Abstract
A rapid, reliable and sensitive LC-MS/MS method for the determination of melatonin in milk was developed and validated. Sample was extracted with dichloromethane and cleaned by passing through Chem Elut solid phase extraction cartridge. The solvent was evaporated to dryness, reconstituted with methanol and analysed by LC-MS/MS on Agilent zorbax Eclipse XDB C-18 rapid resolution analytical column. The analytical procedure was found to be accurate, precise and linear. The method accuracy was 92.2 % (range 90.06–94.58) and the mean precision was 1.55 % and the calibration was linear for 1 to 150 pg mL−1 (R2 > 0.99), the lowest limit of quantification (LLOQ) was 1 pg mL−1. 7-D Melatonin (7-DM) was used as an internal standard. This method was proved to be a promising method for the determination of melatonin for market milk and human milk samples.
Keywords: Melatonin, 7-D melatonin, LC-MS/MS, Milk
Introduction
Melatonin (5-methoxy-N-acetyltryptamine) is an important indole hormone produced in the pineal gland of brain. It controls many function in our daily life’s especially sleep-wake process, circadian rhythms. Melatonin is an excellent antioxidant, protective against many types of cancer (Anisimov et al. 2006) and also contributes in communication of time of day to the infants through breast feed milk (Illnerova et al. 1993). Melatonin has been used successfully for the treatment of jet-lag effects and other sleep disorder (James et al. 1990; Jan and Espezel 1995). It plays very significant role in the regulation of circadian rhythm, reproduction and retinal function (Waldhauser et al. 1984; Berga and Yen 1990; Zimmerman et al. 1990). Melatonin contributes to the development of brain, growth of infant and maturation of circadian system including sleep wake mechanism. But, the secretion of melatonin will start after 3 months of infants’ birth (Kennaway et al. 1992). Meanwhile the hormone is supplied through mother milk or cow milk. Melatonin is not only useful to the infants but, also useful for night hormone to the adults. Hence, there is a need to understand the level of melatonin in market milk supplies and mother milk consumed by the human beings.
There are many methods reported for quantification of melatonin with different kind of matrix like plasma, saliva, pineal gland and biological samples by GC-MS Chemical ionisation(Fourtillan et al. 1994), LC-MS/MS (Eriksson et al. 2003; Cao et al. 2006), GC-ECD (Greiner and Chan 1978), HPLC-FLD (Rizzo et al. 2002; Vitale et al. 1996; Iinuma et al. 1999; Munoz et al. 2009; Lizuka et al. 2007; Itoh et al. 1997), HPLC-Electrochemical detector (Chanut et al. 1998) and HPLC chemiluminesence (Lu et al. 2002). Melatonin in human milk was reported by RIA and the method was validated by GC-MS (Illnerova et al. 1993). ELISA kit was used to determine melatonin in cow milk (Kollmann et al. 2008) and a HPLC-FLD method was used to quantify milk melatonin (Egoshi et al. 2000). The study of melatonin in milk by analytical methods is limited.
The present study is focused on the development of a new analytical method to quantify the amount of melatonin in milk through solid-phase extraction and quantification using LC-MS/MS tandem mass spectrometry. In this method we used 7-D melatonin as internal standard to ensure the extraction efficiency for sample preparation and for method accuracy.
Experimental
Chemicals and materials
Reference standard melatonin (99.9 %) was procured from Sigma Aldrich (St Louis, Missouri USA), dichloromethane and methanol (LC-MS grade) were procured from Lab scan. Deuterium labelled melatonin was purchased from Cerillient Corporation USA. Chem elut SPE cartridge from Agilent Technology USA. Ultrapure ammonium formate (LC-MS grade) procured from Biosolve chemicals Netherland. All the analysis was carried out using ultra pure milli Q water.
Preparation of stock and standard solutions
Standard stock solution of melatonin 1 μg mL−1 was prepared in methanol. From the standard stock solution 1 ng mL−1 of working standard solution was prepared and it was diluted to eight different concentrations from 1 to 150 pg mL−1 for calibration purpose. 400 pg mL−1 of 7-D melatonin working internal standard solution (WIS) was prepared from 1 μg mL−1 stock solution. While preparing calibration standard 100 μl of WIS was added and each standard contained 40 pg mL−1 of 7-D melatonin. All standard stock solutions were kept at −20 °C and working standards solutions were prepared freshly before analysis.
