Abstract
Changes in texture and colour of pidan white as influenced by glucose treatment at levels of 0, 2 and 5% were determined after pickling (week 3) and during the storage up to 12 weeks. Hardness and cohesiveness of pidan white without glucose treatment were more retained but showed a decrease in adhesiveness as storage time increased up to week 12 (P < 0.05). Higher browning intensity and a*-value were noticeable in the pidan white treated with glucose at both levels as the storage time increased (P < 0.05). Thus, glucose could enhance the development of brown colour, mainly via the Maillard reaction with free amino groups of pidan white at alkaline pH, but it could impair the textural property. Pidan white without glucose treatment showed the higher color and appearance likeness score, but lower texture and odour likeness score than commercial counterpart (P < 0.05). Therefore, glucose was not a necessary aid for pidan production.
Keywords: Pidan, Glucose, Colour, Browning, Texture, Egg white
Introduction
Alkaline process has been used in food industry to destroy toxins and to obtain products with a prolonged shelf-life. Pidan is one of the typical examples of such products, which are produced by soaking duck eggs in 4.2% NaOH/5.0% NaCl with 0.2% ZnCl2 solution at room temperature (30 °C) for 3 weeks and ageing for another 3 weeks (Ganasen and Benjakul 2010a). Alkaline treatment can induce the degradation of proteins as well as Maillard reactions during the processing of pidan. Apart from brown colour, gel-like texture of pidan is desirable and has been governed by cations used (Ganasen and Benjakul 2010a). Texture of pidan white pickled with zinc cation was quite similar with those traditionally prepared pidan pickled with lead cation. However the colour of pidan white treated with zinc cation is amber brown colour, compared to lead treated pidan white, which is brown in colour. However, lead is toxic and prohibited for food processing.
Colour of pidan may be due to the Malliard reactions between the glucose and amino acid in egg white (Li and Hsieh 2004). Sankaran et al. (1989) reported that glucose in egg white accounts for 0.4%. Reducing sugars are essential ingredients in Maillard reaction, as they provide the carbonyl groups for interaction with the free amino groups of amino acids, peptides and proteins. Glycosylation or glycation induces the covalent attachment of sugars to α- or ε-NH2 groups of amino acids and protein to form glycated proteins (Friedman 1996; Rossini et al. 2011). The Maillard reaction produces a variety of intermediate products and finally brown pigments (melanoidins) are formed (Van Boekel 1998). Ageing of pidan aids in the development of brown colour during processing of pidan (Ganasen and Benjakul 2010b). In order to enhance the colour development during ageing of pidan pickled with zinc cation, further development process should be focused. The addition of glucose, a precursor for Maillard reaction, would be a means to enhance the development of brown colour, in which the desirable colour can be obtained.
Although egg white contains glucose at some level, it might not be sufficient to accelerate the Maillard reaction. Furthermore, the enhanced Maillard reaction might play a role in textural properties of pidan white. Therefore, the objectives of this study were to investigate the changes in colour and texture of pidan white treated with zinc cation in the absence and presence of glucose at different levels after pickling and during storage for up to 12 weeks.
Materials and methods
Chemicals
Zinc chloride (ZnCl2), sodium hydroxide and sodium chloride were purchased from Lab-Scan (Bangkok, Thailand). 2,4,6-Trinitrobenzenesulfonic acid (TNBS) and L-leucine were obtained from Sigma-Aldrich (St. Louis, MO, USA). Glucose and other chemicals were purchased from Merck (Damstadt, Germany).
Duck egg collection
Fresh eggs of duck (Anas platyrhucus) with the weight range of 65–75 g were obtained within 1 day of laying from a farm in Rathabhum, Songhkla province, Thailand. Duck eggs were cleaned and checked for any crack prior to pickling.
Preparation of pidan
Clean duck eggs were soaked in a pickling solution containing 4.2% NaOH, 5% NaCl and 0.2% ZnCl2. Traditionally prepared pidans using PbO2 were taken as the control. Eggs (60 eggs) were soaked in different pickling solutions (6 l) at room temperature (30–32 °C) for 3 weeks. Pickled pidan was then soaked in the glucose solution at the concentrations of 0, 2 and 5% (w/v) for 48 h. Thereafter, all samples were removed and coated with white clay paste (clay: water, 4:1 (w/v)) to obtain a thickness of 2–3 mm. Coated eggs were left at room temperature (30–32 °C) and stored up to 12 weeks. During storage, the samples were taken for analyses every 2 weeks.
Texture profile analysis (TPA)
Pidan white samples with different treatments stored for different times were subjected to TPA. TPA was performed as described by Bourne (1978) with a TA-XT2i texture analyser (Stable Micro Systems, Surrey, England). Prior to analysis, pidan white samples of various treatments were cut into a cube (1.0×1.0×1.0 cm3). The samples were compressed twice to 50% of their original height with a compression cylindrical aluminum probe (15 mm diameter). Textural analyses were performed at room temperature. Force-distance deformation curves were recorded at cross-head speed of 5 mm/s and the recording speed was 5 mm/s. Hardness, adhesiveness, and cohesiveness were evaluated using the Micro Stable software (Stable Micro Systems, Surrey, England).
