Abstract
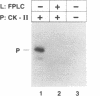
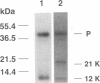
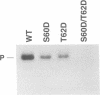
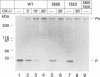
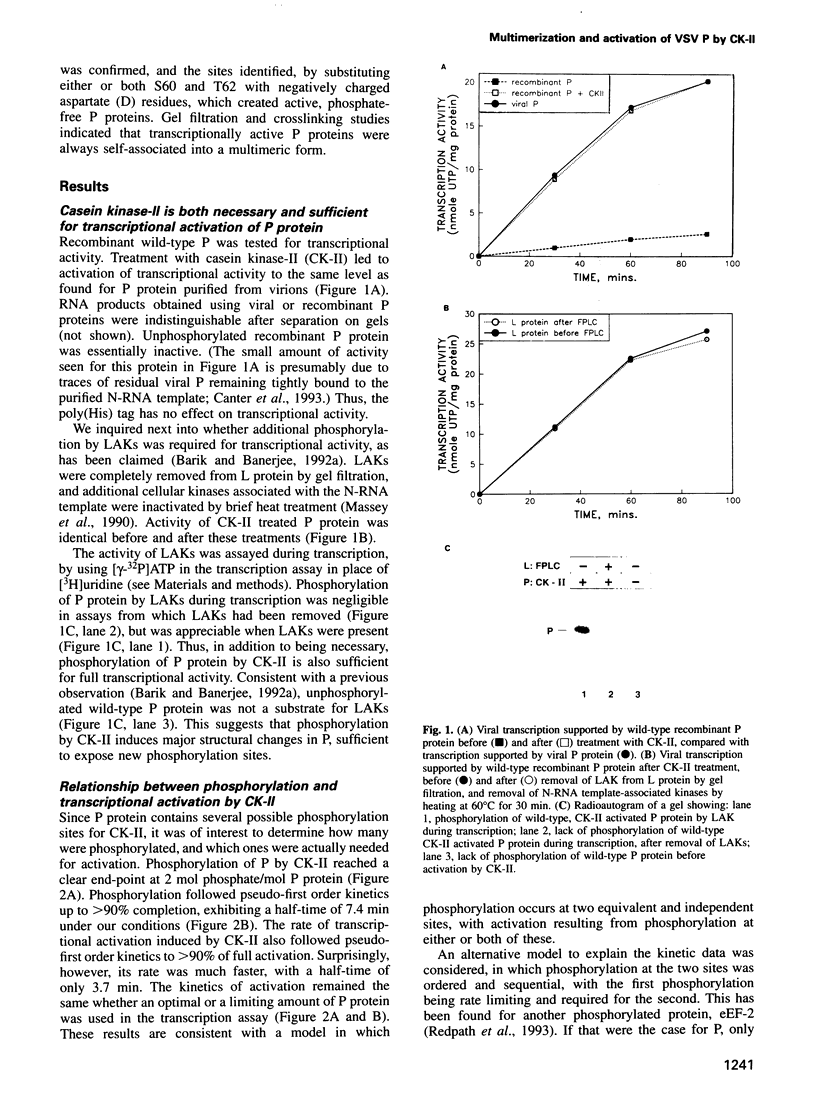
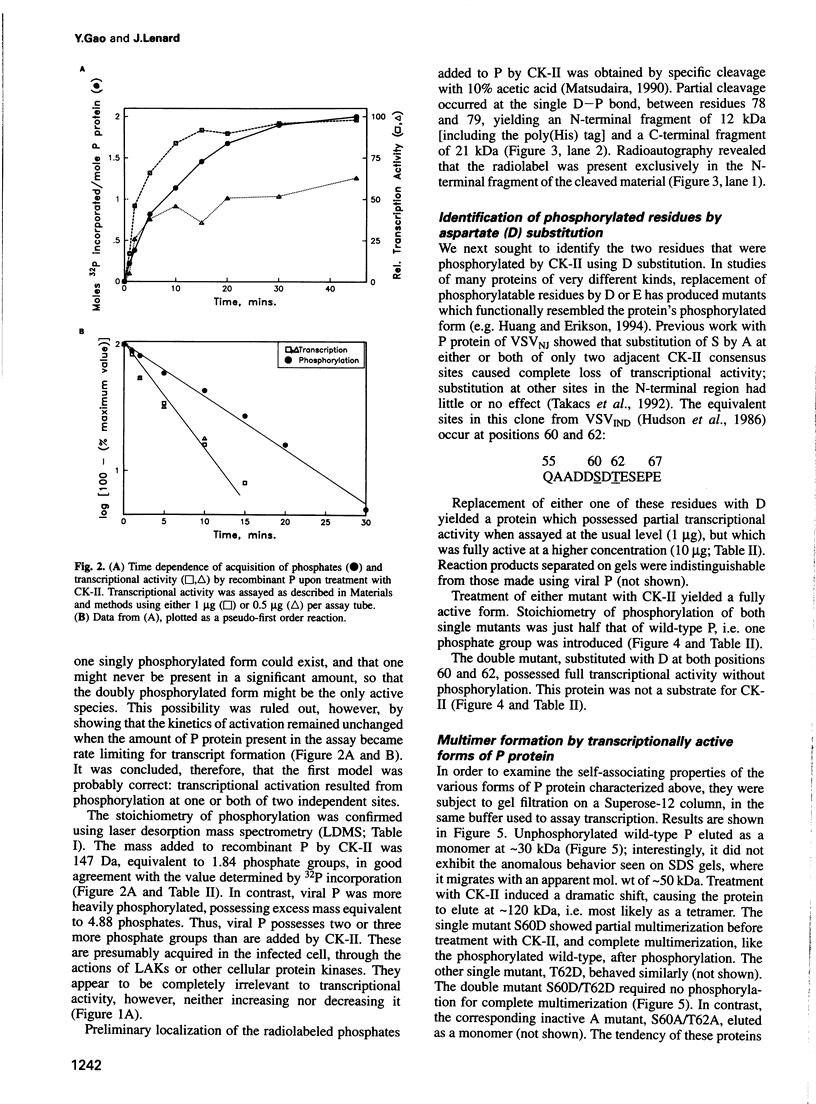
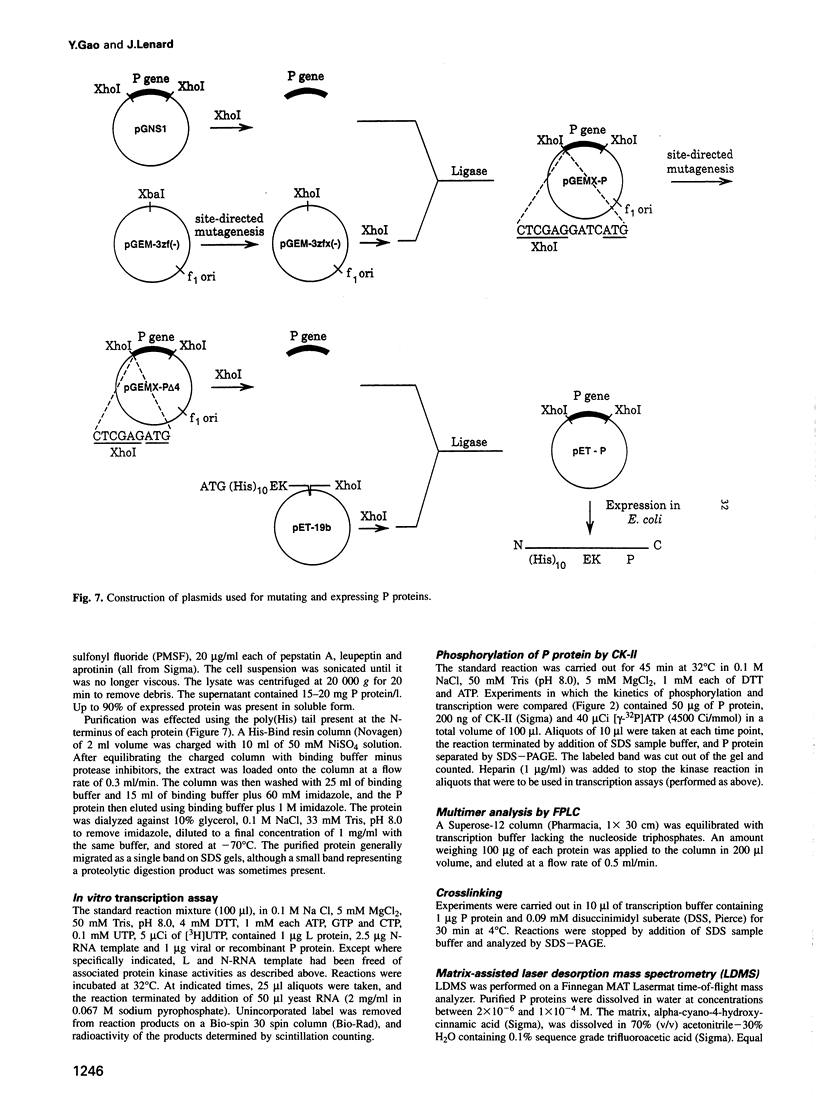
Casein kinase-II (CK-II) is a widely distributed protein kinase, which plays numerous roles in the regulation of transcription through modification of transacting transcription factors. Phosphorylation of vesicular stomatitis virus (VSV) P protein by CK-II was found to be both necessary and sufficient for transcriptional activation. Upon treatment of P by CK-II, activity was acquired faster (t1/2 = 3.7 min) than were total phosphates (t1/2 = 7.4 min). Stoichiometry was 2 mol phosphate/mol P, indicating activation by phosphorylation at either one or both of two independent sites. The sites were identified by substituting aspartate (D) residues at either S60 or T62, producing proteins that were partly active without phosphorylation, but were fully active at higher concentrations; CK-II added only a single phosphate group to each of these, and conferred full activity. P protein doubly substituted with D at S60 and T62 was fully active without phosphorylation, and was not a substrate for CK-II. Active P protein, whether CK-II treated or doubly substituted, was shown by gel filtration and crosslinking to exist as a discretely multimeric, probably tetrameric, structure. The singly substituted mutants were partly multimeric, becoming fully so after CK-II treatment. Phosphorylation by CK-II thus mediates the self-association of P into the multimeric, transcriptionally active form.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barik S., Banerjee A. K. Phosphorylation by cellular casein kinase II is essential for transcriptional activity of vesicular stomatitis virus phosphoprotein P. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6570–6574. doi: 10.1073/pnas.89.14.6570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barik S., Banerjee A. K. Sequential phosphorylation of the phosphoprotein of vesicular stomatitis virus by cellular and viral protein kinases is essential for transcription activation. J Virol. 1992 Feb;66(2):1109–1118. doi: 10.1128/jvi.66.2.1109-1118.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckes J. D., Perrault J. Stepwise phosphorylation of vesicular stomatitis virus P protein by virion-associated kinases and uncoupling of second step from in vitro transcription. Virology. 1992 Jun;188(2):606–617. doi: 10.1016/0042-6822(92)90515-q. [DOI] [PubMed] [Google Scholar]
