Abstract
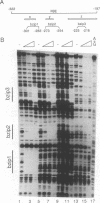
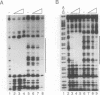
Transcription of the Drosophila yolk protein (Yp) genes is regulated by the somatic sex determination pathway. A gene at the bottom of this pathway, doublesex, encodes the female-specific DSXF and male-specific DSXM proteins that bind to and regulate transcription from several sites in the Yp genes. We report site-directed mutagenesis, protein binding and germline transformation experiments that identify and characterize the activity of a single binding site (dsxA) for the doublesex proteins and two binding sites for other regulatory proteins. A single copy of the three sites is sufficient to direct the sex and fat body specificities of Yp transcription. The sites form an enhancer with two strongly synergistic enhancer elements. One element (22 bp) consists of dsxA and an overlapping site, bzip1, that binds the DmC/EBP (slbo) protein, a member of the bZIP family of transcriptional activators. The other element is an 11 bp binding site (ref1) for an unknown protein. Tissue-specific activation requires strong cooperation between the ref1 site and the bzip1 or dsxA sites. Sex specificity is regulated exclusively by the dsxA site which connects the sex determination pathway to the target gene through DSXM repression and DSXF activation.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belote J. M., Handler A. M., Wolfner M. F., Livak K. J., Baker B. S. Sex-specific regulation of yolk protein gene expression in Drosophila. Cell. 1985 Feb;40(2):339–348. doi: 10.1016/0092-8674(85)90148-5. [DOI] [PubMed] [Google Scholar]
- Bridges C. B. TRIPLOID INTERSEXES IN DROSOPHILA MELANOGASTER. Science. 1921 Sep 16;54(1394):252–254. doi: 10.1126/science.54.1394.252. [DOI] [PubMed] [Google Scholar]
- Brunelle A., Schleif R. F. Missing contact probing of DNA-protein interactions. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6673–6676. doi: 10.1073/pnas.84.19.6673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burtis K. C., Baker B. S. Drosophila doublesex gene controls somatic sexual differentiation by producing alternatively spliced mRNAs encoding related sex-specific polypeptides. Cell. 1989 Mar 24;56(6):997–1010. doi: 10.1016/0092-8674(89)90633-8. [DOI] [PubMed] [Google Scholar]
- Burtis K. C., Coschigano K. T., Baker B. S., Wensink P. C. The doublesex proteins of Drosophila melanogaster bind directly to a sex-specific yolk protein gene enhancer. EMBO J. 1991 Sep;10(9):2577–2582. doi: 10.1002/j.1460-2075.1991.tb07798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burtis K. C. The regulation of sex determination and sexually dimorphic differentiation in Drosophila. Curr Opin Cell Biol. 1993 Dec;5(6):1006–1014. doi: 10.1016/0955-0674(93)90085-5. [DOI] [PubMed] [Google Scholar]
- Coschigano K. T., Wensink P. C. Sex-specific transcriptional regulation by the male and female doublesex proteins of Drosophila. Genes Dev. 1993 Jan;7(1):42–54. doi: 10.1101/gad.7.1.42. [DOI] [PubMed] [Google Scholar]
- Ellenberger T. E., Brandl C. J., Struhl K., Harrison S. C. The GCN4 basic region leucine zipper binds DNA as a dimer of uninterrupted alpha helices: crystal structure of the protein-DNA complex. Cell. 1992 Dec 24;71(7):1223–1237. doi: 10.1016/s0092-8674(05)80070-4. [DOI] [PubMed] [Google Scholar]
- Erdman S. E., Burtis K. C. The Drosophila doublesex proteins share a novel zinc finger related DNA binding domain. EMBO J. 1993 Feb;12(2):527–535. doi: 10.1002/j.1460-2075.1993.tb05684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falb D., Maniatis T. A conserved regulatory unit implicated in tissue-specific gene expression in Drosophila and man. Genes Dev. 1992 Mar;6(3):454–465. doi: 10.1101/gad.6.3.454. [DOI] [PubMed] [Google Scholar]