Determination of pH
At week 3, 6 and 12, pidan white samples with different treatments were determined for pH according to the method of Benjakul et al. (1997).
Measurement of UV-absorbance
UV-absorbance of pidan white samples was measured according to the method of Ajandouz et al. (2001). Prior to measurement, the samples were mixed with 5 volumes of deionised water (w/v). The mixtures were homogenised at a speed of 5,000 rpm for 10 min using a homogeniser (IKA Labortechnik, Selangor, Malaysia) followed by centrifugation at a speed of 10,000×g for 10 min at 27 °C using a refrigerated centrifuge (model J-E Avanti, Beckman Coulter, Inc., Palo Alto, CA, USA). The dilution of 20-fold was made. The absorbance was measured at 294 using a UV-1601 spectrophotometer (Shimadzu, Kyoto, Japan).
Measurement of fluorescence intensity
Fluorescence intensity of pidan white samples was determined as described by Morales and Jimenez-Perez (2001). Twenty-fold diluted samples were prepared as previously described. The fluorescence intensity was measured at an excitation wavelength of 347 nm and an emission wavelength of 415 nm using a RF-1501 Fluorescence spectrophotometer (Shimadzu, Kyoto, Japan).
Measurement of browning intensity
Browning intensity of pidan white samples of all treatments was determined as described by Benjakul et al. (2005). The samples were prepared and 20-fold diluted as described previously. The absorbance was measured at 420 nm using a spectrophotometer.
Determination of free amino group content
Free amino group content was determined according to the method of Benjakul and Morrissey (1997). Pidan white samples (100-fold dilution) (125 μl) were mixed with 2.0 ml of 0.20 M phosphate buffer, pH 8.2, and 1.0 ml of 0.01% TNBS solution was then added. The solutions were mixed thoroughly and placed in a temperature-controlled water bath (Memmert, Bavaria, Germany) at 50 °C for 30 min in the dark. The reaction was terminated by adding 2.0 ml of 0.1 M sodium sulfite. The mixtures were cooled at room temperature for 15 min. The blank was prepared in the same manner as the samples except that distilled water was used instead of 0.01% TNBS. The absorbance was measured at 420 nm. Free amino group content was expressed in terms of L-leucine.
Determination of reducing sugar content
Reducing sugar content was determined according to the method of Chaplin (1994). All reagents were prepared as described by Chaplin (1994). One ml of pidan white samples (100-fold dilution) was mixed with 1.0 ml of reagent C in screw-sealed tubes. The mixtures were heated in a boiling water for 15 min and then cooled with tap water. One ml of reagent D was added and mixed well. Finally, 3 ml of deionised water was added to the mixtures. The absorbance was measured at 520 nm. The reducing sugar content was calculated from the standard curve of glucose ranging from 10 to 100 μM.
Colour measurement
The colour of pidan white was measured using a Hunter Lab Labscan II colourimeter (Hunter Associates Laboratory Inc., Reston, VA, USA) and expressed as L* (lightness), a* (redness/greenness) and b* (yellowness/blueness).
Sensory analysis
The sensory evaluation was performed by 30 untrained panelists, who were the graduate students in Food Science and Technology programme with the age of 25–33 years and were familiar with pidan consumption. The pidan samples were peeled and pidan white was separated. The pidan white was cut into small slices (2 × 2 × 2 cm3) before analysis. The assessment was conducted for the shell appearance, color, appearance, odour flavour, texture and overall likeness using a 9-point hedonic scale (Mailgaard et al. 1999), where 1, dislike extremely; 2, dislike very much; 3, dislike moderately; 4, dislike slightly; 5, neither like nor dislike; 6, like slightly; 7, like moderately; 8, like very much; 9, like extremely.
Statistical analysis
Completely randomised design was used throughout the study. The experiments were run in triplicate using three lots of eggs. Data were presented as mean values with standard deviations. One-way analysis of variance (ANOVA) was carried out and mean comparisons were run by Duncan’s multiple range tests (Steel and Torrie 1980). Statistical analyses were performed with the statistical program (SPSS for windows (Version 10), SPSS Inc, Chicago, IL, USA).
