Abstract
Ethanol, which affects all body organs, exerts a number of cytotoxic effects, most of them independent of cell type. Ethanol treatment leads to increased membrane fluidity and to changes in membrane protein composition. It can also interact directly with membrane proteins, causing conformational changes and thereby influencing their function. The cytotoxic action may include an increased level of oxidative stress. Heat shock protein molecular chaperones are ubiquitously expressed evolutionarily conserved proteins which serve as critical regulators of cellular homeostasis. Heat shock proteins can be induced by various forms of stresses such as elevated temperature, alcohol treatment, or ischemia, and they are also upregulated in certain pathological conditions. As heat shock and ethanol stress provoke similar responses, it is likely that heat shock protein activation also has a role in the protection of membranes and other cellular components during alcohol stress.
Keywords: Ethanol, Membrane proteins, Membrane fluidity, Chaperones, Heat shock proteins, Cell protection, Apoptosis
Why is this topic timely?
Alcoholism is one of the most devastating diseases worldwide with an extremely high incidence. In spite of the tremendous efforts made by different organizations and charities, the problem persists and is a top priority problem in modern societies. In this review, we summarize the harmful effects of alcohol and the natural defensive mechanism against its toxicity.
The effects of alcohol on cellular physiology
Ethanol affects all tissues in the human organism and alcoholism is still one of the most common health, social, and economic problems worldwide. Ethanol exerts a number of cytotoxic effects, most of which are independent of cell type (reviewed in Baker and Kramer 1999). These harmful effects include the hyperfluidization of membranes, the denaturation of proteins, and the production of an elevated level of reactive oxygen species (ROS), which can interact with various cell components and lead to lipid and protein peroxidation and DNA damage. Ethanol can influence the function of mitochondria and their energy metabolism (Cunningham et al. 1990; Hoek 1994), and it has antiproliferative effects (Shoker et al. 1997).
Several lines of evidence indicate that ethanol displays marked effects on biological membranes. Ethanol can disrupt the physical structure of almost any type of membrane, including the plasma membrane, membranes of cell organelles (endoplasmic reticulum and mitochondrium), and liposomes (Goldstein 1986). It can increase membrane fluidity, which contributes directly to its acute toxicological action. Alterations in the lipid environment of membranes can influence the functions of membrane-associated proteins, such as receptors, ion channels, and enzymes, and can therefore alter biological processes, including membrane transport, enzymatic reactions, and signaling pathways (Escribá et al. 2008). Moreover, ethanol can interact directly with membrane proteins and bring about conformational changes, thereby influencing their functions (Fadda and Rossetti 1998). For instance, ethanol can affect the function of voltage-dependent sodium channels (Mullin and Hunt 1987) or NMDA receptors (Lovinger et al. 1990). It has also been found that chronic ethanol consumption leads to profound changes in the membrane protein profile of different tissues. The loss of membrane-associated proteins in the liver (Babu and Vemuri 1990) and the cerebral cortex (Babu et al. 1990) of chronically alcohol-treated rats suggested that this protein loss might be responsible for the membrane disruption caused by ethanol.
Both acute and chronic ethanol administration can give rise to enhanced ROS generation and at the same time to depressed activity of the protective antioxidant system, which results in oxidative stress (Wu and Cederbaum 2003). The free radicals produced can then react with different cellular components, causing the oxidation of lipids and proteins and DNA damage, and leading eventually to cell death. Lipid peroxidation results in significant changes in the structure and organization of membrane lipids, which can cause further alterations in numerous membrane functions, and consequently, in the functions of different membrane proteins (Mason et al. 1997; Vígh et al. 2005; Escribá et al. 2008). ROS can also denature proteins, which results in changes in protein structure leading to protein aggregation and the loss of enzymatic activity. Ethanol treatment of isolated rat hepatocytes causes a dose-dependent increase in ROS level and a mild decrease in cell viability (Bailey et al. 1999). Through the use of mitochondrial inhibitors, that study has revealed that the sites of ethanol-induced ROS production are the NADH dehydrogenase complex and the ubiquinone cycle of complex II. The in vitro ethanol treatment of a liver slice culture was demonstrated to be cytotoxic and to increase the level of lipid peroxidation (Naik et al. 2004). In an elegant in vivo study, chronic ethanol consumption in rats was found to result in oxidative stress, hepatocyte apoptosis, necrosis, inflammation, and a decreased level of glutathione, but these phenomena could be prevented by the overexpression of the mitochondrial isoform of superoxide dismutase (Wheeler et al. 2001). Taken together, these studies suggest that an elevated level of free radical production and oxidative stress play central roles in the pathogenesis of alcoholic liver disease (Wu and Cederbaum 2003). Furthermore, ethanol can also induce oxidative stress and depress the activity of the protective antioxidant system in extrahepatic tissues such as the heart and brain (reviewed in Nordmann et al. 1990). Short-term alcohol treatment led to decreased glutathione levels in heart, liver, and gastrocnemius muscle of mice and reduced plasma superoxide dismutase capacity (Islam et al. 2013). Acute ethanol treatment results in an increased lipid peroxide level and a decreased glutathione level in rat brain homogenates (Uysal et al. 1989). Both in vitro ethanol addition and chronic alcohol consumption have been demonstrated to lead to the enhanced formation of ROS and increased lipid peroxidation in the rat brain (Montoliu et al. 1994). Higher levels of lipid peroxidation in different brain regions and also in the liver were measured after both acute and chronic ethanol administrations in rats (Calabrese et al. 1996; Calabrese et al. 1998). The short-term ethanol treatment of cultured fetal rat cortical neurons resulted in increased ROS formation and a decreased level of glutathione followed by apoptosis (Rathinam et al. 2006). Chronic ethanol feeding decreased the activity levels of SOD-1 and SOD-2 in the brain (Calabrese et al. 1998). The brain is especially sensitive to oxidative injury, due to its high oxygen consumption, the high content of polyunsaturated fatty acid side chains in the neuronal membranes, and the weak antioxidant system in most brain regions (Halliwell 2006).
The cytoskeleton system seems to be another possible target of ethanol toxicity, and this may also be related to oxidative stress. Disruption of the cytoskeletal components can influence the functions of cells. In chronically ethanol-treated rats, the rate of tubulin polymerization and the microtubule reorganization were altered, while the level of expression of tubulin was unchanged in the damaged liver (Yoon et al. 1998). In an ethanol treated astrocyte culture, the actin cytoskeleton and the entire microtubular network appeared to be disrupted and disorganized (Tomas et al. 2003; Tomas et al. 2005). Acute ethanol treatment resulted in disruption of the actin network and enhanced ROS formation in the C6 rat glioma cell line. The finding that different antioxidants can prevent ethanol-induced alterations in actin organization an indication that an elevated ROS level contributes to the cytoskeletal remodeling effect of ethanol (Loureiro et al. 2011).
Ethanol-induced cell death seems to involve apoptosis. Hepatic apoptosis occurs in alcoholic liver disease, but ethanol can also induce apoptosis in the brain and in several other cell types. Aroor and Baker (1997) reported that the apoptotic cell number was significantly elevated in the human HL-60 promyeolocytic cell line after ethanol treatment. After a single day of binge ethanol treatment, Zhou et al. (2001) detected apoptotic cells in the liver, and Fas/Fas ligand system-mediated caspase-3 activation was postulated to play a central role in this apoptotic pathway, but mitochondrial cytochrome c release was also involved. Low-concentration ethanol treatment can also lead to apoptosis, probably through Fas receptor activation in a human hepatocellular carcinoma cell line (Castaneda and Kinne 2001; Kai et al. 2010). Ethanol enhances apoptosis in cerebellar granular cell cultures, involving an increase in caspase activity (Oberdoerster and Rabin 1999). These results were confirmed by several other studies, which demonstrated that ethanol treatment can trigger extensive apoptosis in vivo in the developing mammalian brain during the synaptogenetic period. Neurons are extremely sensitive to the cytotoxic effect of ethanol during this process, which occurs after birth in rats, and prenatally in humans. The acute ethanol treatment of 7-day-old rats led to extensive neuronal apoptosis (Ikonomidou et al. 2000). A similar result was described by Olney et al. (2002), who observed widespread neurodegeneration and caspase-3 activation in many brain regions of alcohol-treated mouse pups. Ethanol-induced apoptosis appears to require the presence of Bax, as acute ethanol treatment did not trigger caspase-3 activation and neurodegeneration in Bax-deficient mice (Young et al. 2003).
Heat shock proteins and their roles in membrane protection
Heat shock proteins (Hsps) are evolutionarily conserved proteins that can be found in every organism examined to date and in almost all cell types (Lindquist 1986). They can be induced by various forms of stress, such as hypoxia, ischemia, heavy metal, or ethanol exposure and infections, and they are also upregulated in several diseases. Hsps are rapidly induced in response to stressors and their most important function is to protect cells from the toxic effects of stress. A mild, sublethal stress that induces Hsp expression may generate resistance to a later severe, normally lethal injury. This phenomenon, called preconditioning, is observed in different cell types and tissues. Besides their general protective functions, Hsps also play roles in normal cellular homeostasis and development. Most of the stress-induced proteins are molecular chaperones and they have crucial functions in the biosynthesis, folding/unfolding, transport and assembly of other proteins (Finka and Goloubinoff 2013). Most of the Hsps are grouped by molecular weight. Human Hsps are classified into five families: Hsp110, Hsp90, Hsp70, Hsp40, and the small Hsps (sHsps; Kampinga et al. 2009).