Sample preparation and extraction
Market milk and human milk samples were collected and stored in the dark at −20 °C. One hour before extraction milk samples were brought to room temperature. To one gram milk sample 100 μl of 400 pg mL−1 of 7-D melatonin internal standard was added and kept for 15 min, then it was transferred to chem elut solid phase extraction (SPE) cartridge and it was allowed to stand for 15 min. 15 ml of dichloromethane (DCM) (3 × 5 ml) was passed through SPE by gravity flow. Collected DCM was evaporated to dryness using nitrogen and reconstituted with methanol.
The reconstituted solution was passed through 0.22 μm PVDF filter and the filtered solution was used for LC-MS/MS analysis.
LC-ESI-MS/MS analysis
Agilent 6460 ESI jet stream LC-MS/MS was employed for all experiments. An Agilent zorbax eclipse XDB C-18 rapid resolution (Lot No: B09176) column 50 mm × 4.6 mm ID × 1.8Μ were used. The mobile phase A was constituted using 5 mM ammonium formate in 80 % water and 20 % methanol and mobile phase B was methanol. The gradient pump program (Table 1) was used with 0.3 mL/min flow and 10 μl of injection volume as analytical condition.
Table 1.
Liquid chromatography pump program
| Time, minute | Mobile phase A | Mobile phase B |
|---|---|---|
| 0.0 to 2.0 | 100 | 0 |
| 2.1 to 6.0 | 10 | 90 |
| 6.1 to 10 | 100 | 0 |
The MS/MS system consisted of a triple quadrupole mass spectrometer equipped with electro spray ionisation (ESI) source with jet stream technology, operated in positive ion mode. Mass Hunter workstation software version B.02.01 was used for instrument control and data acquisition. The optimum MS conditions are summarised in Tables 2 and 3. The multi reaction monitoring (MRM) mode was used for quantification of melatonin and the detector response delta EMV was set at 400 V.
Table 2.
Optimized source parameters
| Source parameter | Optimized result |
|---|---|
| Gas temperature(°C) | 250 |
| Gas flow (L/minute) | 11 |
| Nebulizer gas (psi) | 35 |
| Sheath gas temperature (°C) | 400 |
| Sheath gas flow (L/minute) | 12 |
| Capillary voltage (V) | 2000 |
| Nozzle voltage (V) | 0 |
Table 3.
Optimized transitions for melatonin and 7-D melatonin
| Compound | MRM transitions (m/z) | Fragmentor (V) | C Ea (V) | Dwell time (μs) |
|---|---|---|---|---|
| Melatonin | 233.0 − 174.1 233.0 − 159.1 |
84 84 |
10 26 |
300 300 |
| 7-D Melatonin | 240.0 − 178.1 240.0 − 163.1 |
84 84 |
09 29 |
300 300 |
aCollision energy
Specificity
The specificity was evaluated by analysing 20 blank milk samples. Melatonin free milk samples were extracted for specificity analysis. Melatonin free milk samples were prepared by adding activated charcoal to milk samples for 24 h and the melatonin was absorbed by the charcoal. The melatonin free milk samples (n = 20) were extracted and analysed for specificity.
Linearity and Lower Limit of Quantification (LLOQ)
The eight point calibration curve was constructed by plotting peak area ratio (y) of melatonin to 7-D melatonin versus melatonin nominal concentration(x). Linearity was evaluated through the melatonin concentration range from 1 to 150 pg mL−1. Weighted least-square linear regression analysis was used to determine the slope, intercept and correlation coefficient.
The lower limit of quantification (LLOQ) was defined as the lowest milk melatonin concentration that yield a signal-to-noise (S/N) ratio >10, with acceptable precision and accuracy (<20 %).
Precision and accuracy
The precision and accuracy were determined by replicate analysis (n = 6) of milk samples at four concentration levels of melatonin (1, 10, 50 and 100 pg mL−1). To evaluate intra-assay and inter-assay precision and accuracy, three consecutive batches were analysed. Each batch contained a freshly prepared calibration curve and six replicates of four different concentrations of milk samples. Intra-day accuracy and precision were evaluated by analysis of samples at different times during the same day. Inter-day accuracy and precision were determined by repeated analysis of sample over three consecutive days. The accuracy was expressed as bias through calculating the percentages of difference between measured and nominal value (RME %), whereas the precision was expressed using relative standard deviation (RSD %). Precision and accuracy are acceptable if RSD and RME are ≤15 % (Causon 1997).