Results and discussion
Effect of glucose treatment on textural properties of pidan white during storage
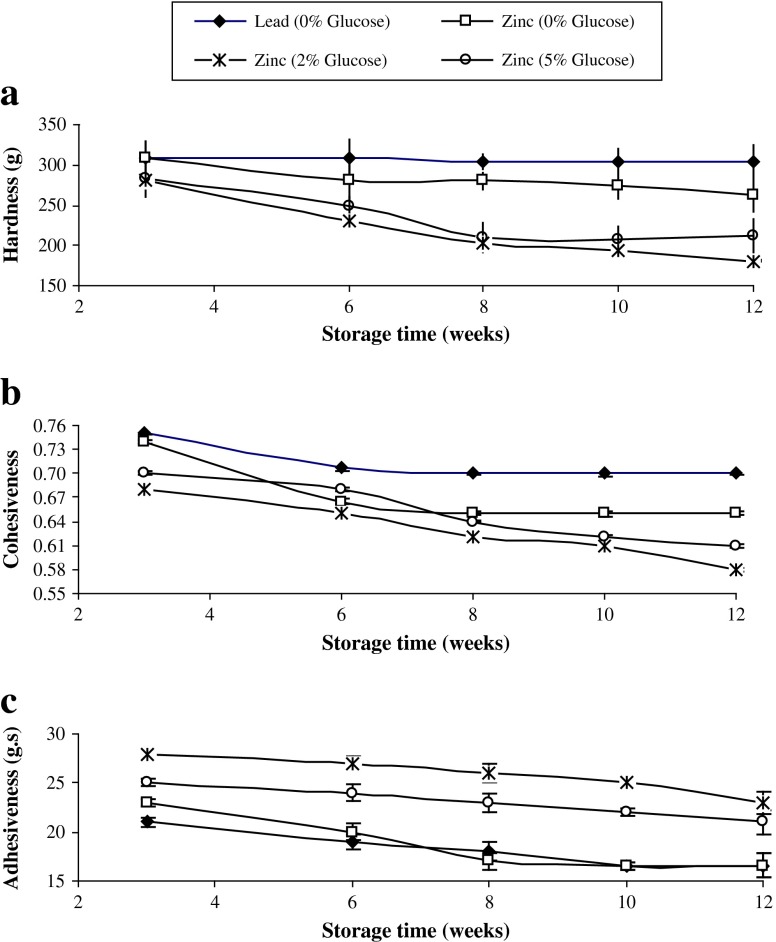
Hardness of pidan white of all treatments gradually decreased up to week 12 of storage (P < 0.05) (Fig. 1a). Hardness of pidan white obtained from all treatments was more resistant to compression at week 3 of pickling, most likely due to the aggregation of egg white proteins in the presence of cations. This was evidenced by the gel-like structure of egg white after 3 weeks of pickling. Proteins with negative charge under alkaline condition could interact each other in the presence of cations via ‘salt-bridge’ mechanism, thereby lowering the repulsive force between protein molecules (Ganasen and Benjakul 2010b). During storage, the decrease in hardness was found in pidan white of all treatments. However, the decrease in hardness of pidan white was more pronounced with samples treated with glucose at both levels (P < 0.05). Ageing of pidan resulted in the weakening of aggregate formed during pickling. Furthermore, glucose penetrated into egg white might enhance Maillard reaction at alkaline pH, competing with cations in forming the bridges between protein molecules. As a result, the gel network formed previously through cations was weakened. However, in the presence of lead or zinc cations (without glucose), the liquefaction was slightly retarded during storage (P < 0.05). Cations might stabilize the protein network, thereby lowering the dissociation of protein network previously formed, though slightly higher alkaline pH was obtained during storage (Ganasen and Benjakul 2010b). Electrostatic attraction between the positively charged Zn2+-water complex and the carboxylic groups of the negatively charged protein played a role in protein aggregation. As a result, gel-like structure was formed (Shi et al. 2008).
Fig. 1.
Changes in texture profile analysis (TPA) of pidan white treated with and without glucose during storage at room temperature. Bars represent the standard deviations (n = 3)
Cohesiveness is often used as an indice of the ability of gel to maintain an intact network structure. Higher values of cohesiveness indicate how well the product withstands intact network structure (Fernandez-Lopez et al. 2006). Cohesiveness of pidan white gradually decreased up to week 12, irrespective of glucose used (P < 0.05) (Fig. 1b). This change was in accordance with that found for hardness of pidan white. The increase in pH of white proteins might lead to the repulsion between protein molecules to some degree (Ganasen and Benjakul 2010b). Glucose soaking can enhance the Maillard reaction during storage, causing partial destabilization of ion-induced gel. Cohesiveness of pidan white treated with lead and zinc ions (without glucose treatment) was slightly higher than that of pidan white obtained from other treatments during storage (P < 0.05). Those cations could maintain an intact gel network effectively along with the continuous dehydration of pidan white (Ganasen and Benjakul 2010b).
Adhesiveness is defined as the work necessary to overcome the attractive forces between the product and a specific surface (Raikos et al. 2007). The gradual decrease in adhesiveness was observed during storage up to week 12 (P < 0.05) (Fig. 1c). High polarity or hydrophilicity of egg proteins at alkaline pH more likely contributed to the stickiness of pidan white (Ganasen and Benjakul 2010b). Among all samples, those treated with lead and zinc ions (without glucose treatment) showed the lower adhesiveness, indicating the less stickiness of pidan white during storage. Gradual decrease in adhesiveness during storage in all treatments might be attributed to the moisture loss of pidan white during prolonged storage (Ganasen and Benjakul 2010b). As a result, pidan white had the less stickiness. However, the adhesiveness of pidan white treated with glucose, irrespective of amount used, was higher during storage (P > 0.05). Glucose treatment probably resulted in the increase in hydrophilicity, in which proteins could bind more water, leading to stickiness of pidan white. Thus glucose treatment caused a slight decrease in the strength of pidan white gel during the extended storage.
