Abstract
Composition, functional properties and in vitro antioxidative activities of protein hydrolysates prepared from muscle of sardinelle (Sardinella aurita) were investigated. Sardinelle protein hydrolysates (SPH) were obtained by treatment with crude enzyme preparations from Bacillus pumilus A1 (SPHA1), Bacillus mojavensis A21 (SPHA21) and crude enzyme extract from sardinelle (Sardinella aurita) viscera (SPHEE). The protein hydrolysates SPHA1, SPHA21 and SPHEE contained high protein content 79.1%, 78.25% and 74.37%, respectively. The protein hydrolysates had an excellent solubility and possessed interfacial properties, which were governed by their concentrations. The antioxidant activities of protein hydrolysates at different concentrations were evaluated using various in vitro antioxidant assays, including 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical method, reducing power assay, chelating activity, β-carotene bleaching and DNA nicking assay. All protein hydrolysates showed varying degrees of antioxidant activity. SPHA21 had the highest DPPH radical scavenging activity (89% at 6 mg/ml) and higher ability to prevent bleaching of β-carotene than SPHA1 and SPHEE (p < 0.05). However, SPHEE exhibited the highest metal chelating activity (89% at 1 mg/ml) and the strongest protection against hydroxyl radical induced DNA breakage (p < 0.05).
Keywords: Sardinelle (Sardinella aurita), Protein hydrolysate, Hydrolysis, Functional properties, Antioxidative activity
Introduction
Marine organisms represent a valuable source of new compounds. The biodiversity of the marine environment and the associated chemical diversity constitute a practically unlimited resource of new active substances in the field of the development of bioactive products.
With marine species comprising approximately a half of the total global biodiversity, the sea offers an enormous resource of novel compounds (De Vries and Beart 1995) and it has been classified as the largest remaining reservoir of natural molecules to be evaluated for drug activity.
Very different kinds of substances have been obtained from marine organisms among other reasons because they are living in a very exigent, competitive, and aggressive surrounding very different in many aspects from the terrestrial environment, a situation that demands the production of quite specific and potent active molecules.
Free radical-mediated reactive oxygen species (ROS) and lipid peroxidation (LPO) have gained considerable attention nowadays (Manso et al. 2008; Seema et al. 2008; Nasirullah et al. 2009). The formation of free radicals such as superoxide anion (O−2) and hydroxyl radical (•OH) is an unavoidable consequence in aerobic organisms during respiration.
Uncontrolled generated free radicals are very unstable, and react rapidly with other chemical groups or substances in the body, leading to cell or tissue injury. In addition, numerous studies have revealed that uncontrolled lipid peroxidation is involved in the occurrence of numerous chronic diseases (Butterfield et al. 2002; Pryor and Ann 1982). In particular, lipid peroxidation in foods affects their nutritive value and may cause disease conditions following the consumption of potentially toxic reaction products.
In humans, uncontrolled generation of ROS can result in oxidative damage of cellular DNA, protein and lipids (Seifried et al. 2007). When there is a lack of antioxidant to quench the excess reactive free radicals, cardiovascular, cancer, neurodegenerative, Alzheimer’s and inflammatory diseases may develop in the body (Shui and Leong 2006). In addition, auto-oxidation of fats and oils in foods triggered by ROS is responsible for flavor deterioration as well as nutrient loss (Saiga et al. 2003). To prevent oxidative deterioration of foods and to provide protection against serious diseases, it is important to inhibit the oxidation of lipids and the formation of free radicals occurring in the foodstuff and living body.
Synthetic antioxidants have been widely used in stabilization of foods. The two most commonly used are butylated hydroxyanisole (BHA) and butylated hydroxytoluene (BHT), which are added to fatty and oil foods to prevent oxidative deterioration (Löliger 1991). However, use of these chemical compounds has begun to be restricted because of their induction of DNA damage and their toxicity (Ito et al. 1986). Moreover, both BHT and BHA appear to be involved in tumor promotion (Botterweck et al. 2000). Therefore, there is great interest in finding new and safe antioxidants from natural sources, especially peptides derived from hydrolyzed food proteins.
Fish protein hydrolysates such as skin gelatin hydrolysate from brownstripe red snapper (Khantaphant and Benjakul 2008) or meat protein hydrolysates from yellow travelly (Klompong et al. 2007; Klompong et al. 2009), round scad (Thiansilakul et al. 2007a,b), mackerel (Wu et al. 2003), loach (You et al. 2010), and smooth hound (Mustelus mustelus) muscle (Bougatef et al. 2009) have been reported to exhibit antioxidant activity. Fish protein hydrolysates can be used in food systems, comparable to other pertinent protein hydrolysates (Kristinsson and Rasco 2000a).
In Tunisia, sardinelle (Sardinella aurita) catches were about 14,200 tons in 2009. During processing, solid wastes including heads and viscera are generated and constitute as much as 30% of the original material. These wastes, which represent an environmental problem to the fishing industries, constitute an important source of proteins. Traditionally, this material is transformed into powdered fish flour for animal feed.
The aims of this work were to study the compositions, functional properties and antioxidative activities of sardinelle muscle protein hydrolysates obtained by treatment with crude enzyme preparations from Bacillus pumilus A1, Bacillus mojavensis A21, and crude enzyme extract from sardinelle (Sardinella aurita) viscera.
Materials and methods
Chemicals
1,1-diphenyl-2-picrylhydrazyl (DPPH), butylated hydroxyanisole (BHA), ethylenediaminetetraacetic acid (EDTA), β-carotene and linoleic acid were purchased from Sigma Chemical Co. (St. Louis, MO, USA). All other chemicals, namely potassium ferricyanide, trichloroacetic acid (TCA), ferric chloride, sodium hydroxide, Folin-Ciocalteu reagent, aluminium chloride, sodium nitrite, sodium carbonate, Tween 40 and other solvents, were of analytical grade. Sodium dodecyl sulphate (SDS) was from Bio-Rad Laboratories (Hercules, CA, USA). All solutions were freshly prepared in distilled water.
Materials
Sardinelle (S. aurita) was purchased from the fish market of Sfax city, Tunisia. Muscle was separated, rinsed with cold distilled water, and then stored in sealed plastic bags at -20 °C until used.
Enzymes
The enzyme preparations used were: alkaline proteases from B. pumilus A1 (Fakhfakh-Zouari et al. 2010) and B. mojavensis A21 (Haddar et al. 2009), and crude enzyme extract from viscera of sardinelle (S. aurita) (Ben khaled et al. 2008). All proteolytic enzymes were prepared in our laboratory. The protease activity in the proteolytic preparations was determined by the method of Kembhavi et al. (1993) using casein as a substrate. One unit of protease activity was defined as the amount of enzyme required to liberate 1 μg of tyrosine per minute under the experimental conditions used.
Preparation of endogenous enzyme extract from sardinelle viscera
Viscera from sardinelle (100 g) were homogenised for 60 s with 200 ml of extraction buffer (10 mM Tris–HCl, pH 8.0). The homogenate was centrifuged at 8500 g for 30 min at 4 °C and the supernatant was collected. The supernatant referred to as the crude enzyme extract was stored at 4 °C prior to analysis. All enzymatic assays were conducted within a week after extraction. For a long conservation, supernatant was lyophilised.
Production of sardinelle protein hydrolysates (SPHs)
S. aurita muscle (500 g), in 1000 ml distilled water was first minced, using a grinder (Moulinex Charlotte HV3, France) and then cooked at 90 °C for 20 min to inactivate endogenous enzymes. The cooked muscle sample was subsequently homogenized in a Moulinex® blender for about 2 min. The samples were adjusted to optimal pH and temperature for each enzyme preparation: proteases from B. mojavensis A21 (10.0, 50 °C), proteases from B. pumilus A1 (8.5, 50 °C), and crude enzyme extract from sardinelle viscera (8.0, 45 °C). The protein solution was allowed to equilibrate for 30 min before hydrolysis was initiated. Control experiments were also performed without enzyme addition.
