Abstract
Background
Ciliopathies are a group of diseases associated with abnormal structure or function of primary cilia. Ciliopathies include polycystic kidney disease (PKD), a pathology associated with vascular hypertension. We previously showed that cilia length regulates cilia function, and cilia function is required for nitric oxide (NO) biosynthesis in endothelial cells. Because patients with PKD show abnormal sensory cilia function, the aim of our current study was to search for a targeted therapy focused on primary cilia, which we refer to as ‘ciliotherapy’.
Methods and Results
In the present studies, our in vitro analyses refined fenoldopam as an equipotent and more specific dopaminergic agonist to regulate cilia length and function. Our in vivo studies indicated that fenoldopam increased cilia length and serum NO thereby reducing blood pressure in a PKD mouse model. Our crossover, multicenter, double-blind and placebo-controlled clinical study further indicated that cilia-targeting therapy showed an overall reduction in mean arterial pressure in PKD patients.
Conclusions
Overall, our studies provide the first evidence of ciliotherapy as an innovative intervention in patients with abnormal primary cilia.
Keywords: Primary cilium, Blood pressure, Mechanosensor, Mechanotransduction, Chemosensor, Fluid-shear stress
1. Introduction
Polycystic kidney disease (PKD) is the most common life-threatening hereditary genetic disease, compared to the combined numbers of individuals affected by cystic fibrosis, Downs syndrome, hemophilia, muscular dystrophy, and sickle cell anemia. One of the most concerning issues in PKD is vascular hypertension. In particular, hypertension occurs early in PKD compared to the general population.[1] Although it has been known that PKD is caused by dysfunction in mechanosensory primary cilia,[2],[3] there is unfortunately no targeted therapy on primary cilia at present.
It has been shown that cilia function can be regulated by cilia length.[4] Furthermore, vascular endothelia require proper cilia function for nitric oxide (NO) biosynthesis, in which primary cilia act as molecular switches to initiate NO biochemical reaction.[5],[6] More importantly, vascular endothelia obtained from patients with PKD show abnormal sensory cilia function.[5],[7] Based on our previous drug screening that dopamine enhances cilia function by extending the length of cilia,[8] the aim of our current study was therefore to search for a targeted therapy focused on primary cilia.
2. Methods
The use of animal cells or tissues was approved by the Animal Care and Use Committee of The University of Toledo. The title of our animal cell protocol was Molecular Biology of Kidney Diseases (No. N-105587), and it was approved on June 2010. Signed and informed consent to participate in our clinical study was obtained from the patients, and our clinical protocols were approved by the Department for Human Research Protections of the Biomedical Institutional Review Board of the University of Baghdad, College of Pharmacy. Our clinical protocols were also posted publicly and approved by the International Clinical Trials Registry Platform of the World Health Organization. The clinical trial registry number was IRCT2012110611384N1. The title of our clinical protocols was Dopamine Receptor in Hypertension with Polycystic Kidney Disease (No. DR-PKD), and it was approved on May 2012. The study participants were recruited in June 1 of 2012, and the study was completed in September 30 of 2012.
2.1. Cell culture
Mouse endothelial cells were previously generated and characterized in our laboratory.[6] Cells were grown in Dulbecco's Modification of Eagle's Media (Cellgro, Inc.) containing 10% fetal bovine serum and 1% penicillin G plus streptomycin sulfate (HyClone, Inc.) at 39°C for 3–5 days until fully differentiated before analysis.
2.2. Endothelial-specific Pkd1 mouse model
Endothelial specific-Cre mice were purchased from German Cancer Research Center (DKFZ) in 2009. These mice were bred with Pkd1flox/flox, as previously described.[9] Only male mice in Balb/C background after backcross of 15 generations were used to reduce complexity in hormonal regulation of blood pressure or genetic background, although female mice shared the same blood pressure as in males (data not shown). The endothelial-specific Cre mice were confirmed with a ROSA model, purchased from the Jackson Laboratory in Maine (Stock Number: 007576). To induce Cre recombinant and thus a gene knockout, one- week old pups were injected intra-peritoneally with 250 μg of 50 µL tamoxifen every day for five consecutive days. Blood pressure reading was taken two weeks later.
2.3. Blood pressure measurement
After inactivation of Pkd1 in Tie2Cre:Pkd1flox/flox at one- week old, blood pressure was taken from three-week old mice showing no apparent renal cyst formation. Blood pressure was measured in conscious, trained mice at room temperature using a non-invasive blood pressure system tail-cuff monitor and a built-in photoelectric sensor (Visitech System, NC). Blood pressure measurements were taken twice daily for the duration of the study after the initial three days acclimating each mouse to the cuff. The data from the tail cuff method was also verified with a more invasive, surgically implanted telemetry probe (data not shown). Per previous literature,[10] our initial dosage of fenoldopam was 2 mg/kg per day; we also tried 1 mg/kg which provided us with a less consistent result (data not shown). The dosage of 1.5 mg/kg per day was finally used as it was sufficient to lower the blood pressure consistently in Pkd1 mice. The 1.5 mg/kg of fenoldopam was slowly administered for 20–30 min duration intravenously every day for the duration of our study. All measurements were performed by two-blinded operators.
2.4. NO measurement
A non-fluorescent, CO2-independent medium for imaging was used (pH 7.4). This medium contained (in mmol/L) CaCl2 1.26, MgSO4 0.81, KCl 5.4, KH2PO4 0.44, NaCl 137, Na2HPO4 0.34, NaHCO3 4.166 and D-glucose 5.6. After incubation with the NO-sensitive probe 4-amino-5-methyl- amino-2′,7′-difluorofluorescein (DAF-FM, 20 µmol/L; Invitrogen, Inc.) for 30 min at 39°C, cells were washed to remove excess probe. DAF-FM images were captured at excitation and emission wavelengths of 495 and 515 nm every 5 s through a Nikon TE2000 microscope equipped with a Coolsnap EZ cooled charge-coupled device monochrome digital camera using Metamorph software (Molecular Devices, Corp.). In some cases, serum NO was measured with nitrate/nitrite fluoremetric assay kit (Cayman Chemical, Corp.) as described previously.[5]
2.5. Immunofluorescence
For cilia length studies, endothelial cells were incubated with fenoldopam (Hospira, Inc.) at 0, 1.0, 10.0, 20.0 and 50.0 µmol/L for 16 h at 39°C. Changes in the cilia length in the endothelial cells of femoral arteries were also measured and compared to age-matched control groups. Cells and tissues were fixed as described previously.[8] Anti-acetylated α-tubulin antibody (1: 10,000 dilution; clone 6-11B-1; Sigma, Inc.), fluorescein isothiocyanate (FITC)-labeled anti-mouse antibody (1: 500 dilution; Vector Lab, Inc.), and 4′,6-diamidino-2-phenylindol (DAPI, Vector Lab, Inc.) were used. All measurements and analyses were performed by two-blinded operators.
