Abstract
Stem cells in adult organism are responsible for cell turnover and tissue regeneration. The study of stem cell stress response contributes to our knowledge on the mechanisms of damaged tissue repair. Previously, we demonstrated that sublethal heat shock (HS) induced apoptosis in human embryonic stem cells. This study aimed to investigate HS response of human adult stem cells. Human mesenchymal stem cells (MSCs) cultivated in vitro were challenged with sublethal HS. It was found that sublethal HS did not affect the cell viability assessed by annexin V/propidium staining. However, MSCs subjected to severe HS exhibited features of stress-induced premature senescence (SIPS): irreversible cell cycle arrest, altered morphology, increased expression of senescence-associated β-galactosidase (SA-β-gal) activity, and induction of cyclin-dependent kinase inhibitor p21 protein. High level of Hsp70 accumulation induced by sublethal HS did not return to the basal level, at least, after 72 h of the cell recovery when most cells exhibited SIPS hallmarks. MSCs survived sublethal HS, and resumed proliferation sustained the properties of parental MSCs: diploid karyotype, replicative senescence, expression of the cell surface markers, and capacity for multilineage differentiation. Our results showed for the first time that in human MSCs, sublethal HS induced premature senescence rather than apoptosis or necrosis. MSC progeny that survived sublethal HS manifested stem cell properties of the parental cells: limited replicative life span and multilineage capacity.
Keywords: Adult stem cells, Heat shock, Heat shock proteins (Hsp), Premature senescence
Introduction
The main properties of stem cells are the capacity for self-renewal and multilineage differentiation. They are divided into two main categories: embryonic and adult stem cells. Embryonic stem cells are pluripotent and ensure the development of the whole organism; adult stem cells are responsible for the growth of new tissues and repair and regeneration of damaged tissues. Both cell types sustain self-renewal in vitro and can be expanded in cultures (Bruder et al. 1997; Pera et al. 2000; Reubinoff et al. 2000). Stem cell plasticity raised the possibility of their application in human therapy that inspired great research interest. Numerous reports demonstrated successful treatment of various diseases with human embryonic stem cells in laboratory and clinical studies (Brederlau et al. 2006; Schwartz et al. 2012). However, ethical concerns and the risk of tumor formation in recipients limit embryonic stem application in cell therapy. It revives the interest to human adult stem cells and their clinical application.
Most, if not all, tissues of adult organisms contain stem cells designated as adult stem cells (Bianco et al. 2008; da Silva Meirelles et al. 2006; Paul et al. 2012). Unlike pluripotent embryonic stem cells, adult stem cells are multipotent, i.e., able to differentiate into multiple but not all cell types (Ramkisoensing et al. 2011). Nevertheless, these cells hold a great promise both for biomedical research and cell therapy (Husein and Thiemermann 2010). Most adult stem cells intended for regenerative therapy are prevalently derived from mesenchymal tissues. The main mesenchymal stem cell (MSC) sources are bone marrow, adipose tissue, umbilical cord blood cells, and menstrual blood. Although there are some differences in MSCs isolated from different sources, they share most of their properties (Dominici et al. 2006; Dmitrieva et al. 2012). MSC biology research has been hampered in part because of a lack of unique definitive human MSC markers. The International Society of Cellular Therapy defined hMSCs based on the following three criteria: firstly, human MSCs must be able to adhere to plastic surface under standard tissue culture conditions; secondly, human MSCs must express certain markers, including CD73, CD90, and CD105, and lack the expression of markers, such as CD45, CD34, CD14, CD79 alpha, or CD19 and HLA-DR surface molecules; and, finally, the cells must be capable for differentiation into osteoblasts, chondroblasts, and adipocytes under appropriate in vitro conditions (Dominici et al. 2006).
Stem cell response to harmful stimuli is a prerequisite for the tissue repair and is under active examination (Cmielova et al. 2012). In order to fulfill the task of tissue repair, stem cells must remain uninjured under unfavorable conditions. Temperature is an important factor that regulates various cellular processes. Sudden changes in the environmental temperature are frequent events. Elevated temperature is observed under various medical conditions, such as inflammation and infectious diseases. Some patients receiving stem cell transplantation develop fever (temperature higher 39 °C) (Spitzer 2001). Cell response to heat stress is one of the most examined cellular stress responses. However, although heat is a common stressor, little is known about the stem cell response to elevated temperature. It is considered that eukaryotic cells mostly die by two fundamental alternatives, necrosis or apoptosis. HS is usually regarded as apoptosis or necrosis inducer. Recently, we have shown that in human embryonic stem cells, sublethal HS induced apoptosis (Alekseenko et al. 2012). Apoptosis seems to be a general outcome in embryonic stem cells subjected to various stress factors (Sokolov and Neumann 2013). On the other hand, a common stress response of various adult stem cells to different insults is stress-induced premature senescence (SIPS) (Toussaint et al. 2000; Brandl et al. 2011a, b; Cmielova et al. 2012; Ko et al. 2012), i.e., appearance of biomarkers of replicative senescence (RS). However, we did not find any indications that HS is also a SIPS trigger. The major RS markers are irreversible arrest of cell division, altered cells' morphology, senescence-associated β-galactosidase (SA-β-gal) activity, and modified expression of certain genes. RS (Hayflick's limit) is a fundamental feature of various normal human cells cultivated in vitro and usually attributed to the steady telomere shortening during cell passaging. Comparison between SIPS and RS indicates that RS is mostly associated with the reduction of telomerase activity and attrition of telomeres, whereas SIPS does not usually require these events. SIPS is considered a form of death that irreversibly arrests division of cells but retains their metabolic activity. The list of SIPS-inducing factors thought to be constantly growing includes oxidative stress, H2O2, tert-butyl hydroperoxide, ethanol, ionizing radiations, and mitomycin C (Toussaint et al. 2000). The goal of this study was to evaluate heat shock response of human MSCs. Since little is known about the fate of MSC progeny subjected to severe insults, we investigated the properties of MSCs that survived sublethal heat shock exposure. It was demonstrated for the first time that sublethal temperature induced SIPS rather than apoptosis in MSCs. MSCs respond to HS by altered cell morphology, irreversible cell cycle arrest, increased SA-β-gal activity, and enhanced expression of p21 protein. We also found that MSC progeny resistant to the applied HS retain the properties of parental cells: diploidy, limited life span in culture (replication senescence), expression of specific surface markers, and capacity for differentiation into multiple cell lineages.
Materials and methods
Cells and treatment
The procedures involved human cells were performed in accordance with the standards of the Declaration of Helsinki (1989) and approved by the Institute of Cytology Ethics Committee.
Human MSC was established from shedding endometrium in menstrual blood (Zemelko et al. 2011). Cells were maintained in Dulbecco's Modified Eagle Medium (DMEM)/F12 (Gibco) supplemented with 10 % fetal calf serum (HyClone), antibiotic–antimycotic mixture, and GlutaMAXTM (Gibco) and subcultured at 1:3–1:4 ratio twice a week. Cells at the 7th–11th passages were used in the experiments. Before heat treatment, exponentially growing cells were seeded in 3-cm Petri dishes at subconfluent density and, the next day, exposed to elevated temperature. Plates sealed with parafilm were heated at 45 °C for 10 min (mild heat shock) or 30 min (sublethal heat shock) in a water bath and then returned for recovery under normal conditions in a CO2 incubator at 37 °C for various times.