- Falb D., Maniatis T. Drosophila transcriptional repressor protein that binds specifically to negative control elements in fat body enhancers. Mol Cell Biol. 1992 Sep;12(9):4093–4103. doi: 10.1128/mcb.12.9.4093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garabedian M. J., Shepherd B. M., Wensink P. C. A tissue-specific transcription enhancer from the Drosophila yolk protein 1 gene. Cell. 1986 Jun 20;45(6):859–867. doi: 10.1016/0092-8674(86)90560-x. [DOI] [PubMed] [Google Scholar]
- Keyes L. N., Cline T. W., Schedl P. The primary sex determination signal of Drosophila acts at the level of transcription. Cell. 1992 Mar 6;68(5):933–943. doi: 10.1016/0092-8674(92)90036-c. [DOI] [PubMed] [Google Scholar]
- Kim J., Tzamarias D., Ellenberger T., Harrison S. C., Struhl K. Adaptability at the protein-DNA interface is an important aspect of sequence recognition by bZIP proteins. Proc Natl Acad Sci U S A. 1993 May 15;90(10):4513–4517. doi: 10.1073/pnas.90.10.4513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McKeown M. Sex differentiation: the role of alternative splicing. Curr Opin Genet Dev. 1992 Apr;2(2):299–303. doi: 10.1016/s0959-437x(05)80288-6. [DOI] [PubMed] [Google Scholar]
- McKnight S. L., Lane M. D., Gluecksohn-Waelsch S. Is CCAAT/enhancer-binding protein a central regulator of energy metabolism? Genes Dev. 1989 Dec;3(12B):2021–2024. doi: 10.1101/gad.3.12b.2021. [DOI] [PubMed] [Google Scholar]
- Milos P. M., Zaret K. S. A ubiquitous factor is required for C/EBP-related proteins to form stable transcription complexes on an albumin promoter segment in vitro. Genes Dev. 1992 Jun;6(6):991–1004. doi: 10.1101/gad.6.6.991. [DOI] [PubMed] [Google Scholar]
- Montell D. J., Rorth P., Spradling A. C. slow border cells, a locus required for a developmentally regulated cell migration during oogenesis, encodes Drosophila C/EBP. Cell. 1992 Oct 2;71(1):51–62. doi: 10.1016/0092-8674(92)90265-e. [DOI] [PubMed] [Google Scholar]
- Nelsen B., Sen R. Regulation of immunoglobulin gene transcription. Int Rev Cytol. 1992;133:121–149. doi: 10.1016/s0074-7696(08)61859-8. [DOI] [PubMed] [Google Scholar]
- Ondek B., Gloss L., Herr W. The SV40 enhancer contains two distinct levels of organization. Nature. 1988 May 5;333(6168):40–45. doi: 10.1038/333040a0. [DOI] [PubMed] [Google Scholar]
- Rørth P., Montell D. J. Drosophila C/EBP: a tissue-specific DNA-binding protein required for embryonic development. Genes Dev. 1992 Dec;6(12A):2299–2311. doi: 10.1101/gad.6.12a.2299. [DOI] [PubMed] [Google Scholar]
- Sellers J. W., Vincent A. C., Struhl K. Mutations that define the optimal half-site for binding yeast GCN4 activator protein and identify an ATF/CREB-like repressor that recognizes similar DNA sites. Mol Cell Biol. 1990 Oct;10(10):5077–5086. doi: 10.1128/mcb.10.10.5077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd B., Garabedian M. J., Hung M. C., Wensink P. C. Developmental control of Drosophila yolk protein 1 gene by cis-acting DNA elements. Cold Spring Harb Symp Quant Biol. 1985;50:521–526. doi: 10.1101/sqb.1985.050.01.064. [DOI] [PubMed] [Google Scholar]
- Spradling A. C., Rubin G. M. Transposition of cloned P elements into Drosophila germ line chromosomes. Science. 1982 Oct 22;218(4570):341–347. doi: 10.1126/science.6289435. [DOI] [PubMed] [Google Scholar]
- Tan S., Richmond T. J. DNA binding-induced conformational change of the yeast transcriptional activator PRTF. Cell. 1990 Jul 27;62(2):367–377. doi: 10.1016/0092-8674(90)90373-m. [DOI] [PubMed] [Google Scholar]
- Taylor B. J. Differentiation of a male-specific muscle in Drosophila melanogaster does not require the sex-determining genes doublesex or intersex. Genetics. 1992 Sep;132(1):179–191. doi: 10.1093/genetics/132.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjian R., Maniatis T. Transcriptional activation: a complex puzzle with few easy pieces. Cell. 1994 Apr 8;77(1):5–8. doi: 10.1016/0092-8674(94)90227-5. [DOI] [PubMed] [Google Scholar]