A subpopulation of Hsps was associated with the surface or was present within cellular membranes. This membrane-associated pool of Hsps can alter certain attributes of the membrane lipid phase, such as fluidity and permeability, and can thereby maintain the membrane stability under stress conditions. A number of studies have documented the membrane localization of sHsps in different species (reviewed in Nakamoto and Vígh 2007 and Horváth et al. 2008). After a heat shock, most of the newly synthesized Hsp17 in Synechocystis PCC 6803 was found to be associated with the thylakoid membranes (Horváth et al. 1998; Glatz et al. 1999), binding especially to certain “heat shock lipids” (Balogi et al. 2005). A noteworthy finding was that an increased level of thylakoid association of Hsp17 provided improved resistance to UV-B damage in Synechocystis (Balogi et al. 2008). The level of membrane association of Lo18 depends on the temperature upshift in Oenococcus oeni (Delmas et al. 2001). In Myobacterium tuberculosis, the major membrane protein is a 16-kDa Hsp (Lee et al. 1992). Hsp29 of Toxoplasma gondii is similarly localized to the membrane (de Miguel et al. 2005). In mammalian cells, HspB2 is associated with the outer membrane of the mitochondria (Nakagawa et al. 2001), and the α-crystallins can also bind to lens plasma membranes (Cobb and Petrash 2000). However, it is not only the sHsps that can bind to cellular membranes: as an example in Escherichia coli a subpopulation of GroEL was found to be associated to the membrane (Newman and Crooke 2000). In various mammalian cell lines, Hsp60 has been found in the mitochondrial outer membrane and also in the plasma membrane (Soltys and Gupta 1996). Following the exposure of tumor cells to nonlethal heat stress, Hsp72 was found to be expressed on the cell surface (Multhoff et al. 1995; Stangl et al. 2011; Multhoff and Hightower 2011).
The role of alcohol on membranes and the induction of the stress response
It seems that ethanol affects primarily the cellular membranes, and some of its other toxic effects (for example impairing protein function, elevated ROS generation) also related to membrane hyperfluidization. Enhanced cell membrane fluidity has been documented in different cell types after in vitro ethanol treatment. Ethanol treatment increases the fluidity of murine erythrocytes and synaptosomal and mitochondrial membranes in a dose-dependent manner (Chin and Goldstein 1977). Ethanol exerts a significant disordering effect on isolated erythrocytes, synaptic plasma membranes, and microsomal membranes of the murine brain (Armbrecht et al. 1983). The in vitro disordering of brain membranes was found to be correlated with an elevated sensitivity to ethanol in vivo (Goldstein et al. 1982). Following ethanol treatment, synaptosomal and erythrocyte membranes derived from ethanol-sensitive mice were more strongly disordered than those from ethanol-resistant mice. The ethanol treatment of a primary culture of murine neural crest cells correlated positively and dose-dependently with the membrane fluidity and the significantly decreased cell viability (Chen et al. 1996).
On the other hand, chronic ethanol consumption leads to ethanol tolerance, which may be a response to its primary membrane-disordering effect. Membranes isolated from chronically ethanol-treated mice proved resistant to the fluidizing effect of ethanol (Chin and Goldstein 1977), and in parallel, the cholesterol content of the membranes was increased (Chin et al. 1978). Chronic ethanol treatment also induced a significantly increased cholesterol level in microsomes isolated from chicken brain and liver (Sanchez-Amate et al. 1991). Erythrocyte membranes isolated from chronic alcoholics exhibited an increase in cholesterol content and a decrease in membrane fluidity (Parmahamsa et al. 2004). These early studies clearly demonstrate that ethanol treatment leads to the physical disruption of lipid membranes in the cells. However, the interaction of ethanol with membranes causes not only a decreased membrane order, but also changes in the membrane protein composition, which can ultimately affect receptor functions and signaling pathways in the cell.
In a study of the association between ROS production and the level of membrane fluidity in isolated rat hepatocytes (Sergent et al. 2005), ethanol treatment resulted in an increase in membrane fluidity after incubation for 30 min, but this was prevented by inhibition of the ethanol metabolism, or by pretreatment with either a ROS scavenger or an antioxidant, indicating that the metabolism of ethanol and the formation of ROS are involved in the elevation of membrane fluidity. An increase in the level of ROS production was detected after only a 15-min ethanol incubation. It also emerged that membrane-stabilizing agents which prevent hyperfluidization decreased the levels of ROS production, lipid peroxidation, and cell death in response to a 5-h incubation, but had no detectable effect on the extent of ROS formation induced by a short, 15-min ethanol incubation. In contrast, membrane fluidizing compounds did enhance the degree of ethanol induced oxidative stress. These data suggested that early ROS formation due to the metabolism of ethanol contributed to the rapid elevation of membrane fluidity, which in turn further amplified the oxidative stress and lipid peroxidation, leading finally to cell death.
The activation of the Hsps is mediated by heat shock elements (HSEs) and transcription factors referred to as heat shock factors (HSFs). The HSEs are located in the promoter region of the Hsps. In mammalian cells, at least four members of the HSF family can be detected. Under non-stressful conditions, HSF1 is sequestered in an inactive form in the cytoplasm through interactions with Hsp90, Hsp70, and Hsp40. Following stress, the denatured, misfolded proteins that emerge compete with HSF for association with the Hsps. Thus, HSF dissociates from the chaperones and translocates to the nucleus; meanwhile, the released HSF undergoes trimerization and phosphorylation. Finally, it binds to the HSE of the promoters of heat shock genes and activates them. This occurs rapidly: activation of the DNA-binding form of HSF can be detected within minutes following heat treatment (Morimoto et al. 1992; Morimoto 2002; Sőti et al. 2005).
However, protein denaturation is not the only way to increase the expression of Hsps. The evidence is increasing which suggests that there is an alternative, membrane-associated “thermosensor” that can initiate a signaling pathway and finally activate heat shock genes. During heat stress, the molecular order of membranes rapidly decreases, and this decrease is probably converted into signals leading to the transcription activation of heat shock genes (Vígh et al. 1998; Balogh et al. 2013). In an earlier study by the Vígh team, two membrane fluidizers, benzyl alcohol (BA) and heptanol, were used to increase the membrane fluidity in K562 cells. Both chemicals induced Hsp70 expression without measurable protein denaturation (Balogh et al. 2005). An immediate increase in intracellular Ca2+ level was also observed. BA is additionally able to induce different Hsp genes, without protein denaturation in B16 (F10) cells, and this transcription activation is mediated by HSF1 (Nagy et al. 2007). BA treatment (and also heat stress) induces the reorganization of cholesterol-rich membrane domains, and this might play a crucial role in the generation of a stress signal. It seems that even small alterations in plasma membrane microdomains can activate stress sensing and signaling proteins in these domains (Vígh et al. 2007a; Vígh et al. 2007b). Similarly, the fatty acid and lipid composition of the B16 cell membrane correlated with the changes in the membrane microdomain organization and the altered stress inducibility (Péter et al. 2012; Csoboz et al. 2013). As recently reviewed (Horváth et al. 2012; Balogh et al. 2013), there is increasing evidence that many stress events cause Hsp induction without commensurate protein denaturation on a scale ranging from bacteria to yeast, plant, and mammalian cells. Besides highlighting the importance of membranes and their lipids in the heat shock response, such observations provide a new perspective for future studies into the mechanisms that mediate cellular and organismal responses to heat stress.
Regardless of whether it is acute or chronic, ethanol exposure is rather stressful and (as in the case of other stressors) can induce the activation of HSFs and increase the level of Hsp expression. Alcohol induces Hsp expression primarily in the liver and brain. An elevated level of Hsp70 expression related to ethanol-induced liver injury has been observed in rats (Nanji et al. 1995), and maternal ethanol consumption likewise resulted in an elevated level of Hsp70 in different brain regions of rat pups (Holownia et al. 1995). Both acute and chronic ethanol treatment induces the expression of Hsp70 in the brain and liver of rats (Calabrese et al. 1996; Calabrese et al. 1998). Acute administration of 5 g/kg ethanol resulted in an increased level of Hsp70 in the hippocampus, cerebellum, cortex, striatum, and liver. In the brain, the amount of Hsp70 protein correlated negatively with the level of lipid peroxidation; the cerebellum and hippocampus displayed the lowest levels of lipid peroxidation, with the highest Hsp70 induction (Calabrese et al. 1996). Chronic (12-week) ethanol administration was found to increase the level of Hsp70 in these cerebral areas and in the liver (Calabrese et al. 1998). Chronic ethanol consumption similarly led to an increased expression level of Hsp70 in the amygdala (Bell et al. 2006). Significant elevations in HSP72 protein and mRNA and in HSF1 protein levels were noted in liver after short-term ethanol administration in mice (Islam et al. 2013). An in vitro study demonstrated that ethanol exposure stimulated the translocation of HSF1 from cytoplasm to the nucleus in cultured cortical neuronal cells (Pignataro et al. 2007), and a rapid increase was detected in the level of expression of the genes of Hsp27, Hsp40, Hsp70, and (with slower kinetics) Hsp90. When adult mice were injected with a single dose of ethanol, the nuclear translocation of HSF1 was enhanced and various Hsp genes were activated in the cerebral cortex (Pignataro et al. 2007). The levels of sHsps were increased in neural stem cells derived from the forebrain of mouse embryos when the cells were differentiated in the presence of 50 mM ethanol, and the expression of the HSF genes was also upregulated (Choi et al. 2011).