Cell viability and apoptosis assays
Cell death (apoptosis, necrosis) was assessed by annexin V/propidium iodide staining. Heated and unheated cells were harvested by trypsin/EDTA, incubated with annexin V–fluorescein isothiocyanate (FITC, BD Pharmingen) and propidium iodide according to the manufacturer's instructions and analyzed on Coulter EPICS XL Flow Cytometer (Beckman Coulter). Apoptosis was also quantified microscopically by counting nuclear fragmentation in 4′,6-diamidino-2-phenylindole (DAPI)-stained cells. Not less than 500 randomly selected cells were surveyed. The data are presented as mean ± SD of three independent experiments.
Analysis of cell cycle and proliferation
Heated and unheated cells were harvested with trypsin/EDTA and processed for the cell cycle analysis. Cell suspension was treated with 200 μg/ml saponin for 30 min at room temperature and then with 50 μg/ml propidium iodide (PI) and 250 μg/ml RNase A for 30 min at 37 °C. Cell cycle analysis was performed with Coulter EPICS XL Flow Cytometer and WinList and ModFit LT software (Verity Software House). Proliferation was also evaluated by growth curve assay. In the growth medium, 105 cells per 3-cm plate were seeded. Cells were harvested at the indicated time, stained with trypan blue, and counted. Experiments were repeated three times.
Immunofluorescence
Cells grown on coverslips were fixed with 4 % formalin in phosphate-buffered saline (PBS) for 15 min, permeabilized with 0.1 % Triton X-100, blocked with 1 % bovine serum albumin for 30 min, treated with primary antibodies for 45 min, washed with PBS/0.1 % Tween-20, treated with secondary antibodies for 45 min, washed with PBS/0.1 % Tween-20, counterstained with 1 μg/ml DAPI, and mounted in PBS/90 % glycerol containing 2 % n-propyl gallate. The primary antibodies used include mouse monoclonal to Hsp70 (Lasunskaia et al. 1997), Ki-67 (Abcam), NF-H (Abcam), NeuN (Millipore), and III β-tubulin (Chemicon). The secondary antibody used was DylightTM-488-conjugated goat anti-mouse (Jackson ImmunoResearch). Images were captured using the laser scanning confocal microscope Leica TCS SP5.
PCR assay
Reverse transcription PCR was performed as described (Alekseenko et al. 2012). Briefly, total RNA was isolated with RNeasy Micro Kit (QIAGEN) according to the manufacturer's instructions. cDNA synthesis was performed with 1 μg of total RNA using RevertAid H Minus First Strand cDNA Synthesis Kit (Fermentas) according to the manufacturer's instructions. Specific genes were amplified by Taq DNA polymerase (Fermentas) with a Cyclo Temp amplificator. Expression of target genes was normalized to actin. Primers and reaction conditions are presented in Table 1. Experiments were repeated at least three times.
Table 1.
Primer sequence and reaction conditions for the reverse transcriptase PCR
| Gene | Primer sequence | Annealing temperature (°C) | PCR product size (bp) | GenBank accession numbers |
|---|---|---|---|---|
| Actin | s 5′ gccgagcgggaaatcgtgcgt 3′ | 70 | 506 | NM_001101.3 |
| as 5′ cggtggacgatggaggggccg 3′ | ||||
| p21 | s 5′ ccacatggtcttcctctgctg 3′ | 55 | 316 | NM_001220778.1 |
| as 5′ gatgtccgtcagaacccatg 3′ | ||||
| Hsp27 | s 5′ cgcgctcagccggcaactcag 3′ | 64 | 419 | NM_001540.3 |
| as 5′ aggggtgggcatccgggctaagg 3′ | ||||
| Hsp70 | s 5′ atgcggccaagaaccaggtg 3′ | 61 | 307 | NM_005345.5 |
| as 5′ gcgctgcgagtcgttgaagt 3′ | ||||
| Hsc70 | s 5′ atccccaagattcagaagct 3′ | 63 | 218 | NM_153201.1 |
| as 5′ ttgatgaggacagtcatga 3′ | ||||
| Hsp90 | s 5′ aatcggaagaagctttcaga 3′ | 55 | 446 | NM_005348.3 |
| as 5′ gtgcttgtgacaatacagca 3′ |
Actin Homo sapiens actin, beta (ACTB), mRNA, p21 Homo sapiens cyclin-dependent kinase inhibitor 1A (p21, Cip1; CDKN1A), Hsp27 Homo sapiens heat shock 27-kDa protein 1 (HSPB1), Hsp70 Homo sapiens heat shock 70-kDa protein 1A (HSPA1A), Hsc70 Homo sapiens heat shock 70-kDa protein 8 (HSPA8), Hsp90 Homo sapiens heat shock protein 90-kDa alpha (cytosolic), class A member 1 (HSP90AA1)
Immunoblotting
Immunoblotting was performed as described (Alekseenko et al. 2012). Briefly, whole cell lysate in the lysis buffer was mixed with electrophoresis sample buffer, separated by SDS-PAGE electrophoresis, transferred to PVDF membranes (Millipore), and incubated with antibodies. Proteins were visualized with a SuperSignal chemiluminescence reagent kit (Pierce). The primary antibodies used were mouse monoclonals to Hsp70 (Lasunskaia et al. 1997), rabbit monoclonals to р21Waf1/Cip1 protein (Cell Signaling), rabbit monoclonals to Hsc70 (Abcam), and rabbit monoclonals to GAPDH (Cell Signaling). The secondary antibodies used include horseradish-conjugated goat anti-rabbit (Cell Signaling) and horseradish-conjugated goat anti-mouse (Sigma). All expression levels were normalized to the level of the GAPDH housekeeping protein. Fold change of gene and protein expression was assessed using the Scion Image software. Experiments were repeated at least three times.
SA-β-gal assay
Cell staining for β-galactosidase (β-gal) activity was performed using Senescence β-Galactosidase Staining Kit (Cell Signaling Technology) according to the manufacturer's protocol and quantified microscopically by counting X-gal-positive cells among not less 500 cells.
Assessment of marker properties in MSC progeny that survived heat shock
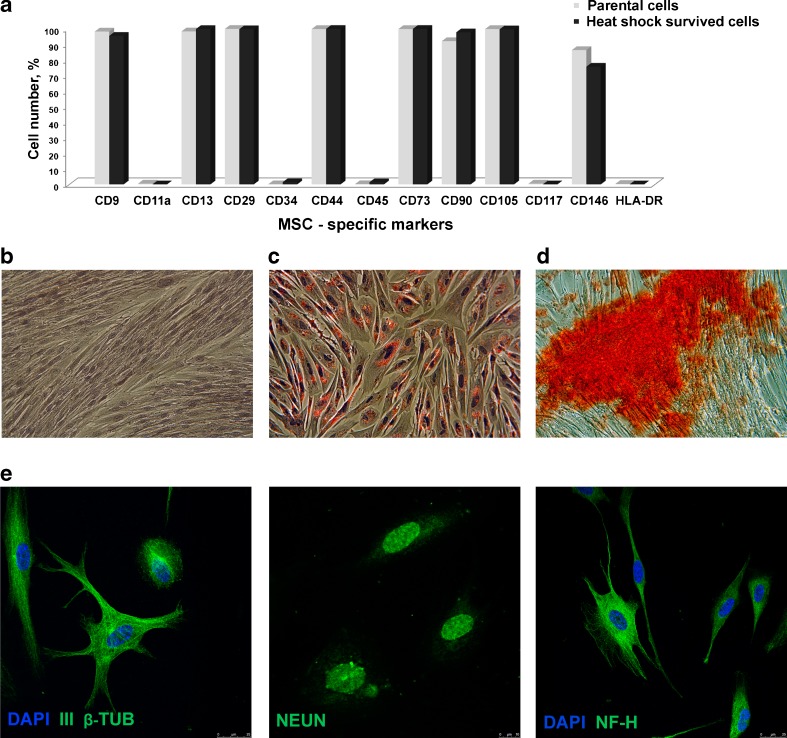
Expression of CD markers was analyzed as described (Zemelko et al. 2011). Briefly, cells were treated with FITC or phycoerythrin-labeled antibodies to CD9, CD11a, CD13 (Beckman Coulter), CD29 (BD Biosciences), CD34, CD45 (Beckman Coulter), CD73 (BD Biosciences), CD90 (Chemicon), CD105, CD117, CD146 (Beckman Coulter), and HLA-DR (Chemicon) and assayed with flow cytometry.