Enzymes were added to the reaction to give an enzyme: substrate (E/S) ratio of 3 U/mg (unit of enzyme: weight of protein). Enzymes were used at the same activity levels to compare hydrolytic efficiencies. During the reaction, the pH of the mixture was maintained constant by continuous addition of 4 N NaOH solution. After the required digestion time, the reaction was stopped by heating the solution for 20 min at 80 °C to inactivate enzymes. The SPHs (sardinelle muscle protein hydrolysate) were then centrifuged at 5000 g for 20 min to separate insoluble and soluble fractions. Finally, the soluble fractions were freeze-dried using a freeze-dryer (Bioblock Scientific Christ ALPHA 1-2, IllKrich-Cedex, France) and stored at −20 °C for further use.
Determination of the degree of hydrolysis
The degree of hydrolysis (DH), defined as the percent ratio of the number of peptide bonds cleaved (h) to the total number of peptide bonds in the protein substrate (htot), was calculated from the amount of base (NaOH) added to keep the pH constant during the hydrolysis (Adler-Nissen 1986) as given below.
 |
where B is the amount of NaOH consumed (ml) to keep the pH constant during the reaction. Nb is the normality of the base, MP is the mass (g) of protein (N × 6.25), and α is the average degree of dissociation of the α-NH2 groups released during hydrolysis expressed as:
 |
where pH and pk are the values at which the proteolysis was conducted. The total number of peptide bonds (htot) in a fish protein hydrolysate was assumed to be 8.6 meq/g (Adler-Nissen 1986).
Chemical analysis
The moisture and ash content were determined according to the (AOAC 2000) standard methods 930.15 and 942.05, respectively. Total nitrogen content of sardinelle protein hydrolysates and undigested sardinelle protein (USP) was determined by using the Kjeldahl method according to the AOAC method number 984.13 (AOAC 2000). Crude protein was estimated by multiplying total nitrogen content by the factor of 6.25. Lipids were determined gravimetrically after Soxhlet extraction of dried samples with hexane. All measurements were performed in triplicate.
Determination of mineral contents
Analyses of calcium (Ca), magnesium (Mg), sodium (Na), potassium (K), chlorure (Cl), nitrate (NO3) and sulphate (SO4) contents in freeze-dried hydrolysates were carried out using the inductively coupled plasma optical emission spectrophotometer (ICP-OES) (Model 4300 DV, Perkin Elmer, Shelton, CT, USA) according to the method of AOAC (1999). Sample (1 g) was mixed well with 1 ml of 70% nitric acid. The mixture was heated on the hot plate until digestion was completed. The digested sample was transferred to a volumetric flask and the volume was made up to 10 ml with deionized water. The solution was then subjected to analysis. The solution was then subjected to analysis. The concentration of minerals was calculated and expressed as mg/kg sample.
Protein concentration
Protein concentration was determined by the method of Bradford (1976) using bovine serum albumin as a standard.
Functional properties
Solubility
Solubility of SPHs was carried out over a wide range of pH value from pH 2.0 to pH 12.0 as described by Tsumura et al. (2005). Briefly, 200 mg of protein hydrolysate sample were suspended in 20 ml of deionized distilled water and the pH of the mixture was adjusted to the desired values with 6 N HCl or 6 N NaOH solutions required. The mixtures were stirred for 30 min at room temperature (25 ± 1 °C) and then centrifuged at 8000 g for 15 min. After appropriate dilution, protein contents in the supernatant were determined using the Bradford method (Bradford 1976), and the percentage of soluble proteins was calculated at each pH value. Solubility analysis was carried out in triplicate.
Emulsifying properties
The emulsifying activity index (EAI) and the emulsion stability index (ESI) of the hydrolysates were determined according to the method of Pearce and Kinsella (1978) with a slight modification. The hydrolysate solutions were prepared by dissolving freeze-dried hydrolysates in distilled water at 60 °C for 30 min. Thirty millilitres of SPH solutions at different concentrations (0.1%, 0.5%, 1%, and 2%) were homogenized with 10 ml of soybean oil for 1 min at room temperature (22 ± 1 °C) using Moulinex_ R62 homogenizer. Aliquots of the emulsion (50 μl) were pipetted from the bottom of the container at 0 and 10 min after homogenization, and diluted 100-fold with 0.1% SDS solution. The absorbance of the diluted solutions was measured at 500 nm using a spectrophotometer (T70, UV/VIS spectrometer, PG Instruments Ltd, China). The absorbances, measured immediately (A0) and 10 min (A10) after emulsion formation, were used to calculate the emulsifying activity index (EAI) and the emulsion stability index (ESI) (Pearce and Kinsella 1978). All determinations are means of at least three measurements.
Foaming properties
Foam expansion (FE) and foam stability (FS) of SPHs were determined according to the method of Shahidi et al. (1995). Twenty milliliters of protein hydrolysates solution at 0.1% (w/v) were homogenized using a Moulinex_ R62 homogenizer to incorporate the air for 1 min at room temperature (25 ± 1 °C). The whipped sample was then immediately transferred into a 50 ml graduated cylinder, and the total volume was measured at 15, 30 and 45 min after whipping. Foam expansion was expressed as percentage of volume increase after homogenization at 0 min, which was calculated according to the following equation:
 |
where A is the volume after whipping (ml); B is the volume before whipping. All determinations are means of at least two measurements.
Foam stability was calculated as the volume of foam remaining after 15, 30 and 45 min.
Determination of antioxidative activities
DPPH radical-scavenging assay
The DPPH radical-scavenging activity of the hydrolysates was determined as described by Bersuder et al. (1998). A volume of 500 μl of each sample at different concentrations (1 to 6 mg/ml) was mixed with 375 μl of 99.5% ethanol and 125 μl of 0.02% DPPH in 99.5% ethanol. The mixtures were then kept at room temperature in the dark for 60 min, and the reduction of DPPH radical was measured at 517 nm using a UV–Visible spectrophotometer (T70, UV/VIS spectrometer, PG Instruments Ltd., China). The control was conducted in the same manner, except that distilled water was used instead of sample. The DPPH radical scavenging activity was calculated as follows:
 |
where Ac is the absorbance of the control reaction and As is the absorbance of the SPHs. DPPH has an absorption at 517 nm which disappear upon reduction by an antiradical compound. Lower absorbance of the reaction mixture indicated higher DPPH radical-scavenging activity. BHA was used as positive control. The test was carried out in triplicate.
Antioxidant assay using the β-carotene bleaching method
The ability of the protein hydrolysates to prevent bleaching of β-carotene was determined as described by Koleva et al. (2002). β-carotene (2 mg) was dissolved in 20 ml of chloroform. A 4 ml aliquot of the chloroform solution was mixed with 40 μl of linoleic acid and 400 μl of Tween 40 in a conical flask. After chloroform was evaporated under vacuum in a rotatory evaporator at 40 °C, 100 ml of distilled water were added by vigorous shaking. The emulsion obtained was freshly prepared before each experiment. To an aliquot of 3 ml of the β-carotene-linoleic acid emulsion, 500 μl of each SPH at different concentrations were added. The emulsion system was immediately placed in water bath and incubated for 2 h at 50 °C and the absorbance of each sample was measured at 470 nm. BHA was used as positive standard. In the case of the control, 0.5 ml of distilled water instead of the sample solution was added to the β-carotene–linoleic acid emulsion. The antioxidant activity of the hydrolysates was evaluated in terms of bleaching of β-carotene using the following formula:
 |
where A0 and A′0 are the absorbances of the test sample and the control measured at time zero, respectively, and At and A′t are the absorbances of the sample and the control, respectively, after incubation for 120 min. Values presented are the mean of triplicate analyses.
