Abstract
‘Lingwu Long’ jujube (Zizyphus jujuba cv. Mill) fruits were harvested at mature-green stages and then treated with 1.0 μL L−1 1-methylcyclopropene (1-MCP), 1.0% CaCl2 or their combination. All treatments were stored at room temperature (22 ± 1 °C) and 80–90% relative humidity (RH) up for 15 days. Results indicated that 1.0 μL L−1 1-MCP, 1.0% CaCl2 or their combination were effective in terms of senescence inhibition, and the storage life was extended by 6, 4 and 9 days, respectively. 1-MCP and CaCl2 treatment had a synergic effect on the inhibition of ethylene production and microbial population of ‘Lingwu Long’ jujube fruit. The combination of 1-MCP and CaCl2 significantly reduced polygalacturonase (PG) and polyphenoloxidase (PPO) activities. It also maintained higher concentrations of titratable acid and ascorbic acid.
Keywords: ‘Lingwu Long’ Jujube, 1-MCP, Calcium chloride, Storage life, Quality maintenance
Introduction
‘Lingwu Long’ Jujube (Zizyphus jujuba cv. Mill) is considered to be a seasonal fruit and is a good source of various minerals and vitamins. This fruit has proven to be a native popular agricultural product that could bring local people more than 1,500,000 dollars each year. However, the ripening and senescence processes of this kind of fruit result in a reduced storage life and a decrease in quality parameters such as firmness, chlorophyll content and nutrients, the occurrence of decay and off-flavors (Valero et al. 2003). Several pre-harvest and post-harvest applications have been used in order to reduce these undesirable changes (Bisen et al. 2011; Goutam et al. 2010; Mehta et al. 1985; Rezaee et al. 2011; Sabιr et al. 2011; Workneh et al. 2011). Moreover, ‘Lingwu Long’ Jujube fruits are easily infected by Alternaria alternata (Fr.) Keissl and the postharvest disease incidence is up to 60%. Thus, there is a need to have alternative technologies to reduce the decay incidence and to decrease the undesirable physic-chemical changes during storage. In this sense, 1-methylcyclopropene (1-MCP) and calcium chloride treatments are considered to be powerful tools.
1-MCP, a strong inhibitor of ethylene perception, now is used extensively by fruit industries to maintain quality, acts at very low concentration for delaying fruit ripening and improving storage quality of many fruits and vegetables (DeEll et al. 2002; Feng et al. 2000; Golding et al. 1998; Ku and Wills 1999). It is odorless, nontoxic and highly effective. It has also been commercially used to extend the storage life of horticultural products.
In recent years, calcium chloride has been widely used in the filed of food preservation. It could help maintain firmness and visual quality resulting in a longer shelf life of the fresh-cut products as well. Pre-harvest applications of calcium compounds and post-harvest dipping in calcium chloride solution have been used in order to prolong the storage life of fruits (Gupta et al. 2011; Mehta et al. 1985).
The main objective of present research is to evaluate the potential effect of 1-MCP, calcium chloride, and their combination on storage life, biochemical changes, microbial growth and quality maintenance of ‘Lingwu Long’ Jujube fruit during storage at room temperature for 15 days.
Materials and methods
Fruit source, treatments and storage condition
‘Lingwu Long’ Jujube (Zizyphus jujuba cv. Mill) fruits used in the experiment were harvested at mature-green stages from a local orchard located in Lingwu, Ningxia Hui Nationality Autonomous Region. The fruits of regular shape and uniform size and color were selected, and the physical damaged ones were removed. Before experimental treatment, the fruits were sanitized in chlorinated water (200 μL L−1) for 2 min and then air-dried.
In preliminary experiments, three doses (0.5, 1.0 and 1.5 μL L−1) for 1-MCP (w/v; SmartFresh™, Rohm and Haas Co., Italy) and three concentrations (0.5%, 1.0% and 1.5%) for calcium chloride (w/v; Sigma–Aldrich) dipping treatments were investigated. The preliminary results showed that 1.0 μL L−1 of 1-MCP and 1.0% of calcium chloride dipping treatments were more effective than other concentrations to retain firmness, reduce decay and maintain quality of ‘Lingwu Long’ Jujube fruit. Thus, 1.0 μL L−1 of 1-MCP and 1.0% of calcium chloride solution were selected for current study.
Fruits were placed inside an airtight chamber of 0.25 m3 and treated with 1.0 μL L−1 1-MCP for 24 h at ambient temperature. Fruits were removed from the chamber after 1-MCP treatment. Then, half of the fruits were dipped in solution containing 1.0% calcium chloride for 2 min and then air-dried with a fan. Fruits were subjected to the same conditions with 0 μL L−1 of 1-MCP and then half of the fruits were treated with calcium chloride solution as described above. Fruit treatment with 0 μL L−1 of 1-MCP and without calcium chloride treatment was taken as control. After each treatment, all fruits were subsequently stored at room temperature (22 ± 1 °C) and 80–90% RH for assessment.
Storage life and firmness assessment
Storage life was estimated as described by Zhong and Xia (2007). Firmness was measured using a TA-XT Plus texture analyzer with 2 mm in diameter for the needle-like probe (Stable Micro System, Scarsdale, NY). The penetration depth into the intact jujube fruit was 5 mm at the penetration rate of 1 mm s−1. Firmness of 12 fruits per replicate was determined.