Ethanol consumption contributes to alterations in the functions of inflammatory cells. In vitro alcohol treatment of macrophages and monocytes promotes the nuclear translocation of HSF1 (but not HSF2) and increases its DNA-binding activity, and acute ethanol administration induces Hsp70 expression in macrophages. On the other hand, the level of Hsp70 is decreased in the course of prolonged ethanol treatment and in parallel the level of Hsp90 is increased (Mandrekar et al. 2008).
Several studies have demonstrated that moderate ethanol exposure can provoke preconditioning in various cell types, which become resistant to a subsequent otherwise lethal stimulus; this might result from the activation of Hsp expression. H9c2 cells treated with 6 % ethanol for 1 h developed thermotolerance and resistance to H2O2, which might be due to the elevated expression of Hsp70 (Su et al. 1998). It has also been revealed that prior administration of ethanol offers a protective effect against neuronal damage due to cerebral ischemia reperfusion in gerbils (Wang et al. 2007). An investigation of the possible neuroprotective effects of ethanol preconditioning in organotypic slice cultures of the maturing rat hippocampal–entorhinal cortex (HEC) complex and in an in vitro culture of rat cerebellar cells demonstrated significant elevations of Hsp70 and Hsp27 after 6 days of moderate ethanol administration, this being correlated with a neuroprotection against the neuronal degeneration provoked by the HIV-1 virus envelope glycoprotein or β-amyloid (Collins et al. 2010). It was observed that suppression of the elevation of Hsp70 and Hsp27 inhibited the neuroprotection, suggesting that both proteins are important for full neuroprotection. Alcohol can activate HSFs and increase the expression of several Hsps, and mild, non-toxic exposure to ethanol can protect cells from various harmful stimuli.
Possible mechanism of Hsp protection and potential therapeutic approaches
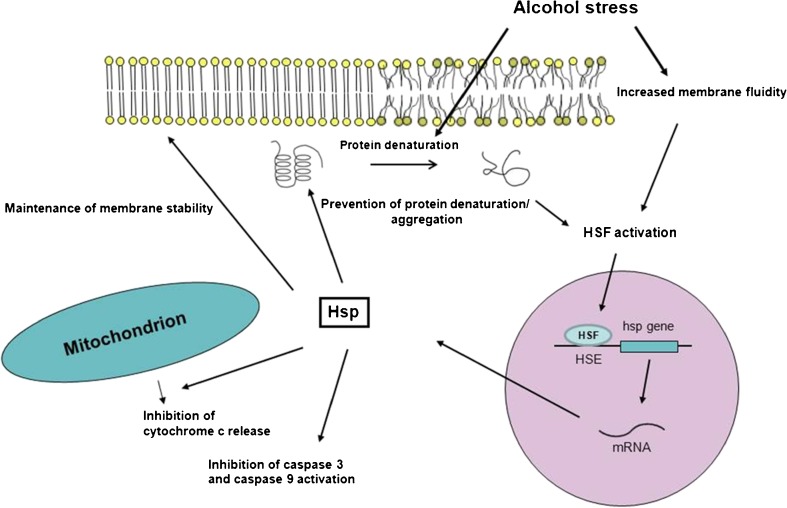
During stress, the cellular plasma membrane is a sensitive target for damage. Stress factors can increase membrane fluidity and denature membrane proteins. Heat shock and ethanol stress responses exhibit extensive similarities (Piper 1995) as both exert a membrane-disruptive effect, and they denature proteins and cause similar changes in the plasma membrane protein composition, as illustrated in Fig. 1.
Fig. 1.
Schematic representation of the chaperone function after alcohol stress. Ethanol treatment can influence the function of different cell components, e.g., it can disrupt the membrane order and impair the structure of proteins. However, these events lead to the activation of HSFs, and subsequently, to the expression of Hsps. The synthesized Hsps finally provide protection against further damage, as they can increase the membrane order, repair the proteins or prevent their aggregation, and block the apoptotic pathway
A number of studies have pointed to a potential link between membrane function and Hsps, which can bind to the membranes, primarily to lipid rafts, and increase the physical order. An early study demonstrated that E. coli GroEL14 can bind to lipid mono- and bilayers and GroEL can increase the molecular order of the lipid bilayers under heat shock conditions (Török et al. 1997). It was later found that Hsp17 of the blue alga Synechocystis is associated with lipid membranes, resulting in an elevated degree of physical order of the membranes and reduced membrane fluidity (Török et al. 2001). αB-crystallin and Synechocystis Hsp17 have been demonstrated to be able to regulate the bilayer–nonbilayer phase equilibrium and to exert a bilayer-stabilizing effect on model membranes in vitro (Tsvetkova et al. 2002). We recently provided evidence of the cholesterol-controlled lipid raft interaction of the mammalian Hspb11 (Hsp16.6; Török et al. 2012). Overall, these data suggest that the membrane-associated pool(s) of Hsps may play a pivotal role in membrane protection under very varied stress conditions (Nakamoto and Vígh 2007; Balogi et al. 2008; Horváth et al. 2008; Horváth and Vígh 2010; Horváth et al. 2012).
As heat shock and ethanol stress provoke similar responses, it is likely that Hsp activation also has a role in membrane protection after ethanol treatment. In the lactic acid bacterium O. oeni, the sHsp Lo18 is mostly associated with the membranes when the cells are heat-shocked or treated with ethanol or BA which can disturb membranes (Coucheney et al. 2005). It also seems that Lo18 can modulate membrane properties by increasing its molecular order, which leads to a diminished membrane fluidity.
The overexpression of different Hsps has a protective effect against oxidative stress. The expression of Hsp27 or αB-crystallin in L929 cells decreased the intracellular level of ROS generated by TNF-α, while the cellular glutathione level was increased and the levels of lipid peroxidation and protein oxidation were lowered (Mehlen et al. 1996). It has been reported that exercise training can enhance the levels of Hsp70 and glutathione (Demirel et al. 1998; Lewis et al. 2013). A 10-week-training program reduced the degree of myocardial lipid peroxidation following short-term ischemia reperfusion, while the level of Hsp70 was largely increased in the left ventricle (Demirel et al. 1998). Heat shock preconditioning can protect the liver from oxidative stress in carbon tetrachloride-induced liver injury (Yamamoto et al. 2000). Oxidative stress led to a suppressed level of protein denaturation, which might be associated with the induction of Hsp70. Similarly, the overexpression of Hsp27 protected the heart from an ischemia-reperfusion injury by decreasing the effects of oxidative stress (Hollander et al. 2004).
Hsps can influence the structure and function of the cytoskeleton, which is one of the targets of ethanol. Chaperones can facilitate the formation of cytoskeletal components and protect them during stress. For example, the overexpression of Hsp70 or αB-crystallin preserved the microtubular integrity after stimulated ischemia in rat neonatal cardiac myocytes (Bluhm et al. 1998). Both αA- and αB-crystallin can block the cytochalasin D-induced depolymerization and heat-induced aggregation of actin filaments (Wang and Spector 1996). The overexpression of Hsp27 can protect cells against the actin fragmentation induced by oxidative stress, and the microfilament stabilization is associated with a higher rate of cell survival following oxidative stress (Huot et al. 1996).
The cytoprotective effect of Hsps is related to their ability to inhibit apoptosis. Various studies have demonstrated that Hsps can interfere with different steps of the apoptotic pathway. Hsp27 can prevent the cytochrome c-dependent activation of procaspase-9 and the subsequent activation of procaspase-3 (Garrido et al. 1999; Bruey et al. 2000), and it inhibits the release of cytochrome c from the mitochondria (Paul et al. 2002). Hsp27 also affects the Fas-induced Daxx-mediated apoptotic pathway (Charette et al. 2000). Hsp70 has been reported to prevent caspase activation (Buzzard et al. 1998); moreover, it can mediate a caspase-independent pathway (Ravagnan et al. 2001). Hsp70 can block formation of the apoptosome (Beere et al. 2000), and similarly to Hsp27, can prevent cytochrome c release from the mitochondria (Stankiewicz et al. 2005). As ethanol treatment can lead to apoptotic cell death, it is likely that Hsps can moderate the cytotoxic effect of ethanol in part through their ability to reduce apoptosis.