Changes in pH, A294, fluorescence intensity and A420 of pidan white during storage
Changes in pH of pidan with different treatments were monitored during pickling and storage. pH of pidan white were 11.20–11.30 at week 3, 12.05–12.14 at week 6 and 12.34–12.45 at week 12. Ganasen and Benjakul (2010b) reported that the final pH of pidan white pickled and aged for 6 weeks were 12.02. The increase in pH indicated the migration of alkali from pickling solution into egg white. Alkaline pH of pidan white had the influence on colour as well as textural development of pidan (Ganasen and Benjakul 2010b). Nevertheless, type of cations and levels of glucose had the negligible effect on the pH of pidan white during storage (P > 0.05).
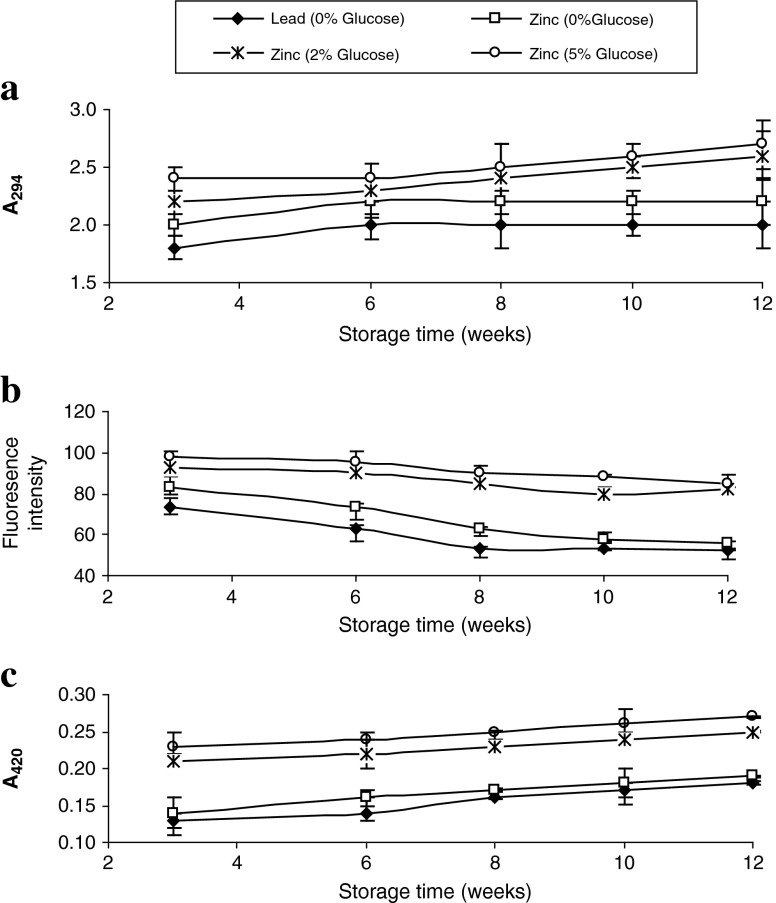
Continuous increases in A294 of pidan white samples were observed during pickling and storage, irrespective of treatments (Fig. 2a). Pidan white treated with zinc cation together with 2% and 5% glucose showed the higher increase in A294, compared with those pickled in solution without glucose (P < 0.05). Glucose could enhance the Maillard reaction at a very high alkaline pH (Li and Hsieh 2004). Absorbance at 294 nm was used to determine the intermediate compounds of the Maillard reaction (Ajandouz et al. 2001; Lerici et al. 1990; Lertittikul et al. 2007). The increase in absorbance at 294 nm suggested the formation of an uncoloured compound, which could be the precursor of the Maillard reaction (Ajandouz et al. 2001). This was more likely due to the higher formation of intermediate generated during a very high alkaline pH. However, the presence of lead cation prevented the formation of colourless intermediate to some extent. Lead has a strong affinity for some ligands, including ε-amino group of lysine, the carboxyl group of glutamic and aspartic acids, the sulfhydryl group of cysteine, and the phenoxy group of tyrosine and imidazole residues. Lead has been reported to interact with proteins (Goering, 1993). As a consequence, amino groups were less available for Maillard reaction. Different intermediate products are formed, either fluorescent or non-fluorescent compounds, during the Maillard reaction (Benjakul et al. 2005). Some intermediate products might undergo conversion to the final brown compounds, while some intermediates were still generated.
Fig. 2.