2.6. Cilia function analysis
For fluid-shear stress experiments, cells grown on glass- bottom plates were installed in custom-made apparatus measured 3 cm × 2.5 mm connected with 2 pumps (Instech, Corp.), one pump for inward fluid flow from a reservoir to the cells and the other for outward fluid flow from the cells to the reservoir, as previously described.[5] For dopamine receptor-5 (DR5) agonist experiments, cells were challenged with dopamine (10 µmol/L; Sigma, Inc.) or fenoldopam (10 µmol/L; Hospira, Inc.). In some experiments, cells were incubated with fenoldopam (10 µmol/L) for 16 h at 39°C before shear stress experiment.
2.7. Patient selection criteria
Signed and informed consent to examine effects of levodopa was obtained from the patients, and blood collection protocols were approved by the Graduate Studies Committee of the University of Baghdad, Baghdad, Iraq. Inclusion of patients was approved by and consulted with the Alkadhumia Academic Hospital and The Renal Diseases and Transplant Center at the Medical City Complex, Baghdad. Thus, the settings and locations where the data were collected were in these hospital and transplant center.
Selection criteria for PKD patients followed official diagnosis criteria,[11],[12] including patient age (25–45 years old), the presence of borderline hypertension, and the number of bilateral renal cysts based on ultrasonography diagnosis. Selection criteria for non-PKD hypertensive (HTN) patients included borderline essential hypertension with no secondary cause. Furthermore, they were selected to be age- and blood pressure- matched to the PKD patients following examination and clearance from consultant physicians. The exclusion criteria for HTN and PKD patients were pregnancy, lactation, diabetes mellitus, myocardial infarction, angina, renal transplantation, renal failure (or creatinine > 3 mg/dL), dialysis, and arrhythmia (according to ECG report). None of the patients had taken any antihypertensive drugs at the time of the study.
2.8. Clinical protocol
The clinical study was a 3-way crossover and double- blind analysis designed to include two groups (Table 1). In each group, patients were further divided into three subgroups randomly. Each patient received treatments three times daily for 7 days. After a washout period of 10 days, each patient was randomly assigned to a different subgroup and received treatments three times daily for 7 days. After a second washout period of 10 days, each patient was put in the last remaining subgroup to receive treatments three times daily for 7 days. For each treatment, patients received placebo, 50 mg or 100 mg levodopa capsules. A 20 mg of domperidone capsule was taken 30 min prior to all treatments. Of note is that domperidone could block D2-like receptor activation by levodopa and thus prevent nausea.[13],[14] The 10-day washout period was to ensure a complete removal of levodopa from the body before the next treatment. As previously reported, the half-life of levodopa is 1–3 h and 7–9 h for domperidone.[15]
Table 1. Clinical study design.
| Group | Sub-group | Treatment |
| Group A (HTN patients) | A1 | Placebo |
| A2 | 50 mg levodopa | |
| A3 | 100 mg levodopa | |
| Group B (PKD patients) | B1 | Placebo |
| B2 | 50 mg levodopa | |
| B3 | 100 mg levodopa |
A 3-way crossover and double-blind analysis was designed to include two groups, hypertensive groups without (HTN) or with polycystic kidney disease (PKD). In each group, patients were further divided into three subgroups randomly for a corresponding treatment.
We chose levodopa as the metabolic precursor of dopamine in borderline hypertensive patients with or without PKD, because fenoldopam was not approved for use by the National Committee for Drug Selection at the Ministry of Health of Iraq. In our clinical study, domperidone was also used as a peripheral D2 dopaminergic antagonist for two reasons. First, it prevents some adverse effects such as nausea induced by levodopa.[13],[14] Second, by blocking D2 receptors, it potentiates activation of the D1 receptors, including DR5, in the peripheral vascular system. From our clinical data (Table 2), the baseline serum creatinine level was significantly higher in the PKD group than in the HTN group. This suggests a progressive deterioration in renal function in PKD, consistent with the imaging studies that indicate the presence of renal cysts in our patients.
