Abstract
The effects of different cooking methods (boiling, microwave cooking, frying and steaming ) on the antioxidant activity of Brassica juncea (BJ) and Moringa oliefera (MO) were assessed by measuring the total phenolic contents (TPC), total flavonoid content (TFC), DPPH radical scavenging activity and Fe2+-chelating ability . TPC (mg gallic acid equivalents per 100 g of dry weight) and TFC (quercetin equivalents per gram of extract) of the fresh, boiled, microwaved, fried and steamed BJ were found to be 23.16, 27.7, 18.7, 35.94, 22.06 and 27.09, 27.8, 24.5, 36.34, 18.01 respectively. For MO it was found to be 34.6, 31.5, 31.6, 39.4, 33.72 and 70.84, 58.13, 55.4, 69.5, 52.78. A proportionate variation in DPPH radical scavenging activity and Fe2+-chelating ability was observed. The results of the present investigation showed that all the cooking methods affected the antioxidant properties of the vegetables; however, frying exhibited less deleterious effects when compared with those of other treatments. Thus an appropriate method might be sought for the processing of such vegetables to retain their antioxidant components at maximum level.
Keywords: Brassica juncea, Moringa oliefera, Processing, Flavonoid, Phenolic
Introduction
Consumers have much interest in taking leafy vegetables as a source of antioxidant for preventive and therapeutic solutions to many diseases including diabetes, cardiovascular, cancer, arthritis and overall aging process (Sasaki et al. 2002; Ren et al. 2003; Tapiero et al. 2002). The free radical scavenging action of dietary foods is attributed to flavonoids and phenolic compounds present in them (Shahidi and Naczk 1995). The antioxidant properties of Moringa oliefera (MO) (Verma et al. 2009) and Brassica juncea (BJ) (Kim et al. 2003) have been widely investigated for their in vitro and in vivo antioxidant activity. Processing of vegetables, particularly cooking can impact their antioxidant potential as it involves the structural integrity of the plant material. Food processing can enhance antioxidant potential by inhibition of enzymatic activity and transformation of antioxidants into more active compounds. It may also reduce antioxidant potential because of loss of certain aspects of their bioactivities when kept at high temperatures (Pedraza-Chaverri et al. 2006) or cooked (Ide and Lau 1997). Knowledge about the effective loss of total antioxidant activity consequent to home processing may have a significant impact on consumers’ food selection and processing.
Therefore the objective of present investigation was to study the in vitro antioxidant effect of the leafy vegetables Brassica juncea and Moringa oliefera after processing them with different cooking methods including boiling, microwave cooking, frying and steaming. Their total phenolic content, total flavonoid content, DPPH radical scavenging activity and Fe2+-chelating ability was studied and correlated with antioxidant action.
Materials and methods
Preparation of vegetable samples
All leafy vegetables (Brassica juncea and Moringa oliefera ) were collected in February 2010 at rural districts of Sambalpur, Orissa , India. DPPH, Folin–Ciocalteu reagent, gallic acid, quercetin and ferrozine were obtained from Sigma Aldrich. Ferrous chloride and sodium carbonate were obtained from Sisco Research Laboratories Pvt. Ltd., India. All other chemicals and solvents used were of analytical grade available commercially.
Vegetables were washed with tap water after removing manually inedible parts with a sharp knife. Vegetables were dried on paper towel and were cut into almost equal small pieces or slices, mixed well. About 1500 g was taken and divided into five portions (300 g for each application). The leafy vegetables were washed under cold running tap water and were blotted. Four 100 g portions of each leafy vegetable were cooked by each of four methods (boiling, frying, microwaving and steaming) in triplicate. Cooking conditions were determined, with a preliminary experiment for vegetable. Three uncooked portions of each vegetable were also tested. For processing by boiling, vegetable (100 g) was added to 150 ml of water that had just reached the boil in a stainless steel pan and cooked for 5 min. The samples were drained off and cooled rapidly on plenty of ice. For processing by microwave cooking, vegetable (100 g) was placed in a glass dish and 5 ml of distilled water was added and microwaved (550 W) for 5 min. Samples were drained off and cooled rapidly on ice. Steaming of vegetables was done by placing vegetable (100 g) on tray in a steam cooker covered with lid and steamed over boiling water for 7.5 min. The samples were rapidly cooled on ice. For frying purpose one part of each sample (100 g) was taken in a frying pan and fried with 3 ml of mustard oil for 5 min and then transferred to a glass container.