Ethylene production
Ethylene production was assayed according to the method of Ergun et al. (2007) with slight modifications. About 1 kg of fruit was sealed in a 2.5 L gas-tight container for 2 h, and 1 mL of headspace gas was withdrawn by means of a gas-tight hypodermic syringe and measured with a gas chromatography equipped with a flame ionization detector (FID) and a Poropak N column (Shimadzu GC-9A, Japan). Ethylene production was expressed as mL kg−1 h−1. The headspace gas sample withdrawn from the same volume of container without fruit was taken as control.
Chemical quality evaluation
Juice samples were obtained by squeezing the fruit from each replicate through four layers of cheesecloth with a hand juicer. Total soluble solids (TSS) of the juice was measured with an Abbe Refractometer, model 10450 (American Optical, Buffalo, NY) and expressed as a percentage. An automatic titrator (Radiometer, Copenhagen, Denmark) equipped with a PHM85 Precision pH meter was used to measure titrable acidity (TA). A 4 g juice sample per replicate was diluted with 20 mL distilled water and titrated with 0.1 mol L−1 NaOH to pH 8.1. TA was calculated as percent malic acid (predominant acid in jujube fruits).
For ascorbic acid (AA) measurement, the frozen peel or flesh tissues were crushed into coarse pieces, and 10 g samples were homogenized with 100 mL of buffer (2% metaphosphoric acid containing 2 mmol L−1 EDTA) using a Waring commercial blender for 3 min. The slurry was centrifuged at 15,000 g for 15 min at 4 ◦C. The AA concentration was evaluated according to the method of Rao and Ormrod (1995). The concentration was expressed as mg per 100 g fresh weight.
Enzymes extraction and assay
Polygalacturonase (PG) was extracted by the method of Pathak and Sanwal (1998). All extractions were performed at 4 °C. Tissue (2 g) from triplicate samples was homogenized in 10 mL of 0.2 mol L−1 acetic acid buffer (pH 6.0) and then centrifuged at 15,000 g for 20 min. The supernatant was used for enzyme assay. PG activity was measured by the method of Zhong and Xia (2007) with slight modifications. The determination of PG activity was based on the hydrolytic release of galacturonic acid from polygalacturonic acid. The reaction mixture contained 0.3 mL of 1.0% (w/v) polygalacturonic acid in 40 mmol L−1 Na–acetate buffer (pH 4.6), 0.1 mL of crude enzyme and 1.9 mL of deionized water. It was incubated at 37 °C for 1 h and the reaction was terminated by the addition of 1.5 mL of dinitrosalicylate reagent and immersion in a boiling water bath for 5 min. The final volume of the sample was adjusted to 25 mL with deionized water. Samples were then cooled to room temperature and the absorbance was measured at 540 nm. The released amount of galacturonic acid from polygalacturonic acid was obtained from the galacturonic acid standard curve. One unit of PG activity was defined as 1 mg of galacturonic acid released per 1 h. Boiled extracts were assayed as controls for the determination.
Polyphenoloxidase (PPO) was extracted by the method of homogenizing 1 g of tissue in 0.1 mol L−1 potassium phosphate buffer (pH 7.0). The homogenate was centrifuged at 15,000 g for 15 min at 4 °C and the supernatant was used as the enzyme source. After native electrophoresis, the gel was equilibrated for 30 min in 0.1% p-phenylene diamine in 0.1 mol L−1 potassium phosphate buffer (pH 7.0) followed by addition of 10 mmol L−1 catechol in the same buffer (Jayaraman et al. 1987).
Microbiological analysis
Change in the microbiological population was studied during storage, as described by Aguayo et al. (2006). Random 10 g samples of jujube fruit were homogenized for 2 min in 10 mL of sterile buffered peptonewater (Difco, Sparks, MD), with a Seward 400 Lab Stomacher (Tekmar, Cincinnati, OH). Dilutions were made in 0.1% peptone water (Hardy Diagnostics, Santa Maria, CA) as needed for plating. The enumeration of particular microbial groups was performed by using the following media and culture conditions: Plate Count Agar (Difco, Sparks, MD) for mesophilic bacteria, incubated at 35 °C for 2 days. Potato Dextrose Agar (Difco, Sparks, MD) with added streptomycin and acidified to pH 3.5 with 10% l-tartaric acid (Fisher, Fair Lawn, NJ), incubated at 29 °C for 2 and 5 days, respectively. All analyses were reported as log10 cfu g−1 (colony forming units per gram of sample).
Statistical analysis
All experiments were replicated three times and data were analyzed using analysis of variance (ANOVA) with SAS statistical software. The least significant difference (LSD) was calculated to compare significant effects at 5% level and only significant differences were discussed unless stated otherwise. Time, treatment, and their interaction were considered the main factors.
