–368. doi: 10.1097/FJC.0b013e3182858f81. [DOI] [PubMed] [Google Scholar]
- 16.Lund LH, Benson L, Dahlström U, et al. Association between use of renin-angiotensin system antagonists and mortality in patients with heart failure and preserved ejection fraction. JAMA. 2012;308:2108–2117. doi: 10.1001/jama.2012.14785. [DOI] [PubMed] [Google Scholar]
- 17.Lund LH, Jurga J, Edner M, et al. Prevalence, correlates, and prognostic significance of QRS prolongation in heart failure with reduced and preserved ejection fraction. Eur Heart J. 2013;34:529–539. doi: 10.1093/eurheartj/ehs305. [DOI] [PubMed] [Google Scholar]
- 18.Andersson SE, Edvinsson ML, Edvinsson L. Reduction of homocysteine in elderly with heart failure improved vascular function and blood pressure control but did not affect inflammatory activity. Basic Clin Pharmacol Toxicol. 2005;97:306–310. doi: 10.1111/j.1742-7843.2005.pto_146.x. [DOI] [PubMed] [Google Scholar]