Table 2. Clinical parameters.
| Hypertension only |
Polycystic kidney disease with hypertension |
||||||||||||||||||
| Placebo (n = 6) |
50 mg levodopa (n = 12) |
100 mg levodopa (n = 8) |
Placebo (n = 12) |
50 mg levodopa (n = 12) |
100 mg levodopa (n = 12) |
||||||||||||||
| Baseline | 2 h | 7 day | Baseline | 2 h | 7 day | Baseline | 2 h | 7 day | Baseline | 2 h | 7 day | Baseline | 2 h | 7 day | Baseline | 2 h | 7 day | ||
| Age, yrs | 36.8 ± 2.7 | 36.8 ± 1.6 | 36.5 ± 1.9 | 37.1 ± 1.9 | 37.4 ± 1.4 | 37.4 ± 1.5 | |||||||||||||
| BMI, kg/m2 | 25.5 ± 1.2 | 25.9 ± 1.1 | 23.1 ± 1.5 | 28.1 ± 1.3 | 27.1 ± 1.2 | 27.1 ± 1.2 | |||||||||||||
| MAP, mmHg | 108.9 ± 1.3 | 106.4 ± 1.0 | 108.6 ± 1.2 | 108.2 ± 1.0 | 104.7 ± 1.3 | 104.7 ± 1.3 | 108.8 ± 0.8 | 104.8 ± 1.2 | 104.4 ± 1.1 | 107.4 ± 0.9 | 106.8 ± 1.0 | 106.7 ± 0.9 | 107.4 ± 0.8 | 104.1 ± 1.5 | 104.9 ± 1.0 | 108.0 ± 0.7 | 107.0 ± 0.8 | 104.1 ± 1.2 | |
| SBP, mmHg | 136.7 ± 1.7 | 136.2 ± 1.1 | 135.8 ± 1.5 | 136.2 ± 1.1 | 132.5 ± 1.3 | 132.5 ± 1.3 | 137.5 ± 1.3 | 133.1 ± 1.6 | 133.1 ± 1.6 | 134.6 ± 1.0 | 133.8 ± 0.9 | 134.2 ± 1.0 | 135.3 ± 0.7 | 133.4 ± 1.0 | 132.8 ± 0.9 | 135.3 ± 0.7 | 135.3 ± 0.7 | 132.8 ± 1.0 | |
| DBP, mmHg | 95.0 ± 1.3 | 93.3 ± 1.1 | 95.0 ± 1.3 | 94.2 ± 1.0 | 90.8 ± 1.4 | 90.8 ± 1.4 | 94.4 ± 0.6 | 90.6 ± 1.1 | 90.0 ± 0.9 | 93.8 ± 1.1 | 93.3 ± 1.1 | 92.9 ± 1.0 | 93.4 ± 0.9 | 89.4 ± 1.0 | 90.9 ± 1.0 | 94.4 ± 0.8 | 92.8 ± 0.9 | 89.7 ± 1.3 | |
| HR, beats/min | 72 ± 3.2 | 71.3 ± 3.4 | 72 ± 3.4 | 72.7 ± 2.9 | 73.2 ± 2.7 | 72.7 ± 2.9 | 75 ± 2.3 | 73.8 ± 2.4 | 73.5 ± 2.5 | 68.5 ± 1.9 | 67.7 ± 1.8 | 68.2 ± 1.9 | 68.8 ± 1.5 | 68.9 ± 1.4 | 67.8 ± 1.3 | 68.3 ± 1.4 | 67.8 ± 1.3 | 71.1 ± 1.1 | |
| Creatinin, mg/dL | 0.75 ± 0.03 | N/A | 0.75 ± 0.03 | 0.79 ± 0.03 | N/A | 0.82 ± 0.02 | 0.81 ± 0.03 | N/A | 0.83 ± 0.03 | 1.32 ± 0.04 | N/A | 1.32 ± 0.04 | 1.28 ± 0.04 | N/A | 1.28 ± 0.03 | 1.19 ± 0.03 | N/A | 1.18 ± 0.04 | |
| Urea, mg/dL | 27.8 ± 1.0 | N/A | 28.3 ± 1.2 | 27.5 ± 1.1 | N/A | 27.6 ± 1.3 | 28.1 ± 0.9 | N/A | 28.6 ± 1.1 | 29.5 ± 0.8 | N/A | 29.7 ± 0.9 | 28.1 ± 0.9 | N/A | 28.4 ± 1.0 | 28.3 ± 0.9 | N/A | 28.6 ± 0.9 | |
| Ca2+, mg/dL | 9.9 ± 0.2 | N/A | 9.8 ± 0.2 | 9.8 ± 0.1 | N/A | 9.8 ± 0.1 | 9.8 ± 0.1 | N/A | 9.8 ± 0.1 | 9.1 ± 0.1 | N/A | 9.0 ± 0.1 | 9.1 ± 0.1 | N/A | 9.1 ± 0.1 | 8.9 ± 0.1 | N/A | 9.1 ± 0.1 | |
| Na+, mEq/L | 145.2 ± 1.1 | N/A | 145.7 ± 1.3 | 144.3 ± 1.0 | N/A | 144.7 ± 1.0 | 143.8 ± 0.8 | N/A | 143.4 ± 1.2 | 142.2 ± 1.2 | N/A | 142.5 ± 1.1 | 142.3 ± 0.9 | N/A | 142.9 ± 1.1 | 142.5 ± 0.9 | N/A | 142.6 ± 0.9 | |
| K+, mEq/L | 4.1 ± 0.1 | N/A | 4.1 ± 0.2 | 4.3 ± 0.1 | N/A | 4.3 ± 0.1 | 4.4 ± 0.1 | N/A | 4.4 ± 0.2 | 4.0 ± 0.1 | N/A | 4.0 ± 0.1 | 4.0 ± 0.1 | N/A | 4.0 ± 0.1 | 4.0 ± 0.1 | N/A | 4.1 ± 0.1 | |
| Albumin, g/dL | 3.6 ± 0.13 | N/A | 3.6 ± 0.04 | 3.6 ± 0.03 | N/A | 3.6 ± 0.02 | 3.6 ± 0.03 | N/A | 3.7 ± 0.03 | 3.5 ± 0.03 | N/A | 3.6 ± 0.03 | 3.5 ± 0.02 | N/A | 3.5 ± 0.03 | 3.5 ± 0.03 | N/A | 3.5 ± 0.03 | |
| ALT, IU/L | 9.8 ± 0.9 | N/A | 9.9 ± 0.9 | 8.3 ± 0.6 | N/A | 8.6 ± 0.7 | 8.9 ± 0.6 | N/A | 8.9 ± 0.5 | 12.1 ± 2.0 | N/A | 12.8 ± 2.0 | 12.2 ± 1.8 | N/A | 12.2 ± 1.8 | 13.3 ± 1.9 | N/A | 13.0 ± 1.6 | |
| AST, IU/L | 9.8 ± 0.8 | N/A | 10.2 ± 0.9 | 9.3 ± 1.0 | N/A | 9.2 ± 0.9 | 9.9 ± 1.0 | N/A | 9.9 ± 0.9 | 10.6 ± 2.0 | N/A | 10.4 ± 1.8 | 10.7 ± 1.9 | N/A | 10.6 ± 1.7 | 11.1 ± 1.6 | N/A | 11.5 ± 1.4 | |
| AP, IU/L | 11.5 ± 1.3 | N/A | 11.9 ± 1.2 | 11.8 ± 0.9 | N/A | 11.6 ± 0.9 | 11.4 ± 0.9 | N/A | 11.2 ± 0.8 | 15.5 ± 2.2 | N/A | 15.4 ± 2.3 | 15.2 ± 2.0 | N/A | 15.0 ± 1.9 | 15.6 ± 1.7 | N/A | 15.4 ± 1.7 | |
| Bilirubin, mg/dL | 0.5 ± 0.02 | N/A | 0.6 ± 0.02 | 0.6 ± 0.02 | N/A | 0.6 ± 0.02 | 0.6 ± 0.04 | N/A | 0.6 ± 0.02 | 0.6 ± 0.02 | N/A | 0.5 ± 0.02 | 0.6 ± 0.02 | N/A | 0.6 ± 0.02 | 0.5 ± 0.02 | N/A | 0.6 ± 0.02 | |
Patient data and serum parameters were tabulated on the table. Mean arterial pressure was calculated as (2 × diastolic + systolic) /3. ALT: alanine aminotransferase; AP: alkaline phosphatase; AST: aspartate aminotransferase; BMI: body mass index; DBP: diastolic blood pressure; SBP: systolic blood pressure; HR: heart rate; MAP: mean arterial pressure; N/A: not available.
