Abstract
Now a days sugar free food are very much popular because of their less calorie content. So food industry uses various artificial sweeteners which are low in calorie content instead of high calorie sugar. U.S. Food and Drug Administration has approved aspartame, acesulfame-k, neotame, cyclamate and alitame for use as per acceptable daily intake (ADI) value. But till date, breakdown products of these sweeteners have controversial health and metabolic effects. On the other hand, rare sugars are monosaccharides and have no known health effects because it does not metabolize in our body, but shows same sweet taste and bulk property as sugar. Rare sugars have no such ADI value and are mainly produced by using bioreactor and so inspite of high demand, rare sugars cannot be produced in the desired quantities.
Keywords: D-allose, D-psicose, Low calorie sweetener, Stevia, Sugar alcohols
Introduction
Obesity is a major problem throughout the world. Surveys consistently show that people are concerned by weight and its health related implications, and for that most individuals are making a concerted effort to either maintain or lose weight. (Serdula et al. 1999; Scott et al. 2006).
Today the major goal of diabetes management is control of blood glucose. So the consumers have a free choice of food products. They must choose the right food to comply with dietary recommendations and at the same time the food industry can considerably contribute to this change by providing adapted food products. This led food industry to discover several forms of alternative intense sweeteners, which have made possible to offer consumer the sweet taste without the calories.
Sugar cannot simply be replaced by these type of intense sweetener because the question of bulk, quality, intensity of sweetness and physical characteristics. Due to these features, rare sugars are desirable for low calorie, as well as bulk sweetener. These sugars tend to have desirable sweetness but are not metabolized in the human body and therefore do not provide calorie intake.
Artificial sweetener
The sensory properties of food is highly influenced by the sensory properties like taste smell texture and appearance (Sorensen et al. 2003). The selection and consumption of food in man play a crucial role in the regulation of human appetite and nutrient intake. A sweetener is a food additive, which mimics the effect of sugar on taste. Therefore, they are called sugar substitutes. Consumers often select those foods, which are composed of low calorie sweetener because they want the taste of sweetness without added calories. The dietary option that such product provides may be especially helpful in the management of obesity or diabetes mellitus.
One group of such sweeteners consists of substances with a very intense sweet taste and is used in small amount to replace the sweetness of a much higher amount of sugar. The sweeteners of this type currently approved for use in the United States are- Aspartame, Acesulfane-K, Neotame, Saccharin, Sucralose, Cyclamate and Alitame. Table 1 summarizes some information about high intensity sweeteners (Godshall 2007).
Table 1.
Properties of high intensity sweeteners*
| Sweetener | Other Names | Sweetness** | Comments |
|---|---|---|---|
| Acesulfame K | Ace K; Sunette; Sweet & Safe; SweetOne | 200 | N-sulfonyl amide structure; approved 2003. |
| Alitame | – | 2000 | Aspartame amide analog (GRAS pending since 1986); limited approval in 4 countries (Mexico, Australia, New Zealand, China) |
| Aspartame | NutraSweet; Equal | 180–200 | Aspartyl-phenylalanine methyl ester; approved 1981 . |
| Cyclamate | Sucaryl, Sugar Twin | 30–50 | Sulfamic acid Na or Ca salt; approved in 50 countries; not USA |
| Neotame | – | 7,000–13,000 Avg 8,000 | Derivative of aspartame, more stable than aspartame; approved 2002 |
| Saccharin | Sweet’n’ Low | 300 | N-sulfonyl amide structure; the first low-cal sweetener |
| Sucralose | Splenda | 600 | Trichlorinated derivative of sucrose; approved 1998. |
** Sucrose = 1 (Relative to a 10% sucrose solution) Different numbers indicate effect in different foods
[Source: Godshall M A (2007)]
Aspartame
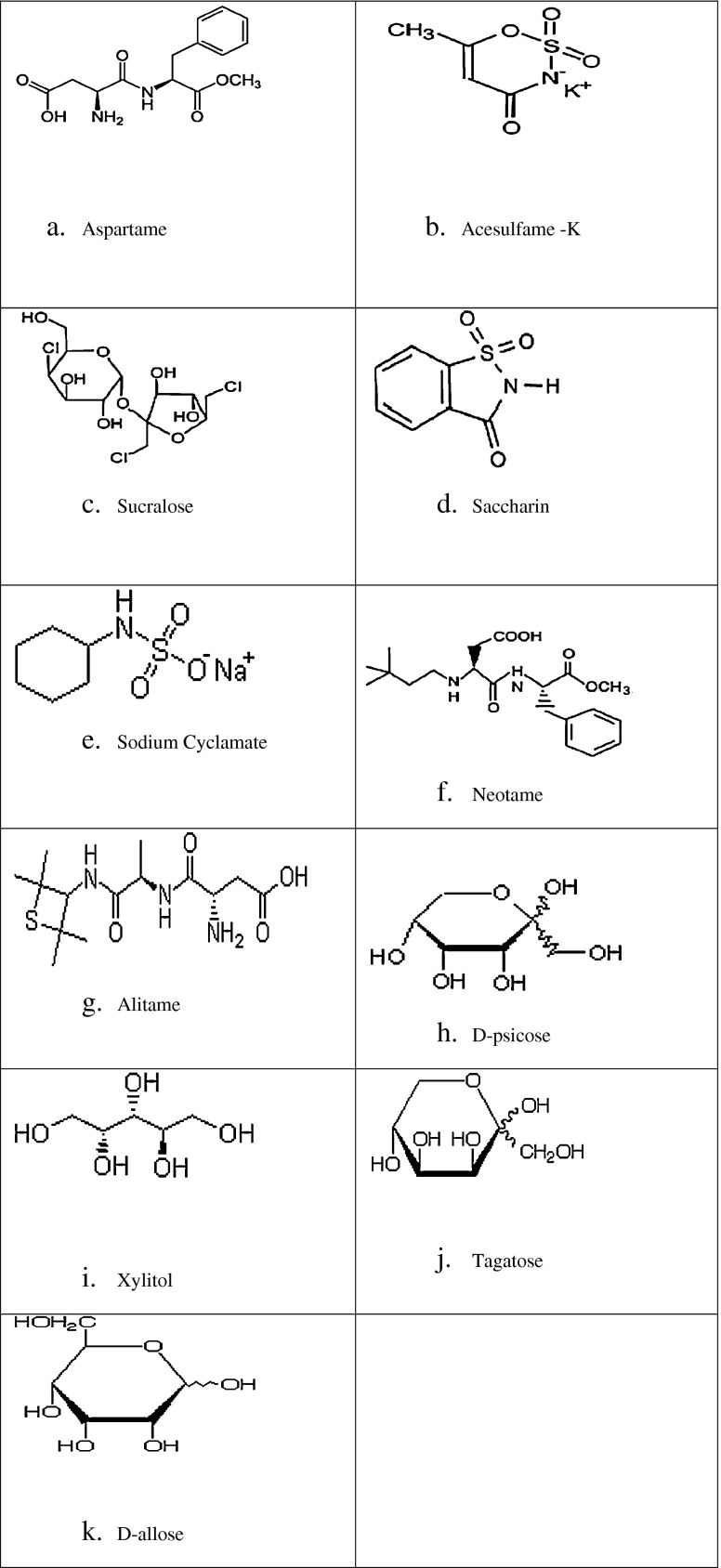
Aspartame (Fig. 1a) was discovered in 1965 by James Schlatter a chemist (Mazur et al. 1970). It is an artificial, non-saccharide sweetener, L-aspertyl-L phenylalanine methyl ester that is a methyl ester of the dipeptide of the amino acids aspartic acid and phenylalanine. Under strongly acidic or alkaline conditions, aspartame may generate methanol by hydrolysis. Under more severe conditions, the peptide bonds are also hydrolyzed, resulting in the free amino acids. It is slightly soluble in water, (about 3gm per 100ml, pH 3 at room temp.). The solubility increases with higher or lower pH as well as with increased temperature. In aqueous solution the relationship between pH and stability of aspartame is a bell-shaped curve with the maximum stability at pH 4.3 (Mazur and Ripper 1979).
