Abstract
Cullin 3, the core subunit of the CRL3 ubiquitin ligase family, is essential for development, but its substrates remain poorly defined. Here, Chen et al. (2009) report that CRL3BACURD targets the RhoA GTPase for degradation thereby maintaining actin cytoskeleton integrity.
Cullin-RING ubiquitin ligases (CRLs) comprise an extensive class of multisubunit enzymes, which promote the ubiquitylation and degradation of a large number of protein substrates. CRLs uniquely exploit combinatorial diversity in order to achieve their unparalleled versatility. Conserved core complexes organized around a cullin (CUL)-like protein - up to eight exist in humans - associate with hundreds of different substrate receptors. Since receptors are known to seek out multiple substrates, the total number of CRL targets is likely in the thousands, predicting that CRLs may impact virtually any cellular process. Actin-based motility is the most recent addition to this repertoire as shown in a report by Chen et al. in this issue of Molecular Cell (Chen et al., 2009).
CUL3 assembles into so-called CRL3 complexes that contain BTB domain proteins as substrate receptors (Geyer et al., 2003). Metazoans typically encode 100 – 200 BTB proteins suspected of being CRL3 substrate receptors, but only 10 or so CRL3 substrates have been discovered to date. This highlights a general predicament in the ubiquitin field, which has found it challenging to come up with efficient methods for matching the plethora of ubiquitylation enzymes with their even greater number and diversity of substrates. Accordingly, three of the best known CRL3 substrates in human cells, CDK2-free cyclin E, the antioxidant transcription factor Nrf2, and the mitotic kinase Aurora B, were discovered by very different strategies. Whereas cyclin E was identified based on its interaction with CUL3 (Singer et al., 1999), the Nrf2-CUL3 connection was made through prior studies that had characterized the BTB protein Keap1 as a negative regulator of Nrf2 activity (Kobayashi and Yamamoto, 2005). Phenotypic characterization of CUL3-deficient cells, which show a failure to complete cytokinesis, pinpointed Aurora B as a mitotic substrate of CRL3 complexes in conjunction with the BTB domain receptors KLHL9 and 13 (Sumara et al., 2007).
A phenotypic strategy has also led Chen et al. (2009) to success in their quest for novel CRL3 substrates. The investigators noticed a striking reorganization of the actin cytoskeleton in CUL3 knockdown cells marked by strong accumulation of abnormal stress fibers. As shown by several complementary assays, this phenotype was dependent on RhoA, a finding of perhaps little surprise, since activation of this small GTPase has long been known as a principal pathway to stress fiber formation. It is striking, however, how directly involved CUL3 was shown to be in controlling RhoA. The protein was dramatically stabilized and accumulated to very high levels in CUL3 knockdown cells, whereas closely related GTPases did not. Since the effect on actin organization was conserved upon knockdown of CUL3 in Drosophila S2 cells, the investigators could take advantage of the considerably smaller stock of BTB proteins in the fly to rapidly screen for RhoA targeting substrate receptors by RNAi. They isolated two BTB proteins whose human homologs they named hBACURD1 and 2. What follows is the entire gamut of biochemical experiments required to prove a bona fide enzyme-substrate relationship between CRL3BACURD and RhoA: BACURDs interact with CUL3 and RhoA at endogenous expression levels, recombinant CRL3BACURD ubiquitylates RhoA in vitro, and expression of dominant negative BACURD mutants induces stress fibers.
Apart from discovering a novel CRL3 substrate, this work touches on a number of interesting aspects regarding CRL biology. First, the finding that knockdown of either hBACURD1 or 2 causes RhoA stabilization suggests that these BTB domain proteins may function in heterodimeric complexes (Figure 1). Although this was not demonstrated in the present report, BTB domains are generally thought to mediate homo- or heterotypic interactions (Stogios et al., 2005). Thus, Aurora B degradation appears to be mediated by a CRL3 complex containing heterodimers of KLHL9 and 13 as substrate receptors (Sumara et al., 2007). The study by Chen et al. therefore reinforces the general notion that CRL complexes function as dimers, perhaps to afford multiple substrate orientations within the same enzymatic complex in order to facilitate the assembly of a multiubiquitin chain (Tang et al., 2007).
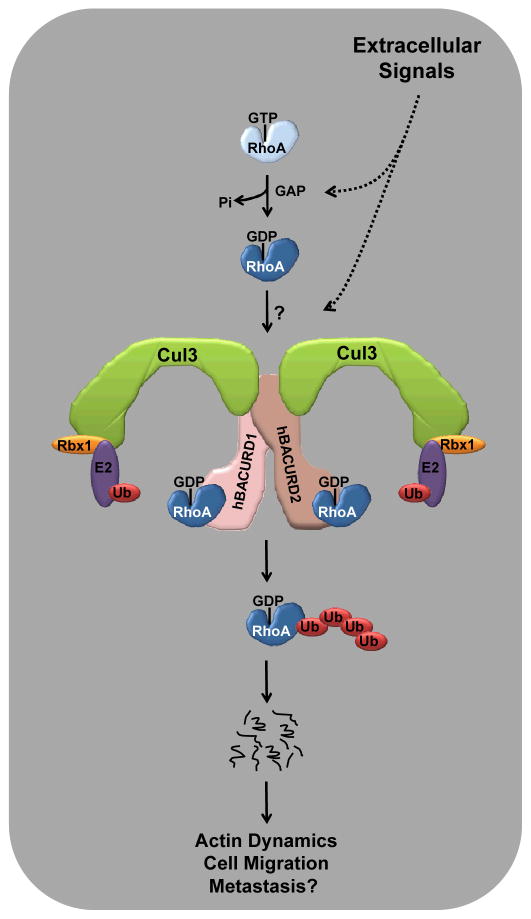
Figure 1. Model of CRL3BACURD-mediated destruction of RhoA.
