Abstract
Morogo (vegetables in Tswana) is a green leafy vegetable from the Amaranthaceae family that can be harvested from wild growing or cultivated. The objective of this study was to determine the nutritional value, the total antioxidant capacity and selected bioactive compounds present in morogo leaves, and evaluate the effect of drying and cooking. Results showed that morogo contained a significant amount of protein (3.6 ± 0.1 g/100 g FW) and minerals which the level exceed 1% of fresh weight. The total antioxidant capacity (μmole TE/ 100 g FW) determined by DPPH and FRAP assays were 118.3 ± 15.3 and 128.4 ± 11.9 respectively. Total polyphenols (109.4 ± 7.5 mg GAE/100 g FW), vitamin C (36.6 ± 1.0 mg /100 g FW) and carotenoids represented by β carotene (25.3 ± 1.3 mg /100 g FW) and xanthophylls (7.48 ± 0.31 mg /100 g FW) formed a significant part of bioactive compounds content of morogo leaves. Since the boiling can cause significant losses of compounds in the boiling water, it can be recommended to avoid cooking methods that can include a boiling step with discard of boiling water.
Keywords: Morogo, Amaranthus hybridus, Total antioxidant, Phytochemicals, Nutritional value, Leafy vegetables
Introduction
The global nutrition transition towards more simplified diets high in energy but poor in micronutrients and functional properties is linked with epidemic increase of non-communicable diseases. For centuries, more than 7000 plant species have been used as food, however, in recent years, it was estimated that 90% of the world’s dietary energy supply is obtained from only 30 species (FAO 1997). Thousands of species and many more varieties fall into a category defined as underutilised or neglected crops which are marginalised by both agriculture and nutrition researchers (Global Forum for Underutilized Species 2009). South Africa (SA) is a country with great biodiversity with many people still using a wide variety of plants in their daily lives for food, water, shelter, fuel, medicine and other needs of life (Van Wyk and Gericke 2000). However, many traditional vegetables are underutilised in favour of introduced non-native vegetables. The availability of indigenous vegetables has declined because of excessive cultivation of field crops, which includes chemical elimination of wild vegetables, habitat change, and the growing ignorance of the young generation about the existence of these foods (Odhav et al. 2007). Traditional vegetables are readily available in the field and have the potential to contribute to reducing malnutrition in the poor segment of the population by providing resources for food diversification and supplementing the diet.
Morogo (vegetables in Tswana, one of the 11 official languages spoken in SA) is a green leafy vegetable from the Amaranthaceae family that can be harvested from wild growing or cultivated plants. The plant is adaptable and grows easily under various weather and soil conditions. The plants are harvested only by hand. Young plants can be pulled up or cut 6 to 8 weeks after sowing when they are 200 mm tall. The leaves are eaten in the same way as spinach; they can be cooked on the same day they were harvested or dried and stored for later preparation.
Although several studies (Nesamvuni et al. 2001; Steyn et al. 2001; Jansen van Rensburg et al. 2004; Odhav et al. 2007; Van der Walt et al. 2009a; Van der Walt et al. 2009b) have examined the nutritional value and the use of traditional and indigenous leafy vegetables in South Africa, there is still a need for data to attract more professional attention to those underutilized foods and highlight their real and potential contribution to nutrition and health. Data on bioactive compounds are particularly lacking for many species. Few studies reported the antioxidant activity of Amaranthus leaves (Odhav et al. 2007; Stangeland et al. 2009).
The discovery of a group of nutrients which have protective effects against cell oxidation has attracted both the scientist and public interest in the last decade. There is growing scientific evidence that dietary antioxidants can play a critical role in preserving human health (Liu 2003). Several epidemiological studies suggest that diets rich in phytochemicals and antioxidants fulfil a protective role in health and disease, and frequent consumption of fruit and vegetables has been associated with a lowered risk of cancer, heart disease, hypertension and stroke (Marco et al. 1997; Vinson et al. 2001; Wolfe and Liu 2003).
The aim of this study was to determine the nutritional value, the total antioxidant capacity and selected bioactive compounds present in the Morogo leafy vegetable, and to evaluate the effect of drying and cooking.
Materials and methods
Sample
About 5 kg of Morogo, Amaranthus hybridus (Grootfontein Herbarium ID 672) leafy material were randomly harvested at physiological maturity, about 8 weeks of age, from several plants in different areas of Qwa-Qwa in the Free State Province of South Africa. The plants were processed the day they were harvested. Healthy green leaves were removed from the stems, thoroughly washed with water, and divided in three groups for fresh, dried and cooked samples respectively.