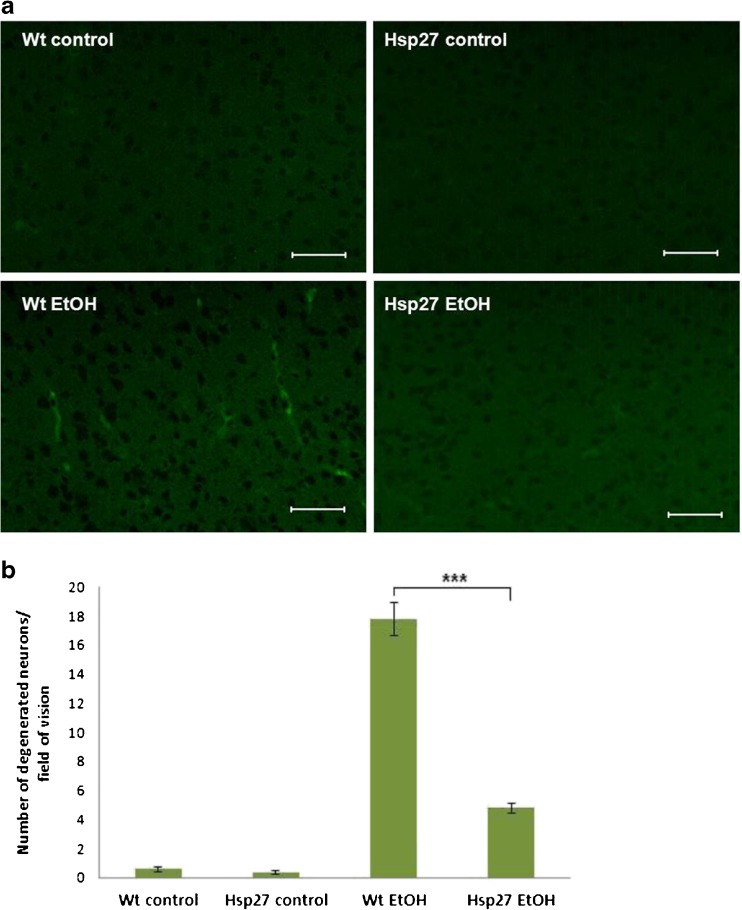
As the overexpression of Hsps can protect cellular components against certain stressors, it might have a beneficial effect in the prevention of different pathological conditions that cause membrane alterations, protein denaturation or apoptosis, like the ethanol-induced syndrome or other neurodegenerative diseases. In our lab, it was demonstrated that Hsp27 displays a neuroprotective effect against acute and chronic ethanol administration. Hsp27-overexpressing transgenic mice and wild-type littermates were injected intraperitoneally with 2 g/kg ethanol, and the motor coordination and muscle strength were then analyzed by means of various behavioral tests, such as the footprint, balance beam, and inverted screen tests. The ethanol treated wild-type mice exhibited ataxia and incoordination, whereas the overexpression of Hsp27 significantly reduced these harmful effects (Toth et al. 2010). When the drinking water of mice was replaced by a 20 % ethanol solution for 5 weeks and neurodegeneration was monitored through the use of Fluoro Jade C staining, a significantly lower number of degenerating neurons were found in the brain of the ethanol-drinking Hsp27 transgenic mice as compared with that of the wild-type mice (Fig. 2; Toth et al. 2010).
Fig. 2.
Investigation of the neuroprotective effects of Hsp27 overexpression during chronic ethanol administration. Following the treatment of mice with 20 % ethanol for 5 weeks, degenerated neurons were detected in frozen brain sections through the use of Fluoro Jade C staining. a Representative picture of Fluoro Jade C staining in the cortex. Scale bar represents 100 μm. b Quantitative comparison of degenerated neurons. Mean ± SEM is shown; ***p < 0.001
Several previous studies have demonstrated that different plant-derived compounds or drugs that have potent antioxidant and anti-inflammatory activities can induce the expression of Hsps. These compounds can be promising therapeutic agents to prevent or moderate the symptoms of diseases accompanied by oxidative damage, inflammation, and apoptosis. For example, xenohormetic plant compounds, such as resveratrol or curcumin, activate the mammalian stress response when ingested and have therapeutic effects against different diseases like cancer, type 2 diabetes, or neurodegenerative diseases and aging (Hooper et al. 2010). These bioactive compounds might have beneficial effects against alcohol induced diseases, such as liver damage and neurodegeneration. Indeed, pretreatment of cells with curcumin or addition of curcumin together with ethanol reduced the level of lipid peroxidation and the toxicity of ethanol (Naik et al. 2004). Celastrol, a triterpene compound isolated from the vine Tripterygium wilfordii of the Celastraceae family, was effective in inducing Hsp70, Hsp27, and Hsp32 in cultures derived from cerebral cortices (Chow et al. 2013). Extracts of T. wilfordii have been used as a traditional herbal therapy in Chinese medicine to treat different illnesses, but recent results have suggested that celastrol could be a potential agent to treat diseases such as Alzheimer disease (Chow et al. 2013; Allison et al. 2001). A study on the potential cytoprotective effect of ginseng indicated that pretreatment with ginseng prevented the ethanol-induced gastric lesions and decreased the level of apoptotic cell death in the rat gastric mucosa (Yeo et al. 2008). Moreover, Hsp27 and Hsp70 were found to be overexpressed in ginseng-pretreated rats. Astaxanthin, a carotenoid of marine animals, is also a powerful antioxidant. It has been shown to decrease the high fructose-fat diet induced ROS production and endoplasmatic reticulum stress in the liver (Bhuvaneswari et al. 2013). Astaxanthin also antagonizes the ethanol effect on cortical spreading depression in the young adult rat brain (Abadie-Guedes et al. 2008). On the other hand, previously astaxanthin was reported to induce the expression of Hsp70 and heme oxygenase in human neuroblastoma cells (Lee et al. 2010). Chitosan, a polysaccharide found in shellfish, krill, fungi, and insects, has membrane stabilizing properties (Filipović-Grcić et al. 2001) and helps to upregulate Hsp70 expression (Khodagholi et al. 2010). Chitosan was reported to be neuroprotective by suppressing Aβ formation (Khodagholi et al. 2010), it can reduce age-related dyslipidemic abnormalities and oxidative stress in aged rats (Anandan et al. 2013), and has anti-ulcerogenic effect against HCl-ethanol induced ulcer (Anandan et al. 2004). Geranylgeranylacetone (GGA) is an antiulcer drug which protects the cells of the gastric mucosa against several forms of stress. The cytoprotective effect of GGA appears to be due, at least in part, to the induction of Hsp expression (Hirakawa et al. 1996). After the treatment of gastric mucosal cell cultures with 7.5 % ethanol for 8 h, apoptotic DNA fragmentation was found (Mizushima et al. 1999). However, pretreatment of the cells with GGA for 3 h decreased the level of DNA fragmentation in a dose-dependent manner. Pretreatment with a low concentration of ethanol also suppressed DNA fragmentation. These results suggest that the Hsp induction by pretreatment with either GGA or a low concentration of ethanol can protect cells against ethanol-induced apoptotic cell death.
These few examples support the notion that certain drugs or bioactive compounds derived from plants and animals that have antioxidant, anti-inflammatory, or anti-apoptotic effects, exert their cytoprotective functions, at least in part, through the induction of Hsp expression. Due to these properties, they might have protective effects in certain pathological conditions, such as alcoholism.
Concluding remarks
The published data clearly reveal the harmful effects of ethanol including the hyperfluidization of cellular membranes, the disruption of proteins, the elevation of ROS products, and the induction of the molecular machinery leading to apoptosis. Acute, low-dose ethanol treatment can induce a heat shock response by activating HSFs, thereby elevating the level of expression of Hsps. In response, the activated Hsps help to counter the cytotoxic effect of ethanol by normalizing membrane disordering, repairing damaged proteins, protecting against oxidative stress, and inhibiting apoptosis.
Acknowledgments
This work was supported by a National Development Agency grant (TAMOP-4.2.2.A-11/1/KONV-2012-0052 to L.V. and M.S).