Reducing power assay
The ability of SPHs to reduce iron (III) was determined according to the method of Yildirim et al. (2001). An aliquot of 1 ml sample of each hydrolysate at different concentrations (1–6 mg/ml) was mixed with 2.5 ml of 0.2 M phosphate buffer (pH 6.6) and 2.5 ml of 1% (w/v) potassium ferricyanide solution. The mixtures were incubated for 30 min at 50 °C. After incubation, 2.5 ml of 10% (w/v) TCA was added and the reaction mixtures were then centrifuged for 10 min at 10,000 g. Finally, 2.5 ml of the supernatant solution from each sample mixture were mixed with 2.5 ml of distilled water and 0.5 ml of 0.1% (w/v) ferric chloride. After a 10 min reaction time, the absorbance of the resulting solutions was measured at 700 nm. Higher absorbance of the reaction mixture indicated higher reducing power.
The control was conducted in the same manner, except that distilled water was used instead of sample. Values presented are the mean of triplicate analyses.
Determination of metal (Fe2+) chelating activity (ferrozine assay)
The chelating activity of the SPHs for Fe2+ was measured according to the method described by Dinis et al. (1994). To 0.5 ml of hydrolysate at different concentrations, 1.6 ml of distilled water and 0.05 ml of FeCl2 (2 mM) was added, followed by the addition of 0.1 ml of ferrozine (5 mM) after 15 min. After a 10 min reaction time at room temperature, the absorbance of the Fe2+-ferrozine complex with red or violet color was measured at 562 nm. The chelating activity of the antioxidant for Fe2+ was calculated according to the following formula:
 |
where A0 is the absorbance of the control (blank); AB is the absorbance of the sample; AR is the absorbance in the presence of the sample. EDTA was used as a standard.
The control was conducted in the same manner, except that distilled water was used instead of sample.
DNA nicking assay
DNA nicking assay was performed using pCRII™TOPO plasmid (invitrogen). A mixture of 10 μl of hydrolysates at the concentration of 1 mg/ml and plasmid DNA (0.5 μg/well) were incubated for 10 min at room temperature followed by the addition of 10 μl of Fenton’s reagent (30 mM H2O2, 50 μM L-ascorbic acid and 80 μM FeCl3). The mixture was then incubated for 5 min at 37 °C. The DNA was analysed on 1% (w/v) agarose gel using ethidium bromide staining.
Statistical analysis
Statistical analyses were performed with Statgraphics ver. 5.1, professional edition (Manugistics Corp., USA) using ANOVA analysis. Differences were considered significant at P < 0.05. All tests were carried out in triplicate.
Results and discussion
Preparation of sardinelle protein hydrolysates using various proteases
It has been demonstrated that functional properties and biological activities of proteins can be increased through hydrolysis with certain enzymes. In this study, various proteases (crude enzyme preparations from B. pumilus A1, B. mojavensis A21, and crude enzyme extract from sardinelle (S. aurita) viscera) were used for the hydrolysis of sardinelle muscle in order to obtain peptides with different molecular weights and different amino acid sequences.
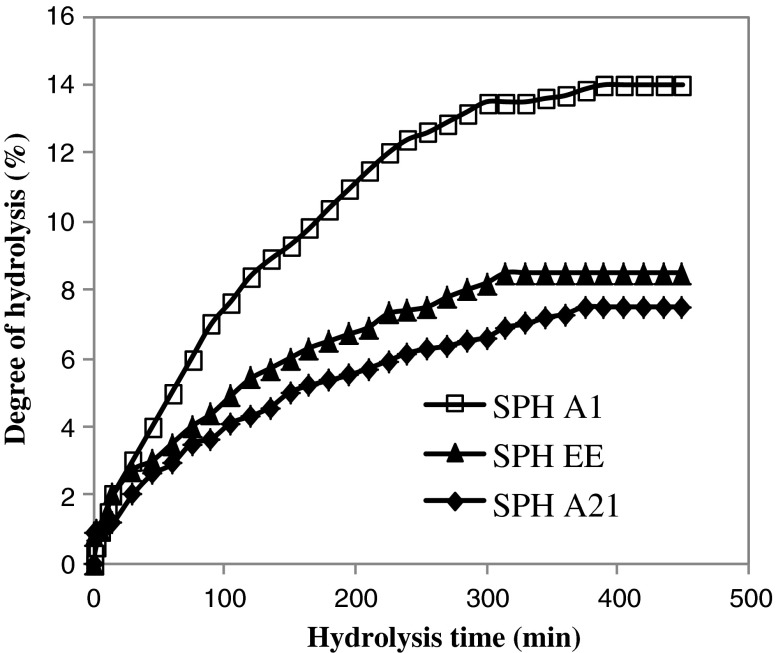
The hydrolysis curves of sardinelle muscle proteins after 6 h of incubation are shown in Fig. 1. The hydrolysis of the sardinelle muscle proteins was characterized by a high rate of hydrolysis for the first 1 h. The rate of enzymatic hydrolysis was subsequently decreased, and then the enzymatic reaction reached the steady-state phase when no apparent hydrolysis took place. Crude enzyme preparation from B. pumilus A1 was the most efficient, while that from B. mojavensis A21 was the lowest efficient. After 6 h of hydrolysis, the DH reached about 14% with crude enzyme preparation from A1 strain and 8.5% with enzyme extract from sardinelle viscera (Fig. 1). The typical hydrolysis curves were also reported for brownstripe red snapper (Lutjanus vitta) (Khantaphant et al. 2011), yellow stripe trevally (Klompong et al. 2007), herring (Liceaga-Gesualdo and Li-Chan 1999), salmon muscle (Kristinsson and Rasco 2000b), sardinelle by-products (Bougatef et al. 2008) and smooth hound muscle (Bougatef et al. 2009).
Fig. 1.
Hydrolysis curves of sardinelle muscle proteins treated with crude enzymes from B. mojavensis A21 (SPHA21), B. pumilus A1 (SPHA1) and crude enzymes extract from sardinelle viscera (SPHEE)
Compositions of protein hydrolysates
The proximate composition of the SPHs is shown in Table 1. The protein hydrolysates had a high protein content (SPHA1: 79.1%; SPHA21: 78.25%; SPHEE: 74.37%) and could be an essential source of proteins. The high protein content was a result of the solubilisation of proteins during hydrolysis, the removal of insoluble undigested non-protein substances and the partial removal of lipid after hydrolysis (Benjakul and Morrissey 1997).
Table 1.
Chemical constituents of SPHs and USP
| Composition (%) | SPH A1 | SPH A21 | SPH EE | USP |
|---|---|---|---|---|
| Protein | 79.1 ± 0.51 | 78.2 ± 0.43 | 74.4 ± 0.31 | 14.7 ± 0.45 |
| Fat | 1.4 ± 0.01 | 0.901 ± 0.74 | 0.97 ± 0.19 | 19 ± 0.86 |
| Moisture | 11.6 ± 0.42 | 10.5 ± 0.87 | 14.6 ± 0.13 | 60.8 ± 0.35 |
| Ash | 11.7 ± 0.53 | 10 ± 0.08 | 10.8 ± 0.04 | 6.4 ± 0.24 |
| Minerals Content (mg/kg) | ||||
| Ca2+ | 254 ± 0.03*** | 191 ± 0.89 | 148 ± 0.46 | ND |
| Na+ | 310 ± 0.15* | 304.2 ± 0.75 | 446.2 ± 0.34 | ND |
| K+ | 1430. ± 0.405* | 1290.7 ± 0.67 | 910 ± 0.96 | ND |
| Mg2+ | 166 ± 0.25*** | 72 ± 0.08 | 74 ± 0.04 | ND |
| Cl− | 281.6 ± 0.09** | 298 ± 0.84 | 234 ± 0.05 | ND |
| NO2−3 | 55.5 ± 1.21** | 43.2 ± 0.13 | 54.3 ± 0.49 | ND |
| SO2−4 | 26.5 ± 0.98*** | 21 ± 0.36 | 32 ± 0.84 | ND |
Values are given as mean±SD from triplicate determinations (n = 3)
ND Not Determinate, USP Undigested sardinelle protein
Sardinelle protein hydrolysates were obtained by treatment with crude enzyme preparations from B. pumilus A1 (SPHA1), B. mojavensis A21 (SPHA21) and crude enzyme extract from sardinelle (S. aurita) viscera (SPHEE).