Confirmation
According to the European commission decision 2002/65/EC (ECD 2002) for the confirmation of analyte by LC-MS methods the following three criteria had to be met. (1) the retention time was 2.5 % of the external standard solution; (2) the signal-to- noise ratio (S/N) for each diagnostic ion should be ≥ 3:1; (3) the relative abundance of two reaction product ions of the sample was within acceptable range relative to the average external standard.
Stability
Stability was determined in two ways: (1) in solvent (stock solution) and (2) in matrix (spiked at milk at 50 pg mL−1).
Results and discussion
Chromatography
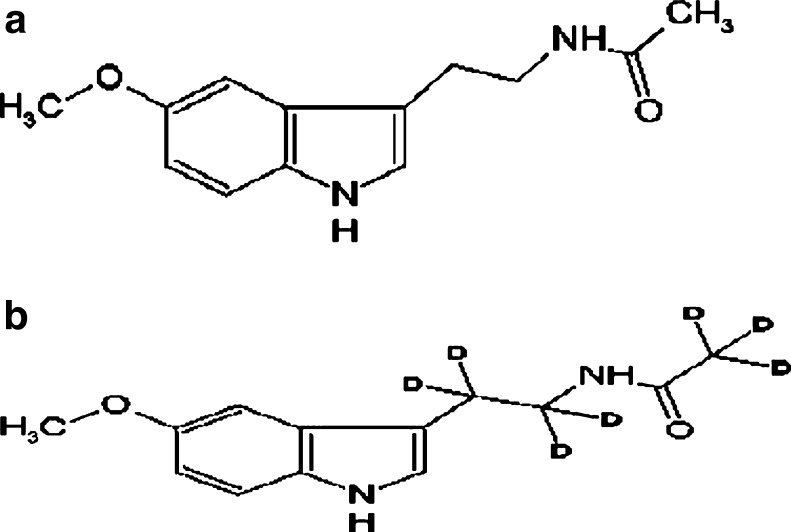
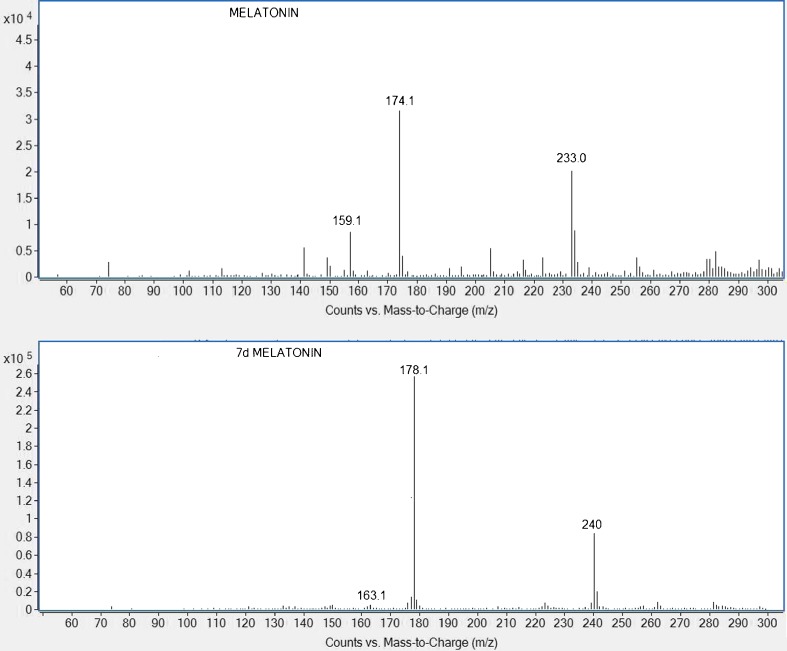
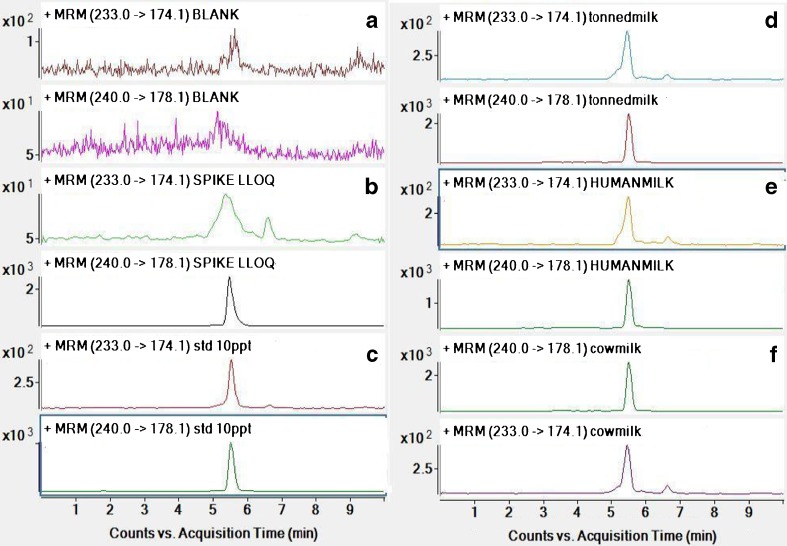
The chemical structure of melatonin and 7-D melatonin (IS) are shown in Fig. 1. Protonated molecular ions of melatonin and IS showed m/z 233.0 and 240.0 ([M + H]+), respectively. The fragmentation mass spectra of ion [M + H]+ from melatonin and IS are shown in Fig. 2. The product ion scan spectra shown high abundance fragment ion at m/z 174.1 and 178.1 for melatonin and IS, respectively. Therefore, multiple reaction monitoring (MRM) using transition at m/z 233.0 to 174.1 and 240.0 to 178.1 was used for quantification of melatonin and IS, respectively. The typical LC-MS/MS MRM chromatograms of blank milk and a milk sample spiked with melatonin at lower limit of quantification (LLOQ) (1 pg mL−1) are shown in Fig. 3. There were no interference peak at the retention time of melatonin and IS, confirming the selectivity of the present method.
Fig. 1.
Chemical structure of melatonin (a) and 7-D melatonin (b)
Fig. 2.
LC-MS/MS fragmentation for melatonin and 7-D melatonin
Fig. 3.
MRM of standard melatonin (233.0 − > 174.1) and 7-D melatonin IS (240.0 − > 178.1) a) Extracted blank milk b) Extracted melatonin in milk at LLOQ level c) Standard melatonin,10 pg/mL d) Extracted toned milk sample e) Extracted human milk sample f) Extracted cow milk sample
Selection of mobile phase and column