References
- Abadie-Guedes R, Santos SD, Cahú TB, Guedes RC, de Souza Bezerra R. Dose-dependent effects of astaxanthin on cortical spreading depression in chronically ethanol-treated adult rats. Alcohol Clin Exp Res. 2008;32:1417–1421. doi: 10.1111/j.1530-0277.2008.00710.x. [DOI] [PubMed] [Google Scholar]
- Allison AC, Cacabelos R, Lombardi VR, Alvarez XA, Vigo C. Celastrol, a potent antioxidant and anti-inflammatory drug, as a possible treatment for Alzheimer’s disease. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25:1341–1357. doi: 10.1016/s0278-5846(01)00192-0. [DOI] [PubMed] [Google Scholar]
- Anandan R, Nair PG, Mathew S. Anti-ulcerogenic effect of chitin and chitosan on mucosal antioxidant defence system in HCl-ethanol-induced ulcer in rats. J Pharm Pharmacol. 2004;56:265–269. doi: 10.1211/0022357023079. [DOI] [PubMed] [Google Scholar]
- Anandan R, Ganesan B, Obulesu T, Mathew S, Asha KK, Lakshmanan PT, Zynudheen AA. Antiaging effect of dietary chitosan supplementation on glutathione-dependent antioxidant system in young andaged rats. Cell Stress Chaperones. 2013;18:121–125. doi: 10.1007/s12192-012-0354-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armbrecht HJ, Wood WG, Wise RW, Walsh JB, Thomas BN, Strong R. Ethanol-induced disordering of membranes from different age groups of C57BL/6NNIA mice. J Pharmacol Exp Ther. 1983;226:387–391. [PubMed] [Google Scholar]
- Aroor AR, Baker RC. Ethanol-induced apoptosis in human HL-60 cells. Life Sci. 1997;61:2345–2350. doi: 10.1016/s0024-3205(97)00938-7. [DOI] [PubMed] [Google Scholar]
- Babu P, Vemuri MC. Liver plasma membrane proteins in chronic ethanol intoxication. Biochern Inr. 1990;20:573–577. [PubMed] [Google Scholar]
- Babu PP, Nagaraju N, Vemuri MC. Differences in the Plasma Membrane Proteins of Chronic Alcoholic Rat Brain. Membr Biochem. 1990;9:227–237. doi: 10.3109/09687689009025843. [DOI] [PubMed] [Google Scholar]
- Bailey SM, Pietsch EC, Cunningham CC. Ethanol stimulates the production of reactive oxygen species at mitochondrial complexes I and III. Free Radic Biol Med. 1999;27:891–900. doi: 10.1016/s0891-5849(99)00138-0. [DOI] [PubMed] [Google Scholar]
- Baker RC, Kramer RE. Cytotoxicity of short-chain alcohols. Annu Rev Pharmacol Toxicol. 1999;39:127–150. doi: 10.1146/annurev.pharmtox.39.1.127. [DOI] [PubMed] [Google Scholar]
- Balogh G, Horváth I, Nagy E, Hoyk Z, Benkő S, Bensaude O, Vígh L. The hyperfluidization of mammalian cell membranes acts as a signal to initiate the heat shock protein response. FEBS J. 2005;272:6077–6086. doi: 10.1111/j.1742-4658.2005.04999.x. [DOI] [PubMed] [Google Scholar]
- Balogh G, Péter M, Glatz A, Gombos I, Török Z, Horváth I, Harwood JL, Vígh L. Key role of lipids in heat stress management. FEBS Lett. 2013;587:1970–1980. doi: 10.1016/j.febslet.2013.05.016. [DOI] [PubMed] [Google Scholar]
- Balogi Z, Török Z, Balogh G, Jósvay K, Shigapova N, Vierling E, Vigh L, Horváth I. “Heat shock lipid” in cyanobacteria during heat/light-acclimation. Arch Biochem Biophys. 2005;436:346–354. doi: 10.1016/j.abb.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Balogi Z, Cheregi O, Giese KC, Juhász K, Vierling E, Vass I, Vígh L, Horváth I. A mutant small heat shock protein with increased thylakoid association provides an elevated resistance against UV-B damage in Synechocystis 6803. J Biol Chem. 2008;283:22983–22991. doi: 10.1074/jbc.M710400200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beere HM, Wolf BB, Cain K, Mosser DD, Mahboubi A, Kuwana T, Tailor P, Morimoto RI, Cohen GM, Green DR. Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nat Cell Biol. 2000;2:469–475. doi: 10.1038/35019501. [DOI] [PubMed] [Google Scholar]
- Bell RL, Kimpel MW, Rodd ZA, Strother WN, Bai F, Peper CL, Mayfield RD, Lumeng L, Crabb DW, McBride WJ, Witzmann FA. Protein expression changes in the nucleus accumbens and amygdala of inbred alcohol-preferring rats given either continuous or scheduled access to ethanol. Alcohol. 2006;40:3–17. doi: 10.1016/j.alcohol.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Bhuvaneswari S, Yogalakshmi B, Sreeja S, Anuradha CV. Astaxanthin reduces hepatic endoplasmic reticulum stress and nuclear factor-κB-mediated inflammation in high fructose and high fat diet-fed mice. Cell Stress Chaperones. 2013 doi: 10.1007/s12192-013-0443-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluhm WF, Martin JL, Mestril R, Dillmann WH. Specific heat shock proteins protect microtubules during simulated ischemia in cardiac myocytes. Am J Physiol. 1998;275:2243–2249. doi: 10.1152/ajpheart.1998.275.6.H2243. [DOI] [PubMed] [Google Scholar]
- Bruey JM, Ducasse C, Bonniaud P, Ravagnan L, Susin SA, Diaz-Latoud C, Gurbuxani S, Arrigo AP, Kroemer G, Solary E, Garrido C. Hsp27 negatively regulates cell death by interacting with cytochrome c. Nat Cell Biol. 2000;2:645–652. doi: 10.1038/35023595. [DOI] [PubMed] [Google Scholar]
- Buzzard KA, Giaccia AJ, Killender M, Anderson RL. Heat shock protein 72 modulates pathways of stress-induced apoptosis. J Biol Chem. 1998;273:17147–17153. doi: 10.1074/jbc.273.27.17147. [DOI] [PubMed] [Google Scholar]
- Calabrese V, Renis M, Calderone A, Russo A, Barcellona ML, Rizza V. Stress proteins and SH-groups in oxidant-induced cell damage after acute ethanol administration in rat. Free Radic Biol Med. 1996;20:391–398. doi: 10.1016/0891-5849(95)02095-0. [DOI] [PubMed] [Google Scholar]
- Calabrese V, Renis M, Calderone A, Russo A, Reale S, Barcellona ML, Rizza V. Stress proteins and SH-groups in oxidantinduced cellular injury after chronic ethanol administration in rat. Free Radic Biol Med. 1998;24:1159–1167. doi: 10.1016/s0891-5849(97)00441-3. [DOI] [PubMed] [Google Scholar]
- Castaneda F, Kinne RK. Apoptosis induced in HepG2 cells by short exposure to millimolar concentrations of ethanol involves the Fas-receptor pathway. J Cancer Res Clin Oncol. 2001;127:418–424. doi: 10.1007/s004320000227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charette SJ, Lavoie JN, Lambert H, Landry J. Inhibition of Daxx-mediated apoptosis by heat shock protein 27. Mol Cell Biol. 2000;20:7602–7612. doi: 10.1128/mcb.20.20.7602-7612.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SY, Yang B, Jacobson K, Sulik KK. The membrane disordering effect of ethanol on neural crest cells in vitro and the protective role of GM1 ganglioside. Alcohol. 1996;13:589–595. doi: 10.1016/s0741-8329(96)00073-0. [DOI] [PubMed] [Google Scholar]
- Chin JH, Goldstein DB. Drug tolerance in biomembranes: a spin label study of the effects of ethanol. Science. 1977;196:684–685. doi: 10.1126/science.193186. [DOI] [PubMed] [Google Scholar]
- Chin JH, Parsons LM, Goldstein DB. Increased cholesterol content of erythrocyte and brain membranes in ethanol-tolerant mice. Biochim Biophys Acta. 1978;513:358–363. doi: 10.1016/0005-2736(78)90204-3. [DOI] [PubMed] [Google Scholar]
- Choi MR, Jung KH, Park JH, Das ND, Chung MK, Choi IG, Lee BC, Park KS, Chai YG. Ethanol-induced small heat shock protein genes in the differentiation of mouse embryonic neural stem cells. Arch Toxicol. 2011;85:293–304. doi: 10.1007/s00204-010-0591-z. [DOI] [PubMed] [Google Scholar]
- Chow AM, Tang DW, Hanif A, Brown IR. Induction of heat shock proteins in cerebral cortical cultures by celastrol. Cell Stress Chaperones. 2013;18:155–160. doi: 10.1007/s12192-012-0364-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb BA, Petrash JM. Characterization of alpha-crystallin-plasma membrane binding. J Biol Chem. 2000;275:6664–6672. doi: 10.1074/jbc.275.9.6664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins MA, Neafsey EJ, Wang K, Achille NJ, Mitchell RM, Sivaswamy S. Moderate Ethanol Preconditioning of Rat Brain Cultures Engenders Neuroprotection Against Dementia-Inducing Neuroinflammatory Proteins: Possible Signaling Mechanisms. Mol Neurobiol. 2010;41:420–425. doi: 10.1007/s12035-010-8138-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coucheney F, Gal L, Beney L, Lherminier J, Gervais P, Guzzo J. A small HSP, Lo18, interacts with the cell membrane and modulates lipid physical state under heat shock conditions in a lactic acid bacterium. Biochim Biophys Acta. 2005;1720:92–98. doi: 10.1016/j.bbamem.2005.11.017. [DOI] [PubMed] [Google Scholar]