Significantly differences between hydrolysates: *P < 0.05; **P < 0.01; *** P < 0.001
All protein hydrolysates had low lipid content (0.9–1.43%). During the hydrolysis process, the muscle cell membranes tend to round up and form insoluble vesicles, leading to the removal of membrane structured lipids (Shahidi et al. 1995). A reduced lipid content was reported in the protein hydrolysate from salmon (Gbogouri et al. 2004), capelin (Shahidi et al. 1995) and herring (Liceaga-Gesualdo and Li-Chan 1999).
From the result, the ash content of protein hydrolysate is between 10 and 11.7%. It was lower than that of protein hydrolysate prepared from round scad (Decapterus maruadsi) (Thiansilakul et al. 2007b) which was 24.56%. The higher ash content of protein hydrolysates may be attributed to the addition of NaOH required for pH adjustment to 8.0 for enzymatic hydrolysis. High ash content has been recognised as a drawback of protein hydrolysate, making applications limited (Shahidi et al. 1995).
Mineral content
The freeze-dried sardinelle protein hydrolysates consisted of different minerals at different levels as shown in Table 1. Na+, K+, Ca2+, Mg2+ and Cl− were found at high concentrations while NO2−3 and SO−4 were found at a low level. The apparent metal ions could act as pro-oxidants in the hydrolysate. Sathivel et al. (2003) reported that K+, Mg2+, Na+ and Ca2+ were abundant in herring and herring by-product hydrolysates and varied with the substrate used.
Fish protein hydrolysates usually contain a moderate NaCl content due to salting for conservation or pH adjustments during the pH shift process.
Determination of functional properties
Solubility
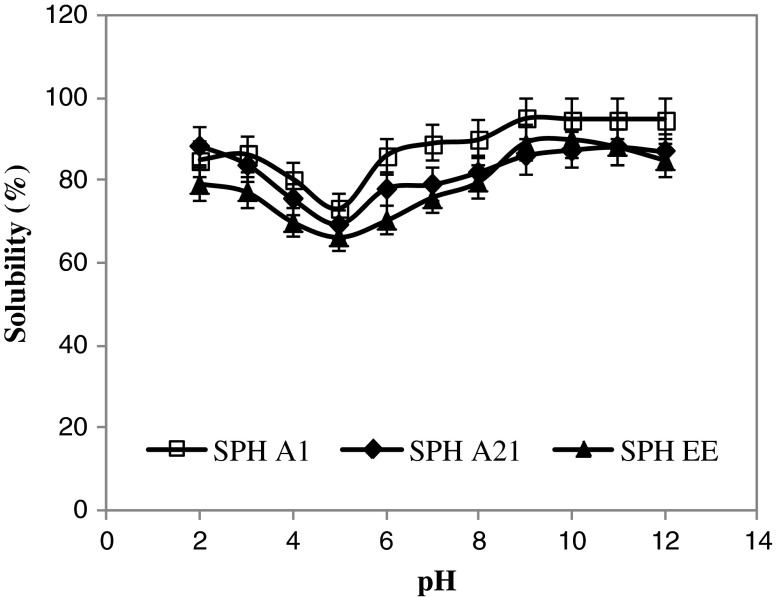
Solubility is one of the most important physicochemical and functional properties of proteins and protein hydrolysates, since it can affect other functional properties such as emulsification and foaming (Kristinsson and Rasco 2000a). Good solubility of proteins is required in many functional applications, especially for emulsions, foams and gels, because soluble proteins provide a homogeneous dispersibility of the molecules in colloidal systems and enhance the interfacial properties (Zayas 1997). The solubility of hydrolysates from the muscle of sardinelle at pH ranging from 2.0 to 12.0 is shown in Fig. 2. Enzymatic hydrolysis considerably improved the solubility of sardinelle proteins at all pH values tested, more than 65% solubility was obtained. The protein solubility of SPHs was minimum at pH 5.0 and increased gradually below and above pH 5.0. The SPHs showed same profiles. In fact, the hydrolysate obtained by treatment with B. pumilus A1 which showed the highest DH (14%) and which may contain lower molecular mass, had excellent solubility in a pH values ranging from 3.0 to 12.0 than the other hydrolysates (p < 0.05). At pH values between 9.0 and 12.0, the solubility of SPHA1 reached about 95%.
Fig. 2.
Solubility profiles of sardinelle protein hydrolysates (SPHs) as a function of pH obtained by treatment with crude enzyme preparations from Bacillus pumilus A1 (SPHA1), Bacillus mojavensis A21 (SPHA21), and crude enzyme extract from sardinelle (S. aurita) viscera (SPHEE)
The difference in solubility observed among hydrolysates can be due to peptide lengh and the ratio of hydrophilic/hydrophobic peptides (Adler-Nissen 1986). The enhancement of solubility of SPHs could be attributed to the release of small soluble peptides and to the exposure of more charged and polar groups to the surrounding water. (Gbogouri et al. 2004 ; Kristinsson and Rasco 2000b).
The high solubility of protein hydrolysates indicates potential applications in formulated food systems by providing attractive appearance and smooth mouth feel to the product (Peterson 1981).
Emulsifying properties
Emulsion activity index (EAI) and emulsion stability index (ESI) of SPHs at different concentrations (0.1%, 0.5%, 1% and 2%) are shown in Table 2. EAI and ESI of all SPHs decreased with increasing concentration. A maximum EAI and ESI values were observed at a concentration of 0.1%. According to Pearce and Kinsella (1978), this concentration refers to the minimum concentration needed to obtain reproducible results for the emulsifying properties and must be established in each case because it depends on the type of protein involved in the emulsifying process. The higher EAI at 0.1% of SPHs was similar to that found by Thiansilakul et al. (2007a).
Table 2.
Emulsifying properties of USP and SPHs at various concentrations
| Hydrolysate concentrations (%) | Emulsifying activity index (m2 g−1) | Emulsion stability index (min) | ||||||
|---|---|---|---|---|---|---|---|---|
| SPHA1 | SPHA21 | SPHEE | USP | SPHA1 | SPHA21 | SPHEE | USP | |
| 0.1 | 76.1 ± 0.09*** | 81.6 ± 0.11 | 86.6 ± 0.32 | 15.1 ± 0.1 | 30 ± 0.22 | 44.4 ± 0.02 | 37.7 ± 0.35 | 1 ± 0.305 |
| 0.5 | 20.1 ± 0.31* | 11.8 ± 0.01 | 7.9 ± 0.14 | 212 ± 0.21 | 28 ± 0.12*** | 40.2 ± 0.04 | 24 ± 0.02 | 6.4 ± 0.11 |
| 1 | 7.3 ± 0.02 | 9.9 ± 0.21 | 11.1 ± 0.09 | 5.5 ± 0.14 | 17 ± 0.02 | 24 ± 0.05 | 16 ± 0.08 | 4.5 ± 0.24 |
| 2 | 5.1 ± 0.08** | 9.2 ± 0.08 | 17.8 ± 0.02 | 6.2 ± 0.3 | 8.2 ± 0.01 | 11.4 ± 0.03 | 15.1 ± 0.19 | 5.3 ± 0.23 |
Values are given as mean±SD from triplicate determinations (n = 3)
Significantly differences between hydrolysates: *P < 0.05; **P < 0.01; *** P < 0.001
USP Undigested sardinelle protein
Sardinelle protein hydrolysates were obtained by treatment with crude enzyme preparations from B. pumilus A1 (SPHA1), B. mojavensis A21 (SPHA21) and crude enzyme extract f rom sardinelle (S. aurita) viscera (SPHEE)
Protein hydrolysates are surface-active materials and promote an oil-in-water emulsion because of their hydrophilic and hydrophobic groups and their charge (Gbogouri et al. 2004; Kristinsson and Rasco 2000a). The dependence of emulsifying activity on the concentration of protein has been explained by adsorption kinetics (Kinsella 1976). The increase in protein–protein interaction resulted in a lower protein concentration at the interface (Lawal 2004). Thus, a thinner film stabilizing the oil droplet was postulated.