Changes in A 294 (a), fluorescence intensity (b) and browning intensity (c) of pidan white treated with and without glucose during storage at room temperature. Bars represent the standard deviations (n = 3)
Fluorescence intensity of all pidan white samples gradually decreased during storage, irrespective of treatments (P < 0.05). Generally, an increase in pH of the system influenced the rate of Maillard reaction and the alkaline condition favoured the reaction. The Maillard reaction is associated with the development of fluorescent compounds formed prior to the generation of brown pigments (Baisier and Labuza 1992; Morales et al. 1996). This fluorescent compounds may be precursors of brown pigments (Labuza and Baisier 1992; Morales and Van Boekel 1997). The lower fluorescence intensity of pidan white during storage was probably caused by the rapid transformation of the intermediates to brown compounds. This led to less remaining fluorescent intermediate products, as shown by the lower fluorescence intensity. Nevertheless, the pidan white treated with glucose at the level of 2% and 5% showed slightly higher fluorescent intensity than those pickled with cations in the absence of glucose. This result was in agreement with that of A294. Addition of glucose therefore enhanced the Maillard reaction at a very high alkaline pH, mainly due to the higher carbonyl group involved in glycation.
Continuous increases in A420 of pidan white samples were observed during storage, irrespective of treatments (Fig. 2c). Maillard reaction is a non-enzymatic browning reaction, which links the carbonyl group of reducing carbohydrates and the amino group of free amino acids, especially lysine residues in proteins (Ajandouz et al. 2001; Kato et al. 1978). Maillard reaction is considered important in pidan white because of the significant amount of glucose naturally present in the egg white proteins (Powrie 1977). Pidan white treated with zinc cation along with glucose at levels of 2 and 5% showed the higher browning intensity of pidan white, compared with other samples during storage up to 12 weeks (P < 0.05). Maillard reaction took place rapidly at a very high alkaline pH. The increases in browning intensity of all samples with increasing storage was most likely due to the production and conversion of fluorescent or non-colour compounds into brown pigment. Therefore, the incorporation of glucose in the pickling solution most likely enhanced the development of brown colour of pidan white.
Changes in free amino group and reducing sugar contents of pidan white during storage
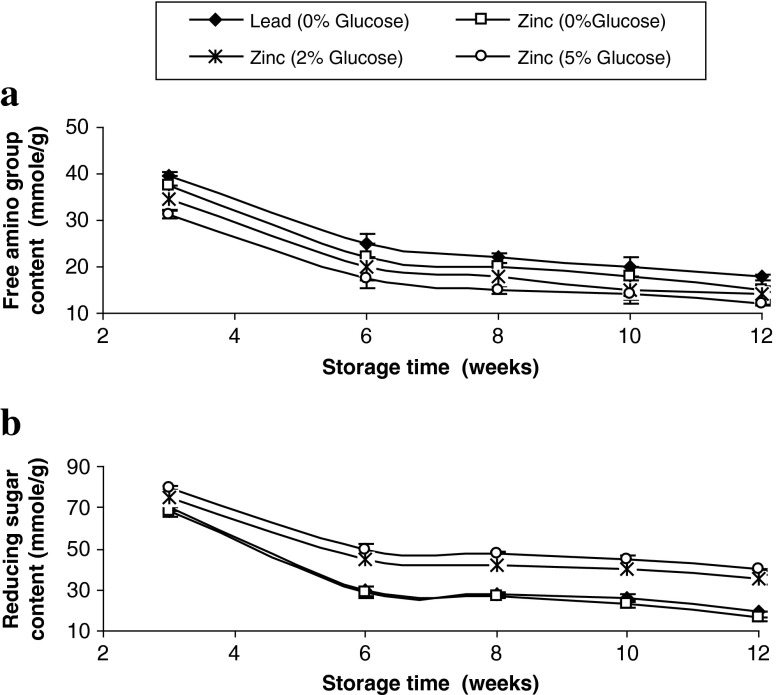
Changes in free amino group and reducing sugar contents of pidan white samples with various treatments are shown in Fig. 3a and b, respectively. Continuous decreases in amino group and reducing sugar content of all pidan white samples were noticeable when the storage time increased up to week 12 (P < 0.05). This result suggested that α- or ε-NH2 groups of amino acids or proteins, which were partially hydrolysed at very high alkaline pH, covalently attached to a sugar to form glycated proteins to a greater extent, particularly when the storage time increased to week 12. The first glycation product, or Schiff base, rearranges to a more stable ketoamine or Amadori product. The Amadori products can then form cross-links between adjacent proteins or with other amino groups, resulting in polymeric aggregates called advanced glycation end-products (Friedman 1996). The decreases in free amino group content were in accordance with the increase in browning (Fig. 2c) and A294 (Fig. 2a) and the decrease in fluorescence intensity (Fig. 2b). This indicated that the increased storage time enhanced the interaction between free amino groups of proteins or peptides and glucose via glycation process (Ganasen and Benjakul 2010b). As a result, intermediate products were formed and further converted to brown pigments, as observed by the increased A420. In general, pidan treated only with glucose at various levels (2 and 5%) was more reactive in forming the glycated product than that treated with cations (without glucose). This was evidenced by the greatest decrease in free amino group and reducing sugar contents with the concomitant increase in browning. The reaction rate of glycation between casein and sugars depended on the percentage of the acyclic form and the electrophilicity of the carbonyl groups (Naranjo et al. 1998; Bunn and Higgins 1981). The difference in reaction rate of sugar in glycation process was possibly governed by the conformation of protein. Under alkaline condition, sugar had the open structure, more favourable for glycation. Nevertheless, lead cation might impede Maillard reaction by higher cross linking and preventing the reaction between amino groups and reducing sugar.