- Csoboz B, Balogh GE, Kusz E, Gombos I, Peter M, Crul T, Gungor B, Haracska L, Bogdanovics G, Torok Z, Horvath I, Vigh L (2013) Membrane fluidity matters: Hyperthermia from the aspects of lipids and membranes. Int J Hyperth (In press) [DOI] [PubMed]
- Cunningham CC, Coleman WB, Spach PI. The effects of chronic ethanol consumption on hepatic mitochondrial energy metabolism. Alcohol Alcohol. 1990;25:127–136. doi: 10.1093/oxfordjournals.alcalc.a044987. [DOI] [PubMed] [Google Scholar]
- de Miguel N, Echeverria PC, Angel SO. Differential subcellular localization of members of the Toxoplasma gondii small heat shock protein family. Eukaryot Cell. 2005;4:1990–1997. doi: 10.1128/EC.4.12.1990-1997.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmas F, Pierre F, Coucheney F, Divies C, Guzzo J. Biochemical and physiological studies of the small heat shock protein Lo18 from the lactic acid bacterium Oenococcus oeni. J Mol Microbiol Biotechnol. 2001;3:601–610. [PubMed] [Google Scholar]
- Demirel HA, Powers SK, Caillaud C, Coombes JS, Naito H, Fletcher LA, Vrabas I, Jessup JV, Ji LL. Exercise training reduces myocardial lipid peroxidation following short-term ischemia-reperfusio. Med Sci Sports Exerc. 1998;30:1211–1216. doi: 10.1097/00005768-199808000-00005. [DOI] [PubMed] [Google Scholar]
- Escribá PV, González-Ros JM, Goñi FM, Kinnunen PK, Vigh L, Sánchez-Magraner L, Fernández AM, Busquets X, Horváth I, Barceló-Coblijn G. Membranes: a meeting point for lipids, proteins and therapies. J Cell Mol Med. 2008;12:829–875. doi: 10.1111/j.1582-4934.2008.00281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadda F, Rossetti ZL. Chronic ethanol consumption: from neuroadaptation to neurodegeneration. Prog Neurobiol. 1998;56:385–431. doi: 10.1016/s0301-0082(98)00032-x. [DOI] [PubMed] [Google Scholar]
- Filipović-Grcić J, Skalko-Basnet N, Jalsenjak I. Mucoadhesive chitosan-coated liposomes: characteristics and stability. J Microencapsul. 2001;18:3–12. doi: 10.1080/026520401750038557. [DOI] [PubMed] [Google Scholar]
- Finka A, Goloubinoff P (2013) Proteomic data from human cell cultures refine mechanisms of chaperone-mediated protein homeostasis. Cell Stress Chaperones : PMID: 23430704 [PubMed - as supplied by publisher] [DOI] [PMC free article] [PubMed]
- Garrido C, Bruey JM, Fromentin A, Hammann A, Arrigo AP, Solary E. HSP27 inhibits cytochrome c-dependent activation of procaspase-9. FASEB J. 1999;13:2061–2070. doi: 10.1096/fasebj.13.14.2061. [DOI] [PubMed] [Google Scholar]
- Glatz A, Vass I, Los DA, Vigh L. The Synechocystis model of stress: From molecular chaperones to membranes. Plant Physiol Biochem. 1999;37:1–12. [Google Scholar]
- Goldstein DB. Effect of alcohol on cellular membranes. Ann Emerg Med. 1986;15:1013–1018. doi: 10.1016/s0196-0644(86)80120-2. [DOI] [PubMed] [Google Scholar]
- Goldstein DB, Chin JH, Lyon RC. Ethanol disordering of spin-labeled mouse brain membranes: correlation with genetically determined ethanol sensitivity of mice. Proc Natl Acad Sci U S A. 1982;79:4231–4233. doi: 10.1073/pnas.79.13.4231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B. Oxidative stress and neurodegeneration: where are we now? J Neurochem. 2006;97:1634–1658. doi: 10.1111/j.1471-4159.2006.03907.x. [DOI] [PubMed] [Google Scholar]
- Hirakawa T, Rokutan K, Nikawa T, Kishi K. Geranylgeranylacetone induces heat shock proteins in cultured guinea pig gastric mucosal cells and rat gastric mucosa. Gastroenterology. 1996;111:345–357. doi: 10.1053/gast.1996.v111.pm8690199. [DOI] [PubMed] [Google Scholar]
- Hoek JB. Mitochondrial energy metabolism in chronic alcoholism. Curr Top Bioenerg. 1994;17:197–241. [Google Scholar]
- Hollander JM, Martin JL, Belke DD, Scott BT, Swanson E, Krishnamoorthy V, Dillmann WH. Overexpression of Wild-Type Heat Shock Protein 27 and a Nonphosphorylatable Heat Shock Protein 27 Mutant Protects Against Ischemia/Reperfusion Injury in a Transgenic Mouse Model. Circulation. 2004;110:3544–3552. doi: 10.1161/01.CIR.0000148825.99184.50. [DOI] [PubMed] [Google Scholar]
- Holownia A, Ledig M, Copin JC, Tholey G. The effect of ethanol on HSP70 in cultured rat glial cells and in brain areas of rat pups exposed to ethanol in utero. Neurochem Res. 1995;20:875–878. doi: 10.1007/BF00969701. [DOI] [PubMed] [Google Scholar]
- Hooper PL, Hooper PL, Tytell M, Vigh L. Xenohormesis: health benefits from an eon of plant stress response evolution. Cell Stress Chaperones. 2010;15:761–770. doi: 10.1007/s12192-010-0206-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horváth I, Vígh L. Cell biology: Stability in times of stress. Nature. 2010;463:436–438. doi: 10.1038/463436a. [DOI] [PubMed] [Google Scholar]
- Horváth I, Glatz A, Varvasovszki V, Török Z, Páli T, Balogh G, Kovács E, Nádasdi L, Benkö S, Joó F, Vígh L. Membrane physical state controls the signaling mechanism of the heat shock response in Synechocystis PCC6803: identification of hsp17 as a “fluidity gene”. Proc Natl Acad Sci U S A. 1998;95:3513–3518. doi: 10.1073/pnas.95.7.3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horváth I, Multhoff G, Sonnleitner A, Vígh L. Membrane-associated stress proteins: more than simply chaperones. Biochim Biophys Acta. 2008;1778:1653–1664. doi: 10.1016/j.bbamem.2008.02.012. [DOI] [PubMed] [Google Scholar]
- Horváth I, Glatz A, Nakamoto H, Mishkind ML, Munnik T, Saidi Y, Goloubinoff P, Harwood JL, Vígh L. Heat shock response in photosynthetic organisms: membrane and lipid connections. Prog Lipid Res. 2012;51:208–220. doi: 10.1016/j.plipres.2012.02.002. [DOI] [PubMed] [Google Scholar]
- Huot J, Houle F, Spitz DR, Landry J. HSP27 phosphorylation-mediated resistance against actin fragmentation and cell death induced by oxidative stress. Cancer Res. 1996;56:273–279. [PubMed] [Google Scholar]
- Ikonomidou C, Bittigau P, Ishimaru MJ, Wozniak DF, Koch C, Genz K, Price MT, Stefovska V, Hörster F, Tenkova T, Dikranian K, Olney JW. Ethanol-induced apoptotic neurodegeneration and fetal alcohol syndrome. Science. 2000;287:1056–1060. doi: 10.1126/science.287.5455.1056. [DOI] [PubMed] [Google Scholar]
- Islam A, Abraham P, Hapner CD, Deuster PA, Chen Y. Tissue specific upregulation of HSP72 in mice following short-term administration of alcohol. Cell Stress Chaperones. 2013;18:215–222. doi: 10.1007/s12192-012-0375-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kai X, Li J, Huang S, Bai Z. Apoptosis Induced by Low-concentration Ethanol in Hepatocellular Carcinoma Cell Strains and Down-regulated AFP and Survivin Analysis by Proteomic Technology. Int J Biol Life Sci. 2010;6:1. [Google Scholar]
- Kampinga HH, Hageman J, Vos MJ, Kubota H, Tanguay RM, Bruford EA, Cheetham ME, Chen B, Hightower LE. Guidelines for the nomenclature of the human heat shock proteins. Cell Stress Chaperones. 2009;14:105–111. doi: 10.1007/s12192-008-0068-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodagholi F, Eftekharzadeh B, Maghsoudi N, Rezaei PF. Chitosan prevents oxidative stress-induced amyloid beta formation and cytotoxicity in NT2 neurons: involvement of transcription factors Nrf2 and NF-kappaB. Mol Cell Biochem. 2010;337:39–51. doi: 10.1007/s11010-009-0284-1. [DOI] [PubMed] [Google Scholar]