Raw and processed vegetables were homogenized in a blender for 2 min. Homogenized samples were dried in a convection oven at 70 °C to constant weight and were kept at 20 °C until analysis. Due to various water content of vegetables, all calculations were made according to dry matter basis.
Determination of total phenolic content
The amount of total phenolic was determined using Folin–Ciocalteu reagent, as described by K Slinkard and V. L Singleton with little modifications (Slinkard and Singleton 1977). About 1 g raw and cooked homogenized samples were extracted with 80% aqueous methanol (4.5 ml) on a mechanical shaker for 2 h. The mixture was centrifuged at 10,000 rpm for 15 min and the supernatant decanted into polypropylene tubes. Supernatants were combined and filtered through Whatman No.1 filter paper. The clear extracts were analyzed both for determination of phenolic content, flavonoid content and antioxidant activity.
Each extract (0.5 ml) was mixed with 2.5 ml of Folin–Ciocalteu reagent and left at room temperature for 5 min. The mixture was kept at room temperature for 1 h after the addition of 2 ml Na2CO3 (250 g/L). The absorbance was measured by UV-visible Spectrophotometer (V-630, Jasco, UK) at 760 nm. Phenolic content was determined from standard curve of gallic acid (Y = 0.0158x + 0.0133, R2 = 0.9943) and expressed as milligram gallic acid equivalents (GAE)/100 g dry weight.
Measurement of Total Flavonoid Content
The aluminum chloride colorimetric method was modified from the procedure reported by Woisky and Salatino (Woisky and Salatino 1998). About 0.5 mL of 10% aluminum chloride solution in ethanol was added to 0.5 ml of sample solution. After 1 h incubation at room temperature the absorbance was measured at 420 nm. Total flavonoid content was determined from the standard curve of quercetin (Y = 0.0227x – 0.0286, R2 = 0.9931) and expressed as milligram quercetin equivalents per gram of extract (mg QE/g).
Determination of total antioxidant activity
Antioxidant activity was determined by the 2,2,-diphenyl-2-picryl-hydrazyl (DPPH) method of Zhang and Hamauzu with some modifications (Turkmen et al. 2005). On dry basis methanol extracts of fresh or cooked vegetables were adjusted to various concentrations (10, 20, 30, 40, 50 μg/ml). An aliquot of 1 ml of 0.1 mmol DPPH radical in methanol was added to a test tube with 1 ml of vegetable extract. Pure methanol was used as control. It was mixed and let to stand at room temperature in the dark for 60 min before the decrease in absorbance at 517 nm was measured. The radical scavenging activity (RSA) was calculated using the equation: 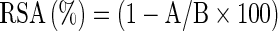 . Where A was the absorbance of sample, and B was the absorbance of the control. The concentration in μg/ml required to scavenge 50% of the radical (IC50) was obtained from the regression equation.
. Where A was the absorbance of sample, and B was the absorbance of the control. The concentration in μg/ml required to scavenge 50% of the radical (IC50) was obtained from the regression equation.
Ferrous ion chelating capacity
The chelating activity of the extracts for ferrous ions Fe2+ was measured according to the reported method (Dinis et al. 1994).To different concentrations of sample extracts (0.5 ml) 0.1 ml of 2 mmol ferrous chloride tetra hydrate, 0.2 ml of 5 mmol ferrozine were added. The volume was made up to 5 ml with methanol. The solutions were mixed and reacted for 10 min. The absorbance at 562 nm was measured. A lower absorbance indicated a higher ferrous iron chelating capacity, which was calculated and the regression equation was obtained from which IC50 was calculated.
The mean values were compared using one-way analysis of variance followed by Duncan’s multiple range tests. Values for fresh leafy vegetable were taken as control. P-values of less than 0.05 were considered significant.
Results and discussion
Phenolic compounds react with Folin–Ciocalteu reagent (FCR) only under basic conditions. Dissociation of a phenolic proton in basic medium leads to a phenolate anion, which is capable of reducing FCR in which the molybdate in the testing system is reduced forming blue colored molybdenum oxide with maximum absorption near 700 nm (Huang et al. 2005). Wide variations was observed in the total phenolic contents of the leaf extracts of BJ ranging from 35.9 mg GAE/100 g for fried samples to 18.7 mg GAE/100 g for samples subjected to microwave cooking (Table 1). The phenolic content was found to be enhanced significantly (p < 0.001) by frying. Frying is believed to enhance the release of these active constituent (Stephen et al. 2010). Microwave cooking reduced the phenolic content significantly (p < 0.001) compared to control. Similar findings were previously reported with 40–80% loss of phenolic content of broccoli (Brassica oleracea L) subjected to boiling and microwave cooking (Vallejo et al. 2003). For MO phenolic content varied from 34.6 mg GAE/100 g for fresh sample to 39.4 mg GAE/100 g for fried sample (Table 2). Thus frying has enhanced the release of phenolic compounds significantly (p < 0.05) but steaming and microwaving has reduced this.