Fig. 3.
Changes in free amino groups (a) and reducing sugar (b) contents of pidan white treated with and without glucose during storage at room temperature. Bars represent the standard deviations (n = 3)
Changes in the colour of pidan white during storage
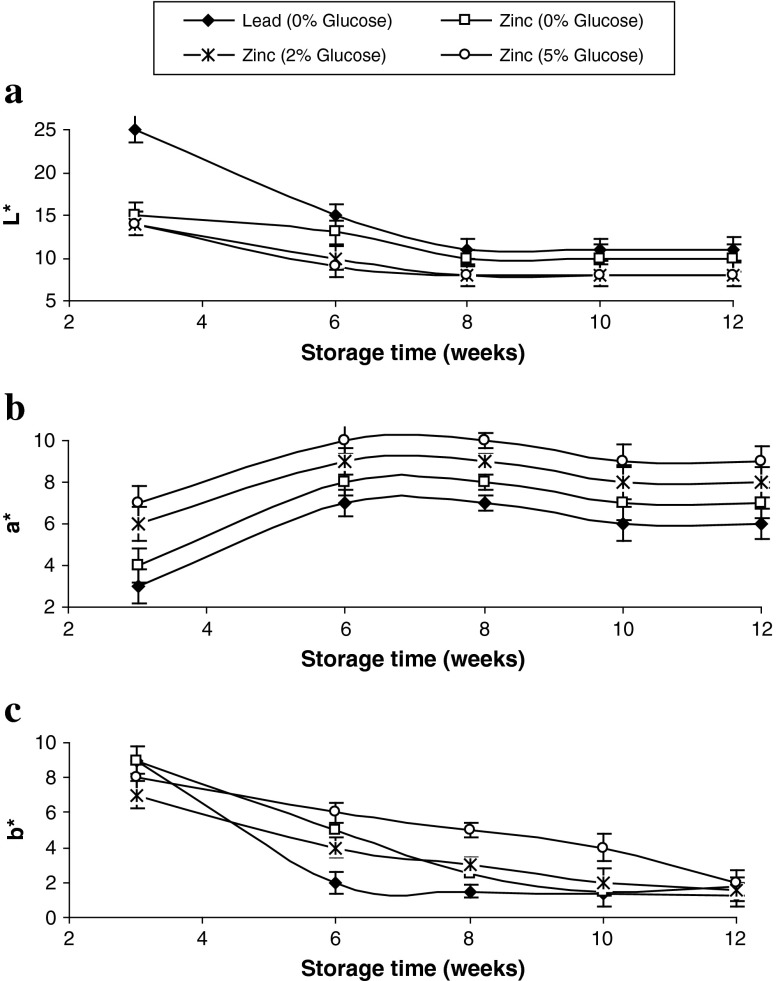
The colour of pidan white of different treatments during storage is shown in Fig. 4. L* and b*-values of pidan white generally decreased, whereas a*-values increased with increasing storage, regardless of glucose treatment (P < 0.05). However, pidan white treated with lead cation showed the higher L* value values, compared to other treatments (P < 0.05). This was probably due to the higher aggregation of proteins, which exhibited the higher light scattering effect. Chantrapornchai and McClements (2002) reported that whey protein gels increased its lightness with increasing protein size. Higher b* and a* values were found in pidan white treated with glucose at the level of 2 and 5% during storage, more likely owing to the formation of yellow or brown pigments. Increased a* values were possibly due to the formation of brown pigments, which might derive from Mailliard reaction of egg white (Ganasen and Benjakul 2010b). This was in accordance with higher browning intensity of pidan white (Fig. 2c). Furthermore, higher a*-values were noticeable in the pidan white treated with glucose at both levels and was coincidental with the increasing A420. Thus glucose treatment had the marked impact on colour of pidan white developed during storage.
Fig. 4.