- Lee BY, Hefta SA, Brennan PJ. Characterization of the major membrane protein of virulent Mycobacterium tuberculosis. Infect Immun. 1992;60:2066–2074. doi: 10.1128/iai.60.5.2066-2074.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DH, Lee YJ, Kwon KH. Neuroprotective effects of astaxanthin in oxygen–glucose deprivation in SH-SY5Y cells and global cerebral ischemia in rat. J Clin Biochem Nutr. 2010;47:121–129. doi: 10.3164/jcbn.10-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis EJ, Ramsook AH, Locke M, Amara CE. Mild eccentric exercise increases Hsp72 content in skeletal muscles from adult and late middle-aged rats. Cell Stress Chaperones. 2013;18:667–673. doi: 10.1007/s12192-013-0412-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist S. The heat-shock response. Ann Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- Loureiro SO, Heimfarth L, Reis K, Wild L, Andrade C, Guma FT, Goncalves CA, Pessoa-Pureur R. Acute ethanol exposure disrupts actin cytoskeleton and generates reactive oxygen species in c6 cells. Toxicol In Vitro. 2011;25:28–36. doi: 10.1016/j.tiv.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Lovinger DM, White G, Weight FF. NMDA Receptor-Mediated Synaptic Excitation Selectively Inhibited by Ethanol in Hippocampal Slice from Adult Rat. J Neurosci. 1990;10:1372–1379. doi: 10.1523/JNEUROSCI.10-04-01372.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrekar P, Catalano D, Jeliazkova V, Kodys K. Alcohol exposure regulates heat shock transcription factor binding and heat shock proteins 70 and 90 in monocytes and macrophages: implication for TNF-α regulation. J Leukoc Biol. 2008;84:1335–1345. doi: 10.1189/jlb.0407256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason RP, Walter MF, Mason PE. Effect of oxidative stress on membrane structure: small-angle X-ray diffraction analysis. Free Radic Biol Med. 1997;23:419–425. doi: 10.1016/s0891-5849(97)00101-9. [DOI] [PubMed] [Google Scholar]
- Mehlen P, Kretz-Remy C, Preville X, Arrigo AP. Human hsp27, Drosophila hsp27, and human αB-crystallin expression-mediated increase in glutathione is essential for the protective activity of these proteins against TNF-α-induced cell death. EMBO J. 1996;15:2695–2706. [PMC free article] [PubMed] [Google Scholar]
- Mizushima T, Tsutsumi S, Rokutan K, Tsuchiya T. Suppression of Ethanol-Induced Apoptotic DNA Fragmentation by Geranylgeranylacetone in Cultured Guinea Pig Gastric Mucosal Cells. Dig Dis Sci. 1999;44:510–514. doi: 10.1023/a:1026692920848. [DOI] [PubMed] [Google Scholar]
- Montoliu C, Vallés S, Renau-Piqueras J, Guerri C. Ethanol-Induced Oxygen Radical Formation and Lipid Peroxidation in Rat Brain: Effect of Chronic Alcohol Consumption. J Neurochem. 1994;63:1855–1862. doi: 10.1046/j.1471-4159.1994.63051855.x. [DOI] [PubMed] [Google Scholar]
- Morimoto RI. Dynamic remodeling of transcription complexes by molecular chaperones. Cell. 2002;110:281–284. doi: 10.1016/s0092-8674(02)00860-7. [DOI] [PubMed] [Google Scholar]
- Morimoto RI, Sarge KD, Abravaya K. Transcriptional Regulation of Heat Shock Genes. J Biol Chem. 1992;267:21987–21990. [PubMed] [Google Scholar]
- Mullin MJ, Hunt WA. Actions of ethanol on voltage-sensitive sodium channels: effects on neurotoxin binding. J Pharmacol Exp Ther. 1987;242:536–540. [PubMed] [Google Scholar]
- Multhoff G, Hightower LE. Distinguishing integral and receptor-bound heat shock protein 70 (Hsp70) on the cell surface by Hsp70-specific antibodies. Cell Stress Chaperones. 2011;16:251–255. doi: 10.1007/s12192-010-0247-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Multhoff G, Botzler C, Wiesnet M, Müller E, Meier T, Wilmanns W, Issels RD. A stress-inducible 72-kDa heat-shock protein (HSP72) is expressed on the surface of human tumor cells, but not on normal cells. Int J Cancer. 1995;61:272–279. doi: 10.1002/ijc.2910610222. [DOI] [PubMed] [Google Scholar]
- Nagy E, Balogi Z, Gombos I, Akerfelt M, Björkbom A, Balogh G, Török Z, Maslyanko A, Fiszer-Kierzkowska A, Lisowska K, Slotte PJ, Sistonen L, Horváth I, Vígh L. Hyperfluidization-coupled membrane microdomain reorganization is linked to activation of the heat shockresponse in a murine melanoma cell line. Proc Natl Acad U S A. 2007;104:7945–7950. doi: 10.1073/pnas.0702557104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik RS, Mujumdar AM, Ghaskadbi S. Protection of liver cells from ethanol cytotoxicity by curcumin in liver slice culture in vitro. J Ethnopharmacol. 2004;95:31–37. doi: 10.1016/j.jep.2004.06.032. [DOI] [PubMed] [Google Scholar]
- Nakagawa M, Tsujimoto N, Nakagawa H, Iwaki T, Fukumaki Y, Iwaki A. Association of HSPB2, a member of the small heat shock protein family, with mitochondria. Exp Cell Res. 2001;271:161–168. doi: 10.1006/excr.2001.5362. [DOI] [PubMed] [Google Scholar]
- Nakamoto H, Vígh L. The small heat shock proteins and their clients. Cell Mol Life Sci. 2007;64:294–306. doi: 10.1007/s00018-006-6321-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanji AA, Griniuviene B, Yacoub LK, Sadrzadeh SM, Levitsky S, McCully JD. Heat-shock gene expression in alcoholic liver disease in the rat is related to the severity of liver injury and lipid peroxidation. Proc Soc Exp Biol Med. 1995;210:12–19. doi: 10.3181/00379727-210-43918. [DOI] [PubMed] [Google Scholar]
- Newman G, Crooke E. DnaA, the initiator of Escherichia coli chromosomal replication, is located at the cell membrane. J Bacteriol. 2000;182:2604–2610. doi: 10.1128/jb.182.9.2604-2610.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordmann R, Riviere C, Rouach H. Ethanol-induced lipid peroxidation and oxidative stress in extrahepatic tissues. Alcohol Alcohol. 1990;25:231–237. doi: 10.1093/oxfordjournals.alcalc.a044996. [DOI] [PubMed] [Google Scholar]
- Oberdoerster J, Rabin RA. Enhanced caspase activity during ethanol-induced apoptosis in rat cerebellar granule cells. Eur J Pharmacol. 1999;385:273–282. doi: 10.1016/s0014-2999(99)00714-1. [DOI] [PubMed] [Google Scholar]
- Olney JW, Tenkova T, Dikranian K, Muglia LJ, Jermakowitz WJ, D’Sa C, Roth KA. Ethanol-induced caspase-3 activation in the in vivo developing mouse brain. Neurobiol Dis. 2002;9:205–219. doi: 10.1006/nbdi.2001.0475. [DOI] [PubMed] [Google Scholar]
- Parmahamsa M, Reddy KR, Varadacharyulu N. Changes in composition and properties of erythrocyte membrane in chronic alcoholics. Alcohol Alcohol. 2004;39:110–112. doi: 10.1093/alcalc/agh034. [DOI] [PubMed] [Google Scholar]
- Paul C, Manero F, Gonin S, Kretz-Remy C, Virot S, Arrigo AP. Hsp27 as a negative regulator of cytochrome C release. Mol Cell Biol. 2002;22:816–834. doi: 10.1128/MCB.22.3.816-834.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Péter M, Balogh G, Gombos I, Liebisch G, Horváth I, Török Z, Nagy E, Maslyanko A, Benkő S, Schmitz G, Harwood JL, Vígh L. Nutritional lipid supply can control the heat shock response of B16 melanoma cells in culture. Mol Membr Biol. 2012;29:274–289. doi: 10.3109/09687688.2012.680203. [DOI] [PubMed] [Google Scholar]
- Pignataro L, Miller AN, Ma L, Midha S, Protiva P, Herrera DG, Harrison NL. Alcohol Regulates Gene Expression in Neurons via Activation of Heat Shock Factor 1. J Neurosci. 2007;27:12957–12966. doi: 10.1523/JNEUROSCI.4142-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper PW. The heat shock and ethanol stress responses of yeast exhibit extensive similarity and functional overlap. FEMS Microbiol Lett. 1995;134:121–127. doi: 10.1111/j.1574-6968.1995.tb07925.x. [DOI] [PubMed] [Google Scholar]
- Rathinam ML, Watts LT, Stark AA, Mahimainathan L, Stewart J, Schenker S, Henderson GI. Astrocyte control of fetal cortical neuron glutathione homeostasis: up-regulation by ethanol. J Neurochem. 2006;96:1289–1300. doi: 10.1111/j.1471-4159.2006.03674.x. [DOI] [PubMed] [Google Scholar]
- Ravagnan L, Gurbuxani S, Susin SA, Maisse C, Daugas E, Zamzami N, Mak T, Jäättelä M, Penninger JM, Garrido C, Kroemer G. Heat-shock protein 70 antagonizes apoptosis-inducing factor. Nat Cell Biol. 2001;3:839–843. doi: 10.1038/ncb0901-839. [DOI] [PubMed] [Google Scholar]