Results and discussion
Storage life
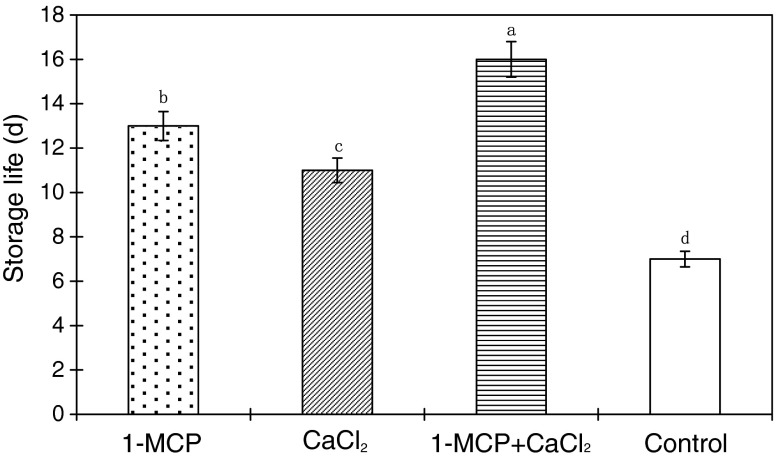
Freshly harvested ‘Lingwu Long’ Jujube fruits were green and their senescence was indicated by chlorophyll loss. Thus, the storage life was defined as the time until the pericarp was uniformly loss of green. ‘Lingwu Long’ Jujube fruit treated with 1-MCP and/or CaCl2 showed extended storage life through a delay in the loss of green color and onset of yellowing compared to the control one (Fig. 1). The extension of storage life of 1-MCP+CaCl2 treatment compared to control was the highest (128.6%), following by 1-MCP treatment (85.7%) and CaCl2 treatment (57.1%).
Fig. 1.
Storage life of ‘Lingwu Long’ Jujube fruit treated with 1-MCP, CaCl2, or their combination during 15 days at room temperature (22 ± 1 °C). Each value is the mean of three replications, and vertical bar represents the standard error of the means (n = 3). Different letters within each column are significantly different (P < 0.05)
Fruit firmness
The best treatment for getting good firmness retention of jujube fruits was 1-MCP plus CaCl2, (Table 1). The synergistic effect among 1-MCP plus CaCl2 on firmness retention was similar to what has been reported for kiwifruit (Vilas-Boas and Kader 2001). Firmness was improved by the CaCl2 treatment. Calcium dip has been used as firming agents to extend postharvest shelf life in whole and in fresh-cut fruit. Rosen and Kader (1989) found that the CaCl2 treated slices of strawberries resulted in higher calcium content and they were firmer than water-dipped slices. Luna-Guzmán and Barrett (2000) also found CaCl2 maintained the firmness throughout storage and less softness in kiwifruit slices dipped in 0.5 or 1.0% CaCl2 was obtained by Agar et al. (1999). Firming and resistance to softening resulting from addition of calcium have been attributed to the stabilization of membrane systems and the formation of Ca-pectates, which increase rigidity of the middle lamella and cell wall to increased resistance to polygalacturonase attack and to improve turgor pressure (Mignani et al. 1995). In the present study, calcium chloride treatment alone played an important role in firmness that even increased after treatment, in particular from day 3 onwards (Table 1).
Table 1.
Firmness and chemical quality evaluations of ‘Lingwu Long’ Jujube fruits treated with 1-MCP, CaCl2, or their combination during 15 days at room temperature (22 ± 1 °C)
| Storage time (d) | Treatment | Firmness (N) | TSS* (%) | TA* (g malic acid/100 g) | AA* (mg/100 g) |
|---|---|---|---|---|---|
| 0 | 15.0 | 34.0 | 0.38 | 408.8 | |
| 3 | 1-MCP | 14.0 | 32.5 | 0.35 | 385.1 |
| CaCl2 | 14.2 | 32.8 | 0.32 | 352.4 | |
| 1-MCP + CaCl2 | 14.5 | 33.0 | 0.37 | 381.4 | |
| Control | 13.8 | 32.5 | 0.31 | 347.3 | |
| 6 | 1-MCP | 13.5 | 31.0 | 0.32 | 343.5 |
| CaCl2 | 14.5 | 31.8 | 0.30 | 311.7 | |
| 1-MCP + CaCl2 | 14.8 | 32.0 | 0.37 | 352.1 | |
| Control | 12.2 | 31.5 | 0.27 | 307.2 | |
| 9 | 1-MCP | 12.1 | 31.0 | 0.28 | 298.9 |
| CaCl2 | 13.0 | 30.0 | 0.28 | 290.7 | |
| 1-MCP + CaCl2 | 13.9 | 31.5 | 0.31 | 318.1 | |
| Control | 11.7 | 30.5 | 0.25 | 284.0 | |
| 12 | 1-MCP | 10.3 | 28.5 | 0.22 | 240.3 |
| CaCl2 | 11.8 | 28.8 | 0.21 | 221.8 | |
| 1-MCP+CaCl2 | 12.4 | 29.0 | 0.30 | 299.2 | |
| Control | 9.8 | 28.0 | 0.16 | 207.5 | |
| 15 | 1-MCP | 8.0 | 25.5 | 0.20 | 201.5 |
| CaCl2 | 10.2 | 25.0 | 0.17 | 184.0 | |
| 1-MCP+CaCl2 | 11.5 | 26.3 | 0.29 | 253.6 | |
| Control | 8.1 | 25.4 | 0.11 | 155.2 | |
| Time | (0.04)a | (1.2)a | (0.08)b | (1.1)c | |
| Treatment | (0.04)c | NS | (1.72)a | (1.0)b | |
| Time × Treatment | (0.09)b | NS | NS | (2.33)b |
Values are the means (n = 3). NS: not significant. L.S.D. values are in brackets
* TSS, total soluble solids; TA, titrable acidity; AA, ascorbic acid
a P < 0.05; b P < 0.01; c P < 0.001
1-MCP had no significant effect on firmness of jujube fruits in related to control (Table 1). Mir et al. (2001) found that a given concentration of 1-MCP had less effect on apple firmness as storage temperature was lowered, and it was hypothesized that lower temperatures might lower the affinity of the binding site for 1-MCP. Mao et al. (2004) held whole seedless watermelon in 10.0 μL L−1 1-MCP and the fresh-cut cylinders obtained were rinsed with 2.0% CaCl2. These authors found that the combination of 1-MCP and CaCl2 retarded the ripening process, as indicated by higher firmness and lower activities of lipolytic enzymes relative to the control. In contrast, Rupasinghe et al. (2005) showed that a post-cut dipping treatment of Nature Seal (6.0% calcium ascorbate) was much more effective than 1-MCP in maintaining firmness of ‘Empire’ and ‘Crispin’ apple slices. And an effective postharvest application of 1-MCP (0.3 μL L−1 for 24 h) was obtained which can delay the ripening of tomato fruits stored at higher temperatures of about 30 and 25 °C (Paul et al. 2010).