- Bell J. C., Prevec L. Phosphorylation sites on phosphoprotein NS of vesicular stomatitis virus. J Virol. 1985 Jun;54(3):697–702. doi: 10.1128/jvi.54.3.697-702.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilsel P. A., Rowe J. E., Fitch W. M., Nichol S. T. Phosphoprotein and nucleocapsid protein evolution of vesicular stomatitis virus New Jersey. J Virol. 1990 Jun;64(6):2498–2504. doi: 10.1128/jvi.64.6.2498-2504.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canter D. M., Jackson R. L., Perrault J. Faithful and efficient in vitro reconstitution of vesicular stomatitis virus transcription using plasmid-encoded L and P proteins. Virology. 1993 Jun;194(2):518–529. doi: 10.1006/viro.1993.1290. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay D., Banerjee A. K. Phosphorylation within a specific domain of the phosphoprotein of vesicular stomatitis virus regulates transcription in vitro. Cell. 1987 May 8;49(3):407–414. doi: 10.1016/0092-8674(87)90293-5. [DOI] [PubMed] [Google Scholar]
- Clinton G. M., Burge B. W., Huang A. S. Phosphoproteins of vesicular stomatitis virus: identity and interconversion of phosphorylated forms. Virology. 1979 Nov;99(1):84–94. doi: 10.1016/0042-6822(79)90039-4. [DOI] [PubMed] [Google Scholar]
- Davis N. L., Arnheiter H., Wertz G. W. Vesicular stomatitis virus N and NS proteins form multiple complexes. J Virol. 1986 Sep;59(3):751–754. doi: 10.1128/jvi.59.3.751-754.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De B. P., Banerjee A. K. Specific interactions of vesicular stomatitis virus L and NS proteins with heterologous genome ribonucleoprotein template lead to mRNA synthesis in vitro. J Virol. 1984 Sep;51(3):628–634. doi: 10.1128/jvi.51.3.628-634.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson S. U., Schubert M. Location of the binding domains for the RNA polymerase L and the ribonucleocapsid template within different halves of the NS phosphoprotein of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5655–5659. doi: 10.1073/pnas.84.16.5655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond D. C., Haley B. E., Lesnaw J. A. Identification and characterization of serine/threonine protein kinase activity intrinsic to the L protein of vesicular stomatitis virus New Jersey. J Gen Virol. 1992 Jan;73(Pt 1):67–75. doi: 10.1099/0022-1317-73-1-67. [DOI] [PubMed] [Google Scholar]
