Abstract
Chronic spinal cord dysfunction occurs in dogs as a consequence of diverse aetiologies, including long-standing spinal cord compression and insidious neurodegenerative conditions. One such neurodegenerative condition is canine degenerative myelopathy (DM), which clinically is a challenge to differentiate from other chronic spinal cord conditions. Although the clinical diagnosis of DM can be strengthened by the identification of the Sod1 mutations that are observed in affected dogs, genetic analysis alone is insufficient to provide a definitive diagnosis. There is a requirement to identify biomarkers that can differentiate conditions with a similar clinical presentation, thus facilitating patient diagnostic and management strategies. A comparison of the cerebrospinal fluid (CSF) protein gel electrophoresis profile between idiopathic epilepsy (IE) and DM identified a protein band that was more prominent in DM. This band was subsequently found to contain a multifunctional protein clusterin (apolipoprotein J) that is protective against endoplasmic reticulum (ER) stress-mediated apoptosis, oxidative stress, and also serves as an extracellular chaperone influencing protein aggregation. Western blot analysis of CSF clusterin confirmed elevated levels in DM compared to IE (p < 0.05). Analysis of spinal cord tissue from DM and control material found that clusterin expression was evident in neurons and that the clusterin mRNA levels from tissue extracts were elevated in DM compared to the control. The plasma clusterin levels was comparable between these groups. However, a comparison of clusterin CSF levels in a number of neurological conditions found that clusterin was elevated in both DM and chronic intervertebral disc disease (cIVDD) but not in meningoencephalitis and IE. These findings indicate that clusterin may potentially serve as a marker for chronic spinal cord disease in the dog; however, additional markers are required to differentiate DM from a concurrent condition such as cIVDD.
Electronic supplementary material
The online version of this article (doi:10.1007/s12192-013-0457-4) contains supplementary material, which is available to authorized users.
Keywords: Dog, Spinal cord disease, Clusterin, Biomarkers, Sod1
Introduction
Cerebrospinal fluid (CSF) has been investigated as a potential source of biomarkers in a range of human and animal neurological disorders (Satoh et al. 2007; Tumani et al. 2008). From a veterinary perspective, a number of neurological conditions can present similar clinical features and therefore the identification of specific biomarkers would greatly facilitate diagnosis, patient treatment and management strategies. Canine degenerative myelopathy (DM) is one such condition that can be difficult to diagnose clinically. It is a spontaneously occurring, adult-onset, progressive neurodegenerative condition that has been recognised as a clinicopathological entity for many years (Averill 1973; Coates and Wininger 2010). The condition is particularly prevalent in German Shepherd dog (Griffiths and Duncan 1975), however a number of other breeds are also affected, including Pembroke Welsh corgis (March et al. 2009), Bernese Mountain dogs (BMD) (Wininger et al. 2011) and boxer dogs (Shelton et al. 2012). Dogs with DM have an insidious onset of progressive upper motor neuron paresis and ataxia of the pelvic limbs that ultimately leads to paraplegia/quadriplegia and euthanasia. Affected dogs that are nursed beyond the paraparetic/plegic state eventually manifest lower motor neuron signs (flaccid paralysis and muscle atrophy) in the pelvic limbs, followed by thoracic limb involvement. Urinary incontinence and brainstem signs such as inability to bark and swallowing difficulty too have been reported in dogs with advanced DM (Coates and Wininger 2010). The principle pathological features of DM are described as a non-inflammatory segmental axonal degeneration and secondary demyelination affecting white matter tracts with the presence of astrocytosis and astrogliosis (Johnston et al. 2000). The white matter lesions are most extensively found in the middle to lower thoracic region. Denervation atrophy of muscle and peripheral neuropathy are also described in dogs with advanced DM (Shelton et al. 2012), implying the involvement of motor neurons. However, specific changes in spinal cord motor neurons are not evident at the light microscopic level (Coates and Wininger 2010). Abnormalities in specific brainstem nuclei including red nucleus have been reported in the brain (Johnston et al. 2000).
The clinical presentation of DM may mimic many acquired spinal cord diseases, some of which can also co-exist with DM, confounding clinical diagnosis. In the early stages of DM, these would most commonly include conditions such as chronic intervertebral disc disease, degenerative lumbosacral syndrome and spinal cord neoplasia (Cherubini et al. 2008). The diagnosis of DM is also complicated by a lack of specific diagnostic tests in the clinical environment which thus relies on the interpretation of case data by the clinician and the necessity of post mortem examination for confirmation. A genetic study has established that the occurrence of DM is strongly associated with a mutation in Sod1 gene (118G > A or E40K) at the same time implying DM is potentially orthologous to human amyotrophic lateral sclerosis (ALS) (Awano et al. 2009). The E40K Sod1 mutation has been recognised as a major risk factor in developing DM, however it does not appear to be specific to DM as the mutation is also seen in a proportion of non-affected individuals and there are rare individuals that do not carry the mutation. In addition, a recent report has identified a novel Sod1 mutation (52A > T) in an affected BMD (Wininger et al. 2011), implying there is the potential for the discovery of further Sod1 mutation(s) in DM. Although sequencing could be employed to detect known mutations and screen for new polymorphisms, in man the detection of a polymorphism in the SOD1 gene is not exclusively synonymous with a clinically significant mutation and may not be specifically diagnostic (Felbecker et al. 2010). Therefore, additional clinical indices, e.g. protein-based biomarkers are required to specifically differentiate DM from other neurological diseases in the clinic, as well as provide new potential insights into disease mechanisms. The successful development of DM biomarkers as an adjunct assay, complementary to genetic marker(s) and the current diagnostic methods used in DM, would be of substantial value to owners and clinicians.
The main aim of this study is to establish potential CSF biomarkers in dogs that could be used to differentiate between chronic spinal conditions and in particular increase the confidence in the clinical diagnosis of DM. We have previously investigated the stability of a number of proteins in canine CSF, including an acute phase protein, haptoglobin and a multifunctional chaperone protein clusterin (Shafie et al. 2013). We now report on the potential for these proteins to serve as biomarkers for chronic canine spinal cord disorders.