Prior to optimization of the mobile phase and column, many mobile phase combinations for the analysis of melatonin were carried out. Among tested mobile phase combinations water: acetonitrile (95:5) mobile phase gave excellent sensitivity for standard melatonin but carryover problem was observed. While water acetonitrile with gradient method had no carryover, but the ion ratio between qualifier and quantifier of extracted milk sample did not match against standard melatonin. By using this method the qualifier ion shows more response compared to quantifier ion due to co eluting compound in the extracted milk sample.
The sensitivity of the method was low when gradient method with ammonium formate and methanol using hypersil gold C-18 150 mm × 4.6 mm ID × 3.0 Μ column. When water: methanol (40:60) isocratic conditions, 50 mm × 4.6 mm ID × 1.8 Μ Agilent Zorbax eclipse XDB C-18 column shown good sensitivity for standard however in the quantifier channel many negative peaks were observed. Using XDB C-18 column gradient with 80 % of 5 mM ammonium formate and methanol gave good sensitivity for melatonin. The ion ratio of melatonin product ions in milk samples were within acceptable range to the standard melatonin.
Sample extraction
Since our method deals with, determination of a single analyte it requires a proper clean up and good chromatographic condition to avoid matrix interferences because the level of melatonin was very low in milk. Two types of clean up procedure can be followed one is solid phase extraction and the other is liquid- liquid extraction followed by dispersive solid phase extraction (DSPE) with suitable sorbent.
Liquid-liquid extraction
Melatonin spiked milk sample extracted with DCM and evaporated to dryness then reconstituted with methanol. Primary secondary amine (PSA) 25 mg was added to the reconstituted solution, shaken well, centrifuged and analysed by LC-MS/MS. PSA was added to remove fatty acids, small proteins etc. Liquid – liquid extraction with DCM followed by dispersive solid phase extraction (DSPE) using PSA only 33 % of IS recovery was obtained. Because of this poor recovery this extraction method was not suitable for further use.
Solid phase extraction
Selection of SPE cartridges is important to get high recovery and less interference. Since milk contains water as major component water absorbing SPE cartridges can be used for extraction.