Ethylene production
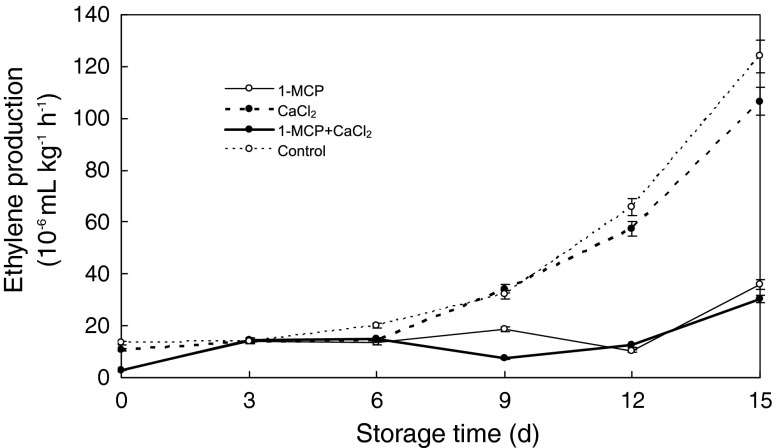
C2H4 production rates were similar among all treatments during the first 6 days at room temperature. Subsequently, the control and CaCl2 treated fruits had higher C2H4 production rates than fruits exposed to 1-MCP, with or without CaCl2 dip (Fig. 2). Aguayo et al. (2006) reported similar results. Lower ethylene production rate was obtained in apple slices prepared from whole 1-MCP (1.0 μL L−1 for 24 h) treated ‘Delicious’, ‘Empire’ and ‘Idared’ fruit (Calderon-Lopez et al. 2005) or ‘Gala’ apples (1-MCP; 1.0 or 0.625 μL L−1 for 18 h) (Bai et al. 2004). Jiang and Joyce (2002) reported that ethylene production derived from 1-MCP-treated apple fruit remained suppressed after 5 and 10 days.
Fig. 2.
Ethylene production of ‘Lingwu Long’ Jujube fruit treated with 1-MCP, CaCl2, or their combination during 15 days at room temperature (22 ± 1 °C). Each value is the mean of three replications, and vertical bar represents the standard error of the means (n = 3)
In the present study, ‘Lingwu Long’ Jujube fruit was a typical of climacteric fruit. Ethylene starts a cascade of events leading to many interactive signaling and metabolic pathways for the progress of ripening in climacteric fruits. Work on climacteric fruit (such as banana) where fruits were treated with 1-MCP after various periods of application of propylene illustrated that ripening related processes once engaged with auto-induced or auto-catalytic ethylene production become partially independent of further ethylene action (Paul et al. 2011). 1-MCP and/or CaCl2 treatment delayed the onset of climacteric ethylene production in ‘Lingwu Long’ Jujube fruit. The magnitude of the ethylene was decreased by the application of 1-MCP (P < 0.05) and the suppressed duration of ethylene was much longer than those without 1-MCP treatment (Fig. 2).
TSS, TA, AA contents
In general, TSS, TA and AA decreased with time of storage ranging from 34.0 to 25.4%, 0.38 to 0.11 g malic acid and 408.8 to 155.2 mg per 100 g fresh weight, respectively. 1-MCP or CaCl2 treatment did not affect TSS but there was a significant influence on TA and AA; 1-MCP plus CaCl2 treatment showed a higher TA and AA than other treatments, particularly control (Table 1). The AA loss during storage is known to be due to its antioxidant activity especially under postharvest storage conditions. The beneficial effect of calcium in preventing decline of AA during storage is due to the regulation of oxidative processes in the cytosol (Hussain et al. 2011). The effects of 1-MCP and CaCl2 treatment on TSS may rely on fruit cultivars and storage conditions. Neither Jiang and Joyce (2002) and Perera et al. (2003) in fresh-cut apples nor Porat et al. (1999) in whole oranges found any significant effects of 1-MCP on TSS. However, TSS was higher in 1-MCP-treated pineapple (Selvarajah et al. 2001) and pear fruits (Mahajan et al. 2010) or reduced in 1-MCP-treated strawberries regardless of the presence or absence of exogenous ethylene (Tian et al. 2000).