Fig. 1.
Chemical structure of low calorie Sweeteners
This sweetener is marketed under a number of trademark names including Equal, Nutrasweet, and Candere and has a good clean sweet taste but its time-intensity profile differs from sucrose.
Synthesis
Chemical synthesis of aspartame involves the coupling of the two amino acid units having appropriate functional group protection with conventional synthetic reagents. The two major processes are known as the Z- and F- processes named after the protecting group used on the aspartyl group. Both of these processes produce some β-coupled products together with the desired α-aspartame.
The Z-process mainly involves the dehydration of the benzyloxycarbonyl-L-aspartic acid with acetic anhydride. The anhydride is then coupled with the methyl ester of L-phenylalanine in toluene to give a mixture of benzyloxy carbonyl α-and β aspartames. The protecting groups are removed by hydrogenolysis and resulting mixture of aspartame isomer yield aspartame upon crystallization (Ager et al. 1998).
The F-process involves the protection of the amino group of aspartic acid with a formyl group and concomitant dehydration to form anhydride. The anhydride is then coupled either with L-phynylalanine or its methyl ester (Hill et al. 1991) and the formyl group removed by acid hydrolysis. The resultant mixture of α and β products are subjected to the esterification conditions of aqueous methanol and preferentially crystallizes out from this mixture and is then neutralized to yield aspartame.
The application of biotechnology and biocatalysts towards the synthesis of the aspartame has been extensively explored. Due to the presence of its dipeptide structure many variations of reverse proteolysis that employ both kinetically and thermodynamically controlled approaches has been investigated with different enzymes and under various reaction condition. Two Japanese companies have reported one formation route to produce aspartame directly by incubating microorganisms with L- aspartic acid and methyl ester of phenylalanine (Ager et al. 1998).
Metabolism and health aspect
Aspartame is a low calorie sweetener used to sweeten a variety of low and reduced calorie foods and beverages including low calorie tabletop sweetener as well as for use in gum, breakfast cereal and other dry products.
Aspartame provides energy of 4 calories per gram. Aspartame is unstable if subjected to prolong heating and therefore cannot be used in baking or cooking. It also decomposes in liquids during storage.
Upon ingestion, aspartame breaks down into natural residual components, including aspartic acid, phenylalanine, methanol and further break down products including formaldehyde, formic acid and diketopiperazine (George et al. 2010; Trocho et al. 1998). Each of which then metabolized just as it would be if derived from other dietary sources and are safe as consumed in normal diets.
Aspartame has been the subject of controversy regarding its safety since its initial approval by the U.S. Food and Drug Administration (FDA) in 1974 (Magnuson et al. 2007).
High level of the naturally occurring essential amino acid phenylalanine is a health hazard to those born with phenylketonuria (PKU) a rare inherited disease. So the phenylalanine level statement or aspartame—sweeten products is for their benefit and has no relevance for general population. Various scientific researches concluded that the effects of aspartame are likely to be attributable to methanol or its metabolites, evidence indicating that fruits and vegetables also contain high level of methanol than aspartame sweetened food and beverage do. But high intake of fruits and vegetables are associated with decrease rather than increase in cancer risk (Heber 2004). Carcinogenicity studies of aspartame were conducted by Nalt Toxicological Programme (NTP) in 2 strains of transgenic mice, and it was concluded that aspartame exposure was associated with increase in cancer in either male or female mice (NTP 2005). Based on government research reviews and recommendations from advisory bodies such as European Commissions Scientific Committee on Food and joint FAO/WHO expert committee on food additives, aspartame has been found to be safe for human consumption by more than ninety countries worldwide (Magnuson et al. 2007).
Acesulfame—k
Acesulfame—k (Fig. 1b) has been developed as sweetener by Hoechst (Clauss and Jensen 1970). This high intensity sweetener is potassium salt of 6-methyl-123-axathiazine-4(3H)-one 2,2-dioxide with molecular formulaC4H4KNO4S and molecular weight of 201.24. It is a white crystalline powder, approximately 120 times sweeter than sucrose and has high water solubility (Rymon Lipinski 1991).
Acesulfame—k is heat stable, so can be used in cooking and baking (Nabors 2002). It may have a bitter after taste when used alone to sweeten food or beverage (Horne et al. 2002) Ace-k is often blended with other sweetener (usually sucralose or aspartame) whereby each sweetener masks the other’s after taste and exhibit a synergistic effects by which the blend is sweeter than its components.
Synthesis
Early methods for Ace-k synthesis used chlorosulfonyl or flurosulfonyl isocyanate with propyne acetone (Clauss and Jensen 1970) and with other chemicals give N-chloro or N-(fluro-sulfonyle) acetoacetamide, which is then cyclized by metabolic potassium hydroxide to give Ace-k. Alternative method involves the treatment of acetoacetamide with at least two equivalents of sulfur trioxide. This results in formation of N-sulfoacetoacetamide, which is then dehydrated by sulfur trioxide to form oxathiaazinone dioxide. Neutralization with potassium hydroxide gives Ace-k. (Clauss et al. 1993).
Metabolism and health aspect
Acesulfame—k is not metabolized in the human body, thus it provides no calories and does not influence potassium intake despite its potassium content (ADA 2004). In 1988 USFDA approved the use of Ace-k in a variety of dry food products and in alcoholic beverages. In 2003 the agency approved its use as a general-purpose sweetener (USFDA 2003). One breakdown product of ace-k is acetoacetamide (George et al. 2010) known to be toxic if consumed in very large doses because human exposure to this breakdown product would be negligible. The USFDA concluded that no further testing of it was necessary.
Sucralose
Sucralose (Fig. 1c) was discovered in 1976. This non-nutritive sweetener is made from sucrose by a process that substitutes 3 chloride atoms for 3 hydroxyl groups on the sucrose molecule (FDA 2006). Sucralose is 450–650 times sweeter than sucrose, has a pleasant sweet taste and its quality and time intensity profile is very close to that of sucrose (Arora et al. 2009). It has a moderate synergy with other nutritive and non-nutritive sweeteners. (Beyts et al. 1995).