Chromosome analysis was done on G-banded metaphase chromosomes by the routine method. Briefly, cells were accumulated in metaphase with 0.01–0.02 μg/ml demecolcine, treated with 0.56 % KCl as hypotonic solution, and stained with Giemsa dye for G-banding. Metaphases were analyzed under a light microscope (Carl Zeiss).
Cell plasticity was evaluated by cell capacity for differentiation into adipogenic, osteogenic, and neural lineages. For induction of osteogenic differentiation, cells were cultured on plastic dishes (Nunc). When cells reached 100 % confluence, they were incubated in a differentiation medium for 4 weeks with the medium being changed every 2 to 3 days. The differentiation medium was as follows: DMEM/F12 supplemented with 10 % fetal calf serum (FCS, HyClone), 10 mM β-glycerophosphate, 100 μg/ml l-ascorbic acid 2-phosphate, and 100 nM dexamethasone. To detect mineralization (calcium deposits), the cells were fixed with ice-cold 70 % ethanol, stained with Alizarin Red S (MP Biomedicals), and counterstained with hematoxylin (Sigma). Images were taken at a ×200 magnification.
For analysis of adipogenic differentiation, cells were grown to 90 % confluence. The cells were then incubated for 5 weeks in the medium composed of 10 % FCS, 10 μg/ml insulin (Sigma), 1 μM dexamethasone (Sigma), 250 μM 3-isobutyl-1-methylxanthine (Sigma), and 200 μM indomethacin (MP Biomedicals). The medium was changed every 3 days. To detect fat deposition, the cells were fixed with 4 % paraformaldehyde (Sigma) for 30 min at room temperature and stained with Oil Red O (MP Biomedicals). Images were taken at a ×200 magnification.
To prove the neural potential, the cells were grown on Matrigel (BD Biosciences)-coated coverslips. Neuronal differentiation was induced by growing the cells in neural basal medium (Gibco) supplemented with 1 % N2 supplement (Gibco), 2 % B27 supplement (Gibco), and 25 ng/ml epidermal growth factor (Calbiochem) for 2 days. Then, the medium was replenished with 1 μM retinoic acid (Sigma), 0.25 mM 3-isobutyl-1-methylxanthine (Sigma), and 1 mM dbcAMP (Sigma). After 7 days of neuronal induction, cells were processed for immunofluorescence experiments.
Results
Cells
Experiments have been performed on MSCs established from human menstrual blood. It is considered that they are derived from shed endometrium. The cells are multipotent; capable for self-renewal; express CD13, CD29, CD44, CD73, CD90, and CD105; and are negative for the hematopoietic markers CD34 and CD45 (Zemelko et al. 2011). These properties make them closely similar to bone marrow-derived MSCs (Allickson and Xiang 2012) that are the most examined human adult stem cells. The advantage of MSCs derived from menstrual blood is the affordable, painless, and noninvasive manner of their isolation.
Sublethal heat shock does not induce MSC apoptosis
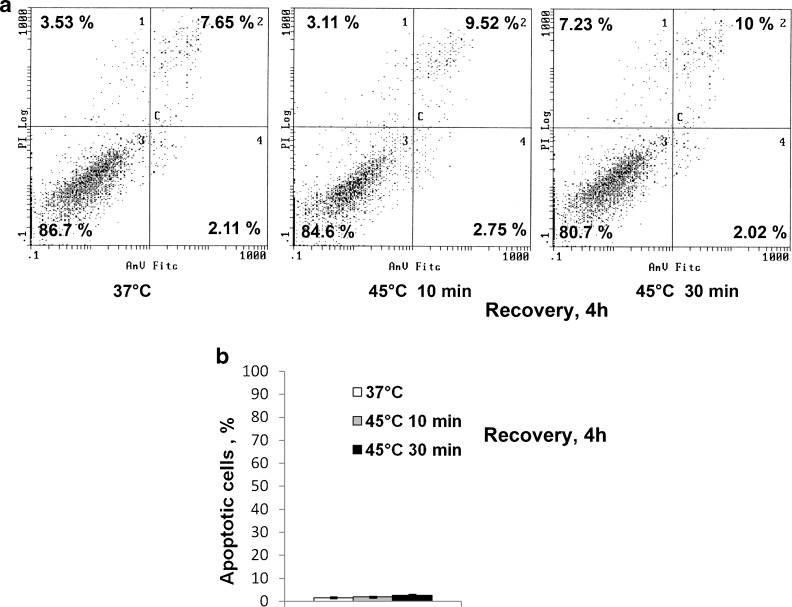
The cell viability was assayed by flow cytometry. Figure 1a demonstrates that elevated temperature did not affect MSC viability measured by annexin V/PI. It is seen that the number of viable or apoptotic cells heated at 45 °C for 10 or 30 min and returned at 37 °C for the recovery for 4 h was not notably changed. The number of apoptotic cells was also measured by the number of cells with fragmented or condensed nuclei in DAPI-stained cultures (Fig. 1b). No difference between heated and unheated cells was observed.
Fig. 1.