PG and PPO activity
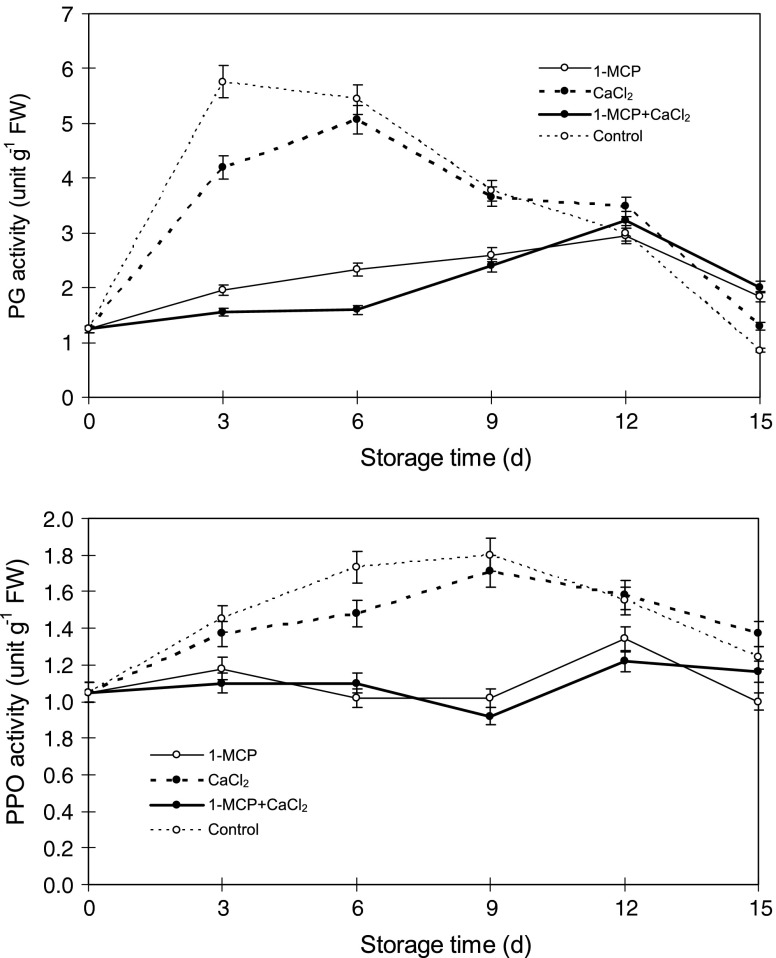
As shown in Fig. 3, PG activity of control jujube fruit increased sharply for the first 3 days during storage and reached a maximum value 4.6-fold higher than initial activity. Thereafter, it decreased sharply. 1-MCP and 1-MCP+CaCl2 treatments greatly retarded PG activity increase in the first 12 days. The maximum PG levels of 1-MCP and 1-MCP+CaCl2 treated fruit were observed on day 12, being 2.4- and 2.6-fold higher than initial levels (P < 0.05), respectively.
Fig. 3.
Polygalacturonase (PG) and Polyphenoloxidase (PPO) activity of ‘Lingwu Long’ Jujube fruit treated with 1-MCP, CaCl2, or their combination during 15 days at room temperature (22 ± 1 °C). Each value is the mean of three replications, and vertical bar represents the standard error of the means (n = 3)
PPO activity of control and CaCl2 treated fruit increased within the early 9 days, and reached a high peak at day 9, and then decreased (Fig. 3). PPO levels of 1-MCP and 1-MCP+CaCl2 treatments were greatly reduced compared to control through the storage period (P < 0.05). Maximum PPO levels of 1-MCP or 1-MCP+CaCl2 treated fruit compared with control and CaCl2 treated fruit was reduced about 24.4% and 28.7%, respectively.
The softening and senescence of fruit were due to damage of cellular membrane integrity and function, which was associated with PG and PPO. The present study showed that 1-MCP treatment significantly reduced PG activity. Polyphenoloxidases had been implicated in a number of physiological functions that may contribute to resistance including cross linking of extension monomers (Everdeen et al. 1988) and lignifications (Walter 1992) and they were also associated with deposition of phenolic compounds into plant cell walls during resistance interactions (Graham and Graham 1991). PPO catalyzed the oxidation of monophenolic and o-diphenolic compounds. Jennings et al. (1969) reported PPO as terminal oxidase in infected plant tissue and the fungi toxins might activate these enzymes. In current study, PPO activity was also suppressed in 1-MCP treated fruit. Therefore, 1-MCP-treated and 1-MCP+CaCl2-treated fruit exhibited an extension storage life and increased by 6 and 5 d compared to control and CaCl2-treated fruit, respectively.
Microbiological analysis
Time of storage significantly increased the mesophilic bacteria and yeast growth, in particular, in control jujube fruit (Table 2). Results showed that other treatments, 1-MCP, CaCl2 treatment or their combination played a positive role in reducing of the microbiological counts. No significant differences among treatments were found in mold growth. Similar effect of 1-MCP, CaCl2 plus CA on fresh-cut strawberries fruit was reported (Aguayo et al. 2006).
Table 2.