It is very much soluble in water and is stable over a wide range of pH and temperature. It does liberate HCl when stored at high temperature and produce some kind of discoloration (Beyts et al. 1995).
Synthesis
The synthesis of sucralose involves a series of selective protection and deprotection steps so that the 4-hydroxyl group can be converted to a chloro atom with inversion of configuration. Treatment of the free hydroxyl groups with sulfuryl chloride produce trichlorodisaccharide which is then deprotected to give the sucralose (Ager et al. 1998). The use of enzymes or microbial cultures to augment synthetic organic chemistry and carry our selected functionalization of complex molecule has been widely documented in the growing field of biocatalysis (Wong and Whitesides 1994).
Metabolism and health aspect
Although sucralose is made from sugar, the human body does not recognize it as a sugar and does not metabolize it therefore it provides no calories. The bulk of sucralose ingested does not leave the gastrointestinal tract and is directly excreted in the feces while 11–27% of it is absorbed (Knight 1993). The amount that is absorbed from the gastro intestinal tract is largely removed from the blood stream by the kidneys and eliminated in the urine. As it is an organo chloride and some of which are known to have significant toxicity (Patel et al. 2006) but sucralose is not known to be toxic. In addition sucralose does not breakdown or dechlorinate. In determining the safety of sucralose, the FDA reviewed data from more than 110 studies in human and animals. Many of the studies were designed to identify possible toxic effects including carcinogenic reproductive and neurological effects but no such effects were found. Food and Drug Administration (FDA) approval is based on the findings that sucralose is safe for human consumption. U.S. Food and Drug Administration (USFDA) approved sucralose as a general-purpose sweetener. The acceptable daily intake (ADI) for sucralose in US is 5mg/kg body weight/day. The estimated daily intake for percentile consumers as calculated by USFDA is 1.6mg/kg body weight/day (USFDA 1999).
Saccharin
Saccharin (Fig. 1d) was discovered by Remson and Fahlberg in 1878 at the Johns Hopkings University, Baltimore. It is a non-nutritive sweetener of 1,2-benzoisothiazol-3-(2H) on 1,1 dioxide. Saccharin has an unpleasant bitter or metallic off taste. As the parent compound is only sparingly soluble in water, the sweetener is usually used as the sodium or calcium salt. Both salts are highly water soluble, 0.67 gms/ml.of water at room temperature (Priebem and Kauffman 1980). It is about 300 times sweeter than sucrose.
Synthesis
Chemical synthesis of saccharin involves the oxidation of the o-toluenesulfonamide with variety of agents like potassium permanganate (Tarbell and Tarbell 1978), chromic acid, electrochemically (Bennett et al. 1992) etc. to the corresponding carboxylic acid. The ortho isomer is dehydrated to give the sweetener. (Bennett et al. 1992; Drasar et al. 1972). Another process involves diazotization of methyl anthranilate and then treatment of the diazonium salt with sulfur dioxide and chloride gas to give the sulfonyl chloride which is then treated with ammonia to give saccharin (Tarbell and Tarbell 1978).
Metabolism and health aspect
The FDA tried to ban saccharin in 1977 because animal studies had showed that it caused cancer in rat (mainly bladder cancer). Many studies have since been performed on saccharin. No study has ever shown a clear casual relationship between saccharin consumption and health risks in human at normal doses. Though some studies have shown a correlation between consumption and cancer incidence (Weihrauch and Diehl 2004). Saccharin is currently permitted for use under an interim regulation that specifies the amounts of saccharin permitted in beverages, processed food, and sugar substitute and requires that the product level must state saccharin in the ingredient declaration and specify the amount used (Kroger et al. 2006).
Cyclamate
Cyclamate (Fig. 1e) was discovered in 1937. It was used as a low calorie sweetener in the United States in 1950s and 1960s. It is a salt of cyclohexylsulfamic acid. Sodium cyclamate is used as non- nutritive sweetener and the analogous calcium salt used specially in low sodium diets. Cyclamate is 30 times sweeter than sucrose. It has a bitter off taste, but has good sweetness synergy with saccharin. It is soluble in water and its solubility can be increased by preparing the sodium or calcium salt (Bopp et al. 1986).
Synthesis
This process begins with the trisaccharide raffinose followed by chemical chlorination to form tetrachloro raffinose TCR. This TCR is then enzymatically treated with a galactosidase to move the 6-chloro-6-deoxygalactosyl moieties from the 6th position to yield cyclamate (Bennett et al. 1992). There are another two methods available for synthesis of saccharin like bioorganic synthesis (Drasar et al. 1972) and regioselective deacylation.
Metabolism and health aspect
Cyclamate itself shows very low toxicity but is metabolized by the gut bacteria to cyclohexylamine which shows greater toxicity (Bopp et al. 1986) because of the nature of cyclamate metabolism. It would be inappropriate to assume that the total daily intake of cyclamate is metabolized to cyclohexylamine. The acceptable daily intake (ADI) for cyclamate was calculated by both the scientific committee of food (SCF) and the joint expert committee on food additives (JECFA) based on the “no observed adverse effect level” (NOAEL). For cyclohexylamine in rats assuming that 18.9% of the daily intake of cyclamate is metabolized to cyclohexylamine each day (SCF 2000). The plasma concentrations of cyclohexylamine following cyclamate intake will depend on both the extent of metabolism by the intestinal flora and the extent of elimination of cyclohexylamine from the circulation.
Scientific research on cyclamate is continuing. Recent studies have provided new data on the extent to which individuals convert cyclamate to cyclohexylamine during long term consumption (Renwick et al. 2004). This study gives first true indication of possible exposure to cyclohexylamine from cyclamate metabolism in humans over a period that is toxicologically relevant to the establishment of ADI for cyclamate.
Neotame
Neotame (Fig. 1f) is a derivative of a dipeptide compound of the amino acids - aspertic acid and phenylalanine. Neotame has been developed as a sweetener with a high degree of sweetness and is obtained by N-alkylating aspartame. Its degree of sweetness varies according to the kind of food and blend composition. It is 7000 to 13,000 times and about 30 to 60 times sweeter than sugar and aspartame respectively (Prakash et al. 2002).
Neotame is an odorless white to gray-white powder with a strong sweetness and is readily soluble in alcohols and slightly soluble in water. The 0.5% aqueous solution of neotame is weakly acidic (pH 5.8) (Prakash et al. 2002).
Synthesis
A chemoenzymatic method used for preparing N-[N-(3-3dimethylbutyl)-L-a aspertyl]-L-phenylamine 1- methyl ester via the enzymatic regioselective hydrolysis of neotame ester using lipases or estarages (Prakash and Zhao 2001).
Another method involve the hydrogenation of L-α-aspartyl –L- phenylalanine I methyl ester and 3–3 dimethylbutyraldehyde produced insitu by the hydrolysis or cleavage of a 3-3-dimethylbutyraldehyde precursor (Prakash 2007).