Chem elute SPE cartridge which contains diatameous earth as sorbent and is able to absorb all the salts and polar components by distribution into a thin films over the solid support. While organic solvent passing through SPE our interest of analyte melatonin was extracted and the other polar and salts interferences remains in the SPE cartridges only. By using this method emulsion problem was rectified which was observed in liquid-liquid extraction.
In this method we used 7-D melatonin as internal standard to ensure the extraction efficiency for sample preparation and for method accuracy.
Performance of the method
A validation procedure was conducted to determine the response linearity, extraction efficiency, lower limit of quantification (LLOQ), precision and accuracy. The MRM mode was used for quantitation. Its specificity for detecting melatonin and 7-D melatonin was illustrated by representative mass chromatograms shown in Fig. 3, which shows no sign of interference from co eluting endogenous compounds in the milk matrix.
The recovery studies of melatonin in milk samples were carried out at four concentration levels (1, 10, 50 and 100 pg mL−1) and the results are summarised in Table 4. The ion ratios of spiked extracted sample have good agreement with the standard melatonin which shows there were no interfering summed ions present. A good linear relationship was found between the peak-area ratios of melatonin to 7-D melatonin versus the concentrations of melatonin ranging 1 to 150 pg mL−1 with eight levels. Linear regression analysis indicated that the correlation coefficient was greater than 0.99. The precision of the method expressed as RSD was below 6 percentages. Heller et al. (2005) reported that gentamicin in milk samples were quantified from 4.5 ng mL−1 by LC-MS/MS method. Durden (2007) reported that doramectin residues in milk samples can be detected upto 60 pg mL−1 by positive electrospray LC-MS/MS method. Martinez-Huelamo et al. (2009) were reported that the limit of detection of pencillins (AMOX, NAFC, OXAC and PENV) were less than 100 pg mL−1 in milk sample by LC-MS/MS. Since our LC-MS/MS method can detect and quantify the melatonin in milk samples upto 1 pg mL−1.
Table 4.
Intra-day and inter-day accuracy and precision (n = 6)
| Added conc. (pg mL−1) | Intra- day assay | Inter- day assay | ||||
|---|---|---|---|---|---|---|
| Found conc. (mean ± S.D.) n = 6 | RSD, % | RME, % | Found conc. (mean ± S.D.) n = 6 | RSD, % | RME, % | |
| 1 | 0.90 ± 0.01 | 0.88 | −10.00 | 0.91 ± 0.01 | 1.09 | −9.08 |
| 10 | 9.12 ± 0.40 | 4.41 | −8.76 | 9.15 ± 0.09 | 0.96 | −8.46 |
| 50 | 46.47 ± 0.49 | 1.04 | −7.07 | 46.05 ± 0.40 | 0.86 | −7.89 |
| 100 | 94.58 ± 0.80 | 0.85 | −5.42 | 94.29 ± 2.16 | 2.29 | −5.71 |
RME = (calculated concentration – nominal concentration)/nominal concentration × 100
RSD = (standard deviation of mean/mean) × 100
Confirmation
The results of confirmative parameter in LC-MS/MS analysis like retention time, ion ratio and S/N ratio for standard and fortified samples are tabulated in Table 5. Li et al. (2011) has used the above confirmatory parameter in the determination of benzimidazoles by LC-MS/MS method.
Table 5.
Confirmation of melatonin in fortified milk sample
| Parameter | Standard | Fortified sample | Limit (ref ECD 2002) |
|---|---|---|---|
| Retention time, minute | 5.479 | 5.454 | ± 2.5 % |
| Ion ratio between qualifier & quantifier ion,% (233.0 − 159.1 & 233.0 − 174.1) | 35.0 | 35.8 | ± 25 % |
| Signal/noise of LLOQ 233.0 − 174.1 | 9.2 : 1 | 9.1 : 1 | ≥ 3:1 |
Internal standard
A known amount of melatonin was added to milk and extraction procedure was carried out without addition of internal standard by liquid -liquid extraction followed by DSPE and by this method the recovery was very poor, but when the extraction was carried out in the dark and the recovery rate was slightly improved. Melatonin is a light sensitive compound and the entire standards and samples were prepared in the amber colored glass wares.