- Sanchez-Amate MC, Zurera JM, Carrasco MP, Segovia JL, Marco C. Ethanol and lipid metabolism. Differential effects on liver and brain microsomes. FEBS Lett. 1991;293:215–218. doi: 10.1016/0014-5793(91)81190-j. [DOI] [PubMed] [Google Scholar]
- Sergent O, Pereira M, Belhomme C, Chevanne M, Huc L, Lagadic-Gossmann D. Role for membrane fluidity in ethanol-induced oxidative stress of primary rat hepatocytes. J Pharmacol Exp Ther. 2005;313:104–111. doi: 10.1124/jpet.104.078634. [DOI] [PubMed] [Google Scholar]
- Shoker AS, Murabit MA, Georges FF, Qualtiere LF, Deneer HG, Prasad K. Inhibition of human lymphocyte function by organic solvents. Mol Cell Biochem. 1997;171:49–58. doi: 10.1023/a:1006882114285. [DOI] [PubMed] [Google Scholar]
- Soltys BJ, Gupta RS. Immunoelectron microscopic localization of the 60-kDa heat shock chaperonin protein (Hsp60) in mammalian cells. Exp Cell Res. 1996;222:16–27. doi: 10.1006/excr.1996.0003. [DOI] [PubMed] [Google Scholar]
- Sőti C, Nagy E, Giricz Z, Vígh L, Csermely P, Ferdinandy P. Heat shock proteins as emerging therapeutic targets. Br J Pharmacol. 2005;146:769–780. doi: 10.1038/sj.bjp.0706396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stangl S, Gehrmann M, Riegger J, Kuhs K, Riederer I, Sievert W, Hube K, Mocikat R, Dressel R, Kremmer E, Pockley AG, Friedrich L, Vigh L, Skerra A, Multhoff G. Targeting membrane heat-shock protein 70 (Hsp70) on tumors by cmHsp70.1 antibody. Proc Natl Acad Sci U S A. 2011;108:733–738. doi: 10.1073/pnas.1016065108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stankiewicz AR, Lachapelle G, Foo CP, Radicioni SM, Mosser DD. Hsp70 inhibits heat-induced apoptosis upstream of mitochondria by preventing Bax translocation. J Biol Chem. 2005;280:38729–38739. doi: 10.1074/jbc.M509497200. [DOI] [PubMed] [Google Scholar]
- Su CY, Chong KY, Owen OE, Dillmann WH, Chang C, Lai CC. Constitutive and Inducible hsp70s are Involved in Oxidative Resistance Evoked by Heat Shock or Ethanol. J Mol Cell Cardiol. 1998;30:587–598. doi: 10.1006/jmcc.1997.0622. [DOI] [PubMed] [Google Scholar]
- Tomas M, Lazaro-Diequez F, Duran JM, Marin P, Renau-Piqueras J, Egea G. Protective effects of lysophosphatidic acid (LPA) on chronic ethanol-induced injuries to the cytoskeleton and on glucose uptake in rat astrocytes. J Neurochem. 2003;87:220–229. doi: 10.1046/j.1471-4159.2003.01993.x. [DOI] [PubMed] [Google Scholar]
- Tomas M, Marin P, Megias L, Egea G, Renau-Piqueras J. Ethanol perturbs the secretory pathway in astrocytes. Neurobiol Dis. 2005;20:773–784. doi: 10.1016/j.nbd.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Török Z, Horvath I, Goloubinoff P, Kovács E, Glatz A, Balogh G, Vígh L. Evidence for a lipochaperonin: Association of active proteinfolding GroESL oligomers with lipids can stabilize membranes under heat shock conditions. Proc Natl Acad Sci U S A. 1997;94:2192–2197. doi: 10.1073/pnas.94.6.2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Török Z, Goloubinoff P, Horvath I, Tsvetkova NM, Glatz A, Balogh G, Varvasovszki V, Los DA, Vierling E, Crowe JH, Vigh L. Synechocystis HSP17 is an amphitropic protein that stabilizes heatstressed membranes and binds denatured proteins for subsequent chaperone-mediated refolding. Proc Natl Acad Sci U S A. 2001;98:3098–3103. doi: 10.1073/pnas.051619498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Török Z, Pilbat A-M, Gombos I, Hocsak E, Sümegi B, Horváth I, Vígh L. Evidence on cholesterol-controlled lipid raft interaction of the small heat shock protein Hspb11. In: Handerson B, Pockley AD, editors. Cellular Trafficking of Cell Stress Proteins in Health and Disease. Dordrecth: Springer; 2012. pp. 75–86. [Google Scholar]
- Toth ME, Gonda S, Vigh L, Santha M. Neuroprotective effect of small heat shock protein, Hsp27, after acute and chronic alcohol administration. Cell Stress Chaperones. 2010;15:807–817. doi: 10.1007/s12192-010-0188-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsvetkova NM, Horvath I, Torok Z, Wolkers WF, Balogi Z, Shigapova N, Crowe LM, Tablin F, Vierling E, Crowe JH, Vigh L. Small heat-shock proteins regulate membrane lipid polymorphism. Proc Natl Acad Sci U S A. 2002;99:13504–13509. doi: 10.1073/pnas.192468399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uysal M, Kutalp G, Ozdemirler G, Aykac G. Ethanol-induced changes in lipid peroxidation and glutathione content in rat brain. Drug Alcohol Depend. 1989;23:227–230. doi: 10.1016/0376-8716(89)90085-9. [DOI] [PubMed] [Google Scholar]
- Vígh L, Maresca B, Harwood JL. Does the membrane’s physical state control the expression of heat shock and other genes? Trends Biochem Sci. 1998;23:369–374. doi: 10.1016/s0968-0004(98)01279-1. [DOI] [PubMed] [Google Scholar]
- Vígh L, Escribá PV, Sonnleitner A, Sonnleitner M, Piotto S, Maresca B, Horváth I, Harwood JL. The significance of lipid composition for membrane activity: new concepts and ways of assessing function. Prog Lipid Res. 2005;44:303–344. doi: 10.1016/j.plipres.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Vígh L, Horváth I, Maresca B, Harwood JL. Can the stress protein response be controlled by 'membrane-lipid therapy'? Trends Biochem Sci. 2007;32:357–363. doi: 10.1016/j.tibs.2007.06.009. [DOI] [PubMed] [Google Scholar]
- Vígh L, Török Z, Balogh G, Glatz A, Piotto S, Horváth I. Membrane-regulated stress response: a theoretical and practical approach. Adv Exp Med Biol. 2007;594:114–131. doi: 10.1007/978-0-387-39975-1_11. [DOI] [PubMed] [Google Scholar]
- Wang K, Spector A. α-crystallin stabilizes actin filaments and prevents cytochalasin-induced depolymerization in a phosphorylation-dependent manner. Eur J Biochem. 1996;242:56–66. doi: 10.1111/j.1432-1033.1996.0056r.x. [DOI] [PubMed] [Google Scholar]
- Wang Q, Sun AY, Simonyi A, Kalogeris TJ, Miller DK, Sun GY, Korthuis RJ. Ethanol preconditioning protects against ischemia/reperfusion-induced brain damage: role of NADPH oxidase-derived ROS. Free Radic Biol Med. 2007;43:1048–1060. doi: 10.1016/j.freeradbiomed.2007.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler MD, Nakagami M, Bradford BU, Uesugi T, Mason RP, Connor HD, Dikalova A, Kadiiska M, Thurman RG. Overexpression of Manganese Superoxide Dismutase Prevents Alcohol-induced Liver Injury in the Rat. J Biol Chem. 2001;276:36664–36672. doi: 10.1074/jbc.M105352200. [DOI] [PubMed] [Google Scholar]
- Wu D, Cederbaum AI. Alcohol, oxidative stress, and free radical damage. Alcohol Res Health. 2003;27:277–284. [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H, Yamamoto Y, Yamagami K, Kume M, Kimoto S, Toyokuni S, Uchida K, Fukumoto M, Yamaoka Y. Heat-shock preconditioning reduces oxidative protein denaturation and ameliorates liver injury by carbon tetrachloride in rats. Res Exp Med. 2000;199:309–318. doi: 10.1007/s004339900040. [DOI] [PubMed] [Google Scholar]
- Yeo M, Kim DK, Cho SW, Hong HD. Ginseng, the Root of Panax ginseng C.A. Meyer, Protects Ethanol-Induced Gastric Damages in Rat through the Induction of Cytoprotective Heat-Shock Protein 27. Dig Dis Sci. 2008;53:606–613. doi: 10.1007/s10620-007-9946-6. [DOI] [PubMed] [Google Scholar]
- Yoon Y, Török N, Krueger E, Oswald B, McNiven MA. Ethanol-induced alterations of the microtubule cytoskeleton in hepatocytes. Am J Physiol. 1998;274:G757–G766. doi: 10.1152/ajpgi.1998.274.4.G757. [DOI] [PubMed] [Google Scholar]
- Young C, Klocke BJ, Tenkova T, Choi J, Labruyere J, Qin YQ, Holtzman DM, Roth KA, Olney JW. Ethanol-induced neuronal apoptosis in vivo requires BAX in the developing mouse brain. Cell Death Differ. 2003;10:1148–1155. doi: 10.1038/sj.cdd.4401277. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Sun X, Kang YJ. Ethanol-induced apoptosis in mouse liver: Fas- and cytochrome c-mediated caspase-3 activation pathway. Am J Pathol. 2001;159:329–338. doi: 10.1016/S0002-9440(10)61699-9. [DOI] [PMC free article] [PubMed] [Google Scholar]