Metabolism and health aspect
Neotame is rapidly metabolized, completely eliminated and does not accumulate in the body. The major metabolic pathway of neotame is hydrolysis of the methyl ester by esterase which is present throughout the body. This yields deesterified neotame, the major metabolite and a significant amount of methanol. Due to the presence of the 3-3-di-methylbutyl group, peptidases which would typically break the peptide bond between the aspartic acid and phenylalanine moieties are essentially blocked, thus reducing the availability of phenylalanine. The amount of methanol derived from neotame is exceedingly small (Neotame 2002). Neotame was approved by the USFDA as a general purpose sweetener in July 2002 (USFDA 2002). It has also been favorably evaluated by JECFA (JFECFA 2004) which established an ADI of 2mg/kg body weight/day. The ADI for neotame in the US is 18 mg/person/day (USFDA 2002).
Alitame
Alitame (Fig. 1g) is an intense sweetener with sweetness potency 200 times greater than that of sucrose. It is a dipeptide of L-aspartic acid and D-alanine with a terminal N-substituted tetra methylthietanyl-amine moiety.
Synthesis
Alitame is prepared by a multi step synthesis involving the reaction between two intermediates (S)-[2-5-dioxo-(4-thiazolidine)] acetic acid and (R) –2- amino-N-(2,2,4-4-tetramethyl-3-thietanyl) propanamides. The final product is isolated and purified by crystallization of an alitame −4-methylbenzenesulfonic acid adduct followed by additional purification steps and finally recrystallization from water (Peter et al. 2002).
Metabolism and health aspect
Alitame is readily absorbed in the GI tract and then rapidly metabolized and excreted. It has two main components, aspartic acid and alanine amide. The aspartic acid component is metabolized normally and the alanine amide passes through the body with minimal metabolic changes. In humans the glucoronic derivative of D-alanine tetramethylthietane amide is the major urinary metabolite. JEFCA reviewed safety data on alitame in 2002. The committee concluded that there was no evidence that alitame is carcinogenic. An ADI of 0–1 mg/kg body weight was allocated on the basis of the NOAEL of 100mg/kg body weight/day to an 18 month study in dogs. Alitame has already been approved in Mexico, Colombia and China as well as Australia and New Zealand (Kroger et al. 2006).
Rare sugar
Rare sugars, which are defined as monosaccharides and their derivatives that are rare in nature (Izumori 2002) have recently attracted a great deal of attention mainly concerning their application. This could provide an alternative to the other sweetener due to its lack of calories. Rare sugars are either not metabolized by the body or metabolized to a lesser extent than natural sugar. Due to these features, rare sugars are well tolerated by diabetes patients. Other advantage of rare sugar is the absence of any objectionable after taste (Zakaria 2001).
D-psicose
D-psicose (Fig. 1h) (D-ribo-2 hexulose), a C-3 epimer of D-fructose is a rare sugar present in small quantities in commercial mixtures of D-glucose and D-fructose obtained from the hydrolysis of sucrose or isomerization of D-glucose (Green and Perlin 1968). D-psicose has 70% of the sweetness of sucrose and has a higher solubility that makes it easy to use for food processing.
It has been reported that the addition of D-psicose in food products improve the gelling behavior and flavor as well as it increases the antioxidant property of the food products (Sun et al. 2006; Sun et al. 2007). Furthermore, food products containing D-psicose maintain a high level of antioxidant effect over a long period of storage, which is able to delay the onset of lipid auto-oxidation and extend the food storage time (Sun et al. 2008). It gives proper sweetness, smooth texture, desirable mouthfeel and great self-stability to food products.
Synthesis
D-psicose has previously been produced by chemical methods from D-fructose using catalytic action of molybdate ions in an acidic aqueous solution (Bilik and Tihlarik 1973) it is also sometimes prepared by boiling D-fructose in ethanol and triethylamine (Doner 1979). All the chemical methods are insufficient in terms of large-scale production.
An improved process for the mass production of D-psicose was developed using D-tagatose −3 epimerase bioreactor. D fructose solution (60%, pH 7.0) was passed at 45°C through a column filled with immobilized D-tagatose-3-epimerage (D-TE) which was produced using recombinant E.Coli. and 25% of the substrate was converted to D-psicose. After epimerization, the substrate D-fructose was removed by treatment with baker’s yeast. The supernatant was concentrated to syrup by evaporation under vacuum and D-psicose was crystallized with ethanol (Takeshita et al. 2002).
Another work was done for mass production of D-psicose using a non characterized gene from Agrobacterium tumifaciens which increase the production 586 fold higher than that of D-TE. The enzyme is D-psicose-3-epimerase. This finding has considerable importance in D-psicose production (Kim et al. 2006).
Metabolism and health aspect
An animal study on the suppression of increase in plasma glucose concentration with D-psicose found significant drop in plasma glucose concentration when maltose and sucrose were used as substrates, but no significant drop when glucose and soluble starch were used as substrate (Matsuo 2006). Another animal study proposed that D-psicose inhibited the hydrolysis of maltose with α-glucosidase in rats (Matsuo and Izumori 2006). The doses of D-psicose at 5g (around 1/15 of carbohydrate intake) would be the minimum effective dose for suppressing the elevation of plasma glucose and insulin concentration for 75g maltodextrin. This study confirmed the improving effects of glucose tolerance. D-psicose is expected to serve as a food material with low glycemic index.
Another study demonstrated that D-psicose inhibits intestinal sucrase and maltase activities in an uncompetitive manner and suppress the plasma glucose increase after sucrose and maltose ingestion. Thus D-psicose may be useful in preventing postprandial hyperglycemia in diabetic patient when food containing sucrose and maltose are ingested (Lida et al. 2008).
Xylitol
Xylitol (Fig. 1i) is a naturally occurring sugar. Xylitol is a five carbon sugar that tastes and looks exactly like sugar.
Synthesis
The synthesis of xylitol from natural product is based on the pentosans occurring in many plants. Xylan, a constituent of pentosan, is a polysaccharide that can be hydrolyzed in to D-xylose. Xylitol can be synthesized by hydrogenation of xylose (Zakaria 2001). Xylitol also can be produced from D-glucose by three steps (Povelainen and Miasnikov 2006). Xylitol production from yeast is an alternative to chemical studies (Lachke and Jeffries 1986).
Metabolism and health aspect
Xylitol metabolises easily and independently from insulin in humans and produces very low amount energy. Xylitol has a recognized glycemic index of 8 and have a caloric value of 2.4 calories/gm. (Sellman 2003)
Xylitol is non-fermentable and therefore cannot be converted to acids by oral bacteria, thus it helps to restore a proper alkaline/acid balance in mouth. Several clinical trials have indicated that xylitol products (chewing gum) are more effective in reducing dental caries.
In 1996, the joint expert committee on Food Additive (JECFA) confirmed the safety of xylitol for human consumption and allocated xylitol an ADI of ‘Not specified’. The scientific committee for food of the European Union (EU) also determined xylitol ‘acceptable’ for dietary uses (Xylitol 2009).