Changes in colour of pidan white treated with and without glucose during storage at room temperature. Bars represent the standard deviations (n = 3)
Sensory properties of white pidan
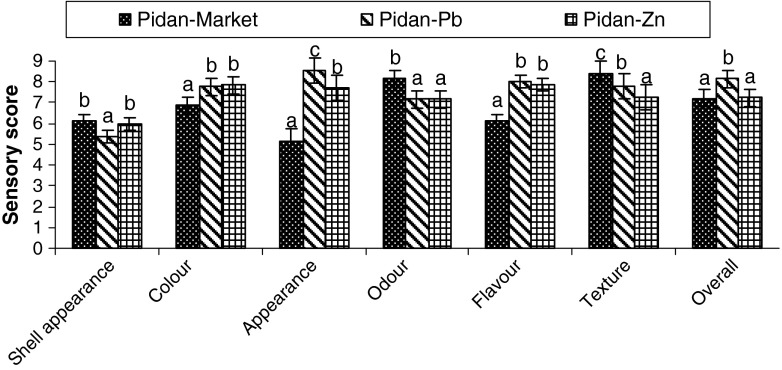
Due to the non-significant difference in colour, especially L* and b* values of pidan without and with glucose treatment at the end of storage and the higher hardness and cohesiveness of pidan treated with ZnCl2 without the additional glucose, the pidan treated with 0.2% ZnCl2 without glucose was selected for sensory evaluation in comparison with pidan treated with 0.2% PbO2 and the commercial pidan. Likeness scores of pidan white treated with 0.2% PbO2 and 0.2% ZnCl2 as compared to those of commercial pidan are shown in Fig. 5. Shell appearance of pidan treated with 0.2% PbO2 showed the low score (P < 0.05). This was mostly due to the presence of black spots on the shell. Wang and Fung (1996) reported that the addition of zinc causes no black spots in egg shell and membrane. Likeness score of colour, appearance and flavour of pidan white was higher than those of pidan obtained from the market (P < 0.05). However texture likeness of pidan white treated with 0.2% ZnCl2 was lower than other pidans. For overall likeness, pidan treated with 0.2% ZnCl2 without glucose showed the similar score to the commercial pidan.
Fig. 5.
Likeness score of different pidan whites. Pidan-Market: commercial pidan; Pidan-Pb: Pidan treated with PbO2; Pb-Zn: Pidan treated with ZnCl2
Conclusion
Glucose had pronounced effect on brown colour development of pidan white, whereas it showed the detrimental effect on textural property of pidan white during storage. Therefore, glucose was not necessary for improvement of pidan quality and it directly increased the production cost of pidan.
Acknowledgements
The authors would like to express their sincere thanks to Graduate School of Prince of Songkla University for the financial support.
References
- Ajandouz EH, Tchiapke LS, Ore FD, Benjibas A, Puigserver A. Effect of pH on caramelisation and Maillard reaction kinetics in fructose-lysine model systems. J Food Sci. 2001;66:926–932. doi: 10.1111/j.1365-2621.2001.tb08213.x. [DOI] [Google Scholar]
- Baisier WM, Labuza TP. Maillard browning kinetics in liquid model system. J Agric Food Chem. 1992;40:707–713. doi: 10.1021/jf00017a001. [DOI] [Google Scholar]
- Benjakul S, Morrissey MT. Protein hydrolysates from Pacific whiting solid waste. J Agric Food Chem. 1997;45:3423–3430. doi: 10.1021/jf970294g. [DOI] [Google Scholar]
- Benjakul S, Seymour TA, Morrissey MT, An H. Physicochemical changes in Pacific whiting muscle proteins during iced storage. J Food Sci. 1997;62:729–733. doi: 10.1111/j.1365-2621.1997.tb15445.x. [DOI] [Google Scholar]
- Benjakul S, Lertittikul W, Bauer F. Antioxidative activity of Maillard reaction products from a porcine plasma protein–sugar model system. Food Chem. 2005;93:189–196. doi: 10.1016/j.foodchem.2004.10.019. [DOI] [Google Scholar]
- Bourne MC. Texture profile analysis. Food Technol. 1978;32(7):62–72. [Google Scholar]
- Bunn HF, Higgins PJ. Reaction of monosaccharides with proteins: possible evolutionary significance. Science. 1981;213:222–224. doi: 10.1126/science.12192669. [DOI] [PubMed] [Google Scholar]
- Chantrapornchai W, McClements DJ. Influence of NaCl on optical properties, large-strain rheology and water holding capacity of heat-induced whey protein isolate gels. Food Hydrocolloid. 2002;16:467–476. doi: 10.1016/S0268-005X(01)00124-2. [DOI] [Google Scholar]
- Chaplin MF. “Carbohydrate analysis.” A practical approach. New York: Oxford University Press; 1994. pp. 1–41. [Google Scholar]
- Fernandez-Lopez J, Martinez A, Fernandez-Gines JM, Sayas-Barbera E, Sendra E, Perez-Alvarez JA. Gelling and colour properties of ostrich (Struthio camelus) egg white. J Food Qual. 2006;29:171–183. doi: 10.1111/j.1745-4557.2006.00065.x. [DOI] [Google Scholar]
- Friedman M. Food browning and its prevention: an overview. J Agric Food Chem. 1996;44:631–653. doi: 10.1021/jf950394r. [DOI] [Google Scholar]