All physicians (Altaei QH, Alshimmari IK, Alsaidi MM and Khammas H) from different clinics or hospitals enrolled participations suitable to our study criteria independently, and they administered the interventions to the participants. The random allocation sequence of participants was generated by Kathem S and supervised by Ismail SH. Thus, neither physicians nor patients knew the subgroup classifications or treatment types. Because the steady state concentration of drug depends of its half-life, levodopa with a short half-life would reaches steady state within two days. As such, effects of levodopa on vasculatures should be apparent within a week of our study, which serves as an endpoint of our study. Blood pressure, heart rate and blood samples were taken before treatment (baseline), two hours after the first treatment, and two hours after the last dose on the seventh day of the treatment period. There was no follow-up after the end of study period.
2.9. Clinical measurements
All measurements were taken after patients were in the sitting position for at least 10 min. Blood pressure and heart rate were measured by the physicians using mercury sphygmomanometer and Radial pulse rate, respectively. Blood samples (4–5 mL) were collected by venepuncture at the clinical laboratories of the hospitals. Creatinine, urea, calcium, sodium, potassium, albumin, alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (AP), and total bilirubin were measured immediately after blood collection before (baseline) and after 7-day treatment. NO and asymmetric dimethylarginine (ADMA) were measured before (baseline), 2-h and 7-day after treatments. ADMA and NO were measured using commercially available kits (Enzo Life sciences, Inc.).
2.10. Data analysis
For NO imaging study, a total of at least 50 cells within a cell population were randomly analyzed for each sample. For immunofluorescence study, cilia length of 30–50 randomly selected cells was measured from one coverslip for each concentration of fenoldopam. All experiments were repeated at least three times on different sets of cell populations (coverslips).
For the HTN group (Group A), 21 patients were recruited in the study. Nine patients were withdrawn from the study because they changed their minds (non-compliant). Thus, their data were excluded from analysis. For the PKD group (Group B), 18 patients were recruited. Two patients were withdrawn from the study due to non-compliance, and their data were not analyzed. The sample size for PKD group was based on the number of patients seen in the clinics within the study period, whereas the sample size of HTN was to match that of PKD group.
All quantifiable experimental values are expressed as mean ± SE, and values of P < 0.05 are considered significant. All comparisons between the two groups were performed with student's t-test. Comparisons of three or more groups were done using ANOVA, followed by Dunnett's posttest analysis. Data analysis was performed using Sigma Plot software version 11.
3. Results
Endothelial cells express dopamine receptor-3 (DR3) and DR5,[8] but only DR5 was localized in the primary cilia of wild-type and Pkd1 endothelial cells (Figure 1A). The chemo-activation of primary cilia with either dopamine (activating both DR3 and DR5) or fenoldopam (activating only DR5) induced NO biosynthesis (Figure 1B). Like dopamine,[8] fenoldopam increased cilia length within 16 h in a dose-dependent manner (Figure 2A). Fenoldopam at an optimal concentration of 10 μmol/L significantly increased cilia length from 2.4 ± 0.1 μm to 3.6 ± 0.2 μm and from 1.3 ± 0.1 μm to 1.6 ± 0.1 μm in wild-type and Pkd1 endothelial cells, respectively (Figure 2B).
Figure 1. Ciliary localized of DR5 plays an important role in NO production (n = 3 for each experiment).
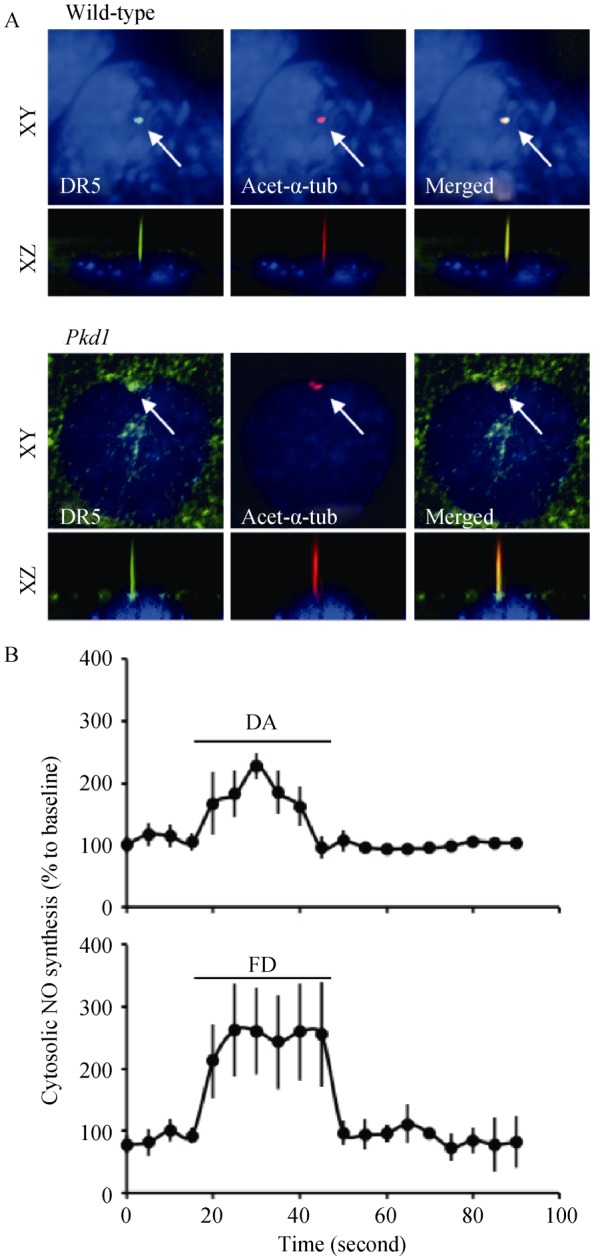
(A): DR5 specifically localized to primary cilia in wild-type and Pkd1 endothelial cells. Specific-DR5 antibody (green) counterstained with acetylated-α-tubulin (acet-α-tub) as a ciliary marker (red) and dapi as nuclear marker (blue) are shown; (B): DA (10 μmol/L) and FD (10 μmol/L) induced cytosolic NO biosynthesis in cultured endothelial cells. Arrows indicate the treatment of the dopaminergic agents. DA: dopamine; DR5: dopamine receptor-type 5; FD: fenoldopam; NO: nitric oxide.
Figure 2. Fenoldopam increased the length of vascular endothelial cilia, n = 3 for each drug concentration.