Mild (45 °C, 10 min) and sublethal heat shock (45 °C, 30 min) did not induce apoptosis in human MSCs. a Flow cytometry assay of apoptosis with annexin V/PI. b Apoptosis measured by counting cells with fragmented nuclei
Heat shock induced MSC cell cycle arrest and premature senescence
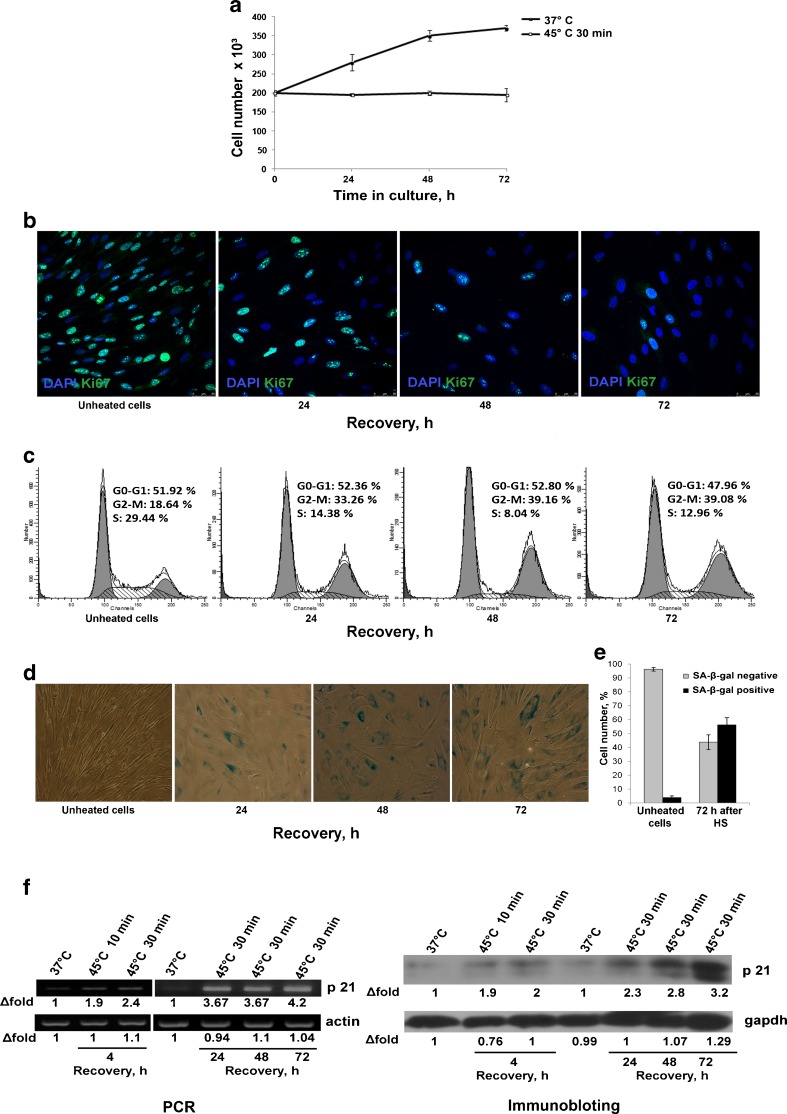
We did not registered reduced viability of cells subjected to heat shock but noticed that heating at 45 °C for 30 min but not 10 min slowed down cell proliferation and had impact on the their morphology. Cell number in MSC cultures exposed to 45 °C for 30 min and returned to the normal conditions was counted during 72 h. Growth curves of unheated and heated MSCs are presented in Fig. 2a. It is seen that the cell number remains unaltered in heated cultures whereas the population of control cells almost doubled during this period. Inhibition of proliferation was confirmed by cell staining with antibodies to Ki-67, a marker of proliferative activity (Fig. 2b). Seventy-two hours after heat shock, only a few cells were Ki-67-positive. Cell cycle analysis by flow cytometry revealed that heated cells were arrested mostly in G2/M phase (Fig. 2c). Cell cycle arrest is a hallmark of stress-induced premature senescence. Other biomarkers of premature senescence are modified cell morphology, increasing SA-β-gal activity, and upregulation of cyclin-dependent kinase inhibitor p21 protein. Figure 2d demonstrates the modified morphology of MSCs subjected to HS: they are enlarged, more flattened, and express X-gal. Three days after HS, the number of SA-β-gal-positive cells increased to about 70 % (Fig. 2e). Figure 2f shows that HS induced expression of p21 protein, a cell cycle inhibitor and major regulator of the senescence program. Four hours after recovery, p21 upregulation was obvious both at mRNA and protein levels. During 72 h after HS, the p21 expression gradually increases up to three times.
Fig. 2.
Heat shock (45 °C, 30 min) induced MSC premature senescence. a Growth curves of heated and unheated cells. MSCs exposed to heat shock stop division. b Immunofluorescence assay of cell proliferation with anti-Ki-67antibodies. Only single cells are Ki-67-positive 72 h after heat shock. c FACS assay of cell cycle distribution. MSCs that were heated at 45 °C for 30 min and returned to the normal culture conditions exhibited cell cycle arrest in G2/M phases. d SA-β-X-gal staining during cell recovery from sublethal HS. The number of X-gal-positive cell increased. e Quantitative assay of X-gal-positive cells. f Expression of cyclin-dependent kinase inhibitor p21 in MSCs exposed to HS. p21 expression is increased along with the augment in X-gal-positive cells (d)
Expression of heat shock proteins in MSCs subjected to HS
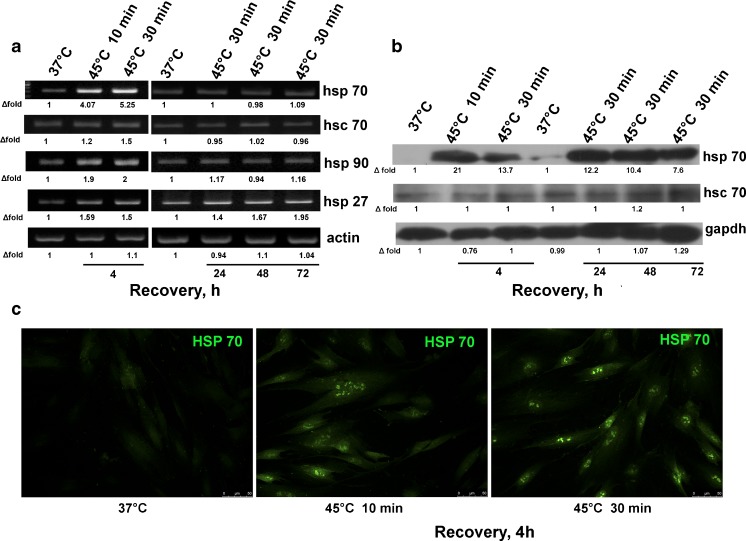
Heat shock response (HSR) in all organisms involves the expression of heat shock proteins (Hsp). We assayed the expression of Hsp by PCR, immunoblot, and immunofluorescence (Fig. 3). It was found (Fig. 3a) that HS induced the expression of a number of Hsp genes (Hsp27, Hsp70, Hsc70, Hsp90) in 4 h. The enhancement (four to five times) was the highest for Hsp70. Twenty-four hours after the cell recovery, the expression of these genes returned to the normal level, except for Hsp27 gene, which remained upregulated, for at least 72 h. Expression of major Hsp, constitutive isoform Hsc70 and inducible Hsp70 isoforms, was assayed by immunofluorescence and immunoblotting. Figure 3c demonstrates that both mild and sublethal HS induced Hsp70 expression and shows Hsp70 redistribution from the cytoplasm into the nucleus. It is the common HSR for many cell types. The results of immunoblotting showed that the level of Hsc70 was basically unchanged whereas the content of Hsp70 was profoundly increased. Robust increase and long-term persistence of Hsp70 protein accumulation was also observed in MSCs treated with subtoxic doses of hydrogen peroxide (data not shown). Although the level of Hsp70 mRNA returned to the initial level already in 24 h, increased level of Hsp70 in cells subjected to sublethal HS was maintained for at least 72 h after recovery when most cells exhibited cell senescence.
Fig. 3.