Microbiological counts of ‘Lingwu Long’ Jujube fruits treated with 1-MCP, CaCl2, or their combination during 15 days at room temperature (22 ± 1 °C)
| Storage time (d) | Treatment | Mesophilic (log10 cfu g−1) | Yeast (log10 cfu g−1) | Mold (log10 cfu g−1) |
|---|---|---|---|---|
| 0 | 2.7 ± 0.22 | 1.2 ± 0.11 | ||
| 6 | 1-MCP | 5.3 ± 0.28 | 2.9 ± 0.69 | |
| CaCl2 | 5.6 ± 0.03 | 3.3 ± 1.20 | <1 | |
| 1-MCP+CaCl2 | 4.7 ± 1.04 | 1.1 ± 0.30 | ||
| Control | 6.8 ± 0.36 | 3.6 ± 0.08 | <1 | |
| 12 | 1-MCP | 6.0 ± 0.47 | 4.2 ± 0.82 | |
| CaCl2 | 6.5 ± 0.60 | 4.4 ± 0.63 | ||
| 1-MCP+CaCl2 | 5.1 ± 2.05 | 2.5 ± 0.37 | ||
| Control | 7.7 ± 1.34 | 4.9 ± 2.11 | <2 | |
| 15 | 1-MCP | 5.8 ± 0.50 | 4.9 ± 0.60 | |
| CaCl2 | 7.3 ± 0.64 | 5.0 ± 1.45 | ||
| 1-MCP+CaCl2 | 5.4 ± 0.98 | 3.1 ± 0.79 | ||
| Control | 8.6 ± 1.02 | 5.5 ± 0.33 | <3 | |
| Time | (1.18)c | (0.98)c | NS | |
| Treatment | (0.92)c | (0.75)c | NS | |
| Time × Treatment | (2.06)b | (2.19)c | NS |
Values are the means ± standard error (n = 3). NS: not significant. L.S.D. values are in brackets
a P < 0.05; b P < 0.01; c P < 0.001
This study also showed that 1-MCP plus CaCl2 treatment could significantly reduce microbial development of ‘Lingwu Long’ Jujube fruit compared to the control (Table 2). There were several reports indicating that the relevance between 1-MCP treatment and decay growth or microbial growth in fruit and vegetable. For cases in which ethylene-sensitive product was exposed to ethylene in mixed storage before processing or in packaged mixes, 1-MCP may be beneficial in controlling senescence-associated decay and microbial growth (Toivonen 2008). In other cases which microorganisms did not elicit a wound ethylene response and there was no exogenous ethylene source, 1-MCP application may have no effect on their growth on fruit and vegetables. The scatter of results in relation to 1-MCP application alone in various fruit and vegetables suggests that factors other than 1-MCP are responsible for determining microbial growth and decay of products. Total microbial growth was decreased by 1-MCP (1.0 μL L−1 for 24 h) in ‘Empire’ apples (Rupasinghe et al. 2005). In strawberries 1-MCP concentrations greater than 15 nL L−1 were associated with increased decay of fruit (Ku and Wills 1999). Exposure of whole strawberries to 0.01, 0.1 or 1.0 μL L−1 1-MCP slightly increased the rate of rot development (Bower et al. 2003). There are also many studies published in the literatures on effect of different levels (0.5, 0.5–1.5, 2.0%, w/v) of CaCl2 treatment on yeast and mold count in ‘Red delicious’ apple fruits after different days of storage (Hussain et al. 2011).
Conclusions
In the present study, the combination of 1-MCP and CaCl2 treatment extended storage life of ‘Lingwu Long’ jujube fruit at room temperature storage by inhibiting of ethylene production and reducing PG and PPO activities. It significantly reduced microbial growth and maintained higher concentrations of titratable acid and ascorbic acid. From this study, it is obvious that the treatment of ‘Lingwu Long’ jujube fruit with 1-MCP, CaCl2 or their combination improved greatly its storage life extension and quality maintenance and can be commercially used as a post-harvest technology.
Acknowledgement
The authors would like to express their appreciation and thanks to the anonymous reviewers and editor of the journal for their valuable comments.
Footnotes
Li Li and Zhaojun Ban contributed equally to this work.
