Abstract
Extracellular heat shock protein 72 (eHsp72) concentration increases during exercise-heat stress when conditions elicit physiological strain. Differences in severity of environmental and exercise stimuli have elicited varied response to stress. The present study aimed to quantify the extent of increased eHsp72 with increased exogenous heat stress, and determine related endogenous markers of strain in an exercise-heat model. Ten males cycled for 90 min at 50 % 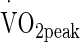 in three conditions (TEMP, 20 °C/63 % RH; HOT, 30.2 °C/51%RH; VHOT, 40.0 °C/37%RH). Plasma was analysed for eHsp72 pre, immediately post and 24-h post each trial utilising a commercially available ELISA. Increased eHsp72 concentration was observed post VHOT trial (+172.4 %) (p < 0.05), but not TEMP (−1.9 %) or HOT (+25.7 %) conditions. eHsp72 returned to baseline values within 24 h in all conditions. Changes were observed in rectal temperature (Trec), rate of Trec increase, area under the curve for Trec of 38.5 and 39.0 °C, duration Trec ≥ 38.5 and ≥39.0 °C, and change in muscle temperature, between VHOT, and TEMP and HOT, but not between TEMP and HOT. Each condition also elicited significantly increasing physiological strain, described by sweat rate, heart rate, physiological strain index, rating of perceived exertion and thermal sensation. Stepwise multiple regression reported rate of Trec increase and change in Trec to be predictors of increased eHsp72 concentration. Data suggests eHsp72 concentration increases once systemic temperature and sympathetic activity exceeds a minimum endogenous criteria elicited during VHOT conditions and is likely to be modulated by large, rapid changes in core temperature.
in three conditions (TEMP, 20 °C/63 % RH; HOT, 30.2 °C/51%RH; VHOT, 40.0 °C/37%RH). Plasma was analysed for eHsp72 pre, immediately post and 24-h post each trial utilising a commercially available ELISA. Increased eHsp72 concentration was observed post VHOT trial (+172.4 %) (p < 0.05), but not TEMP (−1.9 %) or HOT (+25.7 %) conditions. eHsp72 returned to baseline values within 24 h in all conditions. Changes were observed in rectal temperature (Trec), rate of Trec increase, area under the curve for Trec of 38.5 and 39.0 °C, duration Trec ≥ 38.5 and ≥39.0 °C, and change in muscle temperature, between VHOT, and TEMP and HOT, but not between TEMP and HOT. Each condition also elicited significantly increasing physiological strain, described by sweat rate, heart rate, physiological strain index, rating of perceived exertion and thermal sensation. Stepwise multiple regression reported rate of Trec increase and change in Trec to be predictors of increased eHsp72 concentration. Data suggests eHsp72 concentration increases once systemic temperature and sympathetic activity exceeds a minimum endogenous criteria elicited during VHOT conditions and is likely to be modulated by large, rapid changes in core temperature.
Keywords: Heat stress, Heat strain, Heat shock protein, Hyperthermia, Core temperature
Introduction
The human 72 kDa heat shock protein (Hsp72), HSPA1A (Kampinga et al. 2009) is the highly inducible isoform of a large family of proteins with an important role as a molecular chaperone maintaining cellular homeostasis, particularly in response to thermal stimuli (Mizzen and Welch 1988). Research has identified extracellular changes in Hsp72 concentration within whole blood (Marshall et al. 2006; Yamada et al. 2007; Ogura et al. 2008; Magalhães et al. 2010; Périard et al. 2012), and intracellular changes in total protein expression and/or gene transcription in monocytes and systemic tissue (McClung et al. 2008; Selkirk et al. 2009; Magalhães et al. 2010; Amorim et al. 2011) in response to thermal and exercise stress. Hsp72 binds with high affinity to the plasma membrane (Asea et al. 2000) and up-regulates expression of pro-inflammatory cytokines, tumour necrosis factor-α, interleukin-1β and interleukin-6 in human monocytes. Circulating extracellular heat shock protein 72 (eHsp72) acts as an inflammatory molecule and induces cytokine production in immune cells (Asea 2006). The precise biological role of eHsp72 in response to exercise-heat stress has not been fully elucidated; it is believed to contribute to the exercise-related inflammatory reaction (Asea 2003). Acknowledgements have been made by Ogura et al. (2008) that body temperature elevation, and increased circulating catecholamines by supplementation (Whitham et al. 2006) or exercise response (Whitham et al. 2007), in addition to thermal change increase eHsp72. Acute exercise-heat stress presents both thermal and sympathetic challenge and as such, changes in concentration might be used to describe the magnitude of stress presented to an individual or system exercising in different environments.
eHsp72 has been detected in peripheral circulation of healthy individuals (Pockley et al. 1998) and is known to increase in response to single bouts of exercise (Walsh et al. 2001; Febbraio et al. 2002; Fehrenbach et al. 2005). Thermal, oxidative, metabolic and chemical stresses are well reported stimuli for increased concentrations of intracellular (iHsp72), and eHsp72 (Welch 1992; Morimoto et al. 1994). Exercise in hot and humid environments increases physiological strain on the body in comparison with temperate conditions (Galloway and Maughan 1997). Combined with exercise (exercise-heat stress), environmental manipulation to induce hyperthermia (Fehrenbach et al. 2001; Oishi et al. 2002; Moran et al. 2006; Whitham et al. 2007; Sandström et al. 2008; Iguchi et al. 2012) have been reported as stimuli for further increasing eHsp72 compared to exercise alone. Indeed a strong relationship exists between plasma eHsp72 and core temperature (Ruell et al. 2006; Sandström et al. 2009).
Repeated daily exposure to exercise and/or environmental stress results in sequential (i.e. day-on-day) increases in eHsp72 expression (Sandström et al. 2008). In vivo, such a paradigm is utilised in the attainment of a heat acclimated (HA) phenotype (Magalhães et al. 2010; Lorenzo et al. 2010, 2011; Hom et al. 2012), with increases in iHsp72 expression accompanied by “classic” physiological adaptations (e.g. cardiovascular stability; reduced core temperature at rest and during exercise; more rapid sudomotor onset and efficiency, etc.) (Garrett et al. 2011). The response of eHsp72 to environmental factors has not been uniform, with significant increases (Whitham et al. 2007; Yamada et al. 2007; McClung et al. 2008; Magalhães et al. 2010; Périard et al. 2012), or no change (Marshall et al. 2006; Watkins et al. 2007; Hom et al. 2012) from rested basal values reported.
Exercise-heat stress research has largely implemented experimental designs where exogenous (external) factors of exercise intensity and exercise-heat stress conditions are controlled to elicit and measure changes in endogenous (internal) response. It is likely that endogenous factors are more relevant signals for stress response than exogenous variables; eHsp72 accumulation being one indicator of stress (Ruell et al. 2006). Establishment of appropriate endogenous markers and apparent minimum endogenous criteria for eHsp72 release could facilitate economical prescription of repeated exercise-heat sessions with intent of inducing the HA phenotype, a similar notion has been proposed by Gagnon et al. (2013), with regards to investigating heat balance. More efficient procurement of HA typically achieved through exercise-heat stress exposures (≥30 °C) of ≥60 min and repeated for 5–14 sessions (Garrett et al. 2011) would allow researchers and practitioners to prepare individuals most effectively for subsequent work in conditions presenting thermal challenge. At present, the magnitude of expression has not yet been reported directly comparing changes in human eHsp72 following identical exercise in graded exogenous environments with description of changes eHsp72 compared with established endogenous physiological and thermal markers (peak and mean heart rate, core, and muscle temperature). The introduction of novel markers (rate of increase and change in core temperature, area under the curve (AUC) for core temperatures of 38.5 and 39.0 °C, duration spent exercising with core temperature ≥38.5 and ≥39.0 °C) may identify additional criteria for the prescription of exercise-heat stress based upon analysis of the acute response to stress.
The aim of this study was to determine whether increased concentration of eHsp72 were correlated to endogenous markers of heat strain, and to identify the most appropriate endogenous markers for eliciting effective exercise-heat administration in humans. It was hypothesised that a minimum endogenous criteria exists for the appearance of eHsp72 into extracellular spaces during acute exercise-heat stress, and that only exercising in very hot conditions would provide sufficient internal systemic strain for such appearance.
Methods
Volunteers
Ten healthy males (mean ± SD age 21.0 ± 0.5 years, height 172.1 ± 13.9 cm, nude body mass 71.1 ± 8.0 kg, body fat 14.7 ± 4.1 %, peak oxygen uptake (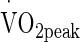 ) 3.81 ± 0.60 L min−1) volunteered to participate in the study.
) 3.81 ± 0.60 L min−1) volunteered to participate in the study.
The confounding variables of smoking (Anbarasi et al. 2006), caffeine (Lu et al. 2008), glutamine (Singleton et al. 2004), generic supplementation (Hillman et al. 2011), thermal exposures (Selkirk et al. 2009), hypoxic exposures (Taylor et al. 2010a), hyperbaric exposures (Taylor et al. 2012) and alcohol (Taylor et al. 2010b) were all controlled in line with previous work in the field (Taylor et al. 2011). Each volunteer was given instructions for dietary requirements in accordance with published guidelines and requested to maintain identical diets in the immediate 48 h prior to each experimental session (Canada 2009).