Expression of heat shock proteins in MSCs subjected to heat shock. Cells were exposed to heat shock, allowed to recover for indicated time, and assayed by PCR (a), immunoblotting (b), or immunofluorescence (c). Densitometry data below each band represent “fold change” after normalization with a loading control (a, b). Hsp70 induced in MSCs by heat shock is localized mostly in the nuclei (c)
Cell properties of MSCs that survived HS
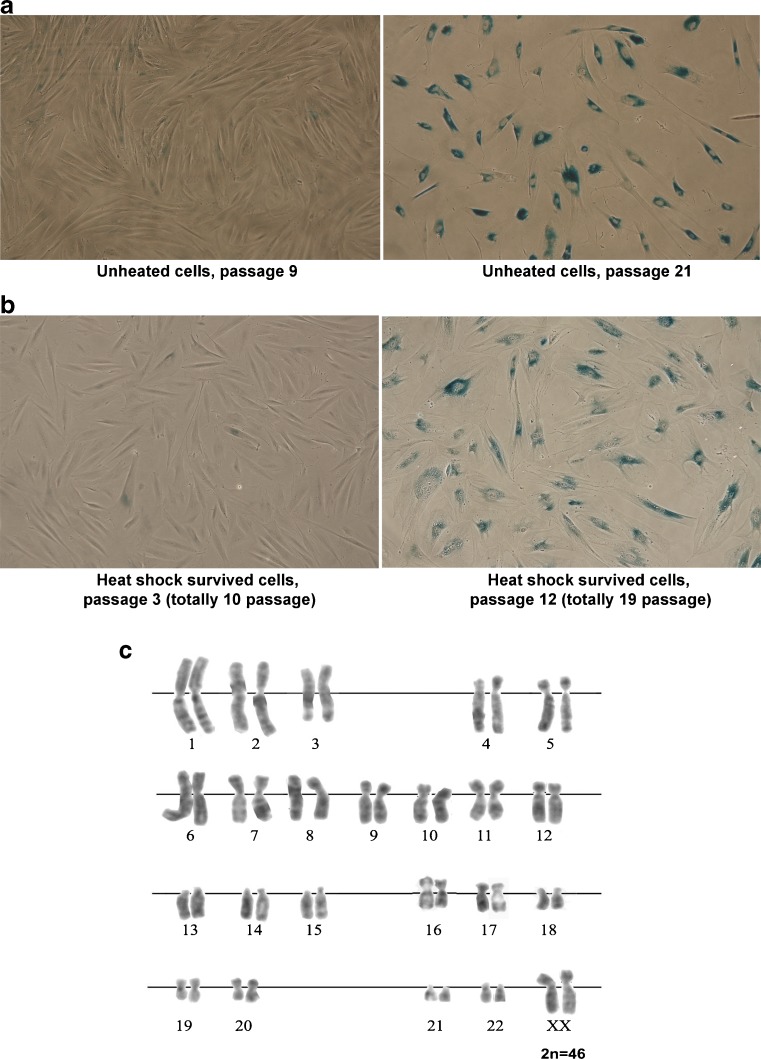
In our study, we employed heat shock conditions (45 °C, 30 min) that were sublethal to MSCs. SIPS was developed only in a part of the cell population. We addressed the fate of SIPS-negative cells. Four days after the heat shock, the cells were plated into new culture dishes. Adhered cells restore the initial fibroblast morphology and resumed proliferation. Figure 4 shows that at the third passage after HS, the cells are morphologically indistinguishable from initial parental cells and retained diploid karyotype. With increasing number of population doublings, the cells recovered from HS exhibited replicative senescence: they became enlarged, slowed down proliferation, and, at last, ceased proliferation, reaching replicative senescence stage after additional 12–14 passages (Fig. 4a, b), i.e., underwent like parental MSCs totally 22–25 passages and died. Parental MSCs as other human stem cells of mesenchymal origin have limited life span in culture. Survived cells retain the expression pattern of stem cell CD markers (Fig. 5a) and exhibit MSC plasticity. They were able to differentiate into adipocytes and osteoblasts (mesoderm lineages; Fig. 5c, d) and transdifferentiate into neuron-like cells (ectoderm lineage; Fig. 5e). Thus, MSC fraction that survived HS retain the properties of parental cells, at least, morphology, profile of CD marker expression, plasticity, and limited replicative life span.
Fig. 4.
MSCs that survived sublethal heat shock exhibit replicative senescence (a, b) and retain normal karyotype (c). MSCs at the seventh passage were heated (45 °C, 30 min) and returned to standard culture conditions. a X-gal staining of parental cells. b X-gal staining of cells that survived heat shock
Fig. 5.
MSCs that survived sublethal heat shock display multilineage potential. Survived cells were assayed for MSC CD marker expression (a) and capacity for differentiation into adipocytes (c), osteoblasts (d), and neural cells (e). b Initial (control) cells were not subjected to differentiation stimuli
Discussion
MSCs are capable for self-renewal and differentiation into various lineages. They exist in various tissues in adult human organisms and are responsible for cell replenishment and regeneration of damaged tissues. Adult stem cells of mesenchymal origin hold a great promise for cell therapy and are the widely used in biomedical research. Examination of stress response of human stem cells cultured in vitro contributes to our knowledge on the tissue repair that is also important for their potential application in cell therapy. In this study, we exploited MSCs isolated from menstrual blood (Zemelko et al. 2011). It is considered that they are derived from shed endometrium. These cells display all the properties of MSCs isolated from other sources and are promising alternatives to widely used bone marrow-derived MSCs (Allickson and Xiang 2012). They are multipotent; capable for self-renewal; express CD13, CD29, CD44, CD73, CD90, and CD105; and are negative for the hematopoietic markers CD34 and CD45 (Zemelko et al. 2011). The advantage of MSCs from menstrual blood is the affordable, painless, and noninvasive manner of their isolation.
Stress response of cultivated human stem cell is under intensive study (Goligorsky et al. 2009; Tower 2012). Cells exposed to stress may respond differently: entry into differentiation, senescence (SIPS), apoptosis, or necrosis. The choice depends on the cell type and stress strength. Mild stress may improve differentiation of stem cells (Stolberg and McCloskey 2009; Hronik-Tupaj et al. 2011). The outcome for unbearable stress is necrosis. Sublethal doses of various stressors produce apoptosis or senescence (SIPS). Oxidative stress may induce senescence in human diploid fibroblasts but apoptosis if the cells are challenged with its higher doses (Bladier et al. 1997).
Temperature is an important environmental and medical factor, and cells often cope with elevated temperature. However, HS response of human MSCs is not well studied. It was demonstrated that mild heat stress promoted myoblast differentiation (Yamaguchi et al. 2010) and MSC osteogenesis (Shui and Scutt 2001; Chen et al. 2013). Severe heat shock conditions occurring during orthopedic procedures and modeled on cultured osteoblast cells induced apoptosis and necrosis (Li et al. 1999; Dolan et al. 2012). It was reported that the heterogenous population of human dental follicle cells may be enriched with stem cells relative to their nonstem counterparts by enhanced temperature (Yao et al. 2011; Rezai Rad et al. 2013). We did not find any evidences that normal human cells exposed to elevated temperature manifested premature senescence phenotype. To our knowledge, we show for the first time that heat shock like many other stressors is a SIPS inducer. Cultured MSCs exposed to enhanced temperature (45 °C, 30 min) exhibited the hallmarks of senescence induced by stress. The cells were G2/M-arrested, had altered morphology, displayed senescence-associated β-galactosidase (SA-β-gal) activity, and increased expression of p21 protein, a cyclin-dependent kinase inhibitor.
HSR is one of the most conserved cellular stress responses. It is characterized by transcription and accumulation of Hsp. Hsp are generally expressed in response to various environmental stresses including heat, oxygen conditions, and toxic agents. Hsp protect cells through their chaperone functions. There is a broad literature on the structure and properties of Hsp, their function in normal and damaged cells, and molecular mechanisms of the expression of Hsp in response to stress. Some evidences indicate that Hsp are involved in the senescence control. Upregulation of Hsp27 in human umbilical cord stem cells delayed their senescence (Liu et al. 2010). Inhibition of Hsp90 (Restall and Lorimer 2010) or Hsp70 depletion (Sherman 2010) in tumor cells induced their premature senescence. Age-related decrease of Hsp27, Hsp70, and Hsp90 basal levels was registered during MSC long-term cultivation (Stolzing et al. 2008). Less is known about the expression of Hsp in normal human cells induced to premature senescence. Recently, it was demonstrated that HSR was suppressed in normal human fibroblasts which underwent premature senescence by DNA-damaging treatments. The HSR suppression was associated with inhibition of both activity and transcription of the heat shock transcription factor Hsf1 (Kim et al. 2012).