For cooked samples, the leaves were boiled in a stainless steel cooking pot for 25 min with minimum water just to cover the leaves and for dried samples, the leaves were air dried at 40 °C for 12 h.
Proximate analysis
Moisture, ash, fat and dietary fibre were analysed using AOAC (1997) methods.
Moisture was determined by air-drying 10 g of sample in oven at 105 °C to a constant weight. Ash content was determined by incinerating 4 g of dried sample in a muffle furnace at 550 °C until the ash turned white. Dietary fibre was analysed by an enzymatic gravimetric method using the Kit TDF-100A, Lot 113 K8803 (Sigma). Crude protein content (N × 6.25) was determined by Kjeldahl digestion technique followed by spectrophotometric determination of the resulting ammonia, using the method of Devani et al. (1989). Total carbohydrates were evaluated by the Anthrone method (Hedge and Hofreiter 1962), after hot digestion with 2.5 N HCl. Fat content was determined by exhaustively extracting dried samples in a Soxhlet apparatus with petroleum ether. The energy value was calculated using Southgate (1981) conversion factors: 4.0 kcal/g for protein, 9.0 kcal/g for fat and 4.0 kcal/g for carbohydrates.
Sample extractions and assays for total antioxidant capacity and selected bioactive compounds
Total antioxidant capacity and total polyphenol
Samples for total antioxidant capacity (TAC) and total polyphenols (TPP) assays were extracted according to the method of Pérez-Jiménez et al. (2008). Ten grams of fresh or 1 g of dry food samples were extracted with 40 ml of methanol/water (50:50, v/v; pH 2.0) at room temperature, using an ultraspeed homogenizer for 5 min. The homogenates were kept at 4 °C for 1 h and then centrifuged at 2500 g for 10 min. The supernatants were recovered and the residue further washed with 40 ml of acetone/water (70:30, v/v) and centrifuged. The resulting supernatants were combined and stored at −20 °C until analysed.
Total antioxidant capacity assays
The TAC was evaluated using the ferric reducing/antioxidant power (FRAP) assays and the 2,2-diphenyl-1-picrylhydrazyl (DPPH).
The FRAP assay was performed using the method described by Benzie and Strain (1996). 2.25 ml of freshly prepared FRAP reagent (containing 8.3 mM 2,4,6-tri(2-pyridyl)-s-triazine (TPTZ), 16.7 mM FeCl3.6H2O and 250 mM acetate buffer, pH 3.6) was mixed with 225 μl distilled water and 75 μl of sample extract. The mixture was incubated at 37 °C and the absorbance read at 593 nm after 4 and 30 min of reaction. The antioxidant capacity was determined based on a calibration curve using Trolox (25–800 μM) standards.
The DPPH assay was performed according to the method of Brand-Williams et al. (1995) as modified by Thaipong et al. (2006). The stock solution of DPPH was prepared by dissolving 24 mg DPPH with 100 ml methanol and then stored at −20 °C until needed. The working solution was obtained by mixing 10 ml stock solution with methanol to get an absorbance of 1.1 ± 0.02 units at 515 nm, using a spectrophotometer. Sample extracts (150 μl) were allowed to react with 2.85 ml of the DPPH solution in the dark for 24 h. Then the absorbance was taken at 515 nm. Results were expressed in Trolox equivalent (μmol TE/g fresh matter) using Trolox (25–800 μM) standard curve.
Total polyphenol assay
The TPP content was determined by the Swain and Hillis (1959) method, using Folin-Ciocalteu reagent. In a test tube, 150 μl of the methanol-acetone extract, 2400 μl of nanopure water, and 150 μl of 0.25 N Folin–Ciocalteu reagent were combined and then mixed well, using a Vortex. The mixture was allowed to react for 3 min after which 300 μl of 1 N Na2CO3 solution was added and mixed well. The solution was incubated at room temperature for 2 h and absorbance measured at 725 nm against a blank. Results were expressed in gallic acid equivalents (GAE; mg/100 g of sample), using gallic acid (0–0.1 mg/mL) standard curve.