Participants were instructed to drink at least 500 ml of water 2 h before all exercise bouts (Sawka et al. 2007). A urine refractometer (Alago Vitech Scientific, Pocket PAL-OSMO, UK) was used to measure the hydration levels of the participants prior to commencement of each trial. A participant was deemed to be euhydrated if urine osmolality was <600 mOsm kg−1 H2O. This experimental control was not violated for any participant for any of the experimental procedures.
After a full description of experimental procedures the protocol was approved by the institutional ethics committee and all subjects completed medical questionnaires and provided signed informed consent following the principles outlined by the Declaration of Helsinki of 1975, as revised in 2008.
Preliminary testing
Prior to undertaking the experimental trials of the study, volunteers attended the laboratories whereby their anthropometric data was collected for height (centimeter) using a fixed stadiometer (Detecto Physicians Scales; Cranlea & Co., Birmingham, UK), and body density using calipers (Harpenden, Burgess Hill, UK) and a four-site skin fold calculation (Durnin and Womersley 1974). Following determination of body density, % body fat was calculated according to the method described by Siri (1956). Nude body mass (NBM) was recorded to 0.01 kg from digital scales (ADAM GFK 150, USA).
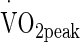 was determined as a means for estimating pre testing aerobic capacity and exercise intensity for the subsequent testing protocols. Volunteers performed an incremental
was determined as a means for estimating pre testing aerobic capacity and exercise intensity for the subsequent testing protocols. Volunteers performed an incremental 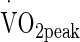 test on a cycle ergometer (Monark e724, Vansbro, Sweden) at a starting intensity of 80 W in temperate laboratory conditions (20 °C, 40 % relative humidity (RH)). Resistance was applied to the flywheel to elicit an increase of 24 W min−1 whilst the volunteer was informed to maintain a constant cadence of 80 rpm. The
test on a cycle ergometer (Monark e724, Vansbro, Sweden) at a starting intensity of 80 W in temperate laboratory conditions (20 °C, 40 % relative humidity (RH)). Resistance was applied to the flywheel to elicit an increase of 24 W min−1 whilst the volunteer was informed to maintain a constant cadence of 80 rpm. The 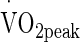 was considered as the highest
was considered as the highest 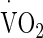 obtained in any 10-s period and in line with the end-point criteria guidelines of the British Association of Sport and Exercise Sciences (Winter et al. 2007). Expired metabolic gas was measured using online gas analysis (Metamax 3X, Cortex, Germany). All preliminary testing was performed on the same ergometer (Monark, e724, Vansbro, Sweden). Heart rate (HR) was recorded during all exercise tests by telemetry (Polar Electro Oyo, Temple, Finland). Power outputs corresponding to 50 %
obtained in any 10-s period and in line with the end-point criteria guidelines of the British Association of Sport and Exercise Sciences (Winter et al. 2007). Expired metabolic gas was measured using online gas analysis (Metamax 3X, Cortex, Germany). All preliminary testing was performed on the same ergometer (Monark, e724, Vansbro, Sweden). Heart rate (HR) was recorded during all exercise tests by telemetry (Polar Electro Oyo, Temple, Finland). Power outputs corresponding to 50 % 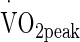 were calculated from the
were calculated from the 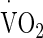 /power output relationship. Saddle position was adjusted by the volunteer to their preferred cycling position and remained unchanged for all trials. During all trials, volunteers wore shorts, socks, and shoes.
/power output relationship. Saddle position was adjusted by the volunteer to their preferred cycling position and remained unchanged for all trials. During all trials, volunteers wore shorts, socks, and shoes.
Experimental protocol
Volunteers presented to the laboratories 60 min prior to testing. Time of day for testing was held constant (10:00 ± 01:00 h) to control for the effects of daily variation in performance (Drust et al. 2005) and HSP expression (Sandström et al. 2009; Taylor et al. 2010b).
Following determination of NBM and hydration status the volunteer then inserted a disposable rectal thermistor (Henleys Medical, UK, Meter logger Model 401, Yellow Springs Instruments, Yellow Springs, MO, USA; accuracy ±0.20 °C) 10 cm past the anal sphincter for measurement of rectal temperature (Trec). Intramuscular temperature (Tmu) was recorded using a muscle temperature probe (Ellab Medical Precision Thermometer, Copenhagen). A 2-g sample of an anaesthetic cream (EMLAi Cream 5 %; AstraZeneca Ltd., Bedfordshire, UK) was applied to the right vastus lateralis muscle 30 min before measurement of resting muscle temperature. With participants seated with the lower leg supported at 90°, a needle (18 G 1.5 in.; BD Microlance 3, Drogheda, Ireland) and a sterile, flexible muscle temperature probe (medical precision thermometer; Ellab, Copenhagen, Denmark) were inserted 4 cm into the belly of the vastus lateralis until a constant temperature was recorded. After removal of the needle, pressure and small adhesive bandage were applied to the entry site to prevent bleeding in accordance with methods described by Duffield et al. (2010).
Volunteers mounted the cycle ergometer located inside a purpose-built environmental chamber with temperature and humidity controlled using automated computer feedback (WatFlow control system; TISS, Hampshire, UK), and were instructed to perform 90 min of continuous cycling exercise at 50 % 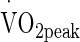 (50 %
(50 % 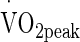 = 1.90 ± 0.30 L min−1, Power at 50 %
= 1.90 ± 0.30 L min−1, Power at 50 % 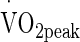 = 120 ± 26 W) in either temperate (TEMP; 20.3 °C ± 0.4 °C, 51.9 ± 14.0 % RH; wet globe bulb temperature (WGBT) 15.8 °C), hot (HOT; 30.2 °C ± 0.1 °C, 52.7 ± 3.0 % RH; WGBT 24.5 °C) and very hot (VHOT; 40.2 °C ± 0.4 °C, 39.0 ± 7.8 % RH; WGBT 31.6 °C) conditions. The sequence was decided by Latin square design.
= 120 ± 26 W) in either temperate (TEMP; 20.3 °C ± 0.4 °C, 51.9 ± 14.0 % RH; wet globe bulb temperature (WGBT) 15.8 °C), hot (HOT; 30.2 °C ± 0.1 °C, 52.7 ± 3.0 % RH; WGBT 24.5 °C) and very hot (VHOT; 40.2 °C ± 0.4 °C, 39.0 ± 7.8 % RH; WGBT 31.6 °C) conditions. The sequence was decided by Latin square design.
During each testing session HR, rating of perceived exertion (RPE, Borg et al. 1985), thermal sensation (TSS, Gagge et al. 1969) and Trec were recorded. Tmu was measured immediately before and after the cessation of each trial. Later, sweat rate was calculated, derived from a change in NBM. Heat strain was calculated using Physiological Strain Index (PSI) (Moran et al. 1998) as follows:
PSI = (5 × (Trec1 − Trec0)/(39.5 − Trec0)) + (5 × (HR1 − HR0) × (180 − HR0)). Where 0 indicates basal values and 1 indicates experimental values.
The Trec area under the curve (AUC) was calculated using a modification to the trapezium rule (Hubbard et al. 1977) when Trec exceeded 38.5 °C (Cheuvront et al. 2008) and 39.0 °C. AUC for Trec > 38.5 °C or AUC for Trec > 39.0 °C was calculated as:
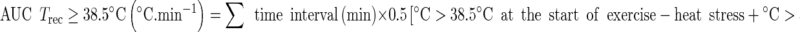 |
In compliance with ethical approval, exercise was terminated if a subject attained a Trec of 39.7 °C.
Blood sampling and analysis
Venous blood samples were taken immediately pre- and post- and 24 h post-test TEMP, HOT and VHOT exercise. A 10-ml whole blood sample was drawn from the antecubital fossa. Each sample was divided equally into 5-ml tubes (Starstedt, Germany) containing EDTA as anticoagulant. Whole blood samples were centrifuged (Eppendorf 5804 R Centrifuge) at 4,500 rpm for a period of 15 min to separate plasma. Plasma was pipetted (Eppendorf Research/Research Pro) into 1.5 ml microtubes (Eppendorf) and stored at −86 °C (Sanyo Ultra Low, VIP Series) until analysis which utilised a commercially available HSP70 high sensitivity enzyme immunometric assay kit (Enzo Life Sciences, Michigan, USA). Quantitative determination of the inducible Hsp72 was performed according to manufacturer’s guidelines. Incubation of the 96 well kit, including the required quality control standards was performed on an orbital shaker (Heidolph Titramax 1000) at 600 rpm, and read by a plate reader using absorption at 450 nm (Elx 800 Universal Microplate reader, Bio-Tek Instruments). Plasma Hsp72 concentrations were corrected for changes in venous plasma volume (Dill and Costill 1974) with haemoglobin collected in duplicate using a microcuvette and analysed using a B-Haemoglobin Photometer (Hemocue Limited, Ängelholm, Sweden) and haematocrit collected in triplicate (∼50 μl) with glass capillary tubes and analysed following centrifugation at 12–14,000 rpm for 3 min (Haemotospin 1300 Centrifuge, Hawksley & Sons Ltd, West Sussex, UK).
Accuracy of the sample data was ensured by plotting a graph for linearity between known sample concentrations and optical density. A linear trendline and equation was used to translate raw plate reader result into Hsp72 units (nanograms per milliliter). The intra/inter-assay variability was 10.5/17.36 %, respectively. The assay sensitivity is described by the manufacturer as 0.09 ng ml−1 and the detection range of the assays were 0.20–12.5 ng ml−1 for Hsp72.