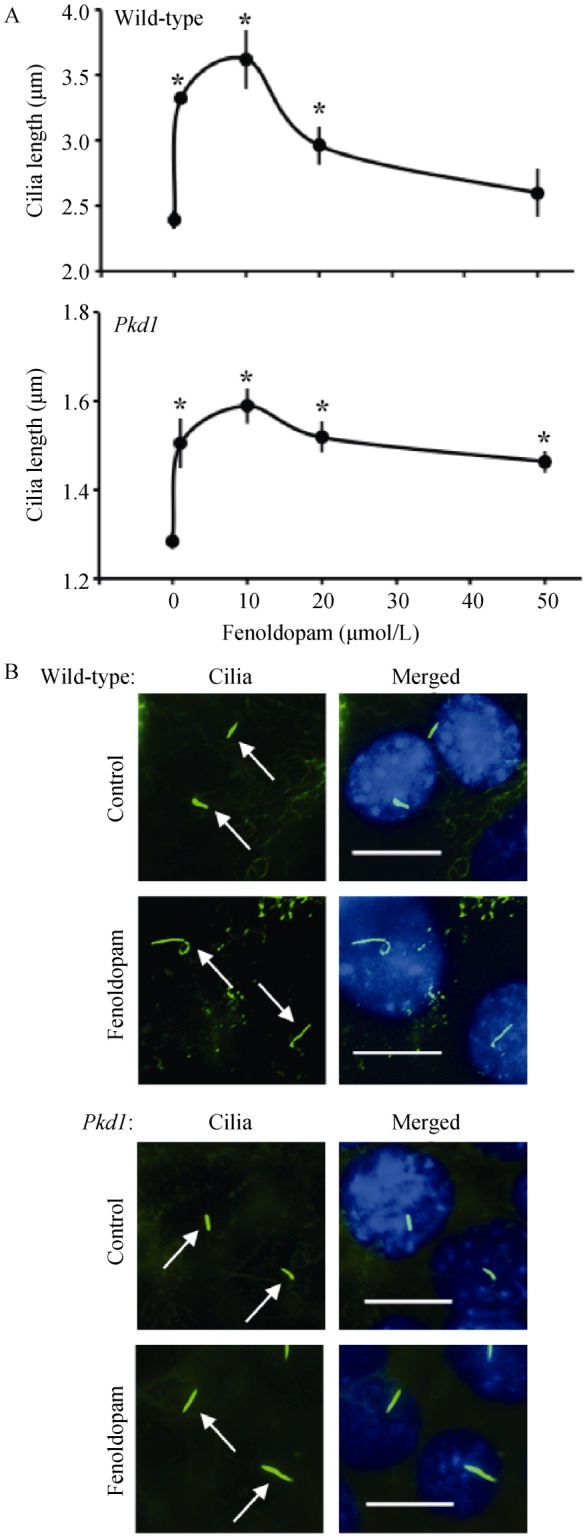
(A): Fenoldopam acting as a DR5 agonist increases ciliary length in a dose-dependent manner in wild-type and Pkd1null/null endothelial cells; (B): Representative fluorescence images show an increase of ciliary length in wild-type and Pkd1 endothelial cells after treatment with fenoldopam (10 μmol/L; 16 h). Asterisks indicate significant difference from the baseline (P < 0.05). Arrows point to cilia. Bar = 10 μm. DR5: dopamine receptor-type 5.
It is commonly known that the mechanosensory cilia can be activated by fluid-shear stress-induced cilia bending.[2] Furthermore, increasing cilia length would enhance the sensitivity of cilia function to fluid shear.[4] To examine the effect of fenoldopam on cilia function, we challenged wild- type and Pkd1 endothelial cells pre-treated without or with fenoldopam (10 μmol/L; 16 h) with fluid-shear (Figure 3A). Cells treated with fenoldopam showed a significantly greater production of NO in response to shear-stress (Figure 3B). While Pkd1 cells are insensitive to fluid-shear stress, our results indicate that dopaminergic activation can partially rescue the mechanosensation properties of primary cilia.
Figure 3. Fenoldopam increased the function of vascular endothelial cilia (n = 3 for each experiment).
(A): Wild-type and Pkdnull/null endothelial cells were challenged with fluid-shear stress to induce cilia activation. Cytosolic NO biosynthesis was measured as readout of ciliary function in cells treated without (control) or with fenoldopam (10 μmol/L; 16 h). (B): Peaks of cytosolic NO biosynthesis were averaged in wild-type and PKD endothelia cells treated without (control) or with fenoldopam. Arrows indicate the start of shear stress (flow). *P < 0.05. NO: nitric oxide; PKD: polycystic kidney disease.
PKD is a ciliopathy associated with hypertension, which has been hypothesized to be a result from the inability of endothelial cells to produce NO.[5],[6] More specifically, primary cilia within vascular-lining endothelia were hypothesized to function as molecular switches for NO biosynthesis in response to changes in blood pressure (shear-stress). To examine the effects of fenoldopam on blood pressure, we thus extended our in vitro finding to an in vivo animal study. To overcome the perinatal lethality of PKD mice, we crossed Pkd1-flox mouse with endothelial-Cre systems of either PdgfβCre or Tie2Cre (Figure 4A). The Rosa26 mouse is used as a reporter system to verify and validate our Cre mice. The innovation of Rosa26 includes its inducible fluorescence reporter system; i.e., all endothelia lining the vasculatures are red fluorescence (non-induced). The red fluorescence will be replaced with green fluorescence upon Cre recombinant (induction). Both Cre systems showed a high efficiency of gene inactivation in the vascular endothelial lining (Figure 4B).
Figure 4. Both Tie2Cre and PdgfβCre models had high efficiency in gene deletion to turn vascular endothelia from red to green fluorescence (n = 4 for each genotype and treatment).
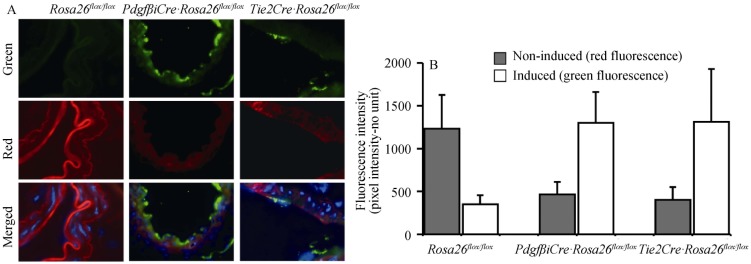
Tie2Cre or PdgfβCre mouse was bred with Gt(ROSA)26Sor (Rosa26) mouse, resulting in either PdgfβCre Rosa26· or Tie2Cre Rosa26· genotype. The Rosa26 mouse was used as a control. These three mouse groups were induced to activate Cre which acts on Rosa26 allele. The Rosa26 genetic background is used as a reporter system to verify and validate the efficiency of the Cre mice. The Rosa26 includes its inducible fluorescence reporter system; i.e., all endothelia lining the vasculatures have red fluorescence (non-induced). The red fluorescence will be replaced with green fluorescence upon Cre recombinant (induction). (A): Abdominal aortas were isolated, stained with nuclear marker (blue), and imaged for their green/red fluorescence; (B): Quantitation analysis of vascular-lining endothelia indicates high efficacy of both PdgfbCre and Tie2Cre backgrounds to delete a specific gene in vascular lining endothelia.