Table 1.
Total phenolic contents (TPC), Total Flavonoid content (TFC), DPPH radical scavenging activity (RSA), Fe2+-chelating ability activity of Brassica juncea leaf extracts prepared by various processing methods
| Fresh | Boil | Microwave | Frying | Steaming | |
|---|---|---|---|---|---|
| TPC(mg GAE/100 g) | 23.1 ± 1.71 | 27.7 ± 0.98 | 18.7 ± 1.13*** | 35.9 ± 1.21*** | 22.06 ± 1.373 |
| TFC(mg QE/g) | 27.09 ± 0.281 | 27.8 ± 1.08 | 24.5 ± 1.55* | 36.3 ± 0.87*** | 18.01 ± 1.254*** |
| DPPH RSA(μg/ml) | 80.03 ± 0.112 | 143.5 ± 1.12*** | 147.5 ± 1.31*** | 79.8 ± 0.49 | 198.9 ± 0.87*** |
| Fe2+-chelation (μg/ml) | 71 ± 1.14 | 120.4 ± 0.23*** | 132.8 ± 1.23*** | 76.9 ± 0.94 | 146.6 ± 0.12*** |
Data are mean±standard deviation values (n = 3).DPPH radical scavenging and Fe2+-chelating ability are expressed as IC50 (μg/ml) indicating concentration in μg/ml required for 50% inhibition. Significant differences at *p < 0.05, ***p < 0.001.
Table 2.
Total phenolic contents (TPC), Total Flavonoid content (TFC), DPPH radical scavenging activity (RSA), Fe2+-chelating ability of Moringa oliefera leaf extracts prepared by various processing methods
| Fresh | Boil | Microwave | Frying | Steaming | |
|---|---|---|---|---|---|
| TPC(mg GAE/100 g) | 34.6 ± 1.44 | 31.5 ± 1.21* | 31.6 ± 0.53 | 39.4 ± 0.66* | 33.7 ± 1.04 |
| TFC(mg QE/g) | 70.8 ± 0.87 | 58.1 ± 0.64* | 55.4 ± 0.18 | 69.5 ± 0.74 | 52.7 ± 1.36*** |
| DPPH RSA(μg/ml) | 51.7 | 103.9 ± 1.13*** | 122.05 ± 1.11*** | 81.9 ± 0.42*** | 198.1 ± 2.46*** |
| Fe2+-chelating (μg/ml) | 62 | 71.4 ± 1.34*** | 70.8 ± 0.67*** | 81.2 ± 1.13*** | 142.07 ± 1.22*** |
Data are mean±standard deviation values (n = 3).DPPH radical scavenging and Fe2+-chelating ability are expressed as IC50 (μg/ml) indicating concentration in μg/ml required for 50% inhibition. Significant differences at *p < 0.05, ***p < 0.001.
The flavones and flavonols react with AlCl3 and form stable colored complexes. Variations were observed for BJ and MO with total flavonoid values ranging from 18.09 mg quercetin equivalents/g for steamed sample to 36.3 mg quercetin equivalents/g for fried sample and 52.7 mg quercetin equivalents/g for steamed extract to 70.8 mg quercetin equivalents/g for fresh extract (Tables 1 and 2). Frying has increased the total flavonoid content of BJ and MO where as steaming reduced it significantly (p < 0.001).This is in agreement with study on phenolic and flavonoid content of MO roots and leaves that reported higher yields in the aqueous organic extracts (Sultana et al. 2009). Boiling of MO (p < 0.05) and microwaving of BJ (p < 0.05) leafy vegetables affected their flavonoid content significantly. There is no previous report on effect of these cooking methods on BJ and MO leaves. However similar studies on Brassica oleracea var. botrytis italica reported decrease in phenolics and antioxidant activity (Zhang and Hamauzu 2004; Gawlik-Dziki 2008).