- Hsu C. H., Morgan E. M., Kingsbury D. W. Site-specific phosphorylation regulates the transcriptive activity of vesicular stomatitis virus NS protein. J Virol. 1982 Jul;43(1):104–112. doi: 10.1128/jvi.43.1.104-112.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W., Erikson R. L. Constitutive activation of Mek1 by mutation of serine phosphorylation sites. Proc Natl Acad Sci U S A. 1994 Sep 13;91(19):8960–8963. doi: 10.1073/pnas.91.19.8960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson L. D., Condra C., Lazzarini R. A. Cloning and expression of a viral phosphoprotein: structure suggests vesicular stomatitis virus NS may function by mimicking an RNA template. J Gen Virol. 1986 Aug;67(Pt 8):1571–1579. doi: 10.1099/0022-1317-67-8-1571. [DOI] [PubMed] [Google Scholar]
- Hunt D. M., Smith E. F., Buckley D. W. Aberrant polyadenylation by a vesicular stomatitis virus mutant is due to an altered L protein. J Virol. 1984 Nov;52(2):515–521. doi: 10.1128/jvi.52.2.515-521.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T., Karin M. The regulation of transcription by phosphorylation. Cell. 1992 Aug 7;70(3):375–387. doi: 10.1016/0092-8674(92)90162-6. [DOI] [PubMed] [Google Scholar]
- Kingsford L., Emerson S. U. Transcriptional activities of different phosphorylated species of NS protein purified from vesicular stomatitis virions and cytoplasm of infected cells. J Virol. 1980 Mar;33(3):1097–1105. doi: 10.1128/jvi.33.3.1097-1105.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretzschmar M., Kaiser K., Lottspeich F., Meisterernst M. A novel mediator of class II gene transcription with homology to viral immediate-early transcriptional regulators. Cell. 1994 Aug 12;78(3):525–534. doi: 10.1016/0092-8674(94)90429-4. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Massey D. M., Deans N., Lenard J. Phosphorylation of NS protein by vesicular stomatitis virus nucleocapsids: lack of effect during RNA synthesis and separation of kinase from L protein. J Virol. 1990 Jul;64(7):3259–3264. doi: 10.1128/jvi.64.7.3259-3264.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters P. S., Banerjee A. K. Resolution of multiple complexes of phosphoprotein NS with nucleocapsid protein N of vesicular stomatitis virus. J Virol. 1988 Aug;62(8):2651–2657. doi: 10.1128/jvi.62.8.2651-2657.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsudaira P. Limited N-terminal sequence analysis. Methods Enzymol. 1990;182:602–613. doi: 10.1016/0076-6879(90)82047-6. [DOI] [PubMed] [Google Scholar]