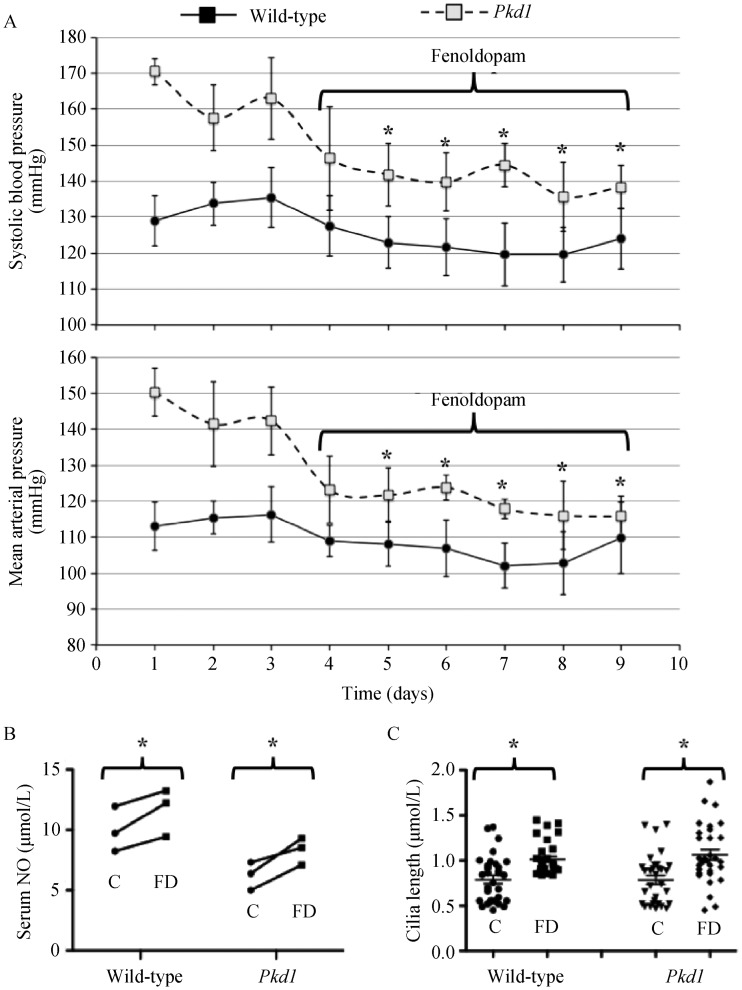
Fenoldopam at 1.5 mg/kg significantly decreased blood pressure in Tie2Cre:Pkd1flox/flox mice (Figure 5A). Compared to serum NO taken at the end of the baseline period, level of NO on the last day of treatment period was significantly increased in both wild-type and Pkd1 mice (Figure 5B). Fenoldopam treatment also significantly increased cilia length (Figure 5C).
Figure 5. Fenoldopam augmented cilia length, increased serum NO and improved blood pressure in Pkd1 mouse model (n = 3 for each experiment; at least 35 double-blind measurements for cilia length were made in each sample.
(A): Baseline blood pressure was measured for at least 3 days in Pkd1flox/flox (wild-type) and Tie2Cre Pkd1flox/flox·(Pkd1) mice followed with intravenous administration of fenoldopam everyday at 1.5 mg/mL per minute for 30 min; (B): Serum NO was measured at day 3 before (control, C) and at day 9 after (fenoldopam, FD) administration of fenoldopam; (C): Cilia length was measured from the femoral arteries before C and after FD in Pkd1flox/flox (wild-type) and Tie2Cre Pkd1flox/flox· mice. *P < 0.05 from the baseline values before fenoldopam treatment. NO: nitric oxide.
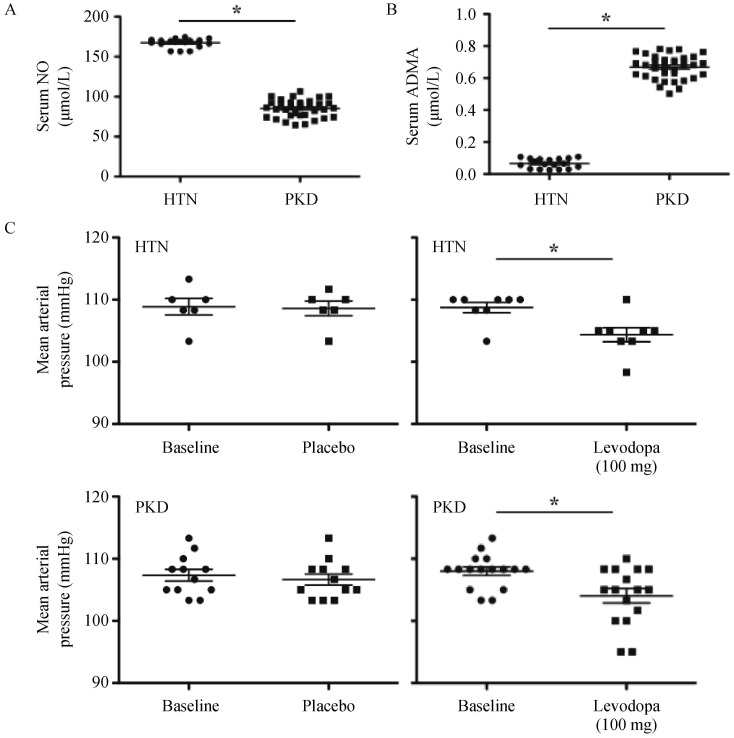
To ensure the clinical relevance of our findings, we recruited a small patient population with borderline hypertension (essential hypertension) and hypertensive-PKD patients. Our hypertensive-PKD patients had a significantly lower NO level than hypertensive-only group (Figure 6A). In addition, the serum level of ADMA was significantly higher in the PKD group than in the hypertensive-only group (Figure 6B).
Figure 6. Serum levels of NO, ADMA and effects of levodopa on hypertensive-only and hypertensive-PKD patients.