- Ganasen P, Benjakul S. Physical properties and microstructure of pidan yolk as affected by different divalent and monovalent cations. LWT-Food Sci Technol. 2010;43:7–85. doi: 10.1016/j.lwt.2009.06.007. [DOI] [Google Scholar]
- Ganasen P, Benjakul S (2010b) Physical properties and microstructure of pidan white as affected by different divalent and monovalent cations. J Food Biochem (Accepted)
- Goering PL. Lead-protein interactions as a basis for lead toxicity. Neurotoxicol. 1993;14:45–60. [PubMed] [Google Scholar]
- Kato Y, Watanabe K, Sato Y. Effect of the Maillard reaction on the attributes of egg white proteins. Agric Biol Chem. 1978;42:2233–223. doi: 10.1271/bbb1961.42.2233. [DOI] [Google Scholar]
- Labuza TP, Baisier WM. The kinetics of nonenzymatic browning. In: Schwartzberg HG, Hartel RW, editors. Physical chemistry of foods. New York: Marcel Dekker; 1992. pp. 595–649. [Google Scholar]
- Lerici CR, Barbanti D, Manzano M, Cherubin S. Early indicators of chemical changes in foods due to enzymic or non enzymic browning reactions. 1: study on heat treated model system. Lebensm Wiss-u Technol. 1990;23:289–294. [Google Scholar]
- Lertittikul W, Benjakul S, Tanaka M. Characteristics and antioxidative activity of Maillard reaction products from a porcine plasma protein–glucose model system as influenced by pH. Food Chem. 2007;2:669–677. doi: 10.1016/j.foodchem.2005.09.085. [DOI] [Google Scholar]
- Li JR, Hsieh YH. Traditional Chinese food technology and cuisine. Asia Pacific J Clin Nutr. 2004;13:147–155. [PubMed] [Google Scholar]
- Mailgaard M, Civille GV, Carr BT. Sensory evaluation techniques. Boca Raton: CRC; 1999. [Google Scholar]
- Morales FJ, Jimenez-Perez S. Free radical scavenging capacity of Maillard reaction products as related to colour and fluorescence. Food Chem. 2001;72:119–125. doi: 10.1016/S0308-8146(00)00239-9. [DOI] [Google Scholar]
- Morales FJ, Van Boekel MAJS. A study on advanced Maillard reaction in heated casein–sugar solution: fluorescence accumulation. Int Dairy J. 1997;7:675–683. doi: 10.1016/S0958-6946(97)00071-X. [DOI] [Google Scholar]
- Morales FJ, Romero C, Jimenez-Perez S. Fluorescence associated with Maillard reaction in milk and milk-resembling system. Food Chem. 1996;67:423–428. doi: 10.1016/0308-8146(95)00245-6. [DOI] [Google Scholar]
- Naranjo GB, Malec LS, Vigo MS. Reducing sugars effect on available lysine loss of casein by moderate heat treatment. Food Chem. 1998;62:309–313. doi: 10.1016/S0308-8146(97)00176-3. [DOI] [Google Scholar]
- Powrie W. Chemistry of eggs and egg products. In: Stadelnan WJ, Cotterill OJ, editors. Egg science and technology. Westport: AVI; 1977. pp. 97–139. [Google Scholar]
- Raikos V, Campbell L, Euston SR. Rheology and texture of hen’s egg protein heat-set gels as affected by pH and the addition of sugar and/or salt. Food Hydrocolloid. 2007;21:237–244. doi: 10.1016/j.foodhyd.2006.03.015. [DOI] [Google Scholar]
- Rossini K, Norena CPZ, Brandelli A. Changes in the color of white chocolate during storage: potential roles of lipid oxidation and non-enzymatic browning reactions. J Food Sci Technol. 2011;48:305–311. doi: 10.1007/s13197-010-0207-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankaran K, Godbole SS, DSouza SF. Preparation of spray-dried, sugar-free egg powder using glucose oxidase and catalase coimmobilized on cotton cloth. Enzyme Microb Technol. 1989;11:617–619. doi: 10.1016/0141-0229(89)90091-4. [DOI] [Google Scholar]
- Shi L, Zhou J, Gunasekaran S. Low temperature fabrication of ZnO-whey protein isolate nanocomposite. Mater Lett. 2008;62:4383–4385. doi: 10.1016/j.matlet.2008.07.038. [DOI] [Google Scholar]
- Steel RD, Torrie JH. Principle and procedures of statistic: a biometrical approach. New York: McGraw-Hill; 1980. [Google Scholar]
- Van Boekel MAJS. Effect of heating on Maillard reaction in milk. Food Chem. 1998;62:403–414. doi: 10.1016/S0308-8146(98)00075-2. [DOI] [Google Scholar]
- Wang J, Fung DYC. Alkaline-fermented foods. A review with emphasis on Pidan fermentation. Crit Rev Microbiol. 1996;22:101–138. doi: 10.3109/10408419609106457. [DOI] [PubMed] [Google Scholar]