Abstract
The localization of yellow fluorescent protein (YFP)-tagged HSP70 proteins was employed to identify stress-sensitive sites in human neurons following temperature elevation. Stable lines of human SH-SY5Y neuronal cells were established that expressed YFP-tagged protein products of the human inducible HSP70 genes HSPA6 (HSP70B′) and HSPA1A (HSP70-1). Following a brief period of thermal stress, YFP-tagged HSPA6 and HSPA1A rapidly appeared at centrioles in the cytoplasm of human neuronal cells, with HSPA6 demonstrating a more prolonged signal compared to HSPA1A. Each centriole is composed of a distal end and a proximal end, the latter linking the centriole doublet. The YFP-tagged HSP70 proteins targeted the proximal end of centrioles (identified by γ-tubulin marker) rather than the distal end (centrin marker). Centrioles play key roles in cellular polarity and migration during neuronal differentiation. The proximal end of the centriole, which is involved in centriole stabilization, may be stress-sensitive in post-mitotic, differentiating human neurons.
Keywords: HSPA6 (HSP70B′), HSPA1A (HSP70-1), SH-SY5Y human neuronal cells, Centrioles, Heat shock
Introduction
Manipulation of the cellular stress response, involving the induction of heat shock proteins (HSPs), has been proposed as a potential therapeutic strategy to combat changes in neural proteins which trigger pathogenic cascades resulting in neurodegenerative diseases (Muchowski and Wacker 2005; Asea and Brown 2008; Ali et al. 2010; Gestwicki and Garza 2012). HSPs are protein repair agents that provide a line of defense against misfolded, aggregation-prone proteins (Muchowski and Wacker 2005; Brown 2007; Kim et al. 2013). As average life expectancy increases, neurodegenerative diseases have become a major problem in the human population; hence, the development of effective treatments and preventive measures is imperative (Asea and Brown 2008; Di Carlo et al. 2012; Murman 2012; Chow et al. 2013). Animal models of neurodegenerative diseases have been constructed in order to investigate the molecular mechanisms of these debilitating neural disorders and to develop potential therapeutic strategies (Hirsch 2007; Phillips et al. 2009; Avila et al. 2011).
HSP70 is a multigene family that includes the stress-inducible members HSPA6 (HSP70B′) and HSPA1A (HSP70-1) (Tavaria et al. 1996; Daugaard et al. 2007; Brocchieri et al. 2008; Kampinga et al. 2009). The human genome includes stress-inducible HSPA6 which is not found in the mouse and rat genome; hence, it is not present as a potential beneficial factor in animal models of neurodegenerative diseases to counter misfolded proteins (Chow and Brown 2007; Noonan et al. 2007a, 2008a). It has been suggested that these HSP70 family members could exhibit differences in their functions (Daugaard et al. 2007; Hageman et al. 2011). HSP70 has been widely studied in the literature (Kiang and Tsokos 1998; Evans et al. 2010; Young 2010). However, information on the cellular expression of HSPA6 is limited with reports on human colon cancer cells (Noonan et al. 2007a, b; 2008a, b) and human macrophages (Smith et al. 2010). In the field of neuroscience, expression of HSPA6 has been studied in our laboratory using human SH-SY5Y neuronal cells (Chow and Brown 2007; Chow et al. 2010).
In the present report, we investigate localization of the yellow fluorescent protein (YFP)-tagged protein products of the HSPA6 and HSPA1A genes following thermal stress in order to identify stress-sensitive “hot spots” in post-mitotic human neuronal cells. Our results indicate that YFP-tagged HSPA6 and HSPA1A rapidly localize to centrioles. These structures play important roles in cellular polarity and migration during neuronal differentiation (Tsai and Gleeson 2005; Higginbotham and Gleeson 2007; de Anda et al. 2010; de Anda and Tsai 2011).
Results and discussion