Tagatose
The ketohexose D-tagatose (Fig. 1j) is structurally similar to D-fructose except for an inverted optically active center. Because of its excellent taste and bulk properties, combined with a possibly very low energy value, D-tagatose has potential for use as a sweetener (Livesey and Brown 1996; Szepesi and Michaelis 1986).
Synthesis
D-tagatose is produced from lactose in two-step. Firstly, lactose is converted to glucose and galactose by hydrolysis and then galactose is isomerizd to D-tagatose by adding calcium hydroxide (Calorie control council 2007).
Metabolism and health aspect
The metabolism of tagatose is identical as fructose but it is incompletely absorbed. The study on small-bowel absorption of tagatose concludes that 15g tagatose/day had a high apparent absorption in the small intestine of humans (Normen et al. 2001). The major part of ingested tagatose is fermented in the colon by indigenous microflora, resulting in the production of short chain fatty acid. The short chain fatty acids are absorbed almost completely and metabolized.
Thus it can be concluded that D-tagatose a carbohydrate with physiological properties potentially valuable for the control of both body weight and symptoms of the metabolic syndrome as seen in diabetics (Livesey and Brown 1996).
Substances such as glucose and especially fructose that promote lopogenesis (Szepesi and Michaelis 1986) and have high glycosylation indices(Mc Phearson et al. 1988) could be replaced with D-tagatose with lower fat accumulation (Levin et al. 1995), lower glycosylation index (Bunn and Higgins 1994) and strong antidiabetic effects founds in rats (Szepsi 1996).
D-allose
D-allose (Fig. 1k), a cis-aldohexose is a non caloric sweetening and bulking agent which have good antioxidant properties. The mass production of D-allose mainly achieved from D-psicose in a batch reaction by crude recombinant L-rhamnose isomerase cross linked with gluteraldehyde (Menavuvu et al. 2006). Studies on D-allose supplementation on Dahl salt sensitive hypertensive rats and spontaneously hypertensive rats suggests the possibility of D-allose supplementation for prevention of salt sensitive hypertension. D-allose has been reported to inhibit segmented neutrophil production and lower platelet count in vivo without other significant detrimental clinical effects in rats (Arnold and Silady 1997). D-allose is also used as potential inhibitor of various glycosides.
Other sweetener
Table 2 summarizes the information about some nutritive sweetener known as sugar alcohol. They are highly soluble and non hygroscopic. The sugar alcohols are non-reducing, temperature stable and more resistant to browning reactions than sucrose (Godshall 2007).
Table 2.
Properties of nutritive sweeteners
| Sweetener | Sweetness | Cal/g | GI* | Type | Source |
|---|---|---|---|---|---|
| Erythritol | 0.7 | 0.2 | 0 | Sugar alcohol | Fermentation of glucose by Moniliella pollinis, a fungus |
| Glucose | 0.5 | 4.0 | 100 | Monosaccharide | Hydrolyzed starch |
| Fructose | 1.5–1.8 | 4.0 | 19–23 | Monosaccharide | Enzymatically isomerized glucose |
| HF CS | 1–1.2 | 4.0 | 60–65 | Mixed glc/fru | Hydrolysis of corn starch and isomerization of glucose |
| HSH | 0.5–0.7 | 2–4 | varies | Mixed polyols | Hydrogenated partially hydrolyzed starch |
| Isomalt/Isomaltitol/Palatinit ™ | 0.45–0.65 | 2.0 | 2 | Sugar alcohol | Hydrogenated isomaltulose; equal mixture of gluco-sorbitol and gluco-mannitol |
| somaltulose/Palatinose ™ | 0.3–0.4 | 2.0 | 32 | Disaccharide | Enzymatic isomerization of sucrose with Protoaminobacter rubrum; GRAS March 2006; a sucrose isomer |
| Lactitol | 0.35–0.4 | 2.4 | 6 | Sugar alcohol | Hydrogenated lactose |
| Lactose | 0.2–0.4 | 4.0 | 46 | Disaccharide | Milk sugar |
| Lactulose | 0.6 | 0.2 | 0 | Disaccharide | Alkaline isomerization of lactose when milk is heated; prebiotic |
| Leucrose | 0.5 | 2.0 | Disaccharide | Dextransucrase action on sucrose and fructose; dextran by- product; a sucrose isomer | |
| Maltitol | 0.5–0.9 | 3.0 | 35–52 | Sugar alcohol | Catalytic hydrogenation of high maltose corn syrup |
| Maltose | 0.4 | 4.0 | 105 | Disaccharide | Enzymatic hydrolysis of starch; long time use |
| Maltulose | 0.3–0.42 | Disaccharide | Alkaline isomerization of maltose; a sucrose isomer; little used | ||
| Mannitol | 0.5–0.72 | 1.6 | 0 | Sugar alcohol | Hydrogenation of invert or fructose; new fermentation process |
| Sorbitol | 0.6 | 2.6 | 9 | Sugar alcohol | Hydrogenated glucose |
| Sucrose | 1.0 | 4.0 | 61–65 | Disaccharide | Cane and beet |
| Tagatose | 0.92 | 1.5 | 0 | Galactose isomer | Hydrolyzed lactose; galactose converted by alkali; GRAS 2001 |
| Trehalose | 0.5–0.7 | 3.6 | 45–50 | Disaccharide | Patented 2-enzyme process from corn starch; GRAS 2000 |
| Trehalulose | 0.5–0.7 | Disaccharide | Sucrose isomer; by-product of palatinose production | ||
| Xylitol | 1.0 | 3.0 | 7–13 | Sugar alcohol | Hydrogenated xylose |
*GI = Glycemic Index
[Source: Godshall M A (2007)]
Erythritol and other polyols
Erythritol is four carbon sugar alcohol (or polyol). It is manufactured by fermentation from glucose and sucrose by Trichosporonoides megachiliensis. It has a sweetness approximately 60–80% of sucrose. Polyols are low digestible carbohydrates which are poorly absorbed from the small intestine. These are also used for their humectant and bulking properties. Excessive consumption has a laxative effect due to unabsorbed polyol increasing the osmotic potential of the gut lumen and other gastrointestinal effect. Erythritol is considered to be of low toxicity. It has been assessed by JECFA which assigned it an ‘ADI not specified’. Studies with human have shown them that ingested doses of erythritol is absorbed from the small intestine and excreted in the urine unchanged (Munro et al. 1998). Another polyol like sorbitol show property close to sugar (Chetana et al. 2010).
Trehalose
Trehalose is a non-reducing disaccharide that consists of two glucose units linked by a 1,1-glycosidic bond. It is with a relative sweetness of 40–45% that of sucrose. Trehalose is produced directly from food-grade starch by a multienzymatic process. This disaccharide is enzymatically hydrolyzed by the enzyme trehalase in the small intestine in to two glucose subunits which are subsequently absorbed and metabolized in a manner similar to maltose. Present study reports that adding trehalose to dehydrated pear cubes could improve aroma retention by 15% (Komes et al. 2006). It has also added advantage of being an antioxidant.