Statistical analysis
All statistical calculations were performed using PASW software version 18.0 (SPSS, Chicago, IL, USA). All outcome variables were assessed for normality of distribution and sphericity prior to further analysis and deemed plausible in all instances unless otherwise stated. A two-way (time × trial) repeated-measures analysis of variance (ANOVA) was performed to test significance between and within trials. One-way ANOVA with repeated measures was used to compare physiological, perceptual and thermal data between exogenous environments, Bonferroni pairwise comparisons compared between separate exogenous temperature conditions.
Stepwise multiple regression analysis was performed for the six dependent variables which yielded the strongest relationship to the increase in eHsp72 concentration (rate of change in Trec (degrees Celsius per hour), peak Trec (degrees Celsius), mean Trec for the final 60 min (degrees Celsius), duration Trec ≥ 39.0 °C (minutes), change in Trec (degrees Celsius), duration Trec ≥ 38.5 °C (minutes)). Nine volunteers’ data were used for the model as no eHsp72 was detected for one volunteer. Data was reported as mean ± SD, with two tailed significance was accepted at p < 0.05.
Results
Physiological and perceptual measures
Mean duration for VHOT trial lasted only 86.5 ± 7.5 min in comparison to TEMP and HOT owing to two participants terminating early as Trec reached 39.7 °C. No difference (f = 2.194, p = 0.140) was reported for the duration exercising in each exogenous temperature condition see Table 1.
Table 1.
Mean ± SD physiological and perceptual measures during exercise-heat stress (n = 10)
| Measure | Duration of exposure (min) | Change in NBM (kg) | Sweat rate (g min−1) | Peak HR (bpm) | Mean HR (bpm) | Peak PSI | Mean PSI | Peak RPE | Mean RPE | Peak TSS | Mean TSS |
|---|---|---|---|---|---|---|---|---|---|---|---|
| TEMP | 90.0 ± 0.0 | −1.02 ± 0.42 | 11.3 ± 4.7 | 141 ± 16 | 132 ± 13 | 4.6 ± 1.3 | 3.5 ± 1.4 | 14.2 ± 1.3 | 12.7 ± 1.1 | 5.2 ± 0.9 | 4.7 ± 0.7 |
| HOT | 90.0 ± 0.0 | −1.26 ± 0.37 | 14.0 ± 4.2 | 155 ± 15a | 142 ± 14a | 6.5 ± 1.7a | 4.8 ± 1.3 | 16.3 ± 2.0a | 13.9 ± 1.5 | 6.8 ± 0.6a | 6.0 ± 0.5a |
| VHOT | 86.5 ± 7.5 | −1.36 ± 0.40 | 15.8 ± 4.3b | 168 ± 14b | 153 ± 14a | 8.6 ± 1.2b | 6.1 ± 1.1b | 18.5 ± 2.0b | 15.2 ± 1.2b | 7.8 ± 0.4b | 6.8 ± 0.4b |
PSI physiological strain index, RPE rating of perceived exertion, TSS thermal sensation scale
aDenotes significantly different to TEMP group (p < 0.05)
bDenotes significantly different to TEMP and HOT group (p < 0.05)
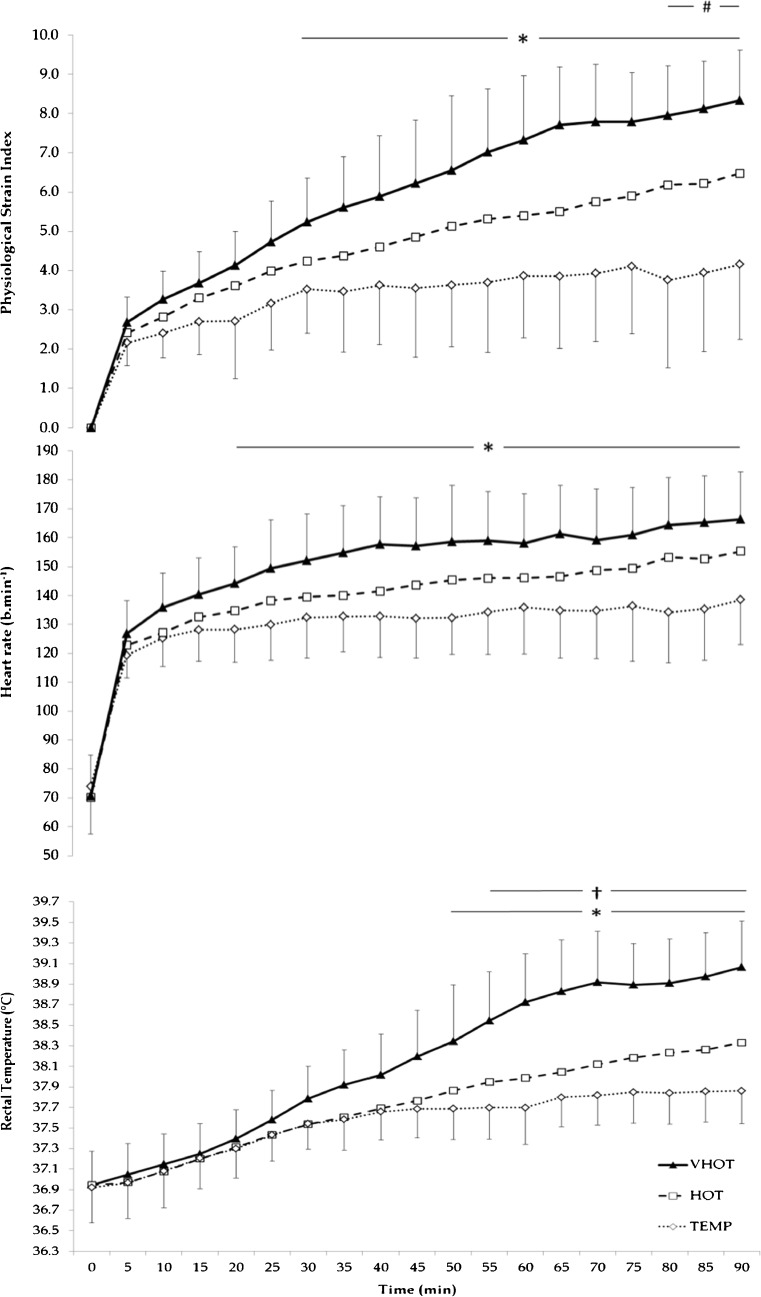
Peak (f = 28.650, p < 0.001) and mean (f = 19.951, p < 0.001) HR were significantly higher in HOT than TEMP conditions (141 ± 16 and 132 ± 13 bpm; p < 0.001), whilst VHOT was significantly higher than TEMP (p = 0.001 and p = 0.001) and HOT (p = 0.018 and p = 0.045) see Fig. 1.
Fig. 1.
Rectal temperature, heart rate and physiological strain index time course for the three exercise-heat stress conditions. Note HOT error bars removed for clarity (HOT mean ± SD; PSI 4.8 ± 1.3, HR 142 ± 14 bpm, T rec 37.71 ± 0.32 °C) number sign significant difference between TEMP and HOT p < 0.05. asterisk significant difference between TEMP and VHOT p < 0.05. dagger symbol significant difference between HOT and VHOT p < 0.05
Calculated sweat rate was significantly different between conditions (f = 4.204, p = 0.032). VHOT (15.8 ± 4.3) was significantly greater (p = 0.042) than TEMP and HOT conditions, no difference existed between TEMP and HOT (p = 0.153), see Table 1.
Perceptual measures RPE and TSS demonstrated significant difference between conditions (RPE f = 103.360, p < 0.001) (TSS f = 71.602, p < 0.001) (Table 1), with peak scores significantly increasing from TEMP to HOT (p = 0.021 and p < 0.001); VHOT was significantly higher (p = 0.008 and p < 0.001) from TEMP and HOT trials. Mean RPE was significantly different between conditions (f = 22.946, p < 0.001), but only significantly greater between VHOT and TEMP and HOT conditions (p = 0.003). Mean TSS was significantly different between all conditions (f = 76.518, p < 0.001), TEMP was significantly lower than HOT (p = 0.000) and VHOT was significantly greater from TEMP (p < 0.001) and HOT (p = 0.001).
Temperature measures
Table 2 reports the values for peak Trec, statistically different between all conditions (f = 59.838, p < 0.001). TEMP was significantly lower than HOT (p = 0.002); VHOT was significantly higher than TEMP (p < 0.001) and HOT (p < 0.001). Mean Trec for the time between 30 and 90 min was significantly different (f = 35.906, p < 0.001) with HOT significantly higher than TEMP (p = 0.028) and VHOT significantly higher than TEMP (p < 0.001) and HOT (p < 0.001).
Table 2.