Flavonols sample extraction
Flavonols were extracted according to the method of Crozier et al. (1997). Ten grams of fresh or 1 g of dry samples were extracted with 40 ml of 62.5% aqueous methanol solution containing 2 g/l of terbutylhydroxyquinone (TBHQ) as antioxidant at room temperature using an ultraspeed homogenizer for 5 min. 10 ml of 6 M HCl was added and the solution refluxed at 90 °C for 2 h. The mixture was cooled, made to 100 ml with methanol, mixed thoroughly and then filtered through 0.5 μm cellulose acetate filter disc before HPLC analysis. The HPLC system was a Series 200 Liquid Chromatography (PerkinElmer, Inc) equipped with a Series 200 LC Quaternary Pump, Series 200 Autosampler, Series 200 Diode Array Detector II and Series 200 Peltier Colum Oven.
Flavonols assay
20 μl of flavonols extracts were injected in a reverse phase C18 (250 mm × 4.6 mm, 5 μm) HPLC column. Two mobile phases was used for elution—(A) 1% formic acid, 99% water and (B) 1% formic acid, 49% water and 50% methanol. The elution profile was 0–4 min, 10% B in A (isocratic), 4–21 min, 10–100% B in A (linear gradient), 42–46 min, 10% B in A (isocratic) with a flow rate of 1 ml/min. Flavonols were detected at 370 nm. The retention times for standard quercetin, myricetin, morin, kaempferol and isorhamnetin were recorded and peak heights used for calculations.
Carotenoids sample extraction
Carotenoids were extracted according to the procedure described by Lako et al. (2007). To 10 g of fresh or 1 g of dry food samples, 1 g of heavy magnesium carbonate (4 MgCO3 Mg[OH]2·5H2O), 20 g sodium sulphate and 30 ml acetone was added, and the sample extracted using a high-speed homogeniser for 5 min. The mixture was filtered through a Whatman N° 4 filter-paper under vacuum. The residue, including the filter-paper, was re-extracted with acetone until no residual is saw in the filter cake. The filtrates was combined and made to volume in either a 100 or 200 ml volumetric flask with acetone depending on the intensity of the colour in the sample. An aliquot of the extract was filtered throuh 0.5 μm nylon filter disc before HPLC analysis. All extractions were conducted in a dark room.
Carotenoids assay
20 μl of carotenoids extracts were injected in a reverse phase C18 (250 mm × 4.6 mm, 5 μm) HPLC column. The mobile phase consisted of 75% acetonitrile, 20% 0.05 M ammonium acetate in methanol, 5% dichloromethane, 0.05% triethylamine and 0.1% BHT with a flow rate of 2.0 ml/min. The carotenoids were detected at 450 nm. Retention times for standard lycopene, xanthophylls and β-carotene were recorded and peak heights used for calculations.
Vitamin C assay
Vitamin C (ascorbic acid) was determined using the method described by Singh et al. (2007). 10 g of fresh or 1 g of dry food samples were homogenized in a blender with 100 ml of 1% m-phosphoric acid. The slurry was adjusted to 250 ml with 1% m-phosphoric acid and filtered through Whatman filter-paper. 1 ml of this filtrate was added to 1 ml of 5% dinitrothreitol, and the volume made up to 10 ml with 1% m-phosphoric acid. The solution was filtered and 20 μl injected on a reverse phase C18 (150 × 4.60 mm, 5 μm) HPLC column. The mobile phase consisted of acetonitrile: 0.05 M KH2PO4 (pH 5.9) in the ration of 75:25 with a flow rate of 1.5 ml/min. The vitamin C was detected at 261 nm. Retention time for standard vitamin C was recorded and peak height used for calculations.
Quality control
Pure reference standards and one certified reference material were used to track method performance and variability over time.
For proximate composition, rye flour CRM-381 from Institute for Reference Material and Measurements (Geel, Belgium) were used. The certified values were 1.56 g/100 g for kjeldahl nitrogen, 1.36 g/100 g for total fat, 72.2 g/100 g for carbohydrates, 8.4 g/100 g for dietary fibre, and 1.08 g/100 g for ash. The recovery percentage ranged form 98.2% to 101% and the coefficient of variation ranged between 1.2% and 1.8%.