(A): Compared to HTN patients, baseline serum NO was significantly lower in hypertensive patients with PKD; (B): Compared to HTN patients, baseline serum ADMA was significantly higher in PKD patients; (C): Blood pressure was measured at baseline and 7 days after placebo or levodopa treatment. Levadopa is effective to improve mean arterial pressure in both HTN and PKD patients. *P < 0.05 between HTN and PKD groups (in A and B), or between different treatments and corresponding baseline groups (in C). ADMA: asymmetric dimethylarginine; HTN: hypertensive-only; NO: nitric oxide; PKD: polycystic kidney disease.
It has been proposed that dopamine receptor agonist is a potential therapeutic option for PKD patients.[16] To examine this possibility, we compared the effects of levodopa in patients with borderline hypertension (essential hypertension) and PKD patients with borderline hypertension (Table 1). Levodopa, a dopaminergic agonist, was selected because it is clinically safe for convenient oral use; however, it functions as a non-specific dopaminergic agonist for DR5 activation. Our double-blind study showed minimal effects of moderate dosages of levodopa (50 or 100 mg) on serum NO (Table 2). Of note, NO regulation in PKD is complicated partly because the increase in basal levels of superoxide and NO-induced peroxynitrite formation in smooth muscle cells.[17] It is therefore conceivable that the NO in our patients had reacted with superoxide to form peroxynitrite.
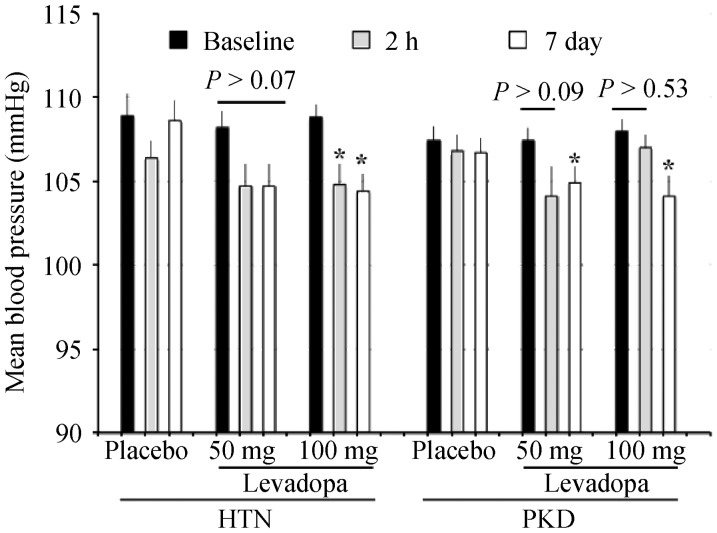
Most importantly, the overall mean arterial pressure was significantly decreased in patients treated with 100 mg levodopa in PKD patients (Figure 6C). Levodopa also showed similar effect on hypertensive-only group (Figure 7). This finding is consistent with our cell culture in vitro and mouse in vivo studies showing that activating DR5 can also increase cilia length and sensitivity to produce NO in normal wild-type control group. It is probable that our hypertensive-only group had a constricted vascular tone that would also be beneficial with dopamine treatment.
Figure 7. Levodopa decreases the overall mean arterial pressure.
Blood pressure was measured at baseline, 2-h and 7 days after placebo or levodopa treatment (placebo vs. 50 mg vs. 100 mg). Levadopa was effective to improve mean arterial pressure in both HTN and hypertensive-PKD patients. *P < 0.05 compared to corresponding baseline groups. HTN: hypertensive-only; PKD: polycystic kidney disease.
4. Discussion
In the present study, our basic science studies indicated that activation of ciliary DR5 with DR5-specific agonist (fenoldopam) increases NO biosynthesis transiently. More importantly, DR5 activation increased cilia length, partially rescued mechanosensitivity of PKD endothelial cells to fluid-shear stress, and thereby decreased the overall mean arterial pressure. In the clinical study, we showed that hypertensive PKD patients had a significantly lower baseline level of NO compared to hypertensive-only patients. More importantly, modest dosage of levodopa at 50 mg or 100 mg decreased the mean arterial blood pressure in the PKD group. We also confirm that the baseline level of ADMA, an endogenous inhibitor for eNOS and a marker for endothelial dysfunction, is significantly higher in the PKD group compared to the hypertensive-only group.
Consistent with the in vitro study, our in vivo data suggest that fenoldopam could extend the length of primary cilia and induce a greater NO production. These would ultimately decrease the overall mean arterial pressure in Pkd1 mouse model. Of note is that fenoldopam at 1.5 mg/kg showed a trend of lower blood pressure in wild-type mice, albeit this decrease was not statistically significant. One possible explanation is that the baseline vascular tone in wild-type mice was already low. As a result, no significant effect of increasing NO on vasodilation was observed. It is worth mentioning that the baseline serum levels of NO were significantly lower in Pkd1 mice compared to wild-type.
ADMA acting as a physiological inhibitor of NO biosynthesis has been used as a marker for endothelial function in the clinical setting.[18],[19] Plasma ADMA levels are highly correlated with the severity of endothelial dysfunction, and high ADMA level further impairs blood flow and accelerates atherogenesis through endothelial dysfunction.[20] Thus, our clinical data indicated that compared to the hypertensive-only group, the PKD group had an abnormality in regulating NO biosynthesis. This is consistent with our previous study indicating that vascular-lining endothelia from patients with PKD are dysfunctional due to their non-responsiveness to flow-induce NO biosynthesis.[5]
In particular, the mechanism of dopamine receptor, especially DR5, in blood pressure regulation still remains unknown. Although it has been propose that peripheral dopaminergic activation increases renal blood flow,[21] we propose that this vasodilation effect of dopamine on renal arteries acts by sensitizing primary cilia function. In our study, levodopa had a minimal effect on individual systolic or diastolic blood pressure (Table 2). Without doubt, a more specific dopaminergic agonist for DR5 may be more suitable for therapeutic management of hypertensive PKD patients. Unfortunately, fenoldopam was not approved for use in our study. We did not seek for a higher dosage of levodopa due to the safety concerns in our patients. Nonetheless, our clinical study sheds light on the possibility for cilia-targeted therapy in PKD patients and possibly in general hypertensive patients.
In summary, our results show that fenoldopam increased cilia length, which further enhanced the mechanosensory function of cilia in NO biosynthesis. Results from a less complex in vivo rodent system with endothelial cilia dysfunction support the idea that a DR5-cilia-NO axis plays an important role in regulating blood pressure in PKD. In a more complex clinical setting, dopaminergic receptor activation showed a potential therapeutic benefit on overall arterial blood pressure. Together, our studies serve as a proof of principle for targeted-clinical therapy on primary cilia as a novel mechanism to modulate the progression of a ciliopathy.