Mean ± SD Measures of rectal and muscle temperature thermal measures during exercise-heat stress (n = 10)
| Measure | Peak rectal temperature (°C) | Mean rectal temperature final 60 min (°C) | Change in rectal temperature (°C) | Rate of rectal temperature increase (°C h−1) | AUC for rectal temperature of 38.5 °C (°C min−1) | AUC for rectal temperature of 39.0 °C (°C min−1) | Duration rectal temperature ≥38.5 °C (min) | Duration rectal temperature ≥39.0 °C (min) | End muscle temperature (°C) | Change in muscle temperature (°C) |
|---|---|---|---|---|---|---|---|---|---|---|
| TEMP | 37.90 ± 0.29 | 37.75 ± 0.29 | +0.98 ± 0.32 | 0.65 ± 0.22 | 0.05 ± 0.16 | 0.00 ± 0.00 | 2.00 ± 6.32 | 0.00 ± 0.00 | 37.0 ± 0.6 | +2.4 ± 1.0 |
| HOT | 38.35 ± 0.52a | 38.00 ± 0.43a | +1.40 ± 0.64 | 0.94 ± 0.42 | 5.84 ± 18.34 | 1.28 ± 4.05 | 6.00 ± 17.29 | 4.00 ± 12.65 | 38.0 ± 0.7a | +3.0 ± 0.5 |
| VHOT | 39.16 ± 0.45b | 38.62 ± 0.40b | +2.22 ± 0.65b | 1.56 ± 0.53b | 14.06 ± 9.53a | 5.11 ± 4.28a | 31.50 ± 14.73a | 20.00 ± 14.53a | 39.7 ± 0.8b | +4.8 ± 1.3b |
AUC area under the curve
aDenotes significantly different to TEMP group (p < 0.05)
bDenotes significantly different to TEMP and HOT group (p < 0.05)
The change in Trec was significantly different between conditions (f = 33.621, p < 0.001), but post hoc analysis only observed significantly greater differences between VHOT, and TEMP and HOT (p < 0.001). This was also true of the rate of Trec increase (f = 37.475, p < 0.001), where VHOT elicited a significantly greater rate compared to TEMP and HOT (p < 0.001).
Area under the curve for Trec of 38.5 °C (f = 4.045, p = 0.035) and 39.0 °C (f = 7.163, p = 0.005) (degrees Celsius per minute) were significantly different between conditions overall, VHOT was significantly greater compared with TEMP and HOT (p = 0.003 and p = 0.013), but no difference was observed between TEMP and HOT.
Duration spent with rectal temperatures of ≥38.5 °C (f = 18.475, p < 0.001) and ≥39.0 °C (f = 9.631, p = 0.001) (minutes) displayed significant main effect difference but was not different between TEMP and HOT, however VHOT was significantly longer than TEMP and HOT (p = 0.014 and p = 0.06).
Main effect for end Tmu was observed as significant (f = 36.381, p < 0.001). Significant difference was also found between TEMP and HOT (p = 0.001); VHOT was significantly higher from TEMP (p < 0.001) and HOT (p = 0.003). The change in Tmu was only significantly greater between VHOT, and TEMP and HOT (p = 0.003) despite overall difference (f = 26.836, p < 0.001). Thermal data for each trial is presented in Table 2.
Overall difference was observed for peak (f = 76.949, p = 0.000) and mean PSI (f = 21.278, p < 0.001) with significantly higher values observed between VHOT, and both TEMP and HOT conditions (p < 0.001 and p = 0.005, respectively), see Table 1. Peak PSI was also significantly lower in TEMP compared to HOT (p = 0.003), no significant difference was observed for mean PSI (p > 0.05). Figure 1 details the change in HR, Trec and PSI for each condition over time.
Extracellular HSP70 expression
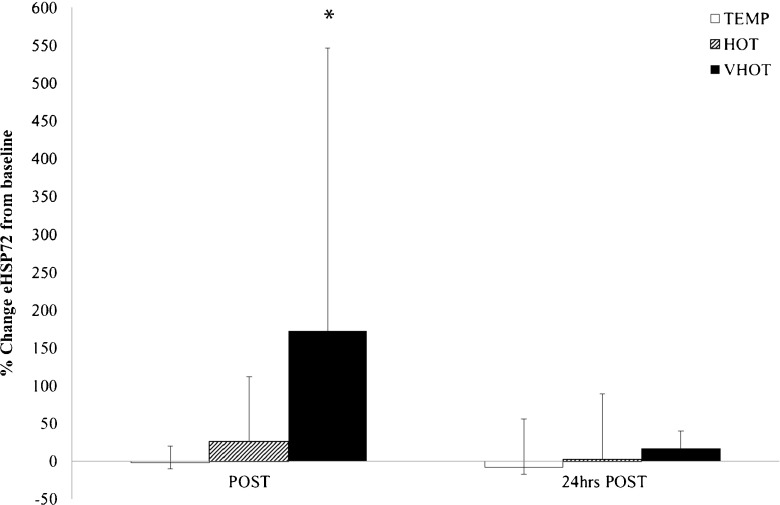
No difference (f = 1.677, p = 0.218) was reported in eHsp72 expression (nanograms per milliliter) for pre testing expression during TEMP, HOT and VHOT experimental sessions. eHsp72 expression (nanograms per milliliter) was observed as significantly different for the main effect (f = 5.928, p = 0.012) with the significant difference observed as an increase from pre to post VHOT (0.266 ± 0.094 to 0.724 ± 0.444). Following post hoc analysis, no difference was found for the effect of temperature or condition in TEMP (p = 1.000) and HOT (p = 0.766) (0.349 ± 0.135 to 0.342 ± 0.165, and 0.299 ± 0.122 to 0.376 ± 0.226, respectively). No significant difference (p > 0.05) was observed between pre and 24 h post in any exercise-heat condition. eHsp72 data presented as a percentage change from baseline, in line with previous work, for post (TEMP −1.9 %; HOT +25.7 %; VHOT +172.4 %) and 24 h post (TEMP −8.6 %; HOT 2.6 %; VHOT 17.1 %) are presented in Fig. 2.
Fig. 2.
Mean ± SD percentage change in eHSP72 from pre, immediately post and 24 h post exercise-heat stress (n = 9) asterisk denotes difference from TEMP and HOT p < 0.05
Relationship between eHSP70, temperature and physiological measures
Rate of change in Trec (r = 0.702), peak rectal temperature (r = 0.655), mean Trec for the final 60 min (r = 0.651), duration Trec ≥39.0 °C (r = 0.635), change in Trec (r = 0.632), peak PSI (r = 0.603), duration Trec ≥38.5 °C (r = 0.559) and peak HR (r = 0.327), were submitted to a stepwise multiple regression to predict post exercise-heat exposure. The first predictor variable to enter the model was rate of change in Trec; the second and final predictor variable to enter the model was change in Trec. The adjusted R2 value for this model was 0.473 and standard error of the estimate 0.228.
Discussion
The aim of this study was to determine the endogenous effects of exercise matched for power output and duration in three exogenous thermal environments on the plasma eHsp72 concentration responses. Significant changes in concentration occurred only pre to post in the VHOT condition, supporting the hypothesis that endogenous thermal and physiological strain elicited only in VHOT conditions provided sufficient stimuli for eHsp72 response during exercise-heat stress. This is in line with other authors with similar experimental designs to the present study (McClung et al. 2008; Magalhães et al. 2010; Périard et al. 2012). Established endogenous physiological and thermoregulatory parameters, particularly those less commonly reported in literature determining eHsp72 changes (rate of Trec increase, area under the curve (AUC) for Trec of 38.5 and 39.0 °C, duration Trec ≥ 38.5 and ≥ 39.0 °C), taken during each condition were analysed to determine whether they could be used to describe more effectively endogenous conditions leading to increased eHsp72 concentration.
The physiological and thermoregulatory responses to each exercise-heat stress condition were as expected for matched exercise in increasing thermal environments (Galloway and Maughan 1997; Maughan et al. 2012). Data observed three levels of strain between TEMP, HOT and VHOT conditions for peak HR, Trec, PSI, and end Tmu suggesting that each exogenous condition was placing independent magnitudes of strain. Other thermoregulatory data (change in Trec, rate of Trec increase, AUC for Trec of 38.5 and 39.0 °C, duration Trec ≥ 38.5 and ≥ 39.0 °C, and change in Tmu) however were in agreement with the experimental rationale, describing two levels, where VHOT was different from TEMP and HOT, but no difference was observed between TEMP and HOT. The thermal and physiological data suggests that VHOT was of greater exercise-heat stress than TEMP and HOT; an observation paralleled by the increased concentration of eHsp72 being only reported in VHOT pre to post exercise. Regrettably, no data was collected that measured skin temperature, this addition in future research studies would allow for the calculation of whole body temperature (Burton 1935) and the inclusion of this descriptor of endogenous strain.
The observation from regression analysis that the rate of increase, and the delta change in Trec are important factors in changing eHsp72 expression is in line with the observations of Périard et al. (2012) for whom exercising at 75 % 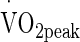 , revealed a relationship emerged between eHsp72 and the rate of increase in Trec. The authors (Périard et al. 2012) suggested that this was possibly due to a greater metabolic demand and energy conversion increasing Trec (i.e., intensity dependent). In the present study it appears despite a lower intensity of work the exogenous conditions were sufficient to elicit different endogenous responses and eHsp72 concentrations. As only two (rate of change in Trec, and change in Trec) of the dependent variables (Tables 1 and 2) were accepted into the regression model, it remains that changes in eHsp72 concentration is multi-factorial and that whilst ensuring endogenous thermal strain is of sufficient onset and magnitude, these determinants are only elements determining the change in concentration. These observations do however, give greater insight into means for facilitating the most economical prescription of exercise-heat sessions.