Milk contains mixture of endogenous components including protein, lipids, minerals, salts and other small and large molecules. These are often present in high amount compared to our interest of analyte (melatonin) and greatly complicate the process of extraction and analysis. To ensure the extraction efficiency in complex milk matrix, we used 7-D melatonin as internal standard from the beginning of sample preparation. Melatonin and 7-D melatonin have similar chemical and physical property which has the advantage that both molecules behave equally during sample extraction, cleanup and in LC-MS/MS analysis.
A known amount of melatonin and 7-D melatonin was added to the milk and extraction was carried out. Recovery was calculated using the ratio of melatonin and 7-D melatonin of the sample to the standard. By this method the recovery was above 90 percentages. Therefore 7-D melatonin is ideally suitable for the analysis of melatonin in milk by LC-MS/MS.
Stability
The stability of melatonin in standard solution and in milk samples were evaluated with different storage conditions and different time periods. The conditions and results are tabulated in Table 6.
Table 6.
Stability study of melatonin
| Analyte | Conc. | Storage condition | Storage period | Result |
|---|---|---|---|---|
| Melatonin stock in methanol | 1 μg mL−1 | −20 °C | 12 months (every month the response of melatonin was compared with initial response) | The response was stable up to 8 month & the response was within 95 to 105 % |
| Melatonin spiked into milk sample | 50 pg mL−1 | −20 °C | 2nd day | 48.5 pg mL−1 |
| 4th day | 48.2 pg mL−1 | |||
| 8th day | 47.8 pg mL−1 | |||
| Melatonin in human milk | 60 pg mL−1 | 10 °C | Initial | 60.0 pg mL−1 |
| 15th hour | 52.2 pg mL−1 | |||
| 60th hour | 38.4 pg mL−1 |
Application to sample (cow and human milk)
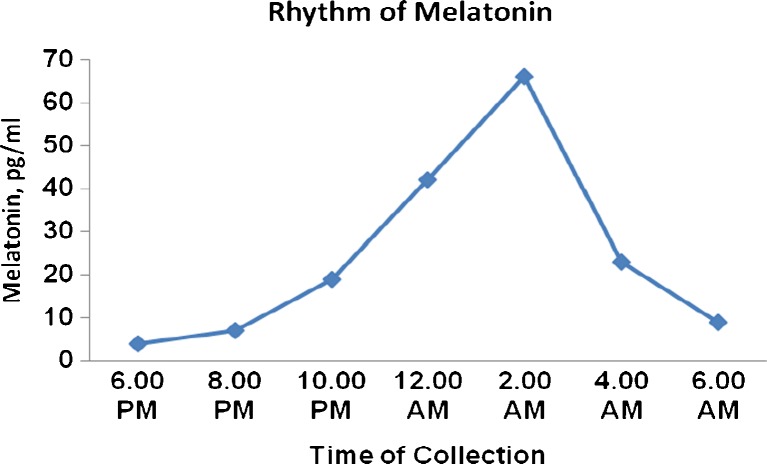
The method was applied to the routine analysis of commercially available market milk sample as well as Indian human milk samples. Human milk samples were collected in different time and analysed. The melatonin level was varied based on date of collection from the delivery day, time of collection and mother health. The secretion of melatonin was high in midnight while compare to other time. The rhythm of melatonin secretion shown in Fig. 4 and the results of few samples are summarized in Table 7.
Fig. 4.
Rhythm of melatonin in human milk
Table 7.
Results of melatonin in milk samples
| Sample | Trial 1 (pg mL−1) | Trial 2 (pg mL−1) | Trial 3 (pg mL−1) | Average | SDa | % RSDb |
|---|---|---|---|---|---|---|
| CMc 1 | 14.34 | 14.44 | 14.57 | 14.45 | 0.12 | 0.81 |
| TMd 1 | 17.85 | 18.51 | 19.11 | 18.41 | 0.62 | 3.40 |
| HMe 1 | 14.74 | 16.50 | 16.52 | 15.92 | 1.02 | 6.44 |
| HM 2 | 5.41 | 5.24 | 5.55 | 5.4 | 0.15 | 2.87 |
aStandard deviation
bRelative standard deviation
cCow milk
dToned milk
eHuman milk, samples were collected in daytime
Conclusion
Earlier reported methods for melatonin in milk was carried out using RIA, ELISA, HPLC-Fluorescent and GC-MS. Sensitivity of the RIA method was very high but the selectivity of the method is questionable, because of cross reactivity of various indolic compounds presents in milk and leads to false positive results. HPLC with fluorescence detector gave a good sensitivity which require derivatization step which is more critical and time consuming. GC-MS method makes identity confirmation possible thus increasing the validity of the results but this method also requires derivatization to enhance the sensitivity. Our LC-MS/MS method is more specific and selective since the detection is based on mass. It gives lower detection limit compare to other analytical techniques without any derivatization step. LC-MS/MS with ESI (+) mode was successfully applied for the determination and quantification of melatonin in cow milk and human milk by following SPE. The analytical results of (extraction and clean up) the method demonstrate that the assay produced is more reliable determination for melatonin in milk samples.