Materials and methods
Clinical material
All dogs included in the CSF biomarker study were presented to the Small Animal Hospital at The University of Glasgow School of Veterinary Medicine for clinical investigation. Ethical approval for the storage and use of CSF samples collected as part of such investigations, and which were excess to the immediate clinical requirements, was granted by the School of Veterinary Medicine Ethics and Welfare Committee of the University of Glasgow. All dogs received complete physical and neurological examination. The neurological examination included the assessment of mental alertness, gait, posture, cranial nerve function, spinal reflexes and responses to stimuli. Magnetic resonance imaging and clinicopathological evaluations comprised of complete blood counts, serum biochemistry and CSF analysis were routinely performed in all cases. CSF (0.5–1.0 ml) was collected into a sterile tube, harvested either from the cerebellomedullary or lumbar cistern under general anaesthesia. The majority of samples for CSF analyses were collected from cerebellomedullary cistern. Whole blood samples were also collected from the jugular vein for the purpose of other investigations and an aliquot stored for genomic DNA (gDNA) extraction. Post mortem examination was not performed in these cases. All clinical samples were temporarily stored at −20°C (maximum 3 days) before being transported on ice to the laboratory, aliquoted and stored at −80°C as has been described previously (Shafie et al. 2013). Since obtaining CSF from healthy dogs is not permitted on ethical grounds, dogs with idiopathic epilepsy (IE) and with the last seizure >3 days from the time of investigation were selected as controls. Samples were also obtained from dogs affected by meningoencephalitis (MEN), which is a neuroinflammatory disorder, and chronic intervertebral disc disease (cIVDD). Majority of CSF samples for this analysis were collected from cistern magna.
A separate archive was utilised for plasma, mRNA and immunohistochemistry (IHC) analyses. Clinical material for these studies was derived from samples collected as part of a study of DM between the period of 1994 and 1998 (Johnston et al. 2000). Plasma was extracted from EDTA-treated blood samples that were stored at −80°C. As part of this study spinal cord and spleen tissue were collected post mortem, snap frozen and stored in liquid nitrogen. IHC analyses were performed using fixed spinal cord tissue. Controls for these analyses were taken from non-neurological cases collected as part of the study by Johnston et al. (2000). CSF was not archived in this study.
All cases were subsequently genotyped based on the presence of a 118G > A mutation in the Sod1 gene (Awano et al. 2009) using a restriction fragment length polymorphism method that was developed in-house (Supplementary data). The selection of DM cases for all experiments was based on the clinical diagnosis of DM and homozygosity for the mutant allele in the Sod1 gene.
Identification of clusterin and haptoglobin as proteins of interest in canine CSF by liquid chromatography–mass spectrometry
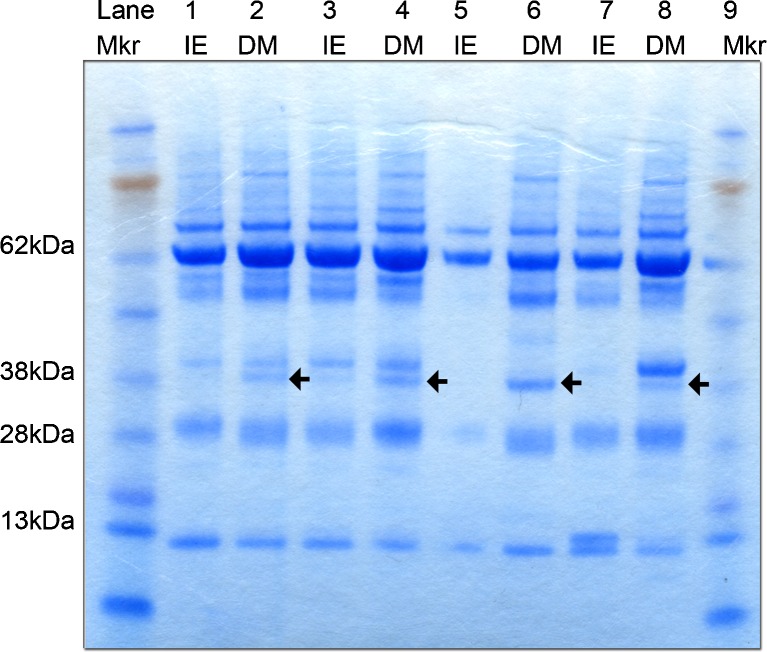
A group of representative DM and IE CSF protein profiles were visualised using Coomassie Blue (SimplyBlue™ SafeStain, Invitrogen, UK) (Fig. 1). The differentially expressed bands were excised for liquid chromatography–mass spectrometry (LC-MS) analysis at the Polyomics facility, University of Glasgow using procedures that have been previously described in detail (Szoor et al. 2013). Protein identifications were assigned using the Mascot search engine (Matrix Science, USA) to interrogate protein sequences in the NCBI Genbank database at 95 % confidence level.
Fig. 1.
SDS-PAGE analysis of IE and DM CSF. SDS-PAGE analysis of IE (n = 4) and DM CSF (n = 4) followed by Coomassie Blue staining revealed an additional protein band at approximately 38 kDa, which was consistently visible in DM CSF (as shown by black arrow), but present at a lower intensity in the IE cases. The comparatively low densities of staining in lane 5 may have been due to a loading error. Mkr pre-stained molecular weight marker, IE idiopathic epilepsy, DM degenerative myelopathy
SDS-PAGE and Western blot
Western blot analysis was performed as previously detailed by (Shafie et al. 2013). In brief, 5 μg of protein from each sample was separated on a 4–12 % Bis–Tris mini gel (NUPAGE Novex, Invitrogen, UK). The samples from each disease condition were loaded alternately across the gel. A CSF sample was aliquoted, stored at −80 °C and included with each gel run to serve as a reference standard (std). Separated proteins were transferred to a nitrocellulose membrane and stained with Ponceau S to assess the consistency of protein loading between samples. Separated proteins were transferred to a nitrocellulose membrane, blocked with 5 % milk powder in Tris-buffered saline (TBS) containing 0.1 % Tween-20 (1× T-TBS), incubated overnight at 4 °C with polyclonal anti clusterin antibody at 1:50,000 dilution (cat. no:ab39991, Abcam, UK) or anti haptoglobin antibody (supplied by Prof David Eckersall, University of Glasgow) in 5 % powdered milk/T-TBS, then with horseradish peroxidise (HRP) conjugated secondary antibody. Immunocomplexes were detected using the enhanced chemiluminescence (ECL) reaction (Thermo Fisher Scientific, UK) and visualised with radiographic film (Hyperfilm ECL, Amersham Biosciences, UK). Immunocomplexes detected by the ECL reagent was quantified by ImageJ (NIH, USA) and the density of protein signal was calculated relative to the reference standard and expressed as relative abundance.
For plasma analysis, EDTA-treated blood was available from the archive material that had been stored at −80 °C and subjected to freeze–thaw cycles which has lead to significant haemolysis resulting in a high haemoglobin content which would compromise the protein assay. Samples were centrifuged at 5,000×g for 20 min, the supernatant removed and subsequently diluted in 1:20 with ultrapure water. A fixed volume of 3 μl of diluted sample was processed for SDS-PAGE and DM and control samples were loaded alternately into the gel. The Western blot procedure was performed as described above using the clusterin antibody at a 1:100,000 dilution. Immunocomplexes quantified for each group using ImageJ were calculated relative to the reference standard and expressed as relative abundance.