, revealed a relationship emerged between eHsp72 and the rate of increase in Trec. The authors (Périard et al. 2012) suggested that this was possibly due to a greater metabolic demand and energy conversion increasing Trec (i.e., intensity dependent). In the present study it appears despite a lower intensity of work the exogenous conditions were sufficient to elicit different endogenous responses and eHsp72 concentrations. As only two (rate of change in Trec, and change in Trec) of the dependent variables (Tables 1 and 2) were accepted into the regression model, it remains that changes in eHsp72 concentration is multi-factorial and that whilst ensuring endogenous thermal strain is of sufficient onset and magnitude, these determinants are only elements determining the change in concentration. These observations do however, give greater insight into means for facilitating the most economical prescription of exercise-heat sessions.
The present study reported eHsp72 as only increasing immediately following the VHOT trial, with values returning to baseline within 24 h (Fig. 2). Increased systemic eHsp72 has been shown to be exercise intensity and duration dependent in temperate conditions (Fehrenbach et al. 2005), with the addition of thermal stress (evidenced by increase Trec) further increasing the magnitude of response (Marshall et al. 2006). Consequently, a heat storage independent threshold of 38.5 °C (Trec) has been postulated (Amorim et al. 2008) and demonstrated central to the magnifying influence of thermal stress on eHsp72 concentrations (Amorim et al. 2008), compared to moderate intensity matched exercise. Data from present study supports this “minimum endogenous criteria” notion (Table 2) from VHOT which elicited a greater internal temperature, rate of internal temperature rise and a greater duration at critical Trec than TEMP and HOT. Our data is supportive of the existence of minimum endogenous criteria for the induction of eHsp72 into the circulation during exercise-heat stress as suggested by Amorim et al. (2008). Supporting the absence of eHsp72 increases in TEMP and HOT (Trec of 37.90 ± 0.29 °C and 38.35 ± 0.52 °C, respectively) in the present study, parallel exercise induced changes in Trec data (mean maximum Trec 38.48 °C) resulting in no change in basal eHsp72 have been reported by Hom et al. (2012) during treadmill walking.
Mechanistically, temperatures >38.5 °C at the hepatosplanchnic viscera are perhaps the most important for increase concentration, with eHsp72 release potentially dependent on the magnitude and duration above this element of the “threshold” (Rhind et al. 2004; Selkirk et al 2008, 2009). Recent evidence (Périard et al. 2012) suggests that the same eHsp72 expression is yielded by short (27.2 min) and longer duration (58.9 min) trials by increasing exercise intensity (from 60 to 75 % of 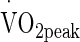 ) with similarity in eHsp72 expression despite differences in peak and mean Trec (39.0 and 39.7 °C, respectively). The data from Périard et al. (2012), at least superficially, indicates that both magnitude and duration above >38.5 °C is irrelevant within normal physiological boundaries (i.e. non-life threatening physical and occupational pursuits) and that it is exceeding Trec of >38.5 °C that is the most potent stimuli of increases in eHsp72 when combined with exercise stress.
) with similarity in eHsp72 expression despite differences in peak and mean Trec (39.0 and 39.7 °C, respectively). The data from Périard et al. (2012), at least superficially, indicates that both magnitude and duration above >38.5 °C is irrelevant within normal physiological boundaries (i.e. non-life threatening physical and occupational pursuits) and that it is exceeding Trec of >38.5 °C that is the most potent stimuli of increases in eHsp72 when combined with exercise stress.
The degree of hyperthermia during exercise-heat stress, be it induced by exogenous environment or prescribed workload, has so far been proposed central to whether Hsp72 is expressed/released, or not. It has been demonstrated that participants exposed to temperatures similar to that of VHOT (Yamada et al. 2007; McClung et al. 2008; Magalhães et al. 2010) where mean calculated heat stress was 32.46 °C (WGBT), elicited largest increases in Hsp72. Marshall et al. (2006) used a greater calculated exogenous heat stress than VHOT (33.1 °C WGBT) combined with lower (38 and 42.5 % 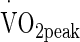 ) exercise intensity, eliciting core temperatures of 38.2 °C. No change in eHsp72 was observed, suggesting that the exercise intensity/workload was insufficient in their experiment to elicit the desired thermal response, and is not presenting sufficient exercise-heat stress.
) exercise intensity, eliciting core temperatures of 38.2 °C. No change in eHsp72 was observed, suggesting that the exercise intensity/workload was insufficient in their experiment to elicit the desired thermal response, and is not presenting sufficient exercise-heat stress.
Attenuation of release may likely occur once Trec returns below “minimum endogenous criteria”, although the precise duration taken for full cessation of Hsp72 release requires further elucidation—the presented data can only suggest this occurring sometime between immediately and 24 h post exercise (Fig. 2). This pattern of elevation and return to baseline in VHOT, as observed during the first tolerance test by Magalhães et al. (2010) or, observed reduction following elevation (Marshall et al. 2006; Périard et al. 2012) from baseline, highlights the transient eHsp72 response to stress followed by removal from the circulation. Caution must be exercised when inferences to a critical endogenous criteria model is made across a broad demographic of exercise capacities (i.e. untrained through to highly trained) as such differences are known to influence eHsp72 release kinetics and magnitudes within thermally challenging environments (Selkirk et al 2008, 2009). Therefore, future work should tightly control this potentially confounding variable.
Hepatosplanchnic, vascular and brain tissue, and peripheral blood mononuclear cells appear the principle sources of Hsp72 release into the systemic circulation (Febbraio et al. 2002; Lancaster et al. 2004; Lancaster and Febbraio 2005b; Johnson and Fleshner 2006). Concise reviews of the proposed active and passive mechanisms of eHsp72 release are presented by Lancaster and Febbraio (2005a), Fleshner and Johnson (2005) and Asea (2007). Briefly, it is proposed (Multhoff and Hightower 1996) that exosomes secreted following the fusion of multivesicular bodies with the plasma membrane, provide the secretory pathway for cells to actively release Hsp72 (Lancaster and Febbraio 2005b). It has also been proposed (Ogawa et al. 2011) that eHsp72 is triggered by circulating ATP during exercise. Further, it has been reported (Johnson and Fleshner 2006) that hormone receptor-mediated pathways exist allowing elevation of eHsp72 during stress. Authors demonstrated that norepinephrine may stimulate a receptor-mediated exocytotic pathway of eHsp72 release. An indirect consequence of exercising at an elevated temperature is that of elevated cardiovascular demand and associated α-adrenergic stimulation as a means for maintaining work rate and required demands to exercising muscle, whilst attempting thermoregulation.
VHOT elicited the greatest heart rate response to the exercise presented; as such this indirect measure of sympathetic activity occurring through physiological and thermal strain, supports an adrenergic receptor-mediated release mechanism. This mechanism is evidenced by the work of Whitham et al. (2006) who observed caffeine supplementation and increase plasma catecholamines as elevating eHsp72. Périard et al. (2012) commented that the release of eHsp72 into extracellular locations is likely to originate from varied tissues and cell types, each potentially affected by specific mechanisms of release and various inducing factors.
The significance of a post exercise increase in eHsp72 remains unclear, proposed immunological functions (Campisi et al. 2003) as a signal for cytokine and inflammatory pathways in response to unaccustomed systemic or whole body stress (Asea et al. 2000), appear most relevant whereby VHOT exercise-heat stress in that trial was of a magnitude sufficient to induce an immunological response which the TEMP and HOT trials were not (Fig. 2).
In a matched thermal environment, exercise intensity contributes to the rate of temperature increase and the degree of hyperthermia (Mora-Rodriguez et al. 2008). Whilst exercise intensity alone has been associated with increased iHsp72 (Liu et al. 1999; Milne and Noble 2002) and eHsp72 (Whitham et al. 2007; Périard et al. 2012), responses to hyperthermia and the sympathetic adrenergic stimulation of exercise offers a further insight into eliciting the greatest response based upon endogenous criteria. Whitham et al. (2006) demonstrated increased eHsp72 was associated with higher plasma levels of catecholamines and heart rate, whilst it has also been observed that following passive heating, neither epinephrine nor norepinephrine was solely responsible for eHsp72 release (Whitham et al. 2007).
Johnson and Fleshner (2006) identified α-adrenergic stimulation, notably through norepinephrine elevation (Nielsen et al. 1997; Ortega et al. 2006; Whitham et al. 2006; Giraldo et al. 2010; Iguchi et al. 2012) as responsible for Hsp72 release into the circulation, this alongside the work of Whitham et al. (2006, 2007) suggest a requirement for individuals to be presented with sustained physiological challenge during exercise-heat stress (Johnson and Fleshner 2006). Exercise intensity, or α-adrenergic stimulation via norepinephrine is likely required to be above an intensity threshold to elicit significant eHsp72 response with the greater exercise intensity data from Périard et al. (2012) leading to data contrasting that of Marshall et al. (2006). The extent to which the adrenergic contribution is required is difficult to determine precisely, indeed it is a limitation of the present study that catecholamine analysis was not performed. It appears with only the VHOT trial eliciting changes in eHsp72 that a mean HR, an indirect measure of sympathetic activation (Rowell et al. 1987), of 153 ± 14 bpm is required from the intensity 50 % of 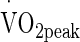 . The intensity of this trial is likely be of greater physiological strain as a result of the increased thermoregulatory requirements which are known to increase proportionally to the cooler conditions of TEMP and HOT (Galloway and Maughan 1997; Maughan et al. 2012). Périard et al. (2012) reported HR values greater than the present study reflecting the elevated work intensity. As with the analysis of Périard et al. (2012), our regression analysis deemed HR responses insufficient predictor elements of change in eHsp72 concentration. The significant difference in HR between VHOT and, TEMP and HOT alongside elevated eHSP72 in only VHOT, despite matched power, is therefore explained by the elevated cardiovascular consequence of increased thermal strain whilst maintaining power output, rather than the thermal strain being a the primary mediator of eHsp72 response.