Foaming properties
Proteins in dispersions cause a lowering of the surface tension at the water/air interface, thus creating foam. Foam expansion (FE) and foam stability (FS) of SPHs are shown in Table 3. As DH increased, SPHs displayed a higher foaming capacity and foam stability. Klompong et al. (2007) reported low foaming capacity and foam stability for yellow stripe trevally (Selaroides leptolepis) when DH increased. However, Shahidi et al. (1995) reported good foaming properties for capelin protein hydrolysates at low DH (12%). Foam expansion after whipping was monitored for 45 min to indicate the foam stability of protein hydrolysates. Within the first 15 min, SPHA1 with a concentration of 0.1% showed the highest foam stability (p < 0.001). Foaming properties are physicochemical characteristics of proteins, allowing them to form and stabilize foams. In the case of surfactants, the stability of foams is a consequence of the well-ordered orientation of the molecules at the interface, where the polar head is located in the aqueous phase and the hydrophobic chain faces the apolar component (Sanchez-Vioque et al. 2001).
Table 3.
The foaming properties of SPHs at 0.1%
| FE% | FS% | |||
|---|---|---|---|---|
| 15 min | 30 min | 45 min | ||
| SPHA1 | 80.1 ± 0.01** | 78.2 ± 0.054*** | 68.9 ± 0.08* | 55.2 ± 0.13** |
| SPHEE | 71.1 ± 0.06 | 68.1 ± 0.12 | 59 ± 0.034 | 51.2 ± 0.087 |
| SPHA21 | 85.1 ± 0.045 | 80.2 ± 0.09 | 62.1 ± 0.20 | 40.3 ± 0.076 |
| USP | 28.4 ± 0.21 | 20.3 ± 0.13 | 12.3 ± 0.03 | 5.5 ± 0.056 |
Values are given as mean±SD from triplicate determinations (n = 3)
Significantly differences between hydrolysates: *P < 0.05; **P < 0.01; *** P < 0.001
USP Undigested sardinelle protein
Sardinelle protein hydrolysates were obtained by treatment with crude enzyme preparations from B. pumilus A1 (SPHA1), B. mojavensis A21 (SPHA21) and crude enzyme extract f rom sardinelle (S. aurita) viscera (SPHEE)
Antioxidant activity of sardinelle muscle hydrolysates
The overall antioxidant action of protein hydrolysates is most likely attributed to the cooperative effects of several mechanisms, including, metal ion chelation, free-radical scavenging and singulet oxygen quenching (Chen et al. 1998). SPHs were assayed for antioxidative activity using various antioxidant assays, including 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical-scavenging activity, reducing power, chelating activity on Fe2+, β-carotene assay and DNA nicking assay.
DPPH radical-scavenging assay
Free radical-scavenging is a primary mechanism by which antioxidants inhibit oxidative processes. The DPPH radical-scavenging assay is a widely used method for evaluating the ability of hydrolysates to scavenge free radicals generated from DPPH reagent. DPPH is a stable free radical that shows maximum absorbance at 517 nm in ethanol. When DPPH radical encounters a proton-donating substrate such as an antioxidant, the radical is scavenged and the absorbance is reduced (Rajapakse et al. 2005). The decrease in absorbance is taken as a measure for radical scavenging activity.
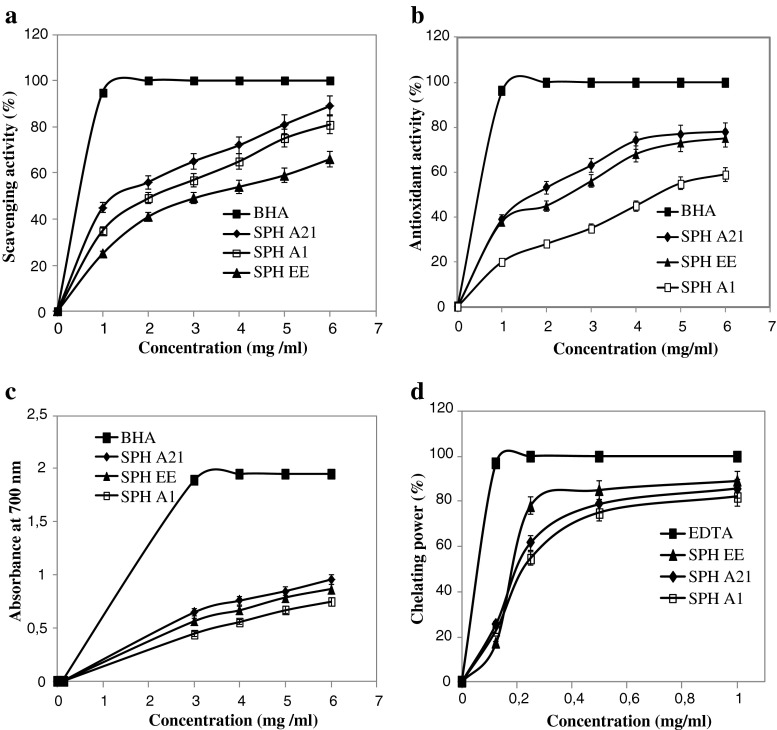
According to Fig. 3a, all the sardinelle protein hydrolysates tested exhibited high antioxidant activity against DPPH and the scavenging activity of all hydrolysates was concentration-dependant. Among all protein hydrolysates SPHA21 showed the highest activity. At 6 mg/ml, the DPPH radical scavenging activity reached 89%, followed by SPHA1 and SPHEE (78% and 66%, respectively) (p < 0.05). However, all hydrolysates showed a lower radical scavenging activity than BHA at the same concentrations.
Fig. 3.
Scavenging effect on DPPH free radical (a), antioxidant activity of SPHs using the β-carotene bleaching method (b), reducing power (c) and metal chelating activity (d) of SPHs at different concentrations. BHA (2.0 mM; 0.36 mg/ml) or EDTA (1 mg/ml) were used as standards (n = 3). Values presented are the mean of triplicate analyses. Sardinelle protein hydrolysates were obtained by treatment with crude enzyme preparations from B. pumilus A1 (SPHA1), B. mojavensis A21 (SPHA21) and crude enzyme extract f rom sardinelle (S. aurita) viscera (SPHEE)
The results obtained suggest that all SPHs contained some peptides that were electron donors and could react with free radicals to convert them to more stable products and terminate the radical chain reaction (Khantaphant and Benjakul 2008). The differences in the radical scavenging ability may be attributed to the difference amino acid composition of peptides within protein hydrolysates. Previous studies have reported that high DPPH radical-scavenging activity for the protein hydrolysates or peptides are usually associated with high hydrophobic amino acid (Rajapakse et al. 2005; Li et al. 2008). Similar results have been reported for protein hydrolysates from mackerel (Scomber austriasicus) (Wu et al. 2003) and round scad (Decapterus maruadsi) (Thiansilakul et al. 2007a) were also reported to possess DPPH radical scavenging activity.
Antioxidant activity measured by the β-carotene bleaching method
The antioxidant assay using the discoloration of β-carotene is widely employed to measure the antioxidant activity of bioactive compounds, because β-carotene is extremely susceptible to free radical-mediated oxidation of linoleic acid (Kumazawa et al. 2002). In this test, β-carotene undergoes rapid discoloration in the absence of antioxidant, which results in a reduction in absorbance of the test solution with increasing reaction time. The presence of antioxidant hinders the extent of bleaching by neutralizing the linoleic hydroperoxyl radicals formed.