Several safety studies on trehalose have been evaluated by JECFA, 2001 and allocated an ADI of ‘not specified’. Trehalose is approved in Japan, Korea, Taiwan, and UK (Kidd and Devorak 1994).
Stevia rebaudiana
Stevia is a natural herb. This zero calorie sweetener mainly containing steviol glycoside which is 10–15 times sweeter than sucrose. Human body does not metabolize these sweet glycosides, so obtains no-calories from stevia. Unlike artificial sweetener, the sweet glycoside does not break down in heat which makes stevia an excellent sweetener for cooking and baking. Studies have indicated that stevia tends to lower the elevated blood pressure. It also significantly improves nutritional status of diabetic patients (Kochhar et al. 2008).
Conclusion
The number of people suffering from diabetes, obesity, hypertension, and heart disease is increasing every year. Increasing amounts of sugars in food, sweets, soft drinks and so on have raised some concern about their health effects. So nowadays artificial sweetener are receiving much more attention. But it gets bad reputation due to their safety issue. On the other hand in spite of demand for rare sugars, their commercial availability, application and usefulness is negligible as they are expensive to prepare and unavailable in nature. So research is required to make natural sugars having the desired quantities of sweetness, low caloric value, and least observed physiological effects.
Acknowledgement
The research work is financially supported by the Centre for Advanced studies (CAS I) programme under University Grants Commission (UGC), Govt. of India and Dept. of Food Processing Industries & Horticulture, Govt. of West Bengal, India, Some facilities have been provided by the centre for Medicinal Food & Applied Nutrition of Jadavpur University, India.
References
- [ADA] American Dietetic Association, Position of the American Dietetic Association: use of nutritive and nonnutritive sweeteners. J Am Diet Assoc. 2004;104:255–275. doi: 10.1016/j.jada.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Ager DJ, Pantaleone DP, Henderson SA, Katritzky AR, Prakash I, Walters DE. Commercial, Synthetic Nonnutritive Sweeteners. Angew Chem Int Edit. 1998;37:1802–1817. doi: 10.1002/(SICI)1521-3773(19980803)37:13/14<1802::AID-ANIE1802>3.0.CO;2-9. [DOI] [Google Scholar]
- Arnold E C, Silady P J (1997) Use of D-allose as an immunosuppressive agent. Patentgenious US Patent 5620960. http://www.patentgenius.com/patent/5620960.html. Accessed 5 March 2010
- Arora S, Singh VP, Sharma V, Wadhwa BK, George V, Singh AK, Sharma GS. Analysis of sucralose and its storage stability in barfi. J Food Sci Technol. 2009;46:114–117. [Google Scholar]
- Bennett C, Dordick JS, Hacking AJ, Cheetham PSJ. Biocatalytic synthesis of disaccharide high-intensity sweetener sucralose via a tetrachlororaffinose intermediate. Biotechnol Bioeng. 1992;39:211–217. doi: 10.1002/bit.260390213. [DOI] [PubMed] [Google Scholar]
- Bilik V, Tihlarik K. Reaction of Saccharides catalyzed by molybdate ions. Epimerization of ketohexoses. Chem Zvesti. 1973;28:106–109. [Google Scholar]
- Bopp BA, Sonders RC, Kesterson JW. Toxicological aspects of cyclamate and cyclohexylamine. Crit Rev Toxicol. 1986;16:213–306. doi: 10.3109/10408448609037465. [DOI] [PubMed] [Google Scholar]
- Bunn HF, Higgins PL. Reactions of monosaccharides with proteins- possible evolutionary significances. Science. 1994;213:222–224. doi: 10.1126/science.12192669. [DOI] [PubMed] [Google Scholar]
- Beyts P K, Lillard D W, Batterman C K (1995) A sucralose compositon. Patentstrom US patent 5380541. http://www.patentstorm.us/patents/5380541/fulltext.html. Accessed 21 August 2009
- Calorie control Council (2007) http://www.caloriecontrol.org/tagatose.html. Accessed 12 February 2009
- Chetana R, Ravi R, Yella Reddy S. Effect of processing variables on quality of milk burfi prepared with and without sugar. J Food Sci Technol. 2010;47:114–118. doi: 10.1007/s13197-010-0005-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clauss K, Jensen H. Cycloadditionen von halogensulfonylisocyanaten an acetylene. Tetrahedron Lett. 1970;11:119–122. doi: 10.1016/S0040-4039(01)97654-3. [DOI] [Google Scholar]
- Clauss J, Schmidt RK, Spiess HW. Determination of domain sizes in heterogeneous polymers by solid-state NMR. Acta Polym. 1993;44:1–17. doi: 10.1002/actp.1993.010440101. [DOI] [Google Scholar]
- Doner LW. Isomerization of D-fructose by base - lequid-cromatographic evaluation and the isolation of D-psicose. Carbohyd Res. 1979;70:209–216. doi: 10.1016/S0008-6215(00)87101-3. [DOI] [Google Scholar]
- Drasar BS, Renwick AG, Williams RT. The role of the gut flora in the metabolism of cyclamate. Biochem J. 1972;129:881–890. doi: 10.1042/bj1290881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [FDA] Food and Drug Administration (2006) Food labeling: health claims; dietary noncariogenic carbohydrate sweeteners and dental caries. Fed Reg 71:15559–15564 http://frwebgate.access.gpo.gov/cgi-bin/getpage.cgi?position=all&page=15559&dbname=2006_register. Accessed 10 June 2010 [PubMed]
- George V, Arora S, Wadhwa BK, Singh AK. Analysis of multiple sweeteners and their degradation products in lassi by HPLC and HPTLC plates. J Food Sci Technol. 2010;47:408–413. doi: 10.1007/s13197-010-0067-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godshall MA. The Expanding World of Nutritive and Non-Nutritive Sweeteners. Sugar J. 2007;69:12–20. [Google Scholar]
- Green GM, Perlin AS. O-Isopropylidene derivatives of D-allose (D-psicose) and D-erythro-hexopyranose-2,3-dilulose. Can J Biochem Cell B. 1968;46:765–770. doi: 10.1139/o68-117. [DOI] [PubMed] [Google Scholar]
- Heber D. Vegetables, fruits and phytoestrogens in the prevention of diseases. J Postgrad Med. 2004;50:145–149. [PubMed] [Google Scholar]