For TAC and bioactive compounds, no commercially available CRM were found. Quality control for both TAC and bioactive compounds was checked by using morogo samples enriched or not enriched with known amount of pure reference standards. Recovery data were generated by spiking samples with pure reference standards before extraction. The percentage of recovery ranged from 85.3% to 106.5%. Coefficient of variations were always < 5% ranging between 1.9% and 4.2%. The limit of detection was determined by measuring 3 times the signal-to-noise ratio of a low concentration of standard and was identical for all compounds at 0.10 μg/100 g.
Statistical analysis
All samples were analysed in triplicate and the mean of the three determinations reported. Statistical analyses of data were performed using SPSS 17.0 software (SPSS Inc., Chicago, IL). Comparisons between dependent variables were determined, using analysis of variance (ANOVA), Duncan’s multiple range test and correlation analysis. Independent-Samples t-test was used to compare fresh and cooked samples. Statistical significance was defined at p ≤ 0.05.
Results and discussion
Proximate composition
Table 1 displays the proximate composition of morogo leafy vegetable. With an average moisture content of 89.8 g/100 g, the leaves of morogo presented moisture content comparable to the values previously reported (Maundu et al. 1999; Kruger et al. 1998) for commercial and indigenous vegetables. According to Alfawaz (2006), leafy vegetables having high moisture content, are tender and succulent in nature while those containing low moisture have a woody texture. The moisture content of morogo is higher than that of spinach (85.1%), but lower than that of lettuce (94.2%) (MRC 2009). The leaves of morogo contained a significant amount of protein (3.6 g/100 g FW) that is higher than the protein content of most commercial leafy vegetables indexed in the South African Food Composition Database (MRC 2009). In contrast, the values for carbohydrates (0.87 g/100 g FW) and fibre (1.81 g/100 g FW) were lower than most of the commercial leafy vegetables. Fat content (0.66 g/100 g FW) was higher than those reported by the South African Food Composition Database (MRC 2009) for spinach (0.4%), cabbage (0.1%) and lettuce (0.1%) and those reported by Van der Walt et al. (2009a) for three dark leafy vegetables grown in South Africa (1.6–2.9% of dry weight), but was within the range reported by Odhav et al. (2007). The values obtained for the mineral content (1.96 g/100 g FW) exceeded 1% of the plant fresh weight and were much higher than the mineral concentration of conventional edible leafy vegetables. In this connection, Van der Walt et al. (2009b) reported relatively high calcium and magnesium concentration in the wild morogo. This agreed with previous studies (Odhav et al. 2007) reporting high concentration of minerals in traditional leafy vegetables and highlighted the potential to use of the leaves of morogo as a mineral source.
Table 1.
The proximate composition (fresh weight basis) of morogo (Amaranthus hybridus) leafy vegetable
| Component | Mean ± SD |
|---|---|
| Moisture (g/100 g) | 89.75 ± 0.36 |
| Ash (g/100 g) | 1.96 ± 0.06 |
| Dietary fiber (g/100 g) | 1.81 ± 0.09 |
| Carbohydrate (g/100 g) | 0.87 ± 0.02 |
| Fat (g/100 g) | 0.66 ± 0.02 |
| Protein (g/100 g) | 3.58 ± 0.09 |
| Energy (kcal/100 g) | 23.70 ± 0.58 |
Three samples in duplicate; n = 3
Antioxidant capacity and phytochemical composition
Table 2 presents the values of TAC, TPP, flavonols, carotenoids and vitamin C, and Figs. 1 and 2 respectively display HPLC profiles related to the flavonoidic and carotenoids fractions detected in the leaves. Because of the methodological limits for antioxidant determination, it was recommended to use different assays, instead of relying on a single assay to assess antioxidant capacity (Pérez-Jiménez et al. 2008). Thus, the TAC (μmole trolox equivalent/100 g FW) of morogo leaves was determined by DPPH and FRAP assays which gave value of 118.3 and 128.4 respectively, suggesting that morogo leaves present significant amounts of antioxidant compounds. This result agreed with previous studies (Odhav et al. 2007; Stangeland et al. 2009) reporting high antioxidant activity for Amaranthus sp. Some studies reported a high correlation between antioxidant capacity and polyphenol content (Pulido et al. 2000; Sanchez-Moreno et al. 2000) suggesting the importance of phenolic compounds as antioxidant. Among polyphenols, flavonoids showed a high antioxidant activity in several systems. Vitamin C and carotenoids are also well known antioxidants (Podsędek 2007).
Table 2.