The antioxidant activities of SPHs as measured by β-carotene bleaching are reported in Fig. 3b. All hydrolysates inhibited the oxidation of β-carotene to different degrees. SPHA21 showed higher ability to prevent bleaching of β-carotene than did SPHA1 and SPHEE (p < 0.05). Furthermore, as can be seen in Fig. 3b, the antioxidant activity of all hydrolysates increased with increasing sample concentration. These results demonstrated that SPHs have strong effects against the discoloration of β-carotene. However, the inhibition of β-carotene bleaching by all hydrolysates, were lower than that obtained by BHA (92.5%).
Reducing power activity
The reducing power assay is often used to evaluate the ability of antioxidant to donate an electron to the free radical (Khantaphant and Benjakul 2008). Samples with higher reducing power have better abilities to donate electron. In this study, the ability of SPHs to reduce Fe3+ to Fe2+ was determined.
Figure 3c shows the reducing power activities of the different hydrolysates at different concentrations. The reducing capacities of all SPHs and BHA are concentration dependent, the values increased with increasing concentration of samples. SPHs obtained by treatment with B. mojavensis A21 proteases exhibited higher reducing power activity than did the other SPHs. However, all hydrolysates showed lower reducing power activities than BHA at the same concentrations.
Different studies have reported that there is a direct correlation between antioxidant activities and reducing powers of some bioactive compounds (Duh 1998; Duh et al. 1999).
Ferrous ion-chelating activity
The chelation of Fe2+ was used to determine the ability of protein hydrolysates in metal-chelating activity. Ferrozine quantitatively forms complexes with Fe2+ ion. In the presence of chelating agents, the complexe formation is disrupted, resulting in the decrease in colour formation (Thiansilakul et al. 2007a). Ferrous chelating activities of SPHs at different concentrations are shown in Fig. 3d. The results indicated that all SPHs were able to chelate Fe2+ ion. Chelating activity against Fe2+ of all SPHs increased with increasing concentration of sample. Among protein hydrolysates, SPHEE exhibited the highest ferrous chelating activity (89% at 1 mg/ml) (p < 0.05). However, SPHs showed lower metal chelating activities than did EDTA, a known metal ion chelator, at all concentrations tested. For example, at 0.25 mg/ml, the metal chelating activities of EDTA, SPHEE, SPHA1 and SPHA21 were 100%, 78%, 54.82% and 61.58% respectively. Therefore, chelation of metal ions by peptides in hydrolysates would retard the oxidative reaction (Klompong et al. 2008). Ferrous chelating activity has been reported for hydrolysate of silver carp (Hypophthalmichthys molitrix) (Dong et al. 2008), round scad (Thiansilakul et al. 2007a) and yellow stripe trevally (Klompong et al. 2008). In fact, chelating activity of SPHs was higher than that of Flavourzyme-hydrolyzed silver carp protein which is 60% at 5 mg/ml (Dong et al. 2008).
The results indicate that hydrolysates can exhibit, to a various extent, antioxidant ability by capturing ferrous ion or other ions.
Transition metals, such as Fe2+ and Cu2+ can catalyse the generation of reactive oxygen species such as hydroxyradical (°OH) and superoxide anion (O−2) (Stohs and Bagchi 1995). Especially, Fe2+ generates °OH by the Fenton reaction, by which the lipid peroxidation chain reaction is accelerated. Therefore, the chelation of metal ions contributes to antioxidation.
Inhibition of supercoiled plasmid DNA scission induced by hydroxyl radicals
Hydroxyl radical is the most reactive free radical. It easily reacts with biomolecules, such as amino acids, proteins and DNA (Cacciuttolo et al. 1993). Therefore, scavenging of the hydroxyl radical is probably one of the most effective defences of a living body against various diseases.
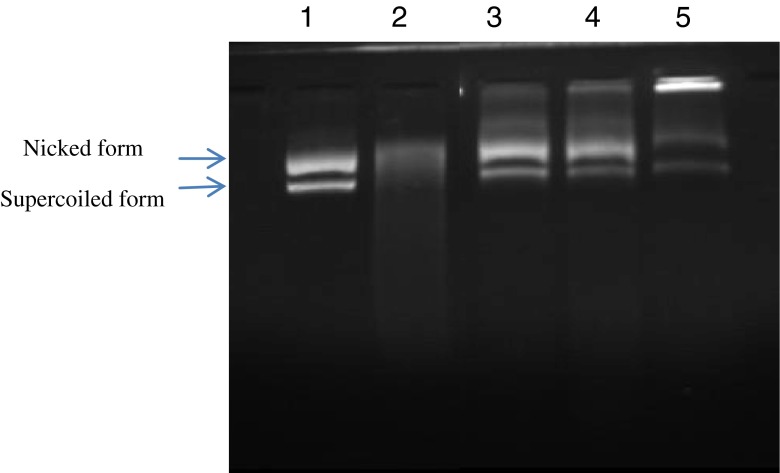
Hydroxyl radicals generated by the Fenton reaction are known to cause oxidative induced breaks in supercoiled plasmid DNA to yield its open circular or relaxed forms. Antioxidative activities of SPHs using DNA nicking assay are reported in Fig. 4. The line 1 represents the untreated plasmid (native DNA) with its two forms: the upper one is open-circular (nicked) DNA and the faster migrating band is supercoiled (closed circular) plasmid. Native DNA contains a high concentration of supercoiled DNA and low concentration of nicked DNA. Lane 2 shows the effect of hydroxyl radical on the native DNA. The reducing of the concentration of both forms indicates that DNA was degraded. Interestingly, all protein hydrolysates exhibited protection against hydroxyl radical induced DNA breakage. SPH EE and SPH A21 exhibited the strongest protection.
Fig. 4.
Gel electrophoresis pattern of the plasmid pCRII™TOPO incubated with Fenton’s reagent in the presence and absence of SPHs. Lane 1: untreated control: native pCRII™TOPO DNA (0.5 μg); lane 2: DNA sample incubated with Fenton’s reagent; lanes 3, 4, 5: Fenton’s reagent+DNA+2 mg SPHs, SPHEE, SPHA21 and SPHA1 respectively. Sardinelle protein hydrolysates were obtained by treatment with crude enzyme preparations from B. pumilus A1 (SPHA1), B. mojavensis A21 (SPHA21) and crude enzyme extract f rom sardinelle (S. aurita) viscera (SPHEE)
Conclusion
The protein hydrolysates obtained from sardinelle muscle may potentially serve as a good source of desirable quality of peptides and amino acids. It could be used as an emulsifier and as a foaming agent with antioxidative activities. Additionally, the solubility of hydrolysates was affected by pH. The results of this study indicate that sardinelle muscle hydrolysates exhibited, to a variable extent, antioxidant activities against various antioxidant systems in vitro, depending on the specificity of the enzyme used for hydrolysates production. Therefore, sardinelle protein hydrolysate can be used in food systems as a natural additive possessing antioxidative properties
Further works should be done to purify and identify antioxidant peptides from SPHs and determine their biological activities in vivo.
Acknowledgement
This work was funded by the Ministry of Higher Education and Scientific Research, Tunisia.