Reverse transcriptase polymerase chain reaction
RNA was extracted from the 12th thoracic spinal cord segment (T12) of archival tissue using a commercial kit (AMS Biotechnology, UK). The reverse transcription reaction was performed as described previously (Al-Saktawi et al. 2003) and clusterin cDNA was amplified using forward (5′-GCC CTT CTT TGA CAT GAT ACA CCA-3′) and reverse (5′-TGCTTC TGG GAT CAT CAC CGT GA-3′) primers (Eurofins, Germany). A housekeeping gene, cyclophilin was utilised as an internal standard. The primers for cyclophilin and PCR conditions were as described (Montague et al. 1997). The PCR products were resolved on a 2 % agarose gel visualised with ethidium bromide staining and the captured images quantified using ImageJ software. The intensity of the mRNA signal was corrected relative to the intensity of cyclophilin products.
Immunohistochemistry
IHC analysis was performed on 4 μm T12 spinal cord sections using Envision+™ System HRP (Dako Cytomation, UK). Sections were initially hydrated and antigen unmasking was performed using 10 mM sodium citrate buffer pH 6.0 in an automated pressure cooker (Menarini Diagnostics, UK). The endogenous peroxidase activity was quenched, followed by the incubation of the primary antibody at a 1:4,000 dilution (cat. no: ab104652, Abcam, UK). Sections were washed and incubated with HRP conjugated antibody. The immunocomplexes were detected with 3,3′-diaminobenzidine chromogen. Sections were dehydrated using a series of degraded alcohol baths and mounted in DPX. The primary antibody was omitted to give a negative control. All sections were reviewed blind and the intensity of staining was recorded based on a subjective scoring system.
Statistical analysis
Statistical analyses were performed using GraphPad Prism version 5.0 (GraphPad Software Inc., USA). The values derived from Western blots and reverse transcriptase polymerase chain reaction (RT-PCR) were assessed for normality using D’Agostino–Pearson omnibus test. Statistical comparison between the control and treated groups was performed using Mann–Whitney U or Kruskal–Wallis with a significance level (α) set at 0.05.
Results
Statistical assessment of normality distribution
All data generated from each group was statistically assessed and failed to meet the requirements of a normal distribution.
Inclusion/exclusion criteria
The Sod1 genotyping protocol was developed and optimised during the course of this study. Genotyping was not completed until after the protein analysis had been performed. For the CSF studies, all cases were genotyped for the 118G > A Sod1 mutation. All DM cases were homozygous for the mutation and had a clinical diagnosis. Affected dogs that were heterozygous for the Sod1 mutation were excluded from the data analysis (marked as H in figures) as these animals did not have a confirmatory pathological diagnosis and the inconsistency of the development DM in heterozygous animals. Control samples for CSF analysis were derived from cases of IE as they were demonstrated to be free of spinal cord conditions and/or significant neurodegenerative diseases and included animals that were either heterozygous or lacking the 118G > A Sod1 mutation. CSF samples from cases representing other disease categories were either heterozygous or lacking the Sod1 mutation. Cases with acute disease (marked as C), e.g. IE (epileptic seizure <3 days prior to sampling) or acute disc disease (<48 h prior to sampling) were excluded as DM is a chronic disease. Further material from DM cases managed with a history of corticosteroid administration was excluded as steroids induce haptoglobin expression (Harvey and West 1987).
For the clusterin plasma level analysis, all cases had a clinical diagnosis of DM backed, for those with appropriate archived tissues, by pathological confirmation and were homozygous for the 118G > A Sod1 mutation. Clusterin mRNA expression and IHC were conducted on the archival material. All DM cases had a pathologically confirmed clinical diagnosis and were homozygous for the 118G > A Sod1 mutation. All control cases were dogs unaffected clinically or pathologically by DM and lacking the Sod1 mutation.
Identification of clusterin and haptoglobin as canine CSF proteins of interest
The CSF protein profile differences between IE (n = 4) and DM (n = 4) was visualised using the Coomassie Blue stain and revealed a protein band estimated at 38 kDa (indicated by the black arrow in Fig. 1) which was consistently present in all DM samples and almost undetectable in IE CSF (Fig. 1). This band was excised and the protein constituents investigated by LC-MS. Two proteins, haptoglobin and clusterin (apolipoprotein J) emerged as constituents of the gel band. Validation of the presence of these two proteins and their relative expression level between the IE and DM groups was then assessed by western blot.
Assessment of haptoglobin and clusterin levels in CSF in DM
The comparative analysis of haptoglobin found that there was no significant difference in the level detected in the DM (n = 5) group compared to the IE (n = 8) group (Fig. 2). The exclusion criteria described above were applied to appropriate cases. In addition, some samples failed to give a quantifiable signal (marked as X in figures). The comparative analysis of CSF clusterin demonstrated that the level of clusterin was significantly elevated in the DM (n = 7) compared to the IE (n = 9) group (p < 0.001) (Fig. 3).
Fig. 2.
Haptoglobin levels in IE and DM CSF. a Western blot analysis of CSF haptoglobin levels in IE (n = 8) and DM (n = 5). Considerable signal intensity variations were detected between samples, and samples marked X were considered to be unquantifiable. Samples marked C and H were also excluded due to acute disease and heterozygosity for the Sod1 (118G > A) mutation. b Vertical scattered graph of data distribution. There was no statistically significant difference between groups. Data presented as median and interquartile range. std reference standard, IE idiopathic epilepsy, DM degenerative myelopathy. Filled upright triangle represents individuals with heterozygosity for Sod1 mutation in IE group
Fig. 3.
Clusterin levels in IE and DM CSF. a Western blot analysis of CSF clusterin levels in IE (n = 9) and DM (n = 7). b Vertical scattered graph of data distribution. Statistical analysis revealed a significant elevation in clusterin between the IE and DM groups (p < 0.001). Samples marked C and H were excluded due to acute disease and heterozygosity for the Sod1 (118G > A) mutation. Sample marked L was collected from lumbar CSF and the protein value from this sample is represented as open square in the vertical scatter graph. Data presented as median and interquartile range. ***p < 0.001; std reference standard, IE idiopathic epilepsy, DM degenerative myelopathy. Filled upright triangle represents individuals with heterozygosity for Sod1 mutation in IE group
Assessment of clusterin levels in plasma
Plasma clusterin levels were examined to determine if the elevated CSF clusterin levels were a consequence of raised plasma clusterin levels. Western blot analysis of controls (n = 8) and DM (n = 8) plasma clusterin detected a protein at approximately 38 kDa and similar to the molecular weight of CSF clusterin (data not shown). Statistical analysis comparing controls and DM cases found that there was no significant difference between these groups (Fig. 4).