Antioxidant capacity and phytochemical composition (fresh weight basis) of morogo (Amaranthus hybridus) leafy vegetable
| Component | Mean ± SD |
|---|---|
| Total Antioxidant Capacity (μmole TE/ 100 g) | |
| DPPH | 118.27 ± 15.34 |
| FRAP | 128.43 ± 11.94 |
| Total polyphenols (mg GAE/100 g) | 109.38 ± 7.52 |
| Flavonols (mg/100 g) | |
| Myricetin | 7.65 ± 0.13 |
| Morin | nd |
| Quercetin | 2.06 ± 0.08 |
| Kaempferol | 1.99 ± 0.07 |
| Isorhamnetin | nd |
| Carotenoids (mg/100 g) | |
| β carotene | 25.30 ± 1.32 |
| Xanthophylls | 7.48 ± 0.31 |
| Lycopene | nd |
| Vitamin C | 36.63 ± 0.98 |
Three samples in duplicate; n = 3
FRAP: ferric reducing/antioxidant power; DPPH: 2,2-diphenyl-1-picrylhydrazyl
nd: not detected (limit of detection = 0.10 μg/100 g)
Fig. 1.
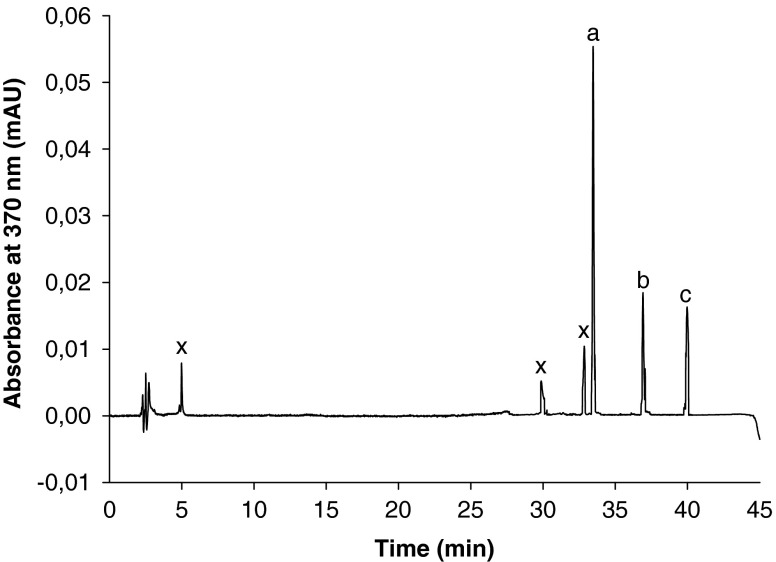
HPLC-DAD chromatogram of morogo (Amaranthus hybridus) leafy vegetable using the conditions described in the flavonol method: a) myricetin; b) quercetin; c) kaempferol; x) unidentified peak
Fig. 2.
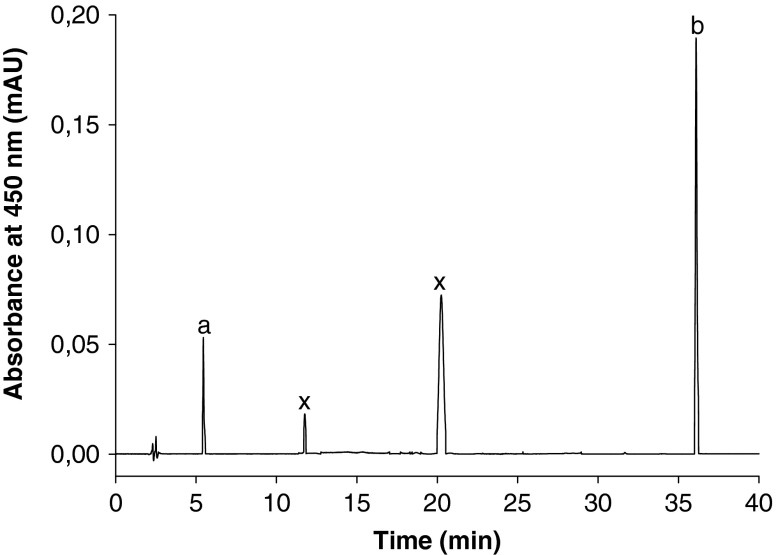
HPLC-DAD chromatogram of morogo (Amaranthus hybridus) leafy vegetable using the conditions described in the carotenoid method: a) xanthophylls; b) β carotene; x) unidentified peak
With a total polyphenol content ranging from 105.5 to 120 mg/100 g FW, the leaves of morogo displayed a significant level of phenolics. These values were comparable to those reported by Van der Walt et al. (2009b) for Amaranthus hybridus from Vhembe, South Africa. Six flavonols including myricetin (7.7 mg/100 g FW), quercetin (2.1 mg/100 g FW), kaempherol (2.0 mg/100 g FW) and three unidentified flavonols were detected in the leaves of morogo; Morin and isorhamnetin were not detected (Fig. 1). Significant levels of carotenoids represented by β carotene (25.3 mg/100 g FW), Xanthophylls (7.5 mg/100 g FW) and two unidentified carotenoids were found in the leaves of morogo; lycopene was, however, not detected (Fig. 2). The values found for β carotene were higher than those reported by Van der Walt et al. (2009b) for total carotenoids in three traditional African green leafy vegetables sampled from different geographical regions in South Africa. The mean value of vitamin C was 36.6 mg/100 g FW, which suggests that morogo leaves contain fair amount of vitamin C; this amount was below the range of 46–126 mg/100 g FW, reported for Amaranthus sp. by Kruger et al. (1998).