References
- Adler-Nissen J. A review of food hydrolysis specific areas. In: Adler-Nissen J, editor. Enzymic hydrolysis of food proteins. Copenhagen: Elsevier Applied Science Publishers; 1986. pp. 57–109. [Google Scholar]
- Official methods of analysis. 16. Washington: Association of Official Analytical Chemists; 1999. [Google Scholar]
- Official methods of analysis. 17. Washington: Association of Official Analytical Chemists; 2000. [Google Scholar]
- Ben Khaled H, Bougatef A, Balti R, Triki-Ellouz Y, Souissi N, Nasri M. Isolation and characterization of trypsin from sardinelle (Sardinella aurita) viscera. J Sci Food Agric. 2008;88:2654–2662. doi: 10.1002/jsfa.3386. [DOI] [Google Scholar]
- Benjakul S, Morrissey MT. Protein hydrolysates from Pacific whiting solid wastes. J Agric Food Chem. 1997;45:3423–3430. doi: 10.1021/jf970294g. [DOI] [Google Scholar]
- Bersuder P, Hole M, Smith G. Antioxidants from a heated histidine–glucose model system. I. Investigation of the antioxidant role of histidine and isolation of antioxidants by high- performance liquid chromatography. J Am Oil Chem Soc. 1998;75:181–187. doi: 10.1007/s11746-998-0030-y. [DOI] [Google Scholar]
- Botterweck AAM, Verhagen H, Goldbohm RA, Kleinjans J, Van den Brandt PA. Intake of butylated hydroxyanisole and butylated hydroxytoluene and stomach cancer risk: results from analyses in the Netherlands cohort study. Food Chem Toxicol. 2000;38:599–605. doi: 10.1016/S0278-6915(00)00042-9. [DOI] [PubMed] [Google Scholar]
- Bougatef A, Nedjar-Arroume N, Ravallec-Plé R, Leroy Y, Guillochon D, Barkia A, Nasri M. Angiotensin I-converting enzyme (ACE) inhibitory activities of sardinelle (Sardinella aurita) by-products protein hydrolysates obtained by treatment with microbial and visceral fish serine proteases. Food Chem. 2008;111:350–356. doi: 10.1016/j.foodchem.2008.03.074. [DOI] [PubMed] [Google Scholar]
- Bougatef A, Hajji M, Balti R, Lassoued I, Triki-Ellouz Y, Nasri M. Antioxidant and free radical-scavenging activities of smooth hound (Mustelus mustelus) muscle protein hydrolysates obtained by gastrointestinal proteases. Food Chem. 2009;114:1198–1205. doi: 10.1016/j.foodchem.2008.10.075. [DOI] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Castenga A, Pocernich CB, Drake J, Scapagnini G, Calabrese V. Nutritional approaches to combat oxidative stress in Alzheimer’s disease. J Nutr Biochem. 2002;13:444–461. doi: 10.1016/S0955-2863(02)00205-X. [DOI] [PubMed] [Google Scholar]
- Cacciuttolo MA, Trinh L, Lumpkin JA, Rao G. Hyperoxia induces DNA damage in mammalian cells. Free Radic Biol Med. 1993;14:267–276. doi: 10.1016/0891-5849(93)90023-N. [DOI] [PubMed] [Google Scholar]
- Chen HM, Muramoto K, Yamauchi F, Fujimoto K, Nokihara K. Antioxidative properties of histidine-containing peptides designed from peptide fragments found in the digests of a soybean protein. J Agric Food Chem. 1998;46:49–53. doi: 10.1021/jf970649w. [DOI] [PubMed] [Google Scholar]
- De Vries DJ, Beart PM. Fishing for drugs from the sea: status and strategies. Trends Pharmacol Sci. 1995;16:275–279. doi: 10.1016/S0165-6147(00)89045-8. [DOI] [PubMed] [Google Scholar]
- Dinis TC, Maderia VM, Almeida LM. Action of phenolic derivatives (acetaminophen, salicylate, and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch Biochem Biophys. 1994;315:161–169. doi: 10.1006/abbi.1994.1485. [DOI] [PubMed] [Google Scholar]
- Dong S, Zeng M, Wang D, Liu Z, Zhao Y, Yang H. Antioxidant and biochemical properties of protein hydrolysates prepared from silver carp (Hypophthalmichthys molitrix) Food Chem. 2008;107:1485–1493. doi: 10.1016/j.foodchem.2007.10.011. [DOI] [Google Scholar]
- Duh PD. Antioxidant activity of burdock (Arctium lappa Linne): it’s scavenging effect on free radical and active oxygen. J Am Oil Chem Soc. 1998;75:455–461. doi: 10.1007/s11746-998-0248-8. [DOI] [Google Scholar]
- Duh PD, Du PC, Yen GC. Action of methanolic extract of mung bean hulls as inhibitors of lipid peroxidation and non-lipid oxidative damage. Food Chem Toxicol. 1999;37:1055–1061. doi: 10.1016/S0278-6915(99)00096-4. [DOI] [PubMed] [Google Scholar]
- Fakhfakh-Zouari N, Haddar A, Hmidet N, Frikha F, Nasri M. Application of statistical experimental design for optimization of keratinases production by Bacillus pumilus A1 grown on chicken feather and some biochemical properties. Process Biochem. 2010;45:617–626. doi: 10.1016/j.procbio.2009.12.007. [DOI] [Google Scholar]
- Gbogouri GA, Linder M, Fanni J, Parmentier M. Influence of hydrolysis degree on the functional properties of salmon byproducts hydrolysates. J Food Sci. 2004;69:615–619. doi: 10.1111/j.1365-2621.2004.tb09909.x. [DOI] [Google Scholar]
- Haddar A, Agrebi R, Bougatef A, Hmidet N, Sellami-Kamoun A, Nasri M. Two detergent stable alkaline serine-proteases from Bacillus mojavensis A21: Purification, characterization and potential application as a laundry detergent additive. Bioresour Technol. 2009;100:3366–3373. doi: 10.1016/j.biortech.2009.01.061. [DOI] [PubMed] [Google Scholar]
- Ito N, Hirose M, Fukushima S, Tsuda H, Shirai T, Tatematsu M. Studies on antioxidants: their carcinogenic and modifying effects on chemical carcinogenesis. Food Chem Toxicol. 1986;24:1071–1081. doi: 10.1016/0278-6915(86)90291-7. [DOI] [PubMed] [Google Scholar]
- Kembhavi AA, Kulkarni A, Pant A. Salt-tolerant and thermostable alkaline protease from Bacillus subtilis NCIM No.64. Appl Biochem Biotechnol. 1993;38:83–92. doi: 10.1007/BF02916414. [DOI] [PubMed] [Google Scholar]
- Khantaphant S, Benjakul S. Comparative study on the proteases from fish pyloric caeca and the use for production of gelatin hydrolysate with antioxidative activity. Comp Biochem Physiol. 2008;151B:410–419. doi: 10.1016/j.cbpb.2008.08.011. [DOI] [PubMed] [Google Scholar]
- Khantaphant S, Benjakul S, Reza Ghomi M. The effects of pretreatments on antioxidative activities of protein hydrolysate from the muscle of brownstripe red snapper (Lutjanus vitta) LWT- Food Sci Technol. 2011;4:1139–1148. doi: 10.1016/j.lwt.2010.10.009. [DOI] [Google Scholar]
- Kinsella JE. Functional properties of proteins in food: a survey. Crit Rev Food Sci Nutr. 1976;8:219–280. doi: 10.1080/10408397609527208. [DOI] [Google Scholar]
- Klompong V, Benjakul S, Kantachote D, Shahidi F. Antioxidative activity and functional properties of protein hydrolysate of yellow stripe trevally (Selaroides leptolepis) as influenced by the degree of hydrolysis and enzyme type. Food Chem. 2007;102:1317–1327. doi: 10.1016/j.foodchem.2006.07.016. [DOI] [Google Scholar]
- Klompong V, Benjakul S, Kantachote D, Hayes KD, Shahidi F. Comparative study on antioxidative activity of yellow stripe trevally protein hydrolysate produced from Alcalase and Flavourzyme. Int J Food Sci Technol. 2008;43:1019–1026. doi: 10.1111/j.1365-2621.2007.01555.x. [DOI] [Google Scholar]
- Klompong V, Benjakul S, Yachai M, Visessanguan W, Shahidi F, Hayes KD. Amino acid composition and antioxidative peptides from protein hydrolysates of yellow stripe trevally (Selaroides leptolepis) J Food Sci. 2009;74:C126–C133. doi: 10.1111/j.1750-3841.2009.01047.x. [DOI] [PubMed] [Google Scholar]
- Koleva II, Van Beek TA, Linssen JPH, De Groot A, Evstatieva LN. Screening of plant extracts for antioxidant activity: a comparative study on three testing methods. Phytochem Anal. 2002;13:8–17. doi: 10.1002/pca.611. [DOI] [PubMed] [Google Scholar]
- Kristinsson HG, Rasco BA. Fish protein hydrolysates: production, biochemical, and functional properties. Crit Rev Food Sci Nutr. 2000;40:43–81. doi: 10.1080/10408690091189266. [DOI] [PubMed] [Google Scholar]
- Kristinsson HG, Rasco BA. Biochemical and functional properties of Atlantic salmon (Salmo salar) muscle hydrolyzed with various alkaline proteases. J Agric Food Chem. 2000;48:657–666. doi: 10.1021/jf990447v. [DOI] [PubMed] [Google Scholar]
- Kumazawa S, Taniguchi M, Suzuki Y, Shimura M, Kwon MS, Nakayama T. Antioxidant activity of polyphenols in carob pods. J Agric Food Chem. 2002;50:373–377. doi: 10.1021/jf010938r. [DOI] [PubMed] [Google Scholar]
- Lawal OS. Functionality of African locust bean (Parkia biglobossa) protein isolate: effects of pH, ionic strength and various protein concentrations. Food Chem. 2004;86:345–355. doi: 10.1016/j.foodchem.2003.09.036. [DOI] [Google Scholar]
- Li Y, Jiang B, Zhang T, Mu W, Liu J. Antioxidant and free radical-scavenging activities of chickpea protein hydrolysate (CPH) Food Chem. 2008;106:444–450. doi: 10.1016/j.foodchem.2007.04.067. [DOI] [Google Scholar]
- Liceaga-Gesualdo AM, Li-Chan ECY. Functional properties of fish protein hydrolysate from herring (Clupea harengus) J Food Sci. 1999;6:1000–1004. doi: 10.1111/j.1365-2621.1999.tb12268.x. [DOI] [Google Scholar]
- Löliger J (1991) The use of antioxidants in foods. In: Aruoma OI, Halliwell B (eds) Free radicals and food additives. London, pp 121–150.