References
- Anisimov VN, Popovich IG, Zabezhinski MA, Anisimov SV, Vesnushkin GM, Vinogradova IA. Melatonin as antioxidant, geroprotector and anticarcinogen. Biochim Biophys Acta. 2006;1757:573–589. doi: 10.1016/j.bbabio.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Berga SL, Yen SSC. Circadian pattern of plasma melatonin concentrations during four phases of the human menstrual cycle. Neuroendocrinol. 1990;51(5):606–612. doi: 10.1159/000125398. [DOI] [PubMed] [Google Scholar]
- Cao J, Murch SJ, Brien RO, Saxena PK. Rapid method for accurate analysis of melatonin, serotonin and auxin in plant samples using liquid chromatography-tandem mass spectrometry. J Chromatogr A. 2006;1134:333–337. doi: 10.1016/j.chroma.2006.09.079. [DOI] [PubMed] [Google Scholar]
- Causon R. validation of chromatographic methods in biomedical analysis viewpoint and discussion. J Chromatogr B. 1997;689(1):175–180. doi: 10.1016/S0378-4347(96)00297-6. [DOI] [PubMed] [Google Scholar]
- Chanut E, Legros JN, Botteri CV, Trouvin JH, Launay JM. Determination of melatonin in rat pineal, plasma and retina by high performance liquid chromatography with electrochemical detection. J Chromatogr B. 1998;709(1):11–18. doi: 10.1016/S0378-4347(98)00041-3. [DOI] [PubMed] [Google Scholar]
- Durden AD. Positive and negative electrospray LC-MS/MS methods for quantitation of the antiparastic endecticide drugs, abamectin, doramectin, emamectin, eprinomectin, ivermectin, moxidectin and selamectin in milk. J Chromatogra B. 2007;850:134–146. doi: 10.1016/j.jchromb.2006.11.014. [DOI] [PubMed] [Google Scholar]
- Egoshi K, Oka T, Yamashita H. Quantitative analysis of melatonin in raw milk by HPLC. Nippon Shokuhin Kagaku Kogaku Kaishi. 2000;54(3):113–117. doi: 10.3136/nskkk.54.113. [DOI] [Google Scholar]
- Eriksson K, Ostin A, Levin J. Quantification of melatonin in human saliva by liquid chromatography-tandem mass spectrometry using stable isotope dilution. J Chromatogr B. 2003;794:115–123. doi: 10.1016/S1570-0232(03)00425-2. [DOI] [PubMed] [Google Scholar]
- European Commission Decision 2002/657/EC (2002) Off J Eur Commun L221
- Fourtillan JB, Gobin P, Faye B, Girault J. A highly sensitive assay of melatonin in human plasma by gas chromatography/negative ion chemical ionization mass spectrometry. Biol Mass Spectrom. 1994;23(8):499–509. doi: 10.1002/bms.1200230807. [DOI] [PubMed] [Google Scholar]
- Greiner AC, Chan SC. Melatonin content of the human pineal gland. Science. 1978;199:83–84. doi: 10.1126/science.199.4324.83. [DOI] [PubMed] [Google Scholar]
- Heller DN, Peggins JO, Nochetto CB, Smith ML, Chiesa OA, Moulton K. LC-MS/MS measurement of gentamicin in bovine plasma, urine, milk and biopsy samples taken from kidneys of standing animals. J Chromatograph B Analyt Technol Biomed Life Sci. 2005;821:22–30. doi: 10.1016/j.jchromb.2005.04.015. [DOI] [PubMed] [Google Scholar]
- Iinuma F, Hamase K, Matsubayashi S, Takahashi M, Watanabe M, Zaitsu K. Sensitive determination of melatonin by precolumn derivatization and reversed-phase high performance liquid chromatography. J Chromatogr A. 1999;835:67–72. doi: 10.1016/S0021-9673(99)00041-2. [DOI] [PubMed] [Google Scholar]
- Illnerova H, Buresova M, Presl J. Melatonin rhythm in human milk. J Clin Endocrinol Metab. 1993;77(3):838–841. doi: 10.1210/jcem.77.3.8370707. [DOI] [PubMed] [Google Scholar]