Effect of drying and cooking
The leaves of morogo are generally cooked before consumption or dried for later preparation, and the effect of drying and cooking was therefore evaluated (Table 3). In general, the data showed no significant effect (p < 0.05) of drying, except for fibre content which significantly increased (7%), and β carotene and vitamin C which significantly decreased at the levels of 19.4% and 13.9% respectively. During cooking, most of the compounds analysed were significantly affected. However, the pattern varied among the compounds. A significant decrease was noted for ash, fibre, TAC, TPP, flavonols, xantophyll and vitamin C, while a significant increase was noted for fat and protein. The compounds mostly affected by the drying and cooking were TAC, TPP and vitamin C which can be lost at a cumulative level of 66.6%, 61.3% and 69.8% respectively. In that connection, (FAO 1990) reported a decrease in ascorbic acid by 19% in cooked amaranth. Since the cooking process used in the present study was boiling, loss of compounds can be explained by the leach of compounds into the boiling water. On the other hand, as suggested by Yamaguchi et al. (2001), the increase of compounds recorded can be credited to the destruction of cell walls and subcellular compartments during boiling that causes the release of compounds.
Table 3.
Effect of drying and cooking on the nutritional and phytochemical composition (dry weight basis) of morogo (Amaranthus hybridus) leafy vegetable
| Component | Effect of drying | Effect of cooking | ||
|---|---|---|---|---|
| t statistic | Changea (%) | t statistic | Changeb (%) | |
| Ash | 0.56 | 0.65 ± 0.32 | 24.58* | 8.18 ± 0.38 |
| Fat | −0.52 | −1.56 ± 0.28 | −19.84* | −17.38 ± 0.26 |
| Carbohydrates | 0.52 | 0.97 ± 0.05 | 2.34 | 7.00 ± 1.39 |
| Protein | 1.85 | 1.86 ± 0.17 | −11.50* | −9.97 ± 0.12 |
| Fiber | −3.14* | −7.34 ± 0.52 | 5.87* | 13.47 ± 1.94 |
| DPPH | 1.55 | 13.00 ± 0.08 | 8.04* | 53.59 ± 0.39 |
| FRAP | 2.32 | 13.07 ± 0.19 | 11.02* | 52.51 ± 0.15 |
| Total polyphenols | 0.41 | 2.98 ± 0.28 | 11.98* | 58.33 ± 0.58 |
| Myricetin | 0.25 | 0.75 ± 1.37 | 38.09* | 51.97 ± 0.38 |
| Quercetin | −0.41 | −1.38 ± 0.89 | 7.42* | 25.44 ± 0.93 |
| Kaempferol | 1.11 | 2.76 ± 0.39 | 17.66* | 40.86 ± 0.57 |
| β carotene | 4.28* | 19.40 ± 2.91 | −0.39 | −0.62 ± 0.03 |
| Xanthophylls | −0.04 | −0.33 ± 1.61 | 6.28* | 20.63 ± 0.26 |
| Vitamin C | 3.13* | 13.91 ± 1.27 | 21.22* | 56.69 ± 0.10 |
Mean ± SD
FRAP: ferric reducing/antioxidant power; DPPH: 2,2-diphenyl-1-picrylhydrazyl
*significant at p ≤ 0.05
a(fresh—dry/fresh) × 100 (a positive value of the change or t reflects a decrease during drying)
b(fresh—cook/fresh) × 100 (a positive value of the change or t reflects a decrease during cooking)
Conclusion
The chemical composition of morogo leaves suggests that the leaves are good sources of proteins, minerals and bioactive compounds. Since most of the compounds can be lost in the boiling water during cooking, it is necessary to avoid cooking methods that can include a boiling step with discard of boiling water. Other compounds in morogo leaves require further research, mainly on the antinutrient and mineral composition.