- Nichol S. T., Rowe J. E., Fitch W. M. Punctuated equilibrium and positive Darwinian evolution in vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1993 Nov 15;90(22):10424–10428. doi: 10.1073/pnas.90.22.10424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peluso R. W. Kinetic, quantitative, and functional analysis of multiple forms of the vesicular stomatitis virus nucleocapsid protein in infected cells. J Virol. 1988 Aug;62(8):2799–2807. doi: 10.1128/jvi.62.8.2799-2807.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redpath N. T., Price N. T., Severinov K. V., Proud C. G. Regulation of elongation factor-2 by multisite phosphorylation. Eur J Biochem. 1993 Apr 15;213(2):689–699. doi: 10.1111/j.1432-1033.1993.tb17809.x. [DOI] [PubMed] [Google Scholar]
- Rigaut K. D., Gao Y., Lenard J. Effects of staurosporine on transcription by vesicular stomatitis virus. Virology. 1993 Jun;194(2):433–440. doi: 10.1006/viro.1993.1282. [DOI] [PubMed] [Google Scholar]
- Sánchez A., De B. P., Banerjee A. K. In vitro phosphorylation of NS protein by the L protein of vesicular stomatitis virus. J Gen Virol. 1985 May;66(Pt 5):1025–1036. doi: 10.1099/0022-1317-66-5-1025. [DOI] [PubMed] [Google Scholar]
- Takacs A. M., Barik S., Das T., Banerjee A. K. Phosphorylation of specific serine residues within the acidic domain of the phosphoprotein of vesicular stomatitis virus regulates transcription in vitro. J Virol. 1992 Oct;66(10):5842–5848. doi: 10.1128/jvi.66.10.5842-5848.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takacs A. M., Das T., Banerjee A. K. Mapping of interacting domains between the nucleocapsid protein and the phosphoprotein of vesicular stomatitis virus by using a two-hybrid system. Proc Natl Acad Sci U S A. 1993 Nov 1;90(21):10375–10379. doi: 10.1073/pnas.90.21.10375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuazon P. T., Traugh J. A. Casein kinase I and II--multipotential serine protein kinases: structure, function, and regulation. Adv Second Messenger Phosphoprotein Res. 1991;23:123–164. [PubMed] [Google Scholar]