In this work, we studied the expression of Hsp in human MSCs underwent SIPS in response to HS. We found that hyperthermia produced upregulation of Hsp27, Hsp70, Hsc70, and Hsp90 gene expression that returned to the initial level 24 h after the cell recovery, except for Hsp27 gene. At the protein level, we assayed the accumulation of the major heat shock protein Hsp70. The cells exhibited typical HSR: robust enhancement of Hsp70 but not Hsc70 expression and Hsp70 relocation from the cytoplasm into the nuclei. Elevated level of Hsp70 protein in MSCs induced by sublethal HS appeared to be regulated at the posttranslational level. Twenty-four hours after HS exposure, the expression of Hsp70 mRNA corresponded to the control level whereas increased Hsp70 accumulation failed to return to the initial level within, at least, 72 h. The accumulation of Hsp70 independent of gene expression was registered in cells challenged with antibodies to CD95 receptor (Concannon et al. 2005). All levels of Hsp70 gene regulation in response to heat stress have been the concern in the review (Silver and Noble 2012). The main function of Hsp70 is to protect cells against damage produced by various stress factors. The higher the Hsp70 content in cells, the higher is their viability under stressful conditions. Stress-vulnerable cells should have low Hsp70 amount. Indeed, Hsp70 content in apoptotic cells is very low (Samali et al. 1999; Lasunskaia et al. 2010). From this point of view, the cells that underwent SIPS might have low level of Hsp70. However, in our experiments, MSCs that underwent premature senescence have profoundly elevated Hsp70 content. Upregulation of Hsp70 expression induced in human fibroblasts by subtoxic doses of heavy metal salts which are potential SIPS inducers was maintained up to 7 days (Strub et al. 2008). On the other hand, Hsp70 level in human fibroblasts with premature senescence phenotype induced by oxidative stress did not noticeably change (Chen et al. 2004) or even decreased (Oh et al. 2008). Further experiments are required to clarify Hsp70 implication in SIPS.
Emerging evidence suggests that SIPS rather than apoptosis is a predominant response of human adult cells challenged with various stressors: hydrogen peroxide (Orciani et al 2010; Brandl et al. 2011a, b), ionizing radiation (Suzuki and Boothman 2008), gamma radiation (Cmielova et al. 2012), doxorubicin (Spallarossa et al. 2010), and tumor necrosis factor alpha (Zhang et al. 2009). It was generally accepted that SIPS is an important tumor suppression mechanism preventing the proliferation of cells that are at risk of malignant transformation. Recently, however, it has become apparent that this process entails more than a simple suppression of cell growth (Rodier and Campisi 2011). Cells that underwent SIPS irreversibly lose the capacity for proliferation; however, they remain metabolically active that may be beneficial or unfavorable for the surrounding cells both in vitro and in vivo. Mitomycin C- or irradiation-treated fibroblasts exhibiting SIPS phenotype are widely utilized as feeder cells for the propagation of embryonic stem cells. It was shown that liver damage in mice with carbon tetrachloride induced hepatic senescence of stellate cells in vivo. Secretion of extracellular matrix-degrading enzymes by these cells was enhanced that limited the extent of fibrosis following the drug application (Krizhanovsky et al. 2008). Myofibroblast senescence is considered as a programmed wound-healing response that functions as a self-limiting mechanism for fibrogenesis (Jun and Lau 2010).
The effect of heat stress on MSCs we observed may be of clinical relevance. There are evidences that MSCs (or their derivatives) are involved in cancer pathology (Karnoub et al. 2007). Tumor progression is strongly influenced by signals derived from its stroma. Hyperthermia (raising the temperature of tumor-loaded tissue) is a therapeutic procedure practical to cancer treatment. This approach is based on the fact that cancer cells are sensitive to elevated temperature. Although the mechanisms of hyperthermia treatment of cancer cells are under active study, there are very few studies regarding the effect of hyperthermia on the tumor-supportive stroma. It has been demonstrated that hyperthermia treatment of human MSCs produced antitumor effect. Cultivation of cancer cells in the conditioned medium collected from heated MSCs exerted a suppressive effect on tumor cell growth, suggesting that hyperthermia enables tumor stromal cells to provide a sensitizing environment for tumor cells (Cho et al. 2009). Further experiments are required to confirm these findings.
The important concern is the fate of cells that survived stress: whether their stemness is compromised or not. A few data are available on the progeny of stem cells subjected to sublethal insults. Adipose-derived human MSCs recovered after genotoxic damage resumed proliferation but underwent parental cell-intrinsic replicative senescence (Altanerova et al. 2009). MSC cultivation under improper conditions (serum deprivation, low oxygen, and especially long cell treatment with trypsin or collagenase) was exploited to enrich MSC populations by putative stem cells (Kuroda et al. 2010; Heneidi et al. 2013). These cells were called multilineage-differentiating stress-enduring (Muse) cells (Kuroda et al. 2010). We demonstrated previously that human embryonic stem cells that survived HS-induced apoptosis restored the initial pluripotent properties (Alekseenko et al. 2012). Here, we demonstrated that MSC progeny that tolerated heat shock exhibited properties of parental cells: expression of MSC markers, diploid set of chromosomes, replicative senescence, and capacity for multilineage differentiation. It is commonly accepted that stem cells are responsible for the damaged tissue repair. We showed experimentally that MSCs subjected to severe stress retained multipotent stem cell properties.
In conclusion, we demonstrated for the first time that in MSC, sublethal HS induced premature senescence rather than apoptosis. The cells that underwent SIPS display a high level of induced Hsp70 that failed to return to the basal content during 72 h. Equally, the novel finding here is that MSC progeny which survived sublethal heat stress retain the properties of parental cells: stemness and replicative senescence.
Acknowledgments
The work was supported by the Russian Foundation for Basic Research (project 11-04-12077), Program of Russian Academy of Sciences “Molecular and Cellular Biology,” and RF President Grant 4957.2012.4.