. The intensity of this trial is likely be of greater physiological strain as a result of the increased thermoregulatory requirements which are known to increase proportionally to the cooler conditions of TEMP and HOT (Galloway and Maughan 1997; Maughan et al. 2012). Périard et al. (2012) reported HR values greater than the present study reflecting the elevated work intensity. As with the analysis of Périard et al. (2012), our regression analysis deemed HR responses insufficient predictor elements of change in eHsp72 concentration. The significant difference in HR between VHOT and, TEMP and HOT alongside elevated eHSP72 in only VHOT, despite matched power, is therefore explained by the elevated cardiovascular consequence of increased thermal strain whilst maintaining power output, rather than the thermal strain being a the primary mediator of eHsp72 response.
Present data fails to elucidate the precise minimum requirement for sympathetic contribution to Hsp72 transcription or translocation as identified by other research (Johnson and Fleshner 2006) through exercise or supplementary pathways. Analysis of plasma norepinephrine would have contributed towards this known mediator regulating the release of Hsp72 in the present study. It is well reported that elevated temperature, derived from external environment, passively or through active means, leads to elevated cardiac strain expressed in the form of increased HR (Galloway and Maughan 1997; Maughan et al. 2012) and as such these two variables cannot be disassociated when considering the whole body response to exercise-heat stress. As regression analysis has failed to accept HR as a predictor of eHsp72 in favour of thermal markers as such we cannot ignore the identification of previous discussed endogenous thermal markers despite early research demonstrating increases in eHsp72 independent of changes in core temperature as a consequence of increased plasma catecholamines. Our data acknowledges the role of HR, and more specifically the elevated cardiac contribution to exercise in the VHOT condition in comparison to HOT and TEMP conditions as a further endogenous descriptor of eHsp72 release. It is therefore proposed that sympathetic activity, most rudimentarily measured from exercising HR, is an important component of the minimum endogenous criteria for increasing eHsp72 during exercise-heat stress, alongside the thermal criteria. Rather than increased temperature directly modulating elevated eHsp72 expression, it appears to be indirectly modulating it via increased HR, a reflection of increased adrenergic/catecholamine contribution to exercise-heat stress (Rowell et al. 1987).
It has been acknowledged by our data and others that core temperature (Ruell et al. 2006; Périard et al. 2012), rate of core temperature increase (Périard et al. 2012), and interestingly, aerobic capacity (Périard et al. 2012) are endogenous factors relating to Hsp72 increases. Périard et al. (2012) observed significant differences in HR despite no difference in eHsp72 increases between groups. In light of this, further work appears warranted to determine the role parasympathetic/sympathetic drive has in determining eHsp72 release during exercise-heat stress.
It is known that training status influences the basal and eHsp72 stress response to exercise-heat stress. In addition, prior HA, or progress towards the phenotype via endurance training may increase the immune response threshold for inducement of eHsp72 via exercise-heat stress. Njemini et al. (2004) also observed that inflammatory status, and its variable nature is also linked to eHsp72. Selkirk et al. (2008, 2009) acknowledged that the threshold for enhanced iHsp72 response, endotoxin leakage and inflammatory activation during exertional heat stress, in similar exogenous conditions to the present study, occurs at a lower temperature in untrained compared with trained subjects and support the endotoxin translocation hypothesis of exertional heat stroke, linking endotoxin tolerance and heat tolerance.
This individual and changing threshold along a continuum modulated by thermotolerance, inflammatory, and training status, suggests that prescription of exercise-heat stress exposure, administered controlling only simple parameters such as exogenous environment and work rate, may ultimately fail to stress sufficiently some individuals. The present data can therefore be used as a guide towards acute exercise-heat stress prescription. It is also important to consider that parameters appropriate for acute interventions shift with repeated exposures, as the HA phenotype and concurrent acquired cellular thermotolerance is enhanced (Sandström et al. 2008; Magalhães et al. 2010; Hom et al. 2012). Based upon these comments and the observation from the regression analysis that the rate of increase in Trec (VHOT 1.56 ± 0.53 °C h−1) and the delta change in Trec (VHOT 2.22 ± 0.65 °C), it may be more appropriate to implement an isothermic (controlled hyperthermia) model of exercise-heat exposure (Garrett et al. 2009, 2011, 2012) where the rate of heat production can be accelerated (Amorim et al. 2008) and proposed minimum endogenous temperatures targeted (Amorim et al. 2011). This model requires greater exercise intensity during the early stages of the exposure, thus ensuring a more rapid increase in Trec and consequently greater change in Trec, followed by a reduction in workload once a desired temperature has been achieved. The benefit of the isothermic model of exercise-heat stress is that specific endogenous temperatures can be targeted, rather than being an uncontrolled response varying on an individual basis, with the potential for more individualised prescription. This model of clamping at a set core temperature is an effective means for mediating increases of circulating stress hormones, which subsequently contribute to induction of circulating cytokine release (Rhind et al. 2004).
The duration in which individuals are in a state of hyperthermia may also be a contributing factor towards increasing eHsp72 concentrations and as such be reflective of a greater overall “dose” of endogenous strain in comparison to a short exposure to extremes of either variable. The more rapid increase in core temperature during the isothermic model could be implemented to ensure a greater percentage of the total exposure time is at or above the desired endogenous threshold for eHsp72 release. Whilst eHsp72 is a useful marker for describing stress it should be noted that no direct role exists between secreted eHsp72 and attainment of HA. Future work should consider the iHsp72 response to exercise-heat stress which might provide greater insight into acquired cellular thermotolerance and the acquirement of HA. Within these experimental designs, the confounding variable of training status and its influence on the prescription of the stress should be controlled to assess the most effective means for increasing iHsp72 gene expression and total protein in tandem with measures of eHsp72. Such data should be used to assess the global HSP response in line with the proposed eHsp72 centric minimum endogenous criteria.
In summary, it appears likely that a minimum endogenous criteria contributes to the multi-factorial release of eHsp72 into the circulation during acute exercise-heat stress, a pathway that may differ from pathological stress resulting in systemic inflammation. Our data observed the endogenous requirement for release as being a core temperature peak of 39.2 °C and a mean of 38.6 °C for a period of 56.5 min alongside mean heart rates of 153 ± 14 bpm, following a rate of increase of 1.6 °C h−1 a core temperature change of 2.2 °C from baseline.
Acknowledgments
The authors would like to thank the volunteers for their participation in this investigation.