Active RhoA·GTP is converted to RhoA·GDP by GAP activity, which may respond to extracellular cues. RhoA·GDP, potentially after additional modification triggered by exogenous stimuli, is then recognized by a dimeric CRL3 complex containing hBACURDs1 and 2 as substrate receptors. Polyubiquitylation leads to proteasomal degradation of RhoA thus affecting actin dynamics, cell migration, and potentially metastasis.
The second issue concerns the substrate targeting mechanism, which is still poorly understood for CRL3 complexes in general. Posttranslational modifications of the substrate, including phosphorylation, hydroxylation, and glycosylation, have been firmly established as triggers for their recognition by CRL1 and CRL2 complexes. In the best studied cases, the so-called “phosphodegrons” of some CRL1/SCF substrates, the phosphate group contributes the essential contacts for a stable interaction with the substrate receptors (Orlicky et al., 2003). Neither CRL3 substrate, cyclin E or Aurora B, was shown to require any such modifications (Singer et al., 1999; Sumara et al., 2007). Likewise, BACURDs and RhoA can interact as proteins recombinantly expressed in Escherichia coli, again suggesting that modifications common in mammalian cells may not be required for targeting RhoA to CRL3 complexes. Intriguingly, however, BACURDs have a strong preference for RhoA·GDP over RhoA·GTP. Thus, the principal substrate recognition mechanism appears to be based on conformation rather than a distinct posttranslational mark in a degron motif (Figure 1). It is not clear, however, whether GTP hydrolysis is sufficient to target RhoA for CRL3BACURD-mediated destruction in vivo. The possibility remains, for example, that modifications that normally constrain the recognition of RhoA·GDP by BACURDs need to be removed first. Such a mechanism, which may respond to extracellular cues (Figure 1), would secure the maintenance of a cellular supply of RhoA·GDP that is ready for activation by GEFs.
A final issue concerns the biological function of CRL3BACURD in regulating the actin cytoskeleton. Rho GTPases control multiple aspects of cell migration, whereby their differential subcellular localization and activation is thought to determine directional movement (Jaffe and Hall, 2005). RhoA appears to act at the rear of the cell to generate contractile forces to propel the cell body forward. Although Chen et al. (2009) show that CRL3BACURD function is required for cell movement in tissue culture and in Xenopus embryos, it is currently unclear whether this involvement occurs through localized control of RhoA abundance by CRL3BACURD. Regardless, since lack of CRL3BACURD function inhibits cell migration, one wonders whether the reverse could also be true, namely that increased CRL3BACURD activity promotes migration and potentially cancer metastasis. If so, the recently observed overexpression of CUL3 in breast cancers (Loignon et al., 2009) could gain significance as a potential tumor drug target. This is particularly intriguing in light of the recent development of a pharmacological agent that can specifically interfere with CRL activity in vivo (Soucy et al., 2009). Clearly, additional work, which may lead to the identification of other CRL3BACURD substrates, is a precondition for gauging future therapeutic opportunities with sincerity, but the present study provides a solid foundation on which to build such efforts.
Contributor Information
Shuangding Wu, Email: swu@burnham.org.
Dieter A. Wolf, Email: dwolf@burnham.org.
References
- Chen Y, Yang Z, Meng M, Zhao Y, Dong N, Yan H, Liu L, Ding MH, Peng HB, Shao F. Mol Cell. 2009 doi: 10.1016/j.molcel.2009.09.004. this issue. [DOI] [PubMed] [Google Scholar]
- Geyer R, Wee S, Anderson S, Yates JRr, Wolf DA. Mol Cell. 2003;12:783–790. doi: 10.1016/s1097-2765(03)00341-1. [DOI] [PubMed] [Google Scholar]
- Jaffe AB, Hall A. Annual Review of Cell and Developmental Biology. 2005;21:247–269. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Yamamoto M. Antioxidants & Redox Signaling. 2005;7:385–394. doi: 10.1089/ars.2005.7.385. [DOI] [PubMed] [Google Scholar]
- Loignon M, Miao W, Hu L, Bier A, Bismar TA, Scrivens JP, Mann K, Basik M, Bouchard A, Fiset PO, et al. Mol Cancer Ther. 2009;8:2432–2440. doi: 10.1158/1535-7163.MCT-08-1186. [DOI] [PubMed] [Google Scholar]
- Orlicky S, Tang X, Willems A, Tyers M, Sicheri F. Cell. 2003;112:243–256. doi: 10.1016/s0092-8674(03)00034-5. [DOI] [PubMed] [Google Scholar]
- Singer JD, Gurian-West M, Clurman B, Roberts JM. Genes Dev. 1999;13:2375–2387. doi: 10.1101/gad.13.18.2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soucy TA, Smith PG, Milhollen MA, Berger AJ, Gavin JM, Adhikari S, Brownell JE, Burke KE, Cardin DP, Critchley S, et al. Nature. 2009;458:732–736. doi: 10.1038/nature07884. [DOI] [PubMed] [Google Scholar]
- Stogios P, Downs G, Jauhal J, Nandra S, Prive G. Genome Biology. 2005;6:R82. doi: 10.1186/gb-2005-6-10-r82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumara I, Quadroni M, Frei C, Olma MH, Sumara G, Ricci R, Peter M. Dev Cell. 2007;12:887–900. doi: 10.1016/j.devcel.2007.03.019. [DOI] [PubMed] [Google Scholar]
- Tang X, Orlicky S, Lin Z, Willems A, Neculai D, Ceccarelli D, Mercurio F, Shilton BH, Sicheri F, Tyers M. Cell. 2007;129:1165–1176. doi: 10.1016/j.cell.2007.04.042. [DOI] [PubMed] [Google Scholar]