- Hill J B, Gelman Y, Dryden Jr HL, Erickson R, Hsu K, Johnson MR (1991) One-pot process for the preparation of α-L-aspartyl-L-phenylalanine methyl ester hydrochloride. JustiaPatents US Patent 5053532. http://patents.justia.com/1991/05053532.html. Accessed 15 June 2010
- Horne J, Lawless HT, Speirs W, Sposato D. Bitter taste of saccharin and acesulfame- K. Chem Senses. 2002;31:8–27. doi: 10.1093/chemse/27.1.31. [DOI] [PubMed] [Google Scholar]
- Izumori K. Bioproduction strategy for rare hexose sugars. Naturwissenschaften. 2002;89:120–124. doi: 10.1007/s00114-002-0297-z. [DOI] [PubMed] [Google Scholar]
- [JFECFA] Joint FAO/WHO Expert Committee on Food Additives (2004) Sixty-third meeting 8 to 17 June. Geneva WHO. http://www.who.int/ipcs/publications/ jecfa/en/Summary63final.pdf. Accessed 3 January 2006
- Kim HJ, Hyun EK, Kim YS, Lee YJ, Oh DK. Characterization of an Agrobacterium Tumifaciens D-psicose 3-epimerase that converts D-fructose to D-psicose. Appl Environ Microbiol. 2006;72:981–985. doi: 10.1128/AEM.72.2.981-985.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komes D, Lovric T, Ganic KK. Aroma of dehydrated pear products. Food Sci Technol. 2006;40:1578–1586. [Google Scholar]
- Kochhar A, Dhindsa S, Sachdeva R. Effect of Stevia Leaf (Stevia rebaudiana) Powder Supplementation and Nutrition Counselling on Anthropometric Parameters and Gain in Knowledge of the Subjects. Ethno-Med. 2008;2:107–113. [Google Scholar]
- Kroger M, Meister K, Kava R. Low-calorie Sweeteners and Other Sugar Substitutes: A Review of the Safety Issues. Compr Rev Food Sci F. 2006;5:35–47. doi: 10.1111/j.1541-4337.2006.tb00081.x. [DOI] [Google Scholar]
- Kidd G, Devorak J. Trehalose is a sweet target for agrobiotech. Biotechnol. 1994;12:1328–1329. doi: 10.1038/nbt1294-1328. [DOI] [Google Scholar]
- Knight I. The development and applications of sucralose, a new high-intensity sweetener. Can J Physiol Pharmacol. 1993;72:435–439. doi: 10.1139/y94-063. [DOI] [PubMed] [Google Scholar]
- Lachke AH, Jeffries TW. Levels of the enzymes of the pentose phosphate pathway in Pachysolen tannophilus Y-2460 and selected mutants. Enzyme Microb Technol. 1986;8:353–359. doi: 10.1016/0141-0229(86)90135-3. [DOI] [Google Scholar]
- Levin GV, Zehner LR, Saunders JP, Beadle IR. Sugar substitutes, their energy values, bulk characteristics and potential health benefits. Am J Clin Nutr. 1995;62:1161–1168. doi: 10.1093/ajcn/62.5.1161S. [DOI] [PubMed] [Google Scholar]
- Lida T, Kishimoto Y, Yoshikawa Y, Hayashi N, Okuma K, Tohi M, Yagi K, Matsuo T, Izumori K. Acute D-psicose Administration Decreases the Glycemic Responses to an Oral Maltodextrin Tolerence Test in Normal Adults. J Nutr Sci Vitaminol. 2008;54:511–524. doi: 10.3177/jnsv.54.511. [DOI] [PubMed] [Google Scholar]
- Livesey G, Brown JC. D-Tagatose Is a Bulk Sweetener with Zero Energy Determined in Rats. J Nutr. 1996;126:1601–1609. doi: 10.1093/jn/126.6.1601. [DOI] [PubMed] [Google Scholar]
- Magnuson BA, Burdock GA, Doull J. Aspartame: a safety evaluation based on current use levels, regulations, and toxicological and epidemiological studies. Crit Rev Toxicol. 2007;37:629–727. doi: 10.1080/10408440701516184. [DOI] [PubMed] [Google Scholar]
- Matsuo T, Izumori K. D-psicose inhibits intestinal α-glucosidase and suppresses glycemic response after carbohydrate ingestion in rats. Tech Bull Fac Agr, Kagawa Univ. 2006;58:27–32. [Google Scholar]
- Matsuo T. Inhibitary effects of D-psicose on glycemic responses after oral carbohydrate tolerance test in rats. J Jpn Soc Nutr Food Sci. 2006;59:247–253. doi: 10.4327/jsnfs.59.119. [DOI] [Google Scholar]
- Mazur RH, Goldkamp AH, James PA, Schlatter JM. Structure-taste relationships of aspartic acid amides. J Med Chem. 1970;6:1217–1221. doi: 10.1021/jm00300a046. [DOI] [PubMed] [Google Scholar]
- Mazur RH, Ripper A. Peptide-based sweeteners. London: Applied Science Publishers; 1979. [Google Scholar]
- Mc Phearson JD, Shilton BH, Walton DJ. Role of fructose in glycosylation and cross-linking of proteins. Biochemistry-US. 1988;27:1901–1907. doi: 10.1021/bi00406a016. [DOI] [PubMed] [Google Scholar]
- Menavuvu BT, Poonperm W, Leang K, Noguchi N, Okada H, Morimoto K, Granstrom TB, Takada G, Izumori K. Efficient biosynthesis of D-allose from D-psicose by cross- linked recombinant L-rhamnose Isomerase: Separation of product by ethanol crystallization. J Biosci Bioeng. 2006;101:340–345. doi: 10.1263/jbb.101.340. [DOI] [PubMed] [Google Scholar]
- Munro IC, Berndt WO, Borzelleca JF, Flamm G, Lynch BS, Kennepohl E, Bar EA, Modderman J. Erythritol: An interpretive summary of biochemical, metabolic, toxicological and clinical data. Food Chem Toxicol. 1998;36:1139–1174. doi: 10.1016/S0278-6915(98)00091-X. [DOI] [PubMed] [Google Scholar]
- Nabors LO. Sweet choices: sugar replacements for foods and beverages. Food Technol. 2002;56:28–32. [Google Scholar]
- Normen L, Laerke HN, Jensen BB, Langkilde AM, Andersson H. Small-bowel absorption of D-tagatose and related effects on carbohydrate digestibility: an ileostomy study. Am J Clin Nutr. 2001;73:105–110. doi: 10.1093/ajcn/73.1.105. [DOI] [PubMed] [Google Scholar]
- [NTP] Natl Toxicology Program (2005) NTP report on the toxicology studies of aspartame in genetically modified (FVB Tg.AC hemizygous) and B6.129- Cdkn2atm1Rdp (N2) deficient mice and carcinogenicity studies of aspartame in genetically modified [B6.129- Trp53tm1Brd (N5) haploinsufficient] mice. October NIH Pub No. 06–4459. http://ntp.niehs.nih.gov/files/ GMM2_Web.pdf. Accessed 9 February 2006 [PMC free article] [PubMed]
- Neotame: Stability overview (2002) http://www.neotame.com/pdf/neotame_stability_overview_US.pdf. Accessed 27 February 2009
- Patel RM, Sharma R, Grimsley E. Popular Sweetener Sucralose as a Migraine Trigger. J Head Face Pain. 2006;46:1303–1304. doi: 10.1111/j.1526-4610.2006.00543_1.x. [DOI] [PubMed] [Google Scholar]