References
- Amorim FT, Yamada PM, Robergs RA, et al. The effect of the rate of heat storage on serum heat shock protein 72 in humans. Eur J Appl Physiol. 2008;104:965–972. doi: 10.1007/s00421-008-0850-3. [DOI] [PubMed] [Google Scholar]
- Amorim F, Yamada P, Robergs R, et al. Effects of whole-body heat acclimation on cell injury and cytokine responses in peripheral blood mononuclear cells. Eur J Appl Physiol. 2011;111:1609–1618. doi: 10.1007/s00421-010-1780-4. [DOI] [PubMed] [Google Scholar]
- Anbarasi K, Kathirvel G, Vani G, et al. Cigarette smoking induces heat shock protein 70 kDa expression and apoptosis in rat brain: modulation by bacoside A. Neuroscience. 2006;138:1127–1135. doi: 10.1016/j.neuroscience.2005.11.029. [DOI] [PubMed] [Google Scholar]
- Asea A. Chaperokine-induced signal transduction pathways. Exerc Immunol Rev. 2003;9:25–33. [PMC free article] [PubMed] [Google Scholar]
- Asea A. Initiation of the immune response by extracellular Hsp72: chaperokine activity of Hsp72. Curr Immunol Rev. 2006;2:209–215. doi: 10.2174/157339506778018514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asea A. Mechanisms of HSP72 release. J Biosci. 2007;32:579–584. doi: 10.1007/s12038-007-0057-5. [DOI] [PubMed] [Google Scholar]
- Asea A, Kraeft SK, Kurt-Jones EA, et al. HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med. 2000;6:435–442. doi: 10.1038/74697. [DOI] [PubMed] [Google Scholar]
- Borg G, Ljunggren G, Ceci R. The increase of perceived exertion, aches and pain in the legs, heart rate and blood lactate during exercise on a bicycle ergometer. Eur J Appl Physiol Occup Physiol. 1985;54:343–349. doi: 10.1007/BF02337176. [DOI] [PubMed] [Google Scholar]
- Burton A. Human calorimetry: II. The average temperature of the tissues of the body * Three figures. J Nutr. 1935;9:261–280. [Google Scholar]
- Campisi J, Leem TH, Fleshner M. Stress-induced extracellular Hsp72 is a functionally significant danger signal to the immune system. Cell Stress Chaperones. 2003;8:272–286. doi: 10.1379/1466-1268(2003)008<0272:SEHIAF>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canada DOF. Nutrition and athletic performance. Med Sci Sports Exerc. 2009;41:709–731. doi: 10.1249/MSS.0b013e31890eb86. [DOI] [PubMed] [Google Scholar]
- Cheuvront SN, Chinevere TD, Ely BR, et al. Serum S-100beta response to exercise-heat strain before and after acclimation. Med Sci Sports Exerc. 2008;40:1477–1482. doi: 10.1249/MSS.0b013e31816d65a5. [DOI] [PubMed] [Google Scholar]
- Dill DB, Costill DL. Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. J Appl Physiol. 1974;37:247–248. doi: 10.1152/jappl.1974.37.2.247. [DOI] [PubMed] [Google Scholar]
- Drust B, Waterhouse J, Atkinson G, et al. Circadian rhythms in sports performance—an update. Chronobiol Int. 2005;22:21–44. doi: 10.1081/CBI-200041039. [DOI] [PubMed] [Google Scholar]
- Duffield R, Green R, Castle P, Maxwell N. Precooling can prevent the reduction of self-paced exercise intensity in the heat. Med Sci Sports Exerc. 2010;42:577–584. doi: 10.1249/MSS.0b013e3181b675da. [DOI] [PubMed] [Google Scholar]
- Durnin JV, Womersley J. Body fat assessed from total body density and its estimation from skinfold thickness: measurements on 481 men and women aged from 16 to 72 years. Br J Nutr. 1974;32:77–97. doi: 10.1079/BJN19740060. [DOI] [PubMed] [Google Scholar]
- Febbraio MA, Ott P, Nielsen HB, et al. Exercise induces hepatosplanchnic release of heat shock protein 72 in humans. J Physiol. 2002;544:957–962. doi: 10.1113/jphysiol.2002.025148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehrenbach E, Niess AM, Veith R, et al. Changes of HSP72-expression in leukocytes are associated with adaptation to exercise under conditions of high environmental temperature. J Leukoc Biol. 2001;69:747–754. [PubMed] [Google Scholar]
- Fehrenbach E, Niess AM, Voelker K, et al. Exercise intensity and duration affect blood soluble HSP72. Int J Sports Med. 2005;26:552–557. doi: 10.1055/s-2004-830334. [DOI] [PubMed] [Google Scholar]
- Fleshner M, Johnson JD. Endogenous extra-cellular heat shock protein 72: releasing signal(s) and function. Int J Hyperthermia. 2005;21:457–471. doi: 10.1080/02656730500088211. [DOI] [PubMed] [Google Scholar]
- Gagge AP, Stolwijk JA, Saltin B. Comfort and thermal sensations and associated physiological responses during exercise at various ambient temperatures. Environ Res. 1969;2:209–229. doi: 10.1016/0013-9351(69)90037-1. [DOI] [PubMed] [Google Scholar]
- Gagnon D, Jay O, Kenny GP. The evaporative requirement for heat balance determines whole-body sweat rate during exercise under conditions permitting full evaporation. J Physiol. 2013;591:2925–2935. doi: 10.1113/jphysiol.2012.248823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway SDR, Maughan RJ. Effects of ambient temperature on the capacity to perform prolonged cycle exercise in man. Med Sci Sports Exerc. 1997;29:1240–1249. doi: 10.1097/00005768-199709000-00018. [DOI] [PubMed] [Google Scholar]
- Garrett AT, Goosens NG, Rehrer NJ, et al. Induction and decay of short-term heat acclimation. Eur J Appl Physiol. 2009;107:659–670. doi: 10.1007/s00421-009-1182-7. [DOI] [PubMed] [Google Scholar]
- Garrett AT, Rehrer NJ, Patterson MJ. Induction and decay of short-term heat acclimation in moderately and highly trained athletes. Sports Med. 2011;41:757–771. doi: 10.2165/11587320-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Garrett AT, Creasy R, Rehrer NJ, et al. Effectiveness of short-term heat acclimation for highly trained athletes. Eur J Appl Physiol. 2012;112:1827–1837. doi: 10.1007/s00421-011-2153-3. [DOI] [PubMed] [Google Scholar]
- Giraldo E, Multhoff G, Ortega E. Noradrenaline increases the expression and release of Hsp72 by human neutrophils. Brain Behav Immun. 2010;24:672–677. doi: 10.1016/j.bbi.2010.02.003. [DOI] [PubMed] [Google Scholar]
- Hillman AR, Vince RV, Taylor L, McNaughton L, Mitchell N, Siegler J (2011) Exercise-induced dehydration with and without environmental heat stress results in increased oxidative stress. Appl Physiol Nutri Metab 36(5):698–706 [DOI] [PubMed]
- Hom LL, Lee EC-H, Apicella JM, et al. Eleven days of moderate exercise and heat exposure induces acclimation without significant HSP70 and apoptosis responses of lymphocytes in college-aged males. Cell Stress Chaperones. 2012;17:29–39. doi: 10.1007/s12192-011-0283-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard RW, Bowers WD, Matthew WT, et al. Rat model of acute heatstroke mortality. J Appl Physiol. 1977;42:809–816. doi: 10.1152/jappl.1977.42.6.809. [DOI] [PubMed] [Google Scholar]
- Iguchi M, Littmann AE, Chang S-H, et al. Heat stress and cardiovascular, hormonal, and heat shock proteins in humans. J Athl Train. 2012;47:184–190. doi: 10.4085/1062-6050-47.2.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JD, Fleshner M. Releasing signals, secretory pathways, and immune function of endogenous extracellular heat shock protein 72. J Leukoc Biol. 2006;79:425–434. doi: 10.1189/jlb.0905523. [DOI] [PubMed] [Google Scholar]
- Kampinga HH, Hageman J, Vos MJ, et al. Guidelines for the nomenclature of the human heat shock proteins. Cell Stress Chaperones. 2009;14:105–111. doi: 10.1007/s12192-008-0068-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster GI, Febbraio MA. Mechanisms of stress-induced cellular HSP72 release: implications for exercise-induced increases in extracellular HSP72. Exerc Immunol Rev. 2005;11:46–52. [PubMed] [Google Scholar]
- Lancaster GI, Febbraio MA. Exosome-dependent trafficking of HSP70: a novel secretory pathway for cellular stress proteins. J Biol Chem. 2005;280:23349–23355. doi: 10.1074/jbc.M502017200. [DOI] [PubMed] [Google Scholar]
- Lancaster GI, Møller K, Nielsen B, et al. Exercise induces the release of heat shock protein 72 from the human brain in vivo. Cell Stress Chaperones. 2004;9:276–280. doi: 10.1379/CSC-18R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Mayr S, Opitz-Gress A, Zeller C, Lormes W, Baur S, Lehmann M, Steinacker JM (1999) Human skeletal muscle HSP70 response to training in highly trained rowers. J Appl Physiol 86(1):101–4 [DOI] [PubMed]
- Lorenzo S, Halliwill JR, Sawka MN, Minson CT. Heat acclimation improves exercise performance. J Appl Physiol. 2010;109:1140–1147. doi: 10.1152/japplphysiol.00495.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo S, Minson CT, Babb TG, Halliwill JR. Lactate threshold predicting time–trial performance: impact of heat and acclimation. J Appl Physiol. 2011;111:221–227. doi: 10.1152/japplphysiol.00334.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu P-Z, Lai C-Y, Chan W-H. Caffeine induces cell death via activation of apoptotic signal and inactivation of survival signal in human osteoblasts. Int J Mol Sci. 2008;9:698–718. doi: 10.3390/ijms9050698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magalhães FDC, Amorim FT, Passos RLF, et al. Heat and exercise acclimation increases intracellular levels of Hsp72 and inhibits exercise-induced increase in intracellular and plasma Hsp72 in humans. Cell Stress Chaperones. 2010;15:885–895. doi: 10.1007/s12192-010-0197-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall HC, Ferguson RA, Nimmo MA. Human resting extracellular heat shock protein 72 concentration decreases during the initial adaptation to exercise in a hot, humid environment. Cell Stress Chaperones. 2006;11:129–134. doi: 10.1379/CSC-158R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maughan RJ, Otani H, Watson P. Influence of relative humidity on prolonged exercise capacity in a warm environment. Eur J Appl Physiol. 2012;112:2313–2321. doi: 10.1007/s00421-011-2206-7. [DOI] [PubMed] [Google Scholar]