The measurement of radical scavenging activity of antioxidant is commonly determined by the DPPH method since it is a quick, reliable and reproducible method to assess the in vitro antioxidant activity of pure compounds as well as plant extracts (Mosquera et al. 2007; Sachindra et al. 2010; Kedare and Singh 2011). The effect of antioxidants on DPPH is based on their ability to donate a hydrogen atom to DPPH, thus converting the radical into a stable molecule. The IC50 value for fried BJ (79.8 μg/ml) was comparable to that of fresh BJ (80.03 μg/ml). This can be attributed to the increased phenolic and flavonoid content of fried BJ. The higher phenolic content of fried BJ has not been able to proportionately increase the antioxidant capacity as compared to fresh BJ (Table 1). This can be explained by the fact that in addition to phenolics, ascorbic acid and hydrolysable tannins contribute to the antioxidant capacity of fresh leafy vegetables. However at higher temperatures (frying) unlike the phenolics these are not stable and do not contribute to the total antioxidant activity (Begum et al. 2009; Koleckar et al. 2008). So the total antioxidant capacity of fried BJ can be attributed only to the phenolics which are stable at higher temperature (Vallejo et al. 2003). Other processing methods significantly (p < 0.001) reduced the antioxidant activity. For MO processing has reduced the radical scavenging activity significantly (p < 0.001) compared to control (Table 2).
Chelating agents that forms bonds with metal are effective as secondary antioxidants because they reduce the redox potential, and thereby stabilize the oxidized form of the metal ion. The fried sample of BJ exhibited marked capacity for iron binding (76.9 μg/ml), compared to fresh BJ (71 μg/ml). This finding supports the previous report showing significant increase in reducing power of some Brassica vegetables because of frying (Sultana et al. 2008). Boiling, microwaving and pressure cooking reduced the capacity of iron chelation significantly (p < 0.001). For MO all the methods of cooking has affected the iron chelating capacity significantly (p < 0.001) compared to control (Table 2).
Conclusion
Of the processing methods examined in the present study frying method significantly increased the phenolic and flavonoid content of BJ. Significant correlation exists between the contents of phenolics or flavonoids and the DPPH radical scavenging ability and Fe2+-chelation ability. For MO frying does not increase the flavonoid content but increases the phenolic content significantly with proportionate increase in DPPH radical scavenging and Fe2+-chelation ability. It is suggested that a proper heat treatment and frying processing could be used to enhance the amount of bioactive compounds and antioxidant capacity of Brassica juncea and Moringa oliefera. The antioxidant potential of these processed vegetables might be used as part of regular diet for protective effect against many diseases including diabetes, cardiovascular, arthritis, overall aging process and chemical carcinogenesis.
Acknowledgement
The authors are thankful to Director, Regional plant resource centre and Dean, School of pharmaceutical sciences for their kind cooperation.
References
- Begum SA, Ahmed MF, Rahman MM. Effect of cooking temperature and storage period on preservation of water soluble vitamin C content in Citrus macroptera and Molinga oleifera lunk. Asian J Food Agro Industry. 2009;2(3):255–261. [Google Scholar]
- Dinis TCP, Madeira VMC, Almeida LM. Action of Phenolic derivatives (Acetaminophen, Salicylate, and 5-Aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch Biochem Biophys. 1994;315(1):161–169. doi: 10.1006/abbi.1994.1485. [DOI] [PubMed] [Google Scholar]
- Gawlik-Dziki U. Effect of hydrothermal treatment on the antioxidant properties of broccoli (Brassica oleracea var. botrytis italica) florets. Food Chem. 2008;109(2):393–401. doi: 10.1016/j.foodchem.2007.12.058. [DOI] [PubMed] [Google Scholar]
- Huang D, Ou B, Prior RL. The chemistry behind antioxidant capacity assays. J Agric Food Chem. 2005;53:1841–1856. doi: 10.1021/jf030723c. [DOI] [PubMed] [Google Scholar]
- Ide N, Lau BH. Garlic compounds protect vascular endothelial cells from oxidized low density lipoprotein-induced injury. J Pharm Pharmacol. 1997;49:908–911. doi: 10.1111/j.2042-7158.1997.tb06134.x. [DOI] [PubMed] [Google Scholar]