Conflict of interest
None
References
- Alekseenko LL, Zemelko VI, Zenin VV, Pugovkina NA, Kozhukharova IV, Kovaleva ZV, Grinchuk TM, Fridlyanskaya II, Nikolsky NN. Heat shock induces apoptosis in human embryonic stem cells but a premature senescence phenotype in their differentiated progeny. Cell Cycle. 2012;11:3260–3269. doi: 10.4161/cc.21595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allickson J, Xiang C. Human adult stem cells from menstrual blood and endometrial tissue. J Zhejiang Univ Sci B. 2012;13:419–420. doi: 10.1631/jzus.B1200062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altanerova V, Horvathova E, Matuskova M, Kucerova L, Altaner C. Genotoxic damage of human adipose-tissue derived mesenchymal stem cells triggers their terminal differentiation. Neoplasma. 2009;56:542–547. doi: 10.4149/neo_2009_06_542. [DOI] [PubMed] [Google Scholar]
- Bianco P, Robey PG, Simmons PJ. Mesenchymal stem cells: revisiting history, concepts, and assays. Cell Stem Cell. 2008;2:313–319. doi: 10.1016/j.stem.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bladier C, Wolvetang EJ, Hutchinson P, de Haan JB, Kola I. Response of a primary human fibroblast cell line to H2O2: senescence-like growth arrest or apoptosis? Cell Growth Differ. 1997;8:589–598. [PubMed] [Google Scholar]
- Brandl A, Hartmann A, Bechmann V, Graf B, Nerlich M, Angele P. Oxidative stress induces senescence in chondrocytes. J Orthop Res. 2011;29:1114–1120. doi: 10.1002/jor.21348. [DOI] [PubMed] [Google Scholar]
- Brandl A, Meyer M, Bechmann V, Nerlich M, Angele P. Oxidative stress induces senescence in human mesenchymal stem cells. Exp Cell Res. 2011;317:1541–1547. doi: 10.1016/j.yexcr.2011.02.015. [DOI] [PubMed] [Google Scholar]
- Brederlau A, Correia AS, Anisimov SV, Elmi M, Paul G, Roybon L, Morizane A, Bergquist F, et al. Transplantation of human embryonic stem cell-derived cells to a rat model of Parkinson's disease: effect of in vitro differentiation on graft survival and teratoma formation. Stem Cells. 2006;24:1433–1440. doi: 10.1634/stemcells.2005-0393. [DOI] [PubMed] [Google Scholar]
- Bruder SP, Jaiswal N, Haynesworth SE. Growth kinetics, self-renewal, and the osteogenic potential of purified human mesenchymal stem cells during extensive subcultivation and following cryopreservation. J Cell Biochem. 1997;64:278–294. doi: 10.1002/(SICI)1097-4644(199702)64:2<278::AID-JCB11>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Chen JH, Stoeber K, Kingsbury S, Ozanne SE, Williams GH, Hales CN. Loss of proliferative capacity and induction of senescence in oxidatively stressed human fibroblasts. J Biol Chem. 2004;279:49439–49446. doi: 10.1074/jbc.M409153200. [DOI] [PubMed] [Google Scholar]
- Chen J, Shi ZD, Ji X, Morales J, Zhang J, Kaur N, Wang S. Enhanced osteogenesis of human mesenchymal stem cells by periodic heat shock in self-assembling. Tissue Eng Part A. 2013;19:716–728. doi: 10.1089/ten.tea.2012.0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho JA, Park H, Kim HK, Lim EH, Seo SW, Choi JS, Lee KW. Hyperthermia-treated mesenchymal stem cells exert antitumor effects on human carcinoma cell line. Cancer. 2009;115:311–323. doi: 10.1002/cncr.24032. [DOI] [PubMed] [Google Scholar]
- Cmielova J, Havelek R, Soukup T, Jiroutová A, Visek B, Suchánek J, et al. Gamma radiation induces senescence in human adult mesenchymal stem cells from bone marrow and periodontal ligaments. Int J Radiat Biol. 2012;88:393–404. doi: 10.3109/09553002.2012.666001. [DOI] [PubMed] [Google Scholar]
- Concannon CG, Fitzgerald U, Holmberg CI, Szegezdi E, Sistonen L, Samali A. CD95-mediated alteration in Hsp70 levels is dependent on protein stabilization. Cell Stress Chaperones. 2005;10:59–65. doi: 10.1379/CSC-69R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006;119:2204–2213. doi: 10.1242/jcs.02932. [DOI] [PubMed] [Google Scholar]
- Dmitrieva RI, Minullina IR, Bilibina AA, Tarasova OV, Anisimov SV, Zaritskey AY. Bone marrow- and subcutaneous adipose tissue-derived mesenchymal stem cells: differences and similarities. Cell Cycle. 2012;11:377–383. doi: 10.4161/cc.11.2.18858. [DOI] [PubMed] [Google Scholar]
- Dolan EB, Haugh MG, Tallon D, Casey C, McNamara LM. Heat-shock-induced cellular responses to temperature elevations occurring during orthopaedic cutting. J R Soc Interface. 2012;9:3503–3513. doi: 10.1098/rsif.2012.0520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- Goligorsky MS, Chen J, Patschan S. Stress-induced premature senescence of endothelial cells: a perilous state between recovery and point of no return. Curr Opin Hematol. 2009;16:215–219. doi: 10.1097/MOH.0b013e32832a07bd. [DOI] [PubMed] [Google Scholar]
- Heneidi S, Simerman AA, Keller E, Singh P, Li X, et al. Awakened by сellular stress: isolation and characterization of a novel population of pluripotent stem cells derived from human adipose tissue. PLoS ONE. 2013;8(6):e64752. doi: 10.1371/journal.pone.0064752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hronik-Tupaj M, Rice WL, Cronin-Golomb M, Kaplan DL, Georgakoudi I. Osteoblastic differentiation and stress response of human mesenchymal stem cells exposed to alternating current electric fields. Biomed Eng Online. 2011;10:19. doi: 10.1186/1475-925X-10-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husein KS, Thiemermann C. Mesenchymal stromal cells: current understanding and clinical status. Stem Cells. 2010;28:585–596. doi: 10.1002/stem.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun JI, Lau LF. Cellular senescence controls fibrosis in wound healing. Aging. 2010;2:627–631. doi: 10.18632/aging.100201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, Richardson AL, Polyak K, Tubo R, Weinberg RA. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–563. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- Kim G, Meriin AB, Gabai VL, Christians E, Benjamin I, Wilson A, Wolozin B, Sherman MY. The heat shock transcription factor Hsf1 is downregulated in DNA damage-associated senescence, contributing to the maintenance of senescence phenotype. Aging Cell. 2012;11:617–627. doi: 10.1111/j.1474-9726.2012.00827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko E, Lee KY, Hwang DS. Human umbilical cord blood-derived mesenchymal stem cells undergo cellular senescence in response to oxidative stress. Stem Cells Dev. 2012;21:1877–1886. doi: 10.1089/scd.2011.0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krizhanovsky V, Yon M, Dickins RA, Hearn S, Simon J, Miething C, Yee H, Zender L, Lowe SW. Senescence of activated stellate cells limits liver fibrosis. Cell. 2008;34:657–667. doi: 10.1016/j.cell.2008.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda Y, Kitada M, Wakao S, Nishikawa K, et al. Unique multipotent cells in adult human mesenchymal cell populations. PNAS. 2010;107:8639–8643. doi: 10.1073/pnas.0911647107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasunskaia EB, Fridlianskaia II, Guzhova IV, Bozhkin VM, Margulis BA. Accumulation of major stress protein 70 kDa protects myeloid and lymphoid cells from death by apoptosis. Apoptosis. 1997;2:156–163. doi: 10.1023/A:1026460330596. [DOI] [PubMed] [Google Scholar]