- McClung JP, Hasday JD, He J-RR, et al. Exercise-heat acclimation in humans alters baseline levels and ex vivo heat inducibility of HSP72 and HSP90 in peripheral blood mononuclear cells. Am J Physiol Regul Integr Comp Physiol. 2008;294:R185–R191. doi: 10.1152/ajpregu.00532.2007. [DOI] [PubMed] [Google Scholar]
- Milne KJ, Noble EG (2002) Exercise-induced elevation of HSP70 is intensity dependent. J Appl Physiol 93(2):561–8 [DOI] [PubMed]
- Mizzen LA, Welch WJ. Characterization of the thermotolerant cell. I. Effects on protein synthesis activity and the regulation of heat-shock protein 70 expression. J Cell Biol. 1988;106:1105–1116. doi: 10.1083/jcb.106.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran DS, Shitzer A, Pandolf KB. A physiological strain index to evaluate heat stress. Am J Physiol. 1998;275:R129–R134. doi: 10.1152/ajpregu.1998.275.1.R129. [DOI] [PubMed] [Google Scholar]
- Moran DS, Eli-Berchoer L, Heled Y, et al. Heat intolerance: does gene transcription contribute? J Appl Physiol. 2006;100:1370–1376. doi: 10.1152/japplphysiol.01261.2005. [DOI] [PubMed] [Google Scholar]
- Mora-Rodriguez R, Del Coso J, Estevez E (2008) Thermoregulatory responses to constant versus variable-intensity exercise in the heat. Med Sci Sports Exerc 40(11):1945–52 [DOI] [PubMed]
- Morimoto R, Tissieres A, Georgopoulos C. The biology of heat shock proteins and molecular chaperones. Plainview: Cold Spring Harbor Laboratory Press; 1994. [Google Scholar]
- Multhoff G, Hightower LE. Cell surface expression of heat shock proteins and the immune response. Cell Stress Chaperones. 1996;1:167–176. doi: 10.1379/1466-1268(1996)001<0167:CSEOHS>2.3.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen B, Strange S, Christensen NJ, et al. Acute and adaptive responses in humans to exercise in a warm, humid environment. Pflügers Arch Eur J Physiol. 1997;434:49–56. doi: 10.1007/s004240050361. [DOI] [PubMed] [Google Scholar]
- Njemini R, Demanet C, Mets T. Inflammatory status as an important determinant of heat shock protein 70 serum concentrations during aging. Biogerontology. 2004;5:31–38. doi: 10.1023/B:BGEN.0000017684.15626.29. [DOI] [PubMed] [Google Scholar]
- Ogawa K, Seta R, Shimizu T, et al. Plasma adenosine triphosphate and heat shock protein 72 concentrations after aerobic and eccentric exercise. Exerc Immunol Rev. 2011;17:136–149. [PubMed] [Google Scholar]
- Ogura Y, Naito H, Akin S, et al. Elevation of body temperature is an essential factor for exercise-increased extracellular heat shock protein 72 level in rat plasma. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1600–R1607. doi: 10.1152/ajpregu.00581.2007. [DOI] [PubMed] [Google Scholar]
- Oishi Y, Taniguchi K, Matsumoto H, et al. Muscle type-specific response of HSP60, HSP72, and HSC73 during recovery after elevation of muscle temperature. J Appl Physiol. 2002;92:1097–1103. doi: 10.1152/japplphysiol.00739.2001. [DOI] [PubMed] [Google Scholar]
- Ortega E, Giraldo E, Hinchado MD, et al. Role of Hsp72 and norepinephrine in the moderate exercise-induced stimulation of neutrophils’ microbicide capacity. Eur J Appl Physiol. 2006;98:250–255. doi: 10.1007/s00421-006-0269-7. [DOI] [PubMed] [Google Scholar]
- Périard JD, Ruell P, Caillaud C, Thompson MW. Plasma Hsp72 (HSPA1A) and Hsp27 (HSPB1) expression under heat stress: influence of exercise intensity. Cell Stress Chaperones. 2012;17:375–383. doi: 10.1007/s12192-011-0313-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pockley AG, Shepherd J, Corton JM. Detection of heat shock protein 70 (Hsp70) and anti-Hsp70 antibodies in the serum of normal individuals. Immunol Invest. 1998;27:367–377. doi: 10.3109/08820139809022710. [DOI] [PubMed] [Google Scholar]
- Rhind SG, Gannon GA, Shephard RJ, et al. Cytokine induction during exertional hyperthermia is abolished by core temperature clamping: neuroendocrine regulatory mechanisms. Int J Hyperth Off J Eur Soc Hyperth Oncol North American Hyperth Group. 2004;20:503–516. doi: 10.1080/02656730410001670651. [DOI] [PubMed] [Google Scholar]
- Rowell LB, Brengelmann GL, Freund PR. Unaltered norepinephrine-heart rate relationship in exercise with exogenous heat. J Appl Physiol. 1987;62:646–650. doi: 10.1152/jappl.1987.62.2.646. [DOI] [PubMed] [Google Scholar]
- Ruell PA, Thompson MW, Hoffman KM, et al. Plasma Hsp72 is higher in runners with more serious symptoms of exertional heat illness. Eur J Appl Physiol. 2006;97:732–736. doi: 10.1007/s00421-006-0230-9. [DOI] [PubMed] [Google Scholar]
- Sandström ME, Siegler JC, Lovell RJ, et al. The effect of 15 consecutive days of heat-exercise acclimation on heat shock protein 70. Cell Stress Chaperones. 2008;13:169–175. doi: 10.1007/s12192-008-0022-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandström ME, Madden LA, Taylor L, et al. Variation in basal heat shock protein 70 is correlated to core temperature in human subjects. Amino Acids. 2009;37:279–284. doi: 10.1007/s00726-008-0144-4. [DOI] [PubMed] [Google Scholar]
- Sawka MN, Burke LM, Eichner ER, et al. American College of Sports Medicine position stand. Exercise and fluid replacement. Med Sci Sports Exerc. 2007;39:377–390. doi: 10.1249/01.mss.0000272779.34140.3b. [DOI] [PubMed] [Google Scholar]
- Selkirk GA, McLellan TM, Wright HE, Rhind SG. Mild endotoxemia, NF-kappaB translocation, and cytokine increase during exertional heat stress in trained and untrained individuals. Am J Physiol Regul Integr Comp Physiol. 2008;295:R611–R623. doi: 10.1152/ajpregu.00917.2007. [DOI] [PubMed] [Google Scholar]
- Selkirk GA, McLellan TM, Wright HE, Rhind SG. Expression of intracellular cytokines, HSP72, and apoptosis in monocyte subsets during exertional heat stress in trained and untrained individuals. Am J Physiol Regul Integr Comp Physiol. 2009;296:R575–R586. doi: 10.1152/ajpregu.90683.2008. [DOI] [PubMed] [Google Scholar]
- Singleton KD, Ziegler TR, Luo M, Fernandez-Estivariz C, Wischmeyer P. Intravenous glutamine increases serum HSP 72 levels in both humans and rodents following systemic inflammation. 27th Annual conference on shock. Halifax, Canada: Lippincott Williams & Wilkins; 2004. p. 250. [Google Scholar]
- Siri WE. The gross composition of the body. Adv Biol Med Phys. 1956;4:239–280. doi: 10.1016/B978-1-4832-3110-5.50011-X. [DOI] [PubMed] [Google Scholar]
- Taylor L, Midgley A, Chrismas B. The effect of acute hypoxia on heat shock protein 72 expression and oxidative stress in vivo. Eur J Appl Physiol. 2010;109:849–855. doi: 10.1007/s00421-010-1430-x. [DOI] [PubMed] [Google Scholar]
- Taylor L, Midgley AW, Chrismas B, et al. Daily quadratic trend in basal monocyte expressed HSP72 in healthy human subjects. Amino Acids. 2010;38:1483–1488. doi: 10.1007/s00726-009-0360-6. [DOI] [PubMed] [Google Scholar]
- Taylor L, Midgley AW, Chrismas B, et al. Daily hypoxia increases basal monocyte HSP72 expression in healthy human subjects. Amino Acids. 2011;40:393–401. doi: 10.1007/s00726-010-0644-x. [DOI] [PubMed] [Google Scholar]
- Taylor L, Midgley AW, Sandstrom ME, et al. The effect of the hyperbaric environment on heat shock protein 72 expression in vivo. Res Sports Med (Print) 2012;20:142–153. doi: 10.1080/15438627.2012.660830. [DOI] [PubMed] [Google Scholar]
- Walsh RC, Koukoulas I, Garnham A, et al. Exercise increases serum Hsp72 in humans. Cell Stress Chaperones. 2001;6:386–393. doi: 10.1379/1466-1268(2001)006<0386:EISHIH>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins AM, Cheek DJ, Harvey AE, et al. Heat shock protein (HSP-72) levels in skeletal muscle following work in heat. Aviat Space Environ Med. 2007;78:901–905. [PubMed] [Google Scholar]
- Welch WJ. Mammalian stress response: cell physiology, structure/function of stress proteins, and implications for medicine and disease. Physiol Rev. 1992;72:1063–1081. doi: 10.1152/physrev.1992.72.4.1063. [DOI] [PubMed] [Google Scholar]
- Whitham M, Walker GJ, Bishop NC. Effect of caffeine supplementation on the extracellular heat shock protein 72 response to exercise. J Appl Physiol. 2006;101:1222–1227. doi: 10.1152/japplphysiol.00409.2006. [DOI] [PubMed] [Google Scholar]
- Whitham M, Laing SJ, Jackson A, et al. Effect of exercise with and without a thermal clamp on the plasma heat shock protein 72 response. J Appl Physiol. 2007;103:1251–1256. doi: 10.1152/japplphysiol.00484.2007. [DOI] [PubMed] [Google Scholar]
- Winter EM, Jones AM, Davison RC, Bromley PD, Mercer TH. The British Association of Sport and Exercise Science guidelines. Oxon: Routledge; 2007. [Google Scholar]
- Yamada PM, Amorim FT, Moseley P, et al. Effect of heat acclimation on heat shock protein 72 and interleukin-10 in humans. J Appl Physiol. 2007;103:1196–1204. doi: 10.1152/japplphysiol.00242.2007. [DOI] [PubMed] [Google Scholar]