References
- Agar IT, Massantini R, Hess-Pierce B, Kader AA. Postharvest CO2 and ethylene production and quality maintenance of fresh-cut kiwifruit slices. J Food Sci. 1999;64:433–440. doi: 10.1111/j.1365-2621.1999.tb15058.x. [DOI] [Google Scholar]
- Aguayo E, Jansasithorn R, Kader AA. Combined effects of 1-methylcyclopropene, calcium chloride dip, and/or atmospheric modification on quality changes in fresh-cut strawberries. Postharvest Biol Technol. 2006;40:269–278. doi: 10.1016/j.postharvbio.2006.01.016. [DOI] [Google Scholar]
- Bai J, Baldwin EA, Soliva-Fortuny RC, Mattheis JP, Stanley R, Perera C, Brecht JK. Effect of pretreatment of intact ‘Gala’ apple with ethanol vapor, heat or 1-methylcyclopropene on quality and shelf life of fresh-cut slices. J Ame Soc Hort Sci. 2004;129:583–593. [Google Scholar]
- Bisen A, Pandey SK, Patel N (2011) Effect of skin coatings on prolonging shelf life of kagzi lime fruits (Citrus aurantifolia Swingle). J Food Sci Technol. doi:10.1007/s13197-010-0214-y [DOI] [PMC free article] [PubMed]
- Bower JH, Biasi WV, Mitcham EJ. Effects of ethylene and 1-MCP on the quality and storage life of strawberries. Postharvest Biol Technol. 2003;28:417–423. doi: 10.1016/S0925-5214(02)00208-9. [DOI] [Google Scholar]
- Calderon-Lopez B, Bartsch JA, Lee CY, Watkins CB. Cultivar effects on quality of fresh-cut apple slices from 1-methylcyclopropene (1-MCP)-treated apple fruit. J Food Sci. 2005;70:221–227. doi: 10.1111/j.1365-2621.2005.tb07161.x. [DOI] [Google Scholar]
- DeEll JR, Murr DP, Porteous MD, Vasantha Rupasinghe HPV. Influence of temperature and duration of 1-methylcyclopropene (1-MCP) treatment on apple quality. Postharvest Biol Technol. 2002;24:349–353. doi: 10.1016/S0925-5214(01)00136-3. [DOI] [Google Scholar]
- Ergun M, Jeong J, Huber DJ, Cantliffe DJ. Physiology of fresh-cut ‘Galia’ (Cucumis melo var. reticulatus) from ripe fruit treated with 1-methylcyclopropene. Postharvest Biol Technol. 2007;44:286–292. doi: 10.1016/j.postharvbio.2006.08.019. [DOI] [Google Scholar]
- Everdeen DS, Kiefer S, Willard JJ, Muldoon EP, Dey PM, Li XB, Lamport DTA. Enzymic cross-linkage of monomeric extensin precursors in vitro. Plant Physiol. 1988;87:616–621. doi: 10.1104/pp.87.3.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng X, Apelbaum A, Sisler EC, Goren R. Control of ethylene responses in avocado fruit with 1-methylcyclopropene. Postharvest Biol Technol. 2000;20:143–150. doi: 10.1016/S0925-5214(00)00126-5. [DOI] [Google Scholar]
- Golding JB, Shearer D, Wyllie SG, McGlasson WB. Application of 1-MCP and propylene to identify ethylene-dependent ripening processes in mature banana fruit. Postharvest Biol Technol. 1998;14:87–98. doi: 10.1016/S0925-5214(98)00032-5. [DOI] [Google Scholar]
- Goutam M, Dhaliwal HS, Mahajan BVC. Effect of pre-harvest calcium sprays on post-harvest life of winter guava (Psidium guajava L.) J Food Sci Technol. 2010;47:501–506. doi: 10.1007/s13197-010-0085-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham MY, Graham TL. Rapid accumulation of anionic peroxidases and phenolic polymers in soybean cotyledon tissues following treatment with Phytophthora megasperma f. sp. glycinea wall glucan. Plant Physiol. 1991;97:1445–1455. doi: 10.1104/pp.97.4.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta N, Jawandha SK, Gill PS. Effect of calcium on cold storage and post-storage quality of peach. J Food Sci Technol. 2011;48:225–229. doi: 10.1007/s13197-010-0116-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain PR, Meena RS, Dar MA, Wani AM (2011) Effect of post-harvest calcium chloride dip treatment and gamma irradiation on storage quality and shelf-life extension of Red delicious apple. J Food Sci Technol. doi:10.1007/s13197-011-0289-0 [DOI] [PMC free article] [PubMed]
- Jayaraman KS, Ramanuja MN, Vijayaraghavan PK, Vaidyanathan CS. Studies on the purification of banana polyphenoloxidase. Food Chem. 1987;24:203–217. doi: 10.1016/0308-8146(87)90152-X. [DOI] [Google Scholar]
- Jennings PH, Brannaman BL, Zscheile FP., Jr Peroxidase and polyphenol oxidase activity associated with Helminthosporium leaf spot of maize. Phytopathology. 1969;59:963–967. [PubMed] [Google Scholar]
- Jiang Y, Joyce DC. 1-Methylcyclopropene treatment effects on intact and fresh-cut apple. J Hort Sci Biotechnol. 2002;77:19–21. [Google Scholar]
- Ku VVV, Wills RBH. Effect of 1-methylcyclopropene on the storage life of broccoli. Postharvest Biol Technol. 1999;17:127–132. doi: 10.1016/S0925-5214(99)00042-3. [DOI] [Google Scholar]
- Luna-Guzmán I, Barrett DM. Comparison of calcium chloride and calcium lactate effectiveness in maintaining shelf stability and quality of fresh-cut cantaloupes. Postharvest Biol Technol. 2000;19:61–72. doi: 10.1016/S0925-5214(00)00079-X. [DOI] [Google Scholar]