- Itoh MT, Ishizuka B, Kudo Y, Fusama S, Amemiya A, Sumi Y. Detection of melatonin and serotonin N-acetyltransferase and hydroxyindole-O-methyltransferase activities in rat ovary. Mol Cell Endocrinol. 1997;136:7–13. doi: 10.1016/S0303-7207(97)00206-2. [DOI] [PubMed] [Google Scholar]
- James SP, Sack DA, Rosenthal NE, Mendelson WB. Melatonin administration in insomnia. Neuropsychopharmacol. 1990;3(1):19–23. [PubMed] [Google Scholar]
- Jan JE, Espezel H. Melatonin treatment of chronic sleep disorders. Dev Med Child Neurol. 1995;37(3):279–280. [PubMed] [Google Scholar]
- Kennaway DJ, Stamp GE, Goble FC. Development of melatonin production in infants and the impact of prematurity. J Clin Endocrinol Metab. 1992;75(2):367–369. doi: 10.1210/jcem.75.2.1639937. [DOI] [PubMed] [Google Scholar]
- Kollmann MT, Locher M, Hirche F, Eder K, Meyer HH, Bruckmaier RM. Effects of tryptophan supplementation on plasma tryptophan and related hormone levels in heifers and dairy cows. Domest Anim Endocrinol. 2008;34(1):14–24. doi: 10.1016/j.domaniend.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Li C, Wu YL, Yang T, Zhang Y. Rapid simultaneous determination of eight benzimidazoles in animal feed by LC-MS/MS. Chromatographia. 2011;73:59–65. doi: 10.1007/s10337-010-1838-9. [DOI] [Google Scholar]
- Lizuka H, Someya K, Yajima T. Hemin-mediated fluorometric determination of melatonin by high-performance liquid chromatography. Int Congr Ser. 2007;1304:409–414. doi: 10.1016/j.ics.2007.07.047. [DOI] [Google Scholar]
- Lu J, Lau C, Lee MK, Kai M. Simple and convenient chemiluminescence method for the determination of melatonin. Anal Chim Acta. 2002;455(2):193–198. doi: 10.1016/S0003-2670(01)01603-8. [DOI] [Google Scholar]
- Martinez-Huelamo M, Jimenez-Gamez E, Hermo MP, Barron D, Barbosa J. Determination of pencillins in milk using LC-UV, LC-MS and LC-MS/MS. J Sep Sci. 2009;32:2385–2393. doi: 10.1002/jssc.200900212. [DOI] [PubMed] [Google Scholar]
- Munoz JLP, Ceinos RM, Soengas JL, Miguez JM. A simple and sensitive method for determination of melatonin in plasma, bile and intestinal tissues by high performance liquid chromatography wit fluorescence detection. J Chromatogr B. 2009;877:2173–2177. doi: 10.1016/j.jchromb.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Rizzo V, Porta C, Moroni M, Scoglio E, Moratti R. Determination of free and total (free plus protein bound) melatonin in plasma and cerebrospinal fluid by high-performance liquid chromatography with fluorescence detection. J Chromatogr B. 2002;774:17–24. doi: 10.1016/S1570-0232(02)00168-X. [DOI] [PubMed] [Google Scholar]
- Vitale AA, Ferrari CC, Aldana H, Affanni JM. Highly sensitive method for the determination of melatonin by normal-phase high-performance liquid chromatography with flurometric detection. J Chromatogr B. 1996;681(2):381–384. doi: 10.1016/0378-4347(96)00051-5. [DOI] [PubMed] [Google Scholar]
- Waldhauser F, Waldhauser M, Lieberman HR, Deng MH, Lynch HJ, Wurtman RJ. Biavailability of oral melatonin in humans. Neuroendocrinol. 1984;39:307–313. doi: 10.1159/000123997. [DOI] [PubMed] [Google Scholar]
- Zimmerman RC, Schroder S, Baars S, Schumacher M, Weise HC. Melatonin and the ovulatory luteinizing hormone surge. Fertil Steril. 1990;54:612–618. [PubMed] [Google Scholar]