Acknowledgments
We are particularly grateful to all the patients participated in our study. Authors would also like to thank Drs. Alaa Abdulrasoul, Qasim Alshamaa, Abdulrasoul Weiss, Ahmed Abbas, Mohammed Hasan, and Intisar Tariq for their assistance. Maki Takahashi and Charisse Montgomery are acknowledged for their technical support and editorial assistance. Sources of funding include NIH DK080640, HL112641, DOD PR130153, HL076709, HL020176 and College of Pharmacy, University of Baghdad. Mohieldin AM is supported by NIH DK096870 predoctoral fellowship.
References
- 1.Kelleher CL, McFann KK, Johnson AM, et al. Characteristics of hypertension in young adults with autosomal dominant polycystic kidney disease compared with the general U.S. population. Am J Hypertens. 2004;17:1029–1034. doi: 10.1016/j.amjhyper.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 2.Nauli SM, Alenghat FJ, Luo Y, et al. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet. 2003;33:129–137. doi: 10.1038/ng1076. [DOI] [PubMed] [Google Scholar]
- 3.Xu C, Rossetti S, Jiang L, et al. Human ADPKD primary cyst epithelial cells with a novel, single codon deletion in the PKD1 gene exhibit defective ciliary polycystin localization and loss of flow-induced Ca2+ signaling. Am J Physiol Renal Physiol. 2007;292:930–945. doi: 10.1152/ajprenal.00285.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abdul-Majeed S, Moloney BC, Nauli SM. Mechanisms regulating cilia growth and cilia function in endothelial cells. Cell Mol Life Sci. 2012;69:165–173. doi: 10.1007/s00018-011-0744-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.AbouAlaiwi WA, Takahashi M, Mell BR, et al. Ciliary polycystin-2 is a mechanosensitive calcium channel involved in nitric oxide signaling cascades. Circ Res. 2009;104:860–869. doi: 10.1161/CIRCRESAHA.108.192765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nauli SM, Kawanabe Y, Kaminski JJ, et al. Endothelial cilia are fluid shear sensors that regulate calcium signaling and nitric oxide production through polycystin-1. Circulation. 2008;117:1161–1171. doi: 10.1161/CIRCULATIONAHA.107.710111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.AbouAlaiwi WA, Ratnam S, Booth RL, et al. Endothelial cells from humans and mice with polycystic kidney disease are characterized by polyploidy and chromosome segregation defects through survivin down-regulation. Hum Mol Genet. 2011;20:354–367. doi: 10.1093/hmg/ddq470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abdul-Majeed S, Nauli SM. Dopamine receptor type 5 in the primary cilia has dual chemo- and mechano-sensory roles. Hypertension. 2011;58:325–331. doi: 10.1161/HYPERTENSIONAHA.111.172080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takakura A, Contrino L, Beck AW, et al. Pkd1 inactivation induced in adulthood produces focal cystic disease. J Am Soc Nephrol. 2008;19:2351–2363. doi: 10.1681/ASN.2007101139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang MZ, Yao B, Fang X, et al. Intrarenal dopaminergic system regulates renin expression. Hypertension. 2009;53:564–570. doi: 10.1161/HYPERTENSIONAHA.108.127035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pei Y, Obaji J, Dupuis A, et al. Unified criteria for ultrasonographic diagnosis of ADPKD. J Am Soc Nephrol. 2009;20:205–212. doi: 10.1681/ASN.2008050507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ravine D, Gibson RN, Walker RG, et al. Evaluation of ultrasonographic diagnostic criteria for autosomal dominant polycystic kidney disease 1. Lancet. 1994;343:824–827. doi: 10.1016/s0140-6736(94)92026-5. [DOI] [PubMed] [Google Scholar]
- 13.Saito I, Kawabe H, Hasegawa C, et al. Effect of L-dopa in young patients with hypertension. Angiology. 1991;42:691–695. doi: 10.1177/000331979104200902. [DOI] [PubMed] [Google Scholar]
- 14.Nishiyama S, Kanno K, Yamaguchi I. Comparison of cardiorenal and emetic effects of dopamine prodrugs docarpamine (TA-870) and levodopa in dogs. J Pharmacobiodyn. 1991;14:120–125. doi: 10.1248/bpb1978.14.120. [DOI] [PubMed] [Google Scholar]
- 15.Brunton LL, Lazo JS, Parker KL. Goodman & Gilman's The Pharmacological Basis of Therapeutics. 12th ed. New York, USA: The McGraw-Hill Companies, Inc; 2011. [Google Scholar]
- 16.Barendregt JN, Florijn KW, Muizert Y, et al. Borderline hypertensive autosomal dominant polycystic kidney disease patients have enhanced production of renal dopamine. Normalization of renal haemodynamics by DOPA infusion. Nephrol Dial Transplant. 1995;10:1332–1341. [PubMed] [Google Scholar]
- 17.Brookes ZL, Ruff L, Upadhyay VS, et al. Pkd2 mesenteric vessels exhibit a primary defect in endothelium-dependent vasodilatation restored by rosiglitazone. Am J Physiol Heart Circ Physiol. 2013;304:H33–H41. doi: 10.1152/ajpheart.01102.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Antoniades C, Demosthenous M, Tousoulis D, et al. Role of asymmetrical dimethylarginine in inflammation-induced endothelial dysfunction in human atherosclerosis. Hypertension. 2011;58:93–98. doi: 10.1161/HYPERTENSIONAHA.110.168245. [DOI] [PubMed] [Google Scholar]
- 19.Juonala M, Viikari JS, Alfthan G, et al. Brachial artery flow- mediated dilation and asymmetrical dimethylarginine in the cardiovascular risk in young Finns study. Circulation. 2007;116:1367–1373. doi: 10.1161/CIRCULATIONAHA.107.690016. [DOI] [PubMed] [Google Scholar]
- 20.Cooke JP. Does ADMA cause endothelial dysfunction? Arterioscler Thromb Vasc Biol. 2000;20:2032–2037. doi: 10.1161/01.atv.20.9.2032. [DOI] [PubMed] [Google Scholar]
- 21.Elkayam U, Ng TM, Hatamizadeh P, et al. Renal vasodilatory action of dopamine in patients with heart failure: magnitude of effect and site of action. Circulation. 2008;117:200–205. doi: 10.1161/CIRCULATIONAHA.107.737106. [DOI] [PubMed] [Google Scholar]