- Mahajan BVC, Singh K, Dhillon WS. Effect of 1-methylcyclopropene (1-MCP) on storage life and quality of pear fruits. J Food Sci Technol. 2010;47:351–354. doi: 10.1007/s13197-010-0058-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao LC, Que F, Donald HJ. 1-Methylcyclopropene and CaCl2 treatments affect lipolytic enzymes in fresh-cut watermelon fruit. Acta Botanica Sinica. 2004;46:1402–1407. [Google Scholar]
- Mehta N, Gupta O, Kachroo A, Yamdagni R. Evaluation of various surfactants on the uptake of calcium in ber fruits. Prog Hort. 1985;17:285–288. [Google Scholar]
- Mignani I, Greve LC, Ben-Arie R, Stotz HU, Li C, Shackel KA, Labavitch JM. The effects of GA3 and divalent cations on aspects of pectin metabolism and tissue softening in ripening tomato pericarp. Physiol Plant. 1995;93:108–115. doi: 10.1034/j.1399-3054.1995.930116.x. [DOI] [Google Scholar]
- Mir NA, Curell E, Khan N, Whitaker M, Beaudry RM. Harvest maturity, storage temperature, and 1-MCP application frequency alter firmness retention and chlorophyll fluorescence of ‘Redchief Delicious’ apples. J Ame Soc Hort Sci. 2001;126:618–624. [Google Scholar]
- Pathak N, Sanwal GG. Multiple forms of polygalacturonase from banana fruits. Phytochem. 1998;48:249–255. doi: 10.1016/S0031-9422(98)00005-3. [DOI] [PubMed] [Google Scholar]
- Paul V, Pandey R, Srivastava GC. Ripening of tomato (Solanum lycopersicum L.). Part I: 1-methylcyclopropene mediated delay at higher storage temperature. J Food Sci Technol. 2010;47:519–526. doi: 10.1007/s13197-010-0090-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul V, Pandey R, Srivastava GC (2011) The fading distinctions between classical patterns of ripening in climacteric and non-climacteric fruit and the ubiquity of ethylene-An overview. J Food Sci Technol. doi:10.1007/s13197-011-0293-4 [DOI] [PMC free article] [PubMed]
- Perera CO, Balchin L, Baldwin E, Stanley R, Tian M. Effect of 1-methylcyclopropene on the quality of fresh-cut apple slices. J Food Sci. 2003;68:1910–1914. doi: 10.1111/j.1365-2621.2003.tb06992.x. [DOI] [Google Scholar]
- Porat R, Weiss B, Cohen L, Daus A, Goren R, Droby S. Effects of ethylene and 1-methylcyclopropene on the postharvest qualities of ‘Shamouti’ oranges. Postharvest Biol Technol. 1999;15:155–163. doi: 10.1016/S0925-5214(98)00079-9. [DOI] [Google Scholar]
- Rao MV, Ormrod DP. Ozone exposure decreases UVB sensitivity in a UVB-sensitive flavonoid mutant of Arabidopsis. Phytochem Photobiol. 1995;61:71–78. doi: 10.1111/j.1751-1097.1995.tb09245.x. [DOI] [PubMed] [Google Scholar]
- Rezaee M, Almassi M, Minaei S, Paknejad F (2011) Impact of post-harvest radiation treatment timing on shelf life and quality characteristics of potatoes. J Food Sci Technol. doi:10.1007/s13197-011-0337-9 [DOI] [PMC free article] [PubMed]
- Rosen JC, Kader AA. Postharvest physiology and quality maintenance of sliced pear and strawberry fruits. J Food Sci. 1989;54:656–659. doi: 10.1111/j.1365-2621.1989.tb04675.x. [DOI] [Google Scholar]
- Rupasinghe HPV, Murr DP, Deell JR, Odumeru J. Influence of 1-methylcyclopropene and natureseal on the quality of fresh-cut ‘Empire’ and ‘Crispin’ apples. J Food Qual. 2005;28:289–307. doi: 10.1111/j.1745-4557.2005.00035.x. [DOI] [Google Scholar]
- Sabιr A, Sabιr FK, Kara Z. Effects of modified atmosphere packing and honey dip treatments on quality maintenance of minimally processed grape cv. Razaki (V. vinifera L.) during cold storage. J Food Sci Technol. 2011;48:312–318. doi: 10.1007/s13197-011-0237-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvarajah S, Bauchot AD, John P. Internal browning in cold-stored pineapples is suppressed by a postharvest application of 1-methylcyclopropene. Postharvest Biol Technol. 2001;23:167–170. doi: 10.1016/S0925-5214(01)00099-0. [DOI] [Google Scholar]
- Tian MS, Prakash S, Elgar HJ, Young H, Burmeister DM, Ross GS. Responses of strawberry fruit to 1-methylcyclopropene (1-MCP) and ethylene. Plant Growth Regul. 2000;32:83–90. doi: 10.1023/A:1006409719333. [DOI] [Google Scholar]
- Toivonen PMA. Application of 1-methylcyclopropene in fresh-cut/minimal processing systems. HortScience. 2008;43:102–105. [Google Scholar]
- Valero D, Martínez-Romero D, Valverde JM, Guillén F, Serrano M. Quality improvement and extension of shelf life by 1-methylcyclopropene in plum as affected by ripening stage at harvest. Innov Food Sci Emerg. 2003;4:339–348. doi: 10.1016/S1466-8564(03)00038-9. [DOI] [Google Scholar]
- Vilas-Boas EV, Kader AA. Effect of 1-MCP on fresh-cut fruits. Perishables Handling Quart. 2001;108:25. [Google Scholar]
- Walter MH (1992) Regulation of lignification in defense. In: Boller T, Meins F (Eds), Genes Involved in. Plant Defense 327–352
- Workneh TS, Osthoff G, Steyn M (2011) Effects of preharvest treatment, disinfections, packaging and storage environment on quality of tomato. J Food Sci Technol. doi:10.1007/s13197-011-0391-3 [DOI] [PMC free article] [PubMed]
- Zhong QP, Xia WS. Effect of 1-methylcyclopropene and/or chitosan coating treatments on storage life and quality maintenance of Indian jujube fruit. LWT - Food Sci Technol. 2007;40:404–411. doi: 10.1016/j.lwt.2006.01.003. [DOI] [Google Scholar]