References
- Alfawaz MA. Chemical composition of hummayd (Rumex vesicarius) grown in Saudi Arabia. J Food Compos Anal. 2006;19:552–555. doi: 10.1016/j.jfca.2004.09.004. [DOI] [Google Scholar]
- Official Methods of Analysis. 16. Washington, DC: Association of Official Analytical Chemists; 1997. [Google Scholar]
- Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Brand-Williams W, Cuvelier ME, Berset C. Use of free radical method to evaluate antioxidant activity. LWT. 1995;28:25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- Crozier A, Lean MEJ, McDonald MS, Black C. Quantitative analysis of flavonoid content of commercial tomatoes, onions, lettuce, and celery. J Agric Food Chem. 1997;45:590–595. doi: 10.1021/jf960339y. [DOI] [Google Scholar]
- Devani MB, Shishoo CJ, Shah AS, Suhgka BN. Spectrophotometric method for microdetermination of nitrogen in kjeldahl digest. JAOAC. 1989;72(6):953–956. [Google Scholar]
- FAO (1990) Utilization of tropical foods: fruits and leaves. FAO Food and Nutrition Paper No. 47/7. Rome, Italy. [PubMed]
- State of the world’s plant genetic resources for food and agriculture. Rome: FAO; 1997. [Google Scholar]
- Global Forum for Underutilized Species (2009) http://www.underutilized-species.org/about_GFU.asp. Accessed 26 November 2010.
- Hedge JE, Hofreiter BT. Estimation of carbohydrate. In: Whistler RL, BeMiller JN, editors. Carbohydrate Chemistry. New York: Academic; 1962. pp. 17–22. [Google Scholar]
- Jansen van Rensburg WS, Venter SL, Netshiluuhi TR, Van der Heever E, Vorster HJ, De Rorde JA. Role of indigenous leafy vegetables in combating hunger and malnutrition. S Afr J Bot. 2004;70:116–123. [Google Scholar]
- Kruger M, Sayed N, Langenhoven M, Holing F (1998) Composition of South African Foods: Vegetables and Fruit. Research Institute for Nutritional Diseases, South African Medical Research Council, South Africa, pp 2–39.
- Lako J, Trenerry C, Wahlqvist M, Wattanapenpaiboon N, Sotheeswaran S, Premier R. Phytochemical flavonols, carotenoids and the total antioxidant properties of a wide selection of Fijian fruit, vegetables and other readily available foods. Food Chem. 2007;101:1727–1741. doi: 10.1016/j.foodchem.2006.01.031. [DOI] [Google Scholar]
- Liu RH. Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. Am J Clin Nutr. 2003;79:727–747. doi: 10.1093/ajcn/78.3.517S. [DOI] [PubMed] [Google Scholar]
- Marco DBF, Joseph V, John K. Mechanisms of disease: antioxidants and atherosclerotic heart disease. N Engl J Med. 1997;337(6):408–416. doi: 10.1056/NEJM199708073370607. [DOI] [PubMed] [Google Scholar]
- Maundu P, Ngugi G, Kabuye CH. Traditional Food Plants of Kenya. Kenya Resource Centre for Indigenous Knowledge (KENRIK) Nairobi: National Museum of Kenya; 1999. [Google Scholar]
- Medical Research Council of South Africa (2009) Food Composition Database. http://databases.mrc.ac.za/FoodComp. Accessed 08 December 2010.