- Kedare SB, Singh RP. Genesis and development of DPPH method of antioxidant assay. J Food Sci Tech. 2011;48(4):412–422. doi: 10.1007/s13197-011-0251-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HY, Yokozawa T, Cho EJ, Cheigh HS, Choi HS, Choi JS, Chung HY. In vitro and in vivo antioxidant effects of mustard leaf (Brassica juncea) Phytother Res. 2003;17(5):465–471. doi: 10.1002/ptr.1179. [DOI] [PubMed] [Google Scholar]
- Koleckar V, Kubikova K, Rehakova Z, Kuc K, Jun D, Opletal L. Condensed and hydrolysable tannins as antioxidants influencing the health. Mini Review Med Chem. 2008;8(5):436–447. doi: 10.2174/138955708784223486. [DOI] [PubMed] [Google Scholar]
- Mosquera OM, Correa YM, Buitrago DC, Nio J. Antioxidant activity of twenty five plants from Colombian biodiversity. Mem Inst Oswaldo Cruz. 2007;102:631–634. doi: 10.1590/S0074-02762007005000066. [DOI] [PubMed] [Google Scholar]
- Pedraza-Chaverri J, Medina-Campos ON, Avila-Lombardo R, Zuniga-Bustos AB, Orozco-Ibarra M. Reactive oxygen species scavenging capacity of different cooked garlic preparations. Life Sci. 2006;78:761–770. doi: 10.1016/j.lfs.2005.05.075. [DOI] [PubMed] [Google Scholar]
- Ren W, Qiao Z, Wang H, Zhu L, Zhang L. Flavonoids: promising anticancer agents. Med Res Rev. 2003;23:519–534. doi: 10.1002/med.10033. [DOI] [PubMed] [Google Scholar]
- Sachindra NM, Airanthi MKWA, Hosokowa M, Miyashita K. Radical scavenging and singlet oxygen quenching activity of extracts from Indian seaweeds. J Food Sci Tech. 2010;47(1):94–99. doi: 10.1007/s13197-010-0022-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki YF, Kawaguchi S, Kamaya A, Ohshita M, Kabasawa K, Iwama K. The comet assay with eight mouse organs: results with 39 currently used food additives. Mutat Res Genet Toxicol Environ Mutagen. 2002;519:103–109. doi: 10.1016/S1383-5718(02)00128-6. [DOI] [PubMed] [Google Scholar]
- Shahidi F, Naczk M. Food phenolics, sources, chemistry, effects, application. Lancaster: Technomic Publishing Co. Inc.; 1995. pp. 75–107. [Google Scholar]
- Slinkard K, Singleton VL. Total phenol analysis: automation and comparison with manual methods. American Journal of Enology and Viticulture. 1977;28:49–55. [Google Scholar]
- Stephen NM, Shakila RJ, Jeyasekaran G, Sukumar D. Effect of different type of heat processing on chemical changes in tuna. J Food Sci Tech. 2010;47(2):174–181. doi: 10.1007/s13197-010-0024-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultana B, Anwar F, Iqbal S. Effect of cooking methods on antioxidant activity of some vegetables from Pakistan. Int J Food Sci Tech. 2008;43(3):560–567. doi: 10.1111/j.1365-2621.2006.01504.x. [DOI] [Google Scholar]
- Sultana B, Anwar F, Ashraff M. Effect of extraction/solvent technique on the antioxidant activity of secected medicinal plants extracts. Molecules. 2009;14:2167–2180. doi: 10.3390/molecules14062167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapiero H, Tew KD, Ba GN, Mathe G. Polyphenol: do they play a role in the prevention of human pathologies? Biomed Pharmacother. 2002;56:200–207. doi: 10.1016/S0753-3322(02)00178-6. [DOI] [PubMed] [Google Scholar]
- Turkmen N, Sari F, Velioglu YS. The effect of cooking methods on total phenolics and antioxidant activity of selected green vegetables. Food Chem. 2005;93(4):713–718. doi: 10.1016/j.foodchem.2004.12.038. [DOI] [Google Scholar]
- Vallejo F, Tomas-Barberan FA, Gracia-Viguera C. Phenolic compound contents in edible parts of broccoli inflorescences after domestic cooking. J Sci Food Agric. 2003;83(14):1511–1516. doi: 10.1002/jsfa.1585. [DOI] [Google Scholar]
- Verma AR, Vijaykumar M, Mathela CS, Rao CV. In vitro and in vivo antioxidant properties of different fractions of Moringa oleifera leaves. Food Chem Toxicol. 2009;47(9):2196–2201. doi: 10.1016/j.fct.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Woisky R, Salatino A. Analysis of propolis:some parameters and procedures for chemical quality control. J Apic Res. 1998;37:99–105. [Google Scholar]
- Zhang D, Hamauzu Y. Phenolics, ascorbic acid, carotenoids and antioxidant activity of broccoli and their changes during conventional and microwave cooking. Food Chem. 2004;88(4):503–509. doi: 10.1016/j.foodchem.2004.01.065. [DOI] [Google Scholar]