- Peter J A, Walker R, Leclercq C (2002) Alitame. WHO Food Additives Series. http://www.inchem.org/documents/jecfa/jecmono/v50je02.htm. Accessed 6 March 2009
- Povelainen M, Miasnikov AN. Production of xylitol by metabolically engineered strains of Bacillus subtilis. J Biotechnol. 2006;128:24–31. doi: 10.1016/j.jbiotec.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Prakash I (2007) Synthesis of N-[N-(3,3-dimethylbutyl)-L-α-aspartyl]-L-phenylalanine 1-methyl ester using 3,3-dimethylbutyraldehyde precursors. Ip.com US Patent 7288670. http://ip.com/patent/US7288670. Accessed 2 June 2010
- Prakash I, Corliss G, Ponakala R, Ishikawa G. Neotame: the next-generation sweetener. Food Technol. 2002;56:36–40. [Google Scholar]
- Prakash I, Zhao R Y (2001) Chemoenzymatic synthesis of neotame, Pub No. WO/2001/085977. http://www.wipo.int/pctdb/en/wo.jsp?wo=2001085977. Accessed 6 March 2009
- Priebem PM, Kauffman GB. Making governmental policy under conditions of scientific uncertainty: A century of controversy about saccharin in congress and the laboratory. Minerva. 1980;18:556–574. doi: 10.1007/BF01096124. [DOI] [PubMed] [Google Scholar]
- Renwick AG, Thompson JP, Shaughnessy M, Walter EJ. The metabolism of cyclamate to cyclohexylamine in humans during long-term administration. Toxicol Appl Pharmacol. 2004;196:367–80. doi: 10.1016/j.taap.2004.01.013. [DOI] [PubMed] [Google Scholar]
- Rymon Lipinski GW. Sensory Properties of Acesulfame-K. New York: Marcel Dekker; 1991. [Google Scholar]
- [SCF] Scientific Committee on Food (2000) Revised opinion of the Scientific Committee on Food on cyclamic acid and its sodium and calcium salts http://ec.europa.eu/food/fs/sc/scf/out53_en.pdf. Accessed 5 May 2000
- Scott SK, Rabito F, Price PD, Butler NN, Schwartzbaum JA, Jackson BM, Love RL, Harris RE. Comorbidity among the morbidity obese: a comparative study of 2002 U.S hospital patient discharge. Surg Obes Relat Dis. 2006;2:105–111. doi: 10.1016/j.soard.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Serdula MK, Mokdad AH, Williamson DF, Galuska DA, Mendlein JM, Heath GW. Prevalence of attempting weight loss and strategies for controlling weight. J Am Med Assoc. 1999;282:1353–1358. doi: 10.1001/jama.282.14.1353. [DOI] [PubMed] [Google Scholar]
- Sellman S (2003) Xylitol: Our Sweet Salvation? Nexus New Times: Jan-Feb http://www.lib .umich.edu/dentlib/nihcdc/abstracts/hayes.html. Accessed 16 March 2009
- Sorensen LB, Mella P, Flint A, Martens M, Raben A. Effect of sensory perception of food in appetite and food intake. A review of studies on humans. Int J Obes. 2003;27:1152–1166. doi: 10.1038/sj.ijo.0802391. [DOI] [PubMed] [Google Scholar]
- Sun Y, Hayakawa S, Jiang H, Ogawa M, Izumori K. Rheological characteristics of heat-induced custard pudding gels with high antioxidative activity. Biosci Biotech Biochem. 2006;70:2859–2867. doi: 10.1271/bbb.60256. [DOI] [PubMed] [Google Scholar]
- Sun Y, Hayakawa S, Ogawa M, Izumori K. Antioxidant properties of custard pudding dessert containing rare hexose, D-psicose. Food Control. 2007;18:220–227. doi: 10.1016/j.foodcont.2005.09.019. [DOI] [Google Scholar]
- Sun Y, Hayakawa S, Ogawa M, Fukada K, Izumori K. Influence of a Rare Sugar, d-Psicose, on the Physicochemical and Functional Properties of an Aerated Food System Containing Egg Albumen. J Agricult Food Chem. 2008;56:4789–4796. doi: 10.1021/jf800050d. [DOI] [PubMed] [Google Scholar]
- Szepesi B, Michaelis OE. Disaccharide effect - comparison of metabolic effects of the intake of disaccharide and of their monosaccharide equivalents. In: Macdonald I, Vrana A, editors. Metabolic Effects of Dietary Carbohydrates. 4. Switzerland: Karger S Basel; 1986. pp. 192–219. [PubMed] [Google Scholar]
- Szepsi B. Carbohydrates. Washington: ILSI Press; 1996. [Google Scholar]
- Tarbell DS, Tarbell AT. The discovery of saccharin. J Chem Educ. 1978;55:161–162. doi: 10.1021/ed055p161. [DOI] [Google Scholar]
- Takeshita K, Suga A, Takada G, Izumori K. Mass production of D- psicose from D-fructose by a continuous bioreaction system using immobilized D-tagatose 3 epimerage. J Biosci Bioeng. 2002;90:453–455. doi: 10.1016/s1389-1723(01)80018-9. [DOI] [PubMed] [Google Scholar]
- Trocho C, Pardo R, Rafecas I, Virgili JX, Remesar X, Fernandez-Lopez JA, Alemany M. Formaldehyde derived from dietary aspartame binds to tissue components in vivo. Life Sci. 1998;63:337–349. doi: 10.1016/S0024-3205(98)00282-3. [DOI] [PubMed] [Google Scholar]
- [USFDA] US Food and Drug Administration (2003) Food additives permitted for direct addition to food for human consumption; acesulfame potassium Final rule. FedReg 68:75411–75413.http://frwebgate.access.gpo.gov/cgi-bin/getpage.cgi?position=all&page=75411&dbname=2003_register. Accessed 22 May 2006
- [USFDA] US Food and Drug Administration (2002) Food additives permitted for direct addition to food for human consumption;Neotame. Fed Reg 67:45300–45310 http://frwebgate.access.gpo.gov/cgi-bin/getpage.cgi?position=all&page=45300&dbname=2002_register. Accessed 22 May 2006
- [USFDA] US Food and Drug Administration (1999) Food additives permitted for direct addition to food for human consumption: sucralose. Fed Reg 64:43908–43909. http://www.fda.gov/ohrms/dockets/98fr/081299b.txt. Accessed 7 March 2009
- Weihrauch MR, Diehl V. Artificial sweeteners - do they bear a carcinogenic risk. Ann Oncol. 2004;15:1460–1465. doi: 10.1093/annonc/mdh256. [DOI] [PubMed] [Google Scholar]
- Wong CH, Whitesides GM. Enzymes in Synthetic Organic Chemistry. Oxford: Elsevier; 1994. [Google Scholar]
- Xylitol (2009) Polyols information sourace. http://www.polyol.org/fap/fap_xylitol.html. Accessed 5 February 2009
- Zakaria A. Production of natural and rare pentoses using microorganisms and their enzymes. Electron J Biotechnol. 2001;4:103–111. [Google Scholar]