- Nesamvuni C, Steyn NP, Potgieter MJ. Nutritional value of wild, leafy plants consumed by the Vhavenda. S Afr J Sci. 2001;97:51–54. [Google Scholar]
- Odhav B, Beekrum S, Akula U, Baijnath H. Preliminary assessment of nutritional value of traditional leafy vegetables in KwaZulu-Natal, South Africa. J Food Compos Anal. 2007;20:430–435. doi: 10.1016/j.jfca.2006.04.015. [DOI] [Google Scholar]
- Pérez-Jiménez J, Arranz S, Tabernero M, Diaz-Rubio ME, Serrano J, Goni I, Saura-Calixto F. Updated methodology to determine antioxidant capacity in plant foods, oils and beverages: Extraction, measurement and expression of results. Food Res Int. 2008;41:274–285. doi: 10.1016/j.foodres.2007.12.004. [DOI] [Google Scholar]
- Podsędek A. Natural antioxidants and antioxidant capacity of Brassica vegetables: A review. LWT. 2007;40:1–11. doi: 10.1016/j.lwt.2005.07.023. [DOI] [Google Scholar]
- Pulido R, Bravo L, Saura-Calixto F. Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power essay. J Agric Food Chem. 2000;48:3396–3402. doi: 10.1021/jf9913458. [DOI] [PubMed] [Google Scholar]
- Sanchez-Moreno C, Jiménez-Escrig A, Saura-Calixto F. Study of low-density lipoprotein oxidizability indexes to measure the antioxidant activity of polyphenols. Nut Res. 2000;20:941–943. doi: 10.1016/S0271-5317(00)00185-8. [DOI] [Google Scholar]
- Singh J, Upadhyay AK, Prasad K, Bahadur A, Rai M. Variability of carotenes, vitamin C, E and phenolics in Brassica vegetables. J Food Compos Anal. 2007;20:106–112. doi: 10.1016/j.jfca.2006.08.002. [DOI] [Google Scholar]
- Southgate DAT (1981) The relationship between food composition and available energy. Provisional Agenda Item 4.1.3, Joint FAO/WHO/UNU Expert Consultation on Energy and Protein Requirements, Rome, 5 to 17 October 1981.
- Stangeland T, Remberg SF, Lye KA. Total antioxidant activity in 35 Ugandan fruits and vegetables. Food Chem. 2009;113:85–91. doi: 10.1016/j.foodchem.2008.07.026. [DOI] [Google Scholar]
- Steyn NP, Olivier J, Wirter P, Burger S, Nesamvuni C. A survey of wild, green, leafy vegetables and their potential in combating micronutrient deficiencies in rural populations. S Afr J Sci. 2001;97:276–278. [Google Scholar]
- Swain T, Hillis WE. The phenolic constituents of Prunus domestica I—the quantitative analysis of phenolic constituents. J Sci Food Agr. 1959;10:63–68. doi: 10.1002/jsfa.2740100110. [DOI] [Google Scholar]
- Thaiponga K, Boonprakoba U, Crosbyb K, Cisneros-Zevallos L, Byrnec DH. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J Food Compos Anal. 2006;19:669–675. doi: 10.1016/j.jfca.2006.01.003. [DOI] [Google Scholar]
- Van der Walt AM, Ibrahim MIM, Bezuidenhout CC, Loots DT. Linolenic acid and folate in wild-growing African dark leafy vegetables (morogo) Public Health Nutr. 2009;12:525–530. doi: 10.1017/S1368980008002814. [DOI] [PubMed] [Google Scholar]
- Van der Walt AM, Loots DT, Ibrahim MIM, Bezuidenhout CC. Minerals, trace elements and antioxidant phytochemicals in wild African dark-green leafy vegetables (morogo) S Afr J Sci. 2009;105:444–448. [Google Scholar]
- Van Wyk BE, Gericke N. People’s Plants: a guide to useful plants of southern Africa. Pretoria, South Africa: Briza Publications; 2000. [Google Scholar]
- Vinson J, Su X, Zubik L, Bose P. Phenol antioxidation quantity and quality in foods: fruit. J Agric Food Chem. 2001;49(11):5315–5321. doi: 10.1021/jf0009293. [DOI] [PubMed] [Google Scholar]
- Wolfe KWX, Liu RH. Antioxidant activity of apple peels. Journal of Agricultural and Food Chem. 2003;51(3):609–614. doi: 10.1021/jf020782a. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Mizobuchi T, Kajinawa H, Miyabe F, Terao J, Takamura H, Matoba T. Radical-scavenging activity of vegetables and the effect of cooking on their activity. Food Sci Tech Res. 2001;7:250–257. doi: 10.3136/fstr.7.250. [DOI] [Google Scholar]