- Manso MA, Miguel M, Even J, Hernández R, Aleixandre A, LópezFandińo R. Effect of the long-term intake of an egg white hydrolysate on the oxidative status and blood lipid profile of spontaneously hypertensive rats. Food Chem. 2008;109:361–367. doi: 10.1016/j.foodchem.2007.12.049. [DOI] [PubMed] [Google Scholar]
- Nasirullah, Jeyarani T, Rakshitha D. Isolation and antioxidant efficacy of nutraceutical concentrates from sesame and flax seed oils. J Food Sci Technol. 2009;46:66–69. [Google Scholar]
- Pearce KN, Kinsella JE. Emulsifying properties of proteins: evaluation of a turbidimetric technique. J Agric Food Chem. 1978;26:716–723. doi: 10.1021/jf60217a041. [DOI] [Google Scholar]
- Peterson BR. The impact of the enzymatic hydrolysis process on recovery and use of proteins. In: Birch GG, Blakebrough N, Parker KJ, editors. Enzymes and food processing. London: Elsevier Applied Science Publishers; 1981. pp. 269–299. [Google Scholar]
- Pryor WA, Ann NY. Free radical biology: xenobiotics, cancer, and aging. Acad Sci. 1982;393:1–22. doi: 10.1111/j.1749-6632.1982.tb31228.x. [DOI] [PubMed] [Google Scholar]
- Rajapakse N, Mendis E, Byun HG, Kim SK. Purification and in vitro antioxidative effects of giant squid muscle peptides on free radical-mediated oxidative systems. J Nutr Biochem. 2005;16:562–569. doi: 10.1016/j.jnutbio.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Saiga A, Tanabe S, Nishimura T. Antioxidant activity of peptides obtained from porcine myofibrillar proteins by protease treatment. J Agric Food Chem. 2003;51:3661–3667. doi: 10.1021/jf021156g. [DOI] [PubMed] [Google Scholar]
- Sanchez-Vioque R, Bagger CL, Rabiller C, Gueguen J. Foaming properties of acylated rapeseed (Brassica napus L.) hydrolysates. J Colloid Interface Sci. 2001;244:386–393. doi: 10.1006/jcis.2001.7932. [DOI] [Google Scholar]
- Sathivel S, Bechtel P, Babbitt J, Smiley S, Crapro C, Reppond K, et al. Biochemical and functional properties of herring (Clupea harengus) byproduct hydrolysates. J Food Sci. 2003;68:2196–2200. doi: 10.1111/j.1365-2621.2003.tb05746.x. [DOI] [Google Scholar]
- Seema MN, Jayaprakasha GK, Singh RP. Antioxidant activity of custard apple (Annona squamosa) peel and seed extracts. J Food Sci Technol. 2008;45:349–352. [Google Scholar]
- Seifried HE, Anderson DE, Fisher EI, Milner JA. A review of the interaction among dietary antioxidants and reactive oxygen species. J Nutr Biochem. 2007;18:567–579. doi: 10.1016/j.jnutbio.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Shahidi F, Xiao-Qing H, Synowiecki J. Production and characteristics of protein hydrolysates from capelin (Mallotus villosus) Food Chem. 1995;53:285–293. doi: 10.1016/0308-8146(95)93934-J. [DOI] [Google Scholar]
- Shui G, Leong LP. Residue from star fruit as valuable source of functional food ingredients and antioxidant nutraceuticals. Food Chem. 2006;97:277–284. doi: 10.1016/j.foodchem.2005.03.048. [DOI] [Google Scholar]
- Stohs SJ, Bagchi D. Oxidative mechanisms in the toxicity of metal ions. Free Radical Biol Med. 1995;18:321–336. doi: 10.1016/0891-5849(94)00159-H. [DOI] [PubMed] [Google Scholar]
- Thiansilakul Y, Benjakul S, Shahidi F. Compositions, functional properties and antioxidative activity of protein hydrolysates prepared from round scad (Decapterus maruadsi) Food Chem. 2007;103:1385–1394. doi: 10.1016/j.foodchem.2006.10.055. [DOI] [Google Scholar]
- Thiansilakul Y, Benjakul S, Shahidi F. Antioxidative activity of protein hydrolysate from round scad muscle using alcalase and flavourzyme. J Food Biochem. 2007;31:266–287. doi: 10.1111/j.1745-4514.2007.00111.x. [DOI] [Google Scholar]
- Tsumura K, Saito T, Tsuge K, Ashida H, Kugimiya W, Inouye K. Functional properties of soy protein hydrolysates obtained by selective proteolysis. LWT. 2005;38:255–261. doi: 10.1016/j.lwt.2004.06.007. [DOI] [Google Scholar]
- Wu HC, Chen HM, Shiau CY. Free amino acids and peptides as related to antioxidant properties in protein hydrolysates of mackerel (Scomber austriasicus) Food Res Inter. 2003;36:949–957. doi: 10.1016/S0963-9969(03)00104-2. [DOI] [Google Scholar]
- Yildirim A, Mavi A, Kara AA. Determination of antioxidant and antimicrobial activities of Rumex crispus L. extracts. J Agric Food Chem. 2001;49:4083–4089. doi: 10.1021/jf0103572. [DOI] [PubMed] [Google Scholar]
- You L, Zhao M, Regenstein JM, Ren J. Changes in the antioxidant activity of loach (Misgurnus anguillicaudatus) protein hydrolysates during a simulated gastrointestinal digestion. Food Chem. 2010;120:810–816. doi: 10.1016/j.foodchem.2009.11.018. [DOI] [Google Scholar]
- Zayas JF (1997) Solubility of proteins. In Functionality of proteins in food. Springer-Verlag, Berlin, pp 6–22