- Lasunskaia EB, Fridlianskaia I, Arnholdt AV, Kanashiro M, Guzhova I, Margulis B. Sub-lethal heat shock induces plasma membrane translocation of 70-kDa heat shock protein in viable, but not in apoptotic, U-937 leukaemia cells. APMIS. 2010;118:179–187. doi: 10.1111/j.1600-0463.2009.02576.x. [DOI] [PubMed] [Google Scholar]
- Li S, Chien S, Brånemark PI. Heat shock-induced necrosis and apoptosis in osteoblasts. J Orthop Res. 1999;17:891–899. doi: 10.1002/jor.1100170614. [DOI] [PubMed] [Google Scholar]
- Liu SP, Ding DC, Wang HJ, Su CY, Lin SZ, Li H, Shyu WC. Nonsenescent Hsp27-upregulated MSCs implantation promotes neuroplasticity in stroke model. Cell Transplant. 2010;19:1261–1279. doi: 10.3727/096368910X507204. [DOI] [PubMed] [Google Scholar]
- Oh S, Lee E, Lee J, Lim Y, Kim J, Woo S. Comparison of the effects of 40% oxygen and two atmospheric absolute air pressure conditions on stress-induced premature senescence of normal human diploid fibroblasts. Cell Stress Chaperones. 2008;13:447–458. doi: 10.1007/s12192-008-0041-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orciani M, Gorbi S, Benedetti M, Di Benedetto G, Mattioli-Belmonte M, Regoli F, Di Primio R. Oxidative stress defense in human-skin-derived mesenchymal stem cells versus human keratinocytes: different mechanisms of protection and cell selection. Free Radic Biol Med. 2010;49:830–838. doi: 10.1016/j.freeradbiomed.2010.06.007. [DOI] [PubMed] [Google Scholar]
- Paul G, Ozen I, Christophersen NS, Reinbothe T, Bengzon J, et al. The adult human brain harbors multipotent perivascular mesenchymal stem cells. PLoS ONE. 2012;7(4):e35577. doi: 10.1371/journal.pone.0035577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pera MF, Reubinoff B, Trounson A. Human embryonic stem cells. J Cell Sci. 2000;113:5–10. doi: 10.1242/jcs.113.1.5. [DOI] [PubMed] [Google Scholar]
- Ramkisoensing AA, Pijnappels DA, Askar SFA, Passier R, Swildens J, et al. Human embryonic and fetal mesenchymal stem cells differentiate toward three different cardiac lineages in contrast to their adult counterparts. PLoS ONE. 2011;6(9):e24164. doi: 10.1371/journal.pone.0024164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restall IJ, Lorimer IAJ. Induction of premature senescence by Hsp90 inhibition in small cell lung cancer. PLoS ONE. 2010;5(6):e11076. doi: 10.1371/journal.pone.0011076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reubinoff BE, Pera MF, Fong CY, Trounson A, Bongso A. Embryonic stem cell lines from human blastocysts: somatic differentiation in vitro. Nat Biotechnol. 2000;18:399–404. doi: 10.1038/74447. [DOI] [PubMed] [Google Scholar]
- Rezai Rad M, Wise GE, Brooks H, Flanagan MB, Yao S. Activation of proliferation and differentiation of dental follicle stem cells (DFSCs) by heat stress. Cell Prolif. 2013;46:58–66. doi: 10.1111/cpr.12004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodier F, Campisi J. Four faces of cellular senescence. J Cell Biol. 2011;192:547–556. doi: 10.1083/jcb.201009094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samali A, Holmberg CI, Sistonen L, Orrenius S. Thermotolerance and cell death are distinct cellular responses to stress: dependence on heat shock proteins. FEBS Lett. 1999;461:306–310. doi: 10.1016/S0014-5793(99)01486-6. [DOI] [PubMed] [Google Scholar]
- Schwartz SD, Hubschman JP, Heilwell G, Franco-Cardenas V, Pan CK, Ostrick RM, Mickunas E, Gay R, Klimanskaya I, Lanza R. Embryonic stem cell trials for macular degeneration: a preliminary report. Lancet. 2012;379:713–720. doi: 10.1016/S0140-6736(12)60028-2. [DOI] [PubMed] [Google Scholar]
- Sherman M. Major heat shock protein Hsp72 controls oncogene-induced senescence. Ann N Y Acad Sci. 2010;1197:152–157. doi: 10.1111/j.1749-6632.2010.05196.x. [DOI] [PubMed] [Google Scholar]
- Shui C, Scutt A. Mild heat shock induces proliferation, alkaline phosphatase activity, and mineralization in human bone marrow stromal cells and Mg-63. Cells In Vitro J Bone Miner Res. 2001;16:731–741. doi: 10.1359/jbmr.2001.16.4.731. [DOI] [PubMed] [Google Scholar]
- Silver JT, Noble TG. Regulation of survival gene hsp70. Cell Stress Chaperones. 2012;1:1–9. doi: 10.1007/s12192-011-0290-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolov M, Neumann R. Lessons learned about human stem cell responses to ionizing radiation exposures: a long road still head of us. Int J Mol Sci. 2013;14:15695–15723. doi: 10.3390/ijms140815695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spallarossa P, Altieri P, Barisione C, Passalacqua M, et al. p38 MAPK and JNK antagonistically control senescence and cytoplasmic p16INK4A expression in doxorubicin-treated endothelial progenitor cells. PLoS ONE. 2010;5(12):e15583. doi: 10.1371/journal.pone.0015583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer TR. Engraftment syndrome following hematopoietic stem cell transplantation. Bone Marrow Transplant. 2001;27:893–898. doi: 10.1038/sj.bmt.1703015. [DOI] [PubMed] [Google Scholar]
- Stolberg S, McCloskey KE. Can shear stress direct stem cell fate? Biotechnol Progress. 2009;25:10–19. doi: 10.1002/btpr.124. [DOI] [PubMed] [Google Scholar]
- Stolzing A, Jones E, McGonagle D, Scutt A. Age-related changes in human bone marrow-derived mesenchymal stem cells: consequences for cell therapies. Mech Ageing Dev. 2008;129:163–173. doi: 10.1016/j.mad.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Strub GM, Depcrynski A, Elmore LW, Holt SE. Recovery from stress is a function of age and telomere length. Cell Stress Chaperones. 2008;4:475–482. doi: 10.1007/s12192-008-0047-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Boothman DA. Stress-induced premature senescence (SIPS)—influence of SIPS on radiotherapy. J Radiat Res. 2008;49:105–112. doi: 10.1269/jrr.07081. [DOI] [PubMed] [Google Scholar]
- Toussaint O, Medrano EE, von Zglinicki T. Cellular and molecular mechanisms of stress-induced premature senescence (SIPS) of human diploid fibroblasts and melanocytes. Exp Gerontol. 2000;35:927–945. doi: 10.1016/S0531-5565(00)00180-7. [DOI] [PubMed] [Google Scholar]
- Tower J. Stress and stem cells. Wiley Interdiscip Rev Dev Biol. 2012;1:789–802. doi: 10.1002/wdev.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T, Suzuki T, Arai H, Tanabe S, Atomi Y. Continuous mild heat stress induces differentiation of mammalian myoblasts, shifting fiber type from fast to slow. Am J Physiol Cell Physiol. 2010;298:140–148. doi: 10.1152/ajpcell.00050.2009. [DOI] [PubMed] [Google Scholar]
- Yao S, Gutierrez DL, He H, Dai Y, Liu D, Wise GE. Proliferation of dental follicle-derived cell populations in heat-stress conditions. Cell Prolif. 2011;44:486–493. doi: 10.1111/j.1365-2184.2011.00778.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemelko VI, Grinchuk TM, Domnina AP, Artzibasheva IV, Zenin VV, Kirsanov AA, Bichevaia NK, Korsak VS, Nikolsky NN. Multipotent mesenchymal stem cells of desquamated endometrium: isolation, characterization, and application as a feeder layer for maintenance of human embryonic stem cells. Tsitologiia. 2011;53:919–929. [PubMed] [Google Scholar]
- Zhang Y, Herbert BS, Rajashekhar G, Ingram DA, Yoder MC, Clauss M, Rehman J. Premature senescence of highly proliferative endothelial progenitor cells is induced by tumor necrosis factor-alpha via the p38 mitogen-activated protein kinase pathway. FASEB J. 2009;23:1358–1365. doi: 10.1096/fj.08-110296. [DOI] [PMC free article] [PubMed] [Google Scholar]