Fig. 4.
Plasma clusterin levels in control (non-neurological disorders) and DM samples. a Western blot analysis of plasma clusterin levels in control (n = 8) and DM (n = 8) cases b Plasma clusterin signals were plotted in vertical scatter plot. Statistical analysis revealed no significant difference. Data presented as median and interquartile range. std reference standard, Ctrl control, DM degenerative myelopathy
Analysis of clusterin expression in archived canine spinal cord
The archive of DM (n = 4) and control (n = 4) material from a previous study (Johnston et al. 2000) was further analysed by genotyping for the 118G > A Sod1 mutation. Material from cases with a pathologically confirmed diagnosis of DM and homozygous for the 118G > A Sod1 mutation were included for further analysis. Material from cases with a pathological confirmation of diseases other than DM and lacking evidence of 118G > A Sod1 mutation were excluded for further analysis.
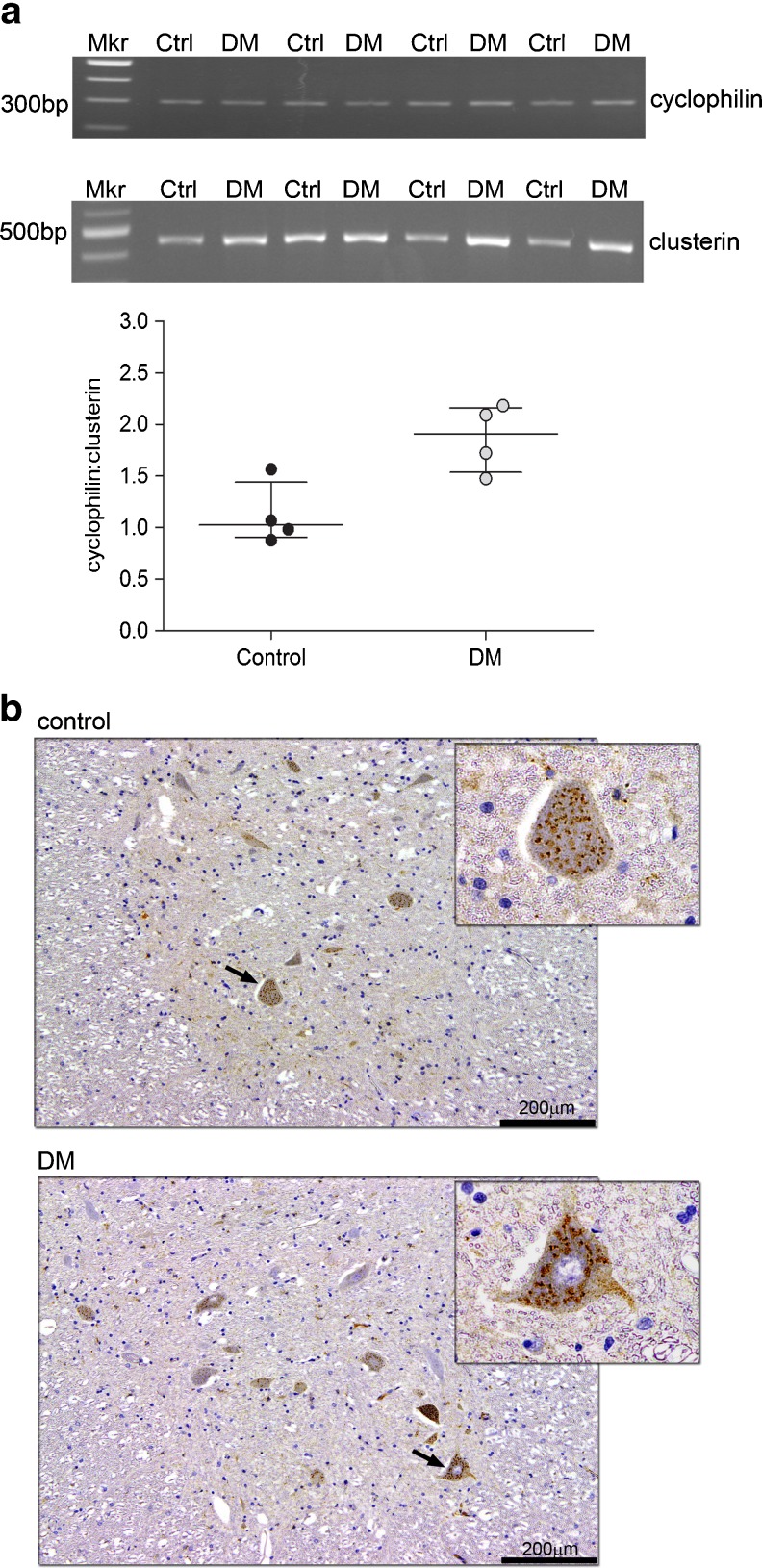
An analysis of clusterin mRNA level from selected spinal cord material was performed. Clusterin mRNA level, expressed relative to the house keeping gene cyclophilin, was found to be elevated in the DM group relative to the control group (Fig. 5a). This difference bordered on statistical significance (p = 0.05)
Fig. 5.
Analysis of clusterin mRNA levels and cellular distribution in control and DM spinal cords. a The relative signal of clusterin and cyclophilin RT-PCR amplicons observed on ethidium bromide stained agarose gels are shown in the top panels. The signals for clusterin mRNA were normalised relative to cyclophilin (cyclophilin:clusterin) and shown graphically. The statistical analysis revealed no significant difference between two groups (exact p value = 0.05); however, the mean of clusterin mRNA in the DM group (n = 4) was found to be elevated by 42 % compared to the control group (n = 4). Data presented as median and interquartile range. b Clusterin immunostaining in T12 spinal cord sections in a representative control and DM case demonstrated a dark, punctate staining pattern localised in the neuronal cytoplasm (as marked by arrow) but not in the nucleus as seen at ×60 magnification (see top right insert). The staining intensity of clusterin in neuronal cell bodies was assessed by a subjective scoring system, but no significant difference was evident between control (n = 4) and DM (n = 5) groups. Mkr molecular weight marker, Ctrl control, DM degenerative myelopathy
The cellular expression of clusterin was then examined in the spinal cord by IHC using archival cases that had been formalin fixed and paraffin embedded. Clusterin IHC demonstrated strong immunoreactivity in both control and DM cases and demonstrated a punctate pattern within the neuronal cytoplasm (Fig. 5b). Semi-quantitative assessment using a scoring system to define the staining pattern consistently found that positive staining was strictly confined within neuronal cell bodies; however, no significant difference in staining intensity was detected between control and DM groups (data not shown).
Clusterin CSF levels in a range of spinal cord conditions
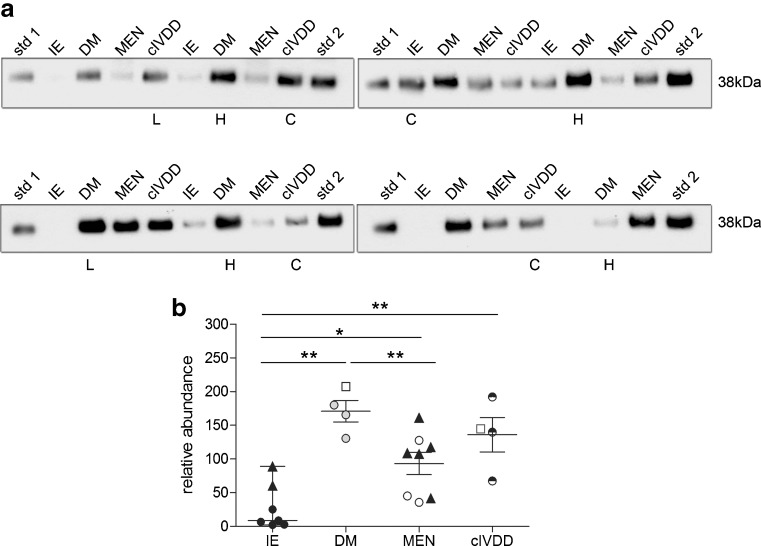
To determine if the high CSF clusterin levels observed in DM is specific to this disorder, samples were analysed from a variety of neurological conditions that routinely undergo CSF collection (Fig. 6). The number of cases available for this comparative study was limited. CSF clusterin was significantly elevated in DM (n = 4) compared to IE (n = 7; p < 0.001), which was consistent with the previous finding and when compared to the MEN (n = 8) group (p < 0.05). However, a similar pattern of CSF clusterin elevation was also observed in cIVDD (n = 4) cases (p < 0.01) compared to IE cases. No significant difference in CSF clusterin levels was detected between DM and cIVDD groups.
Fig. 6.
The comparative analysis of clusterin CSF in various neurological disorders. a Clusterin signals obtained from Western blot analyses. b Signals were quantified and shown graphically. Statistical analysis found that clusterin was significantly elevated in DM (n = 4) and cIVDD (n = 4) compared to IE (n = 7) (DM vs. IE, p < 0.001; cIVDD vs. IE, p < 0.01) and meningitis (n = 8) (DM vs. meningitis, p < 0.05; cIVDD vs. meningitis, p > 0.05). There was no significant difference in CSF clusterin between DM and cIVDD. Samples marked X were excluded from the statistical analysis. The sample marked L was collected from the lumbar cistern and the protein value from this sample is represented as open square in the vertical scatter graph. Data presented as median and interquartile range. *p < 0.05, **p < 0.01, ***p < 0.001; std reference standard, IE idiopathic epilepsy, DM, degenerative myelopathy, MEN meningoencephalitis, cIVDD chronic intervertebral disc disease. Filled upright triangle represents individuals with heterozygosity for Sod1 mutation in control groups
Discussion
In this investigation, we sought to use CSF to identify biomarkers that can differentiate DM with other neurological disorders with similar clinical features, yet distinct underlying aetiologies. We have identified haptoglobin and clusterin as components of a protein band that appeared elevated in DM compared to IE. Validation analysis found that haptoglobin levels were not altered between these disease groups, yet clusterin was elevated in DM CSF. However, the lack of a statistically significant difference in clusterin between DM and cIVDD suggests that clusterin is not a specific biomarker for DM. However, it was noted however that the levels of clusterin were elevated by 20 % in DM CSF compared to cIVDD cases and a comparison of a larger group size is warranted. We are currently exploring other potential CSF biomarkers to assess clusterin as a member of a panel of biomarkers for specific neurological disorders.
In order to understand the significance of CSF clusterin with regards to the disease mechanisms and also appreciate its potential value as a biomarker, it is imperative that the source of this protein is identified. Clusterin is a highly conserved glycoprotein that is ubiquitously expressed in a wide range of tissues and biological fluids (Jones and Jomary 2002). It can bind a variety of ligands, giving great diversity of role for clusterin in cellular activities (Calero et al. 2005). Clusterin has also been proposed to act as a chaperone molecule involved in the regulation of extracellular protein folding (Nuutinen et al. 2009; Wyatt et al. 2009), has been shown to be a target for β-amyloid neurotoxicity pathways (Killick et al. 2012) and can also influence the formation of extracellular B-amyloid aggregates (Narayan et al. 2012). In addition, there is strong evidence that clusterin can have a protective role during oxidative stress (Calero et al. 2005; Carnevali et al. 2006), ER stress-mediated apoptosis (Wang et al. 2013) and may function by facilitating the clearance of misfolded proteins (Poon et al. 2002; Wyatt et al. 2011). However, it remains to be established if clusterin functional activity is disrupted in DM. It is possible that in DM, the stress associated with a mutation in the Sod1 gene is sufficient to trigger the up-regulation and secretion of clusterin into the CSF. Indeed, of particular interest is a recent report that clusterin is elevated in the spinal cord of a symptomatic transgenic model of ALS mediated by the expression of a mutated human SOD1G93A gene (Zinkie et al. 2013). In addition, clusterin has also been widely implicated in human neurodegenerative diseases including Alzheimer’s disease (Calero et al. 2005), Parkinson’s disease (Sasaki et al. 2002) and ALS (Grewal et al. 1999). Clusterin has been found to be highly expressed in Alzheimer’s brain tissue (Lidstrom et al. 1998); however, the CSF clusterin levels described in Alzheimer’s patients have been inconsistent (Sihlbom et al. 2008) or unchanged (Lidstrom et al. 2001). The elevation of clusterin expression also has been reported in acute spinal cord injury (Klimaschewski et al. 2001).
It remains possible that the elevation of clusterin in CSF could be a consequence of blood-derived clusterin being transported to the CSF pathways through the blood-CSF-barrier (Reiber and Peter 2001) (Fig. 7a). Although clusterin levels are robust in plasma, there was no significant difference between the control and DM, which diminishes the possibility of plasma being the source of elevated clusterin in DM CSF. Interestingly, clusterin elevation in plasma has been reported in Alzheimer’s disease (Nilselid et al. 2006; Schrijvers et al. 2011), there are however no reports describing the plasma status of clusterin in ALS.
Fig. 7.
The potential underlying mechanisms leading to CSF clusterin elevation in DM. a A cartoon illustrating the blood, CSF and brain interfaces. CSF clusterin elevation may reflect changes in the blood clusterin levels. The protein may leave the blood vessels and enter the CSF pathways through the tight junctions between the ependymal cells of the choroid plexus. b Compartment model of CSF and spinal cord parenchyma interfaces. Increased clusterin mRNA expression with a concomitant increase of clusterin (CLU) distribution in DM motor neurons may lead to an elevation in CSF clusterin. The potential mechanism involves the movement of clusterin from motor neurons or potentially astrocytes into the subarachnoid space via the Virchow–Robin spaces. Clusterin is subsequently disseminated throughout the CSF pathway
It is tempting to speculate that elevated CSF clusterin may be derived from CNS parenchyma and indeed we observed a raised level of clusterin mRNA in spinal cord from DM cases. There are several reports of an elevated mRNA clusterin level that correlates with an increase in protein abundance (Lidstrom et al. 1998; Grewal et al. 1999). Due to a current lack of tissue from IVDD cases, the basis for elevated CSF clusterin in IVDD has not yet been explored. Interestingly, a 40 % elevation in frontal cortex clusterin mRNA has been reported in sporadic ALS cases relative to controls (Grewal et al. 1999). Similarly, in situ hybridisation also demonstrated that clusterin mRNA was increased in the anterior horn of the spinal cord grey matter in sporadic ALS patients, a region of the spinal cord that is severely affected by neurodegeneration (Grewal et al. 1999). Although the comparison of clusterin staining intensity by IHC between archival control and DM groups found no significant difference, it is possible that the rate and/or quantity of clusterin secretion is the significant cellular event. Clusterin may be secreted by motor neurons (Zinkie et al. 2013), but it is has also been shown that astrocytes/reactive astrocytes can secrete clusterin and may contribute to CSF levels (Cordero-Llana et al. 2011; Zinkie et al. 2013), given that gliosis is a consistent pathological feature of DM (Johnston et al. 2000).
The movement of molecules between the spinal cord parenchyma and CSF is complex and remains speculative (Brodbelt and Stoodley 2007). There is evidence of a potential CSF flow into the spinal cord parenchyma through the Virchow–Robin space, and conversely from the parenchyma into the CSF (Stoodley et al. 1996). Since clusterin is a secreted protein, it would be expected to accumulate in the extracellular milieu, and this may provide an explanation for how clusterin from motor neurons can accumulate in the CSF. This proposal is summarised in Fig. 7b.
Conclusion
Clusterin is elevated in the CSF of chronic spinal cord disorders of the dog compared to meningitis, which is a neuroinflammatory disorder (MEN), and idiopathic epilepsy.
Electronic supplementary material
We have demonstrated that the RLFP technique could be used to differentiate three Sod1 genotypes; wild type (WT), heterozygous (het) and homozygous (homo). Mixtures of wild type and homozygous PCR products were generated to represent each of the genotypes: wild type (100% WT: 0% homozygous), heterozygous (50% WT: 50% homozygous), and homozygous (0% WT: 100% homozygous). Intermediate ratios were also included; 75% WT: 25% homozygous and 25% WT: 75% homozygous. The heterozygous genotype displayed two bands at equal intensities (236 bp and 204 bp) in a mixture containing 50% wild type and 50% homozygous. In 100% wild type mixture, a prominent band was observed at 204bp, suggesting DNA fragments are completely digested whereas in 100% homozygous sample, a single band was observed at 236bp, which is comparable with undigested (UD) PCR product. (JPEG 6 kb)
Sod1 genotyping of canine spleen and blood-derived DNA. HpyAV digestion for 30 minutes in spleen and blood-derived DNA are depicted below. Partial digestion observed in control sample from spleen (C1 and C2), a single band (204 bp) consistent with wild type profile was detected in B1 blood sample, indicating a complete digestion had occurred with 100 ng of PCR product. The heterozygous profile was detected in B3 blood sample, demonstrating two bands at almost equal intensities (236bp and 204bp) and therefore showing a clear-cut differentiation between partially digested DNA products as observed in spleen-derived DNA. Two blood samples (B2 and B4) and a spleen sample demonstrated a homozygous profile, which represented a single band with size corresponding to undigested (UD) sample (236bp). (JPEG 8 kb)
(DOCX 14 kb)
Acknowledgments
This study was funded by the Ministry of Higher Education of Malaysia (MOHE), University Putra Malaysia and the British Small Animal Veterinary Association PetSavers. The authors are grateful to Professor David Eckersall for supplying haptoglobin antibody and also clinicians and staff of the neurology service in SAHGUVS for their help and cooperation with this study.
Declaration for conflict of interest
The authors of this manuscript do not have any financial or personal relationship with other people or organisations that could inappropriately influence or bias the content of the manuscript.
Footnotes
Intan N.F. Shafie and Mark McLaughlin contributed equally to this work.
References
- Al-Saktawi K, McLaughlin M, Klugmann M, Schneider A, Barrie JA, McCulloch MC, Montague P, Kirkham D, Nave KA, Griffiths IR. Genetic background determines phenotypic severity of the Plp rumpshaker mutation. J Neurosci Res. 2003;72:12–24. doi: 10.1002/jnr.10561. [DOI] [PubMed] [Google Scholar]
- Averill DR. Degenerative myelopathy in the aging German Shepherd dog: clinical and pathologic findings. J. Am. Vet. Med. Assoc. 1973;162:1045–1051. [PubMed] [Google Scholar]
- Awano T, Johnson GS, Wade CM, Katz ML, Johnson GC, Taylor JF, Perloski M, Biagi T, Baranowska I, Long S, March PA, Olby NJ, Shelton GD, Khan S, O’Brien DP, Lindblad-Toh K, Coates JR. Genome-wide association analysis reveals a Sod1 mutation in canine degenerative myelopathy that resembles amyotrophic lateral sclerosis. Proc Natl Acad Sci U S A. 2009;106:2794–2799. doi: 10.1073/pnas.0812297106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodbelt A, Stoodley M. CSF pathways: a review. Br J Neurosurg. 2007;21:510–520. doi: 10.1080/02688690701447420. [DOI] [PubMed] [Google Scholar]
- Calero M, Rostagno A, Frangione B, Ghiso J. Clusterin and Alzheimer’s disease. Subcell. Biochem. 2005;38:273–298. doi: 10.1007/0-387-23226-5_14. [DOI] [PubMed] [Google Scholar]
- Carnevali S, Luppi F, D’Arca D, Caporali A, Ruggieri MP, Vettori MV, Caglieri A, Astancolle S, Panico F, Davalli P, Mutti A, Fabbri LM, Corti A (2006) Clusterin decreases oxidative stress in lung fibroblasts exposed to cigarette smoke. Am J Res Crit Care Med 174:393–399 [DOI] [PubMed]
- Cherubini GB, Lowrie M, Anderson TJ. Pelvic limb ataxia in the older dog 1. assessment and non-painful conditions. In Practice. 2008;30:386–391. doi: 10.1136/inpract.30.7.386. [DOI] [Google Scholar]
- Coates JR, Wininger FA. Canine degenerative myelopathy. Vet. Clin. North Am. Small Anim. Pract. 2010;40:929–950. doi: 10.1016/j.cvsm.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Cordero-Llana O, Scott SA, Maslen SL, Anderson JM, Boyle J, Chowhdury RR, Tyers P, Barker RA, Kelly CM, Rosser AE, Stephens E, Chandran S, Caldwell MA. Clusterin secreted by astrocytes enhances neuronal differentiation from human neural precursor cells. Cell Death Differ. 2011;18:907–913. doi: 10.1038/cdd.2010.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felbecker A, Camu W, Valdmanis PN, Sperfeld AD, Waibel S, Steinbach P, Rouleau GA, Ludolph AC, Andersen PM. Four familial ALS pedigrees discordant for two SOD1 mutations: are all SOD1 mutations pathogenic? J Neurol Neurosurg Psychiatry. 2010;81:572–577. doi: 10.1136/jnnp.2009.192310. [DOI] [PubMed] [Google Scholar]
- Grewal RP, Morgan TE, Finch CE. C1qB and clusterin mRNA increase in association with neurodegeneration in sporadic amyotrophic lateral sclerosis. Neurosci Lett. 1999;271:65–67. doi: 10.1016/S0304-3940(99)00496-6. [DOI] [PubMed] [Google Scholar]
- Griffiths IR, Duncan ID. Chronic degenerative radiculomyelopathy in the dog. J. Small Anim. Pract. 1975;16:461–471. doi: 10.1111/j.1748-5827.1975.tb05773.x. [DOI] [PubMed] [Google Scholar]
- Harvey JW, West CL. Prednisone-induced increases in serum alpha-2-globulin and haptoglobin concentrations in dogs. Vet Pathol. 1987;24:90–92. doi: 10.1177/030098588702400115. [DOI] [PubMed] [Google Scholar]
- Johnston PEJ, Barrie JA, McCulloch MC, Anderson TJ, Griffiths IR. Central nervous system pathology in 25 dogs with chronic degenerative radiculomyelopathy. Vet. Rec. 2000;146:629–633. doi: 10.1136/vr.146.22.629. [DOI] [PubMed] [Google Scholar]
- Jones SE, Jomary C. Clusterin. Int. J. Biochem. Cell Biol. 2002;34:427–431. doi: 10.1016/S1357-2725(01)00155-8. [DOI] [PubMed] [Google Scholar]
- Killick R, Ribe EM, Al-Shawi R, Malik B, Hooper C, Fernandes C, Dobson R, Nolan PM, Lourdusamy A, Furney S, Lin K, Breen G, Wroe R, To AW, Leroy K, Causevic M, Usardi A, Robinson M, Noble W, Williamson R, Lunnon K, Kellie S, Reynolds CH, Bazenet C, Hodges A, Brion JP, Stephenson J, Paul Simons J, Lovestone S (2012) Clusterin regulates β-amyloid toxicity via Dickkopf-1-driven induction of the wnt-PCP-JNK pathway. Mol Psychiatry. doi:10.1038/mp.2012.163 [DOI] [PMC free article] [PubMed]
- Klimaschewski L, Obermuller N, Witzgall R. Regulation of clusterin expression following spinal cord injury. Cell Tissue Res. 2001;306:209–216. doi: 10.1007/s004410100431. [DOI] [PubMed] [Google Scholar]
- Lidstrom AM, Bogdanovic N, Hesse C, Volkman I, Davidsson P, Blennow K. Clusterin (apolipoprotein J) protein levels are increased in hippocampus and in frontal cortex in Alzheimer’s disease. Exp Neurol. 1998;154:511–521. doi: 10.1006/exnr.1998.6892. [DOI] [PubMed] [Google Scholar]
- Lidstrom AM, Hesse C, Rosengren L, Fredman P, Davidsson P, Blennow K. Normal levels of clusterin in cerebrospinal fluid in Alzheimer’s disease, and no change after acute ischemic stroke. J. Alzheimer’s Dis. 2001;3:435–442. doi: 10.3233/jad-2001-3501. [DOI] [PubMed] [Google Scholar]
- March PA, Coates JR, Abyad RJ, Williams DA, O’Brien DP, Olby NJ, Keating JH, Oglesbee M. Degenerative myelopathy in 18 Pembroke Welsh Corgi dogs. Vet Pathol. 2009;46:241–250. doi: 10.1354/vp.46-2-241. [DOI] [PubMed] [Google Scholar]
- Montague P, Dickinson PJ, McCallion AS, Stewart GJ, Savioz A, Davies RW, Kennedy PG, Griffiths IR. Developmental expression of the murine Mobp gene. J Neurosci Res. 1997;49:133–143. doi: 10.1002/(SICI)1097-4547(19970715)49:2<133::AID-JNR2>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Narayan P, Orte A, Clarke RW, Bolognesi B, Hook S, Ganzinger KA, Meehan S, Wilson MR, Dobson CM, Klenerman D. The extracellular chaperone clusterin sequesters oligomeric forms of the amyloid-beta(1–40) peptide. Nat Struct Mol Biol. 2012;19:79–83. doi: 10.1038/nsmb.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilselid AM, Davidsson P, Nagga K, Andreasen N, Fredman P, Blennow K. Clusterin in cerebrospinal fluid: analysis of carbohydrates and quantification of native and glycosylated forms. Neurochem Int. 2006;48:718–728. doi: 10.1016/j.neuint.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Nuutinen T, Suuronen T, Kauppinen A, Salminen A (2009) Clusterin: a forgotten player in Alzheimer’s disease. Brain Res Rev 61:89–104 [DOI] [PubMed]
- Poon S, Treweek TM, Wilson MR, Easterbrook-Smith SB, Carver JA (2002) Clusterin is an extracellular chaperone that specifically interacts with slowly aggregating proteins on their off-folding pathway. FEBS Lett 513:259–266 [DOI] [PubMed]
- Reiber H, Peter JB. Cerebrospinal fluid analysis: disease-related data patterns and evaluation programs. J Neurol Sci. 2001;184:101–122. doi: 10.1016/S0022-510X(00)00501-3. [DOI] [PubMed] [Google Scholar]
- Sasaki K, Doh-ura K, Wakisaka Y, Iwaki T. Clusterin/apolipoprotein J is associated with cortical Lewy bodies: immunohistochemical study in cases with alpha-synucleinopathies. Acta Neuropathol. 2002;104:225–230. doi: 10.1007/s00401-002-0546-4. [DOI] [PubMed] [Google Scholar]
- Satoh H, Yamato O, Asano T, Yonemura M, Yamauchi T, Hasegawa D, Orima H, Arai T, Yamasaki M, Maede Y. Cerebrospinal fluid biomarkers showing neurodegeneration in dogs with GM1 gangliosidosis: possible use for assessment of a therapeutic regimen. Brain Res. 2007;1133:200–208. doi: 10.1016/j.brainres.2006.11.039. [DOI] [PubMed] [Google Scholar]
- Schrijvers EM, Koudstaal PJ, Hofman A, Breteler MM. Plasma clusterin and the risk of Alzheimer disease. J. Am. Med. Assoc. 2011;305:1322–1326. doi: 10.1001/jama.2011.381. [DOI] [PubMed] [Google Scholar]
- Shafie INF, Anderson TJ, Penderis J, Eckersall PD, McLaughlin M (2013) A protocol for the management of canine cerebrospinal fluid for the proteomic assessment of putative biomarkers. Vet J. In Press. doi:10.1016/j.tvjl.2013.05.039 [DOI] [PubMed]
- Shelton GD, Johnson GC, O’Brien DP, Katz ML, Pesayco JP, Chang BJ, Mizisin AP, Coates JR. Degenerative myelopathy associated with a missense mutation in the superoxide dismutase 1 (SOD1) gene progresses to peripheral neuropathy in Pembroke Welsh Corgis and Boxers. J Neurol Sci. 2012;318:55–64. doi: 10.1016/j.jns.2012.04.003. [DOI] [PubMed] [Google Scholar]
- Sihlbom C, Davidsson P, Sjogren M, Wahlund LO, Nilsson CL. Structural and quantitative comparison of cerebrospinal fluid glycoproteins in Alzheimer’s disease patients and healthy individuals. Neurochem Res. 2008;33:1332–1340. doi: 10.1007/s11064-008-9588-x. [DOI] [PubMed] [Google Scholar]
- Stoodley MA, Jones NR, Brown CJ. Evidence for rapid fluid flow from the subarachnoid space into the spinal cord central canal in the rat. Brain Research. 1996;707:155–164. doi: 10.1016/0006-8993(95)01228-1. [DOI] [PubMed] [Google Scholar]
- Szoor B, Ruberto I, Burchmore R, Matthews KR. A novel phosphatase cascade regulates differentiation in Trypanosoma brucei via a glycosomal signaling pathway. Genes Dev. 2013;24:1306–1316. doi: 10.1101/gad.570310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumani H, Teunissen C, Sussmuth S, Otto M, Ludolph AC, Brettschneider J. Cerebrospinal fluid biomarkers of neurodegeneration in chronic neurological diseases. Expert Rev Mol Diagn. 2008;8:479–494. doi: 10.1586/14737159.8.4.479. [DOI] [PubMed] [Google Scholar]
- Wang C, Jiang K, Gao D, Kang X, Sun C, Zhang Q, Li Y, Sun L, Zhang S, Guo K, Liu Y. Clusterin protects hepatocellular carcinoma cells from endoplasmic reticulum stress induced apoptosis through GRP78. PLoS One. 2013;8:e55981. doi: 10.1371/journal.pone.0055981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wininger FA, Zeng R, Johnson GS, Katz ML, Johnson GC, Bush WW, Jarboe JM, Coates JR. Degenerative myelopathy in a Bernese Mountain Dog with a novel SOD1 missense mutation. J. Vet. Intern. Med. 2011;25:1166–1170. doi: 10.1111/j.1939-1676.2011.0760.x. [DOI] [PubMed] [Google Scholar]
- Wyatt AR, Yerbury JJ, Wilson MR (2009) Structural characterization of clusterinchaperone client protein complexes. J Biol Chem 284:21920–21927 [DOI] [PMC free article] [PubMed]
- Wyatt AR, Yerbury JJ, Berghofer P, Greguric I, Katsifis A, Dobson CM, Wilson MR (2011) Clusterin facilitates in vivo clearance of extracellular misfolded proteins. Cell Mol Life Sci 68:3919–3931 [DOI] [PMC free article] [PubMed]
- Zinkie S, Gentil BJ, Minotti S, Durham HD (2013) Expression of the protein chaperone, clusterin, in spinal cord cells constitutively and following cellular stress, and upregulation by treatment with Hsp90 inhibitor. Cell Stress Chaperones [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
We have demonstrated that the RLFP technique could be used to differentiate three Sod1 genotypes; wild type (WT), heterozygous (het) and homozygous (homo). Mixtures of wild type and homozygous PCR products were generated to represent each of the genotypes: wild type (100% WT: 0% homozygous), heterozygous (50% WT: 50% homozygous), and homozygous (0% WT: 100% homozygous). Intermediate ratios were also included; 75% WT: 25% homozygous and 25% WT: 75% homozygous. The heterozygous genotype displayed two bands at equal intensities (236 bp and 204 bp) in a mixture containing 50% wild type and 50% homozygous. In 100% wild type mixture, a prominent band was observed at 204bp, suggesting DNA fragments are completely digested whereas in 100% homozygous sample, a single band was observed at 236bp, which is comparable with undigested (UD) PCR product. (JPEG 6 kb)
Sod1 genotyping of canine spleen and blood-derived DNA. HpyAV digestion for 30 minutes in spleen and blood-derived DNA are depicted below. Partial digestion observed in control sample from spleen (C1 and C2), a single band (204 bp) consistent with wild type profile was detected in B1 blood sample, indicating a complete digestion had occurred with 100 ng of PCR product. The heterozygous profile was detected in B3 blood sample, demonstrating two bands at almost equal intensities (236bp and 204bp) and therefore showing a clear-cut differentiation between partially digested DNA products as observed in spleen-derived DNA. Two blood samples (B2 and B4) and a spleen sample demonstrated a homozygous profile, which represented a single band with size corresponding to undigested (UD) sample (236bp). (JPEG 8 kb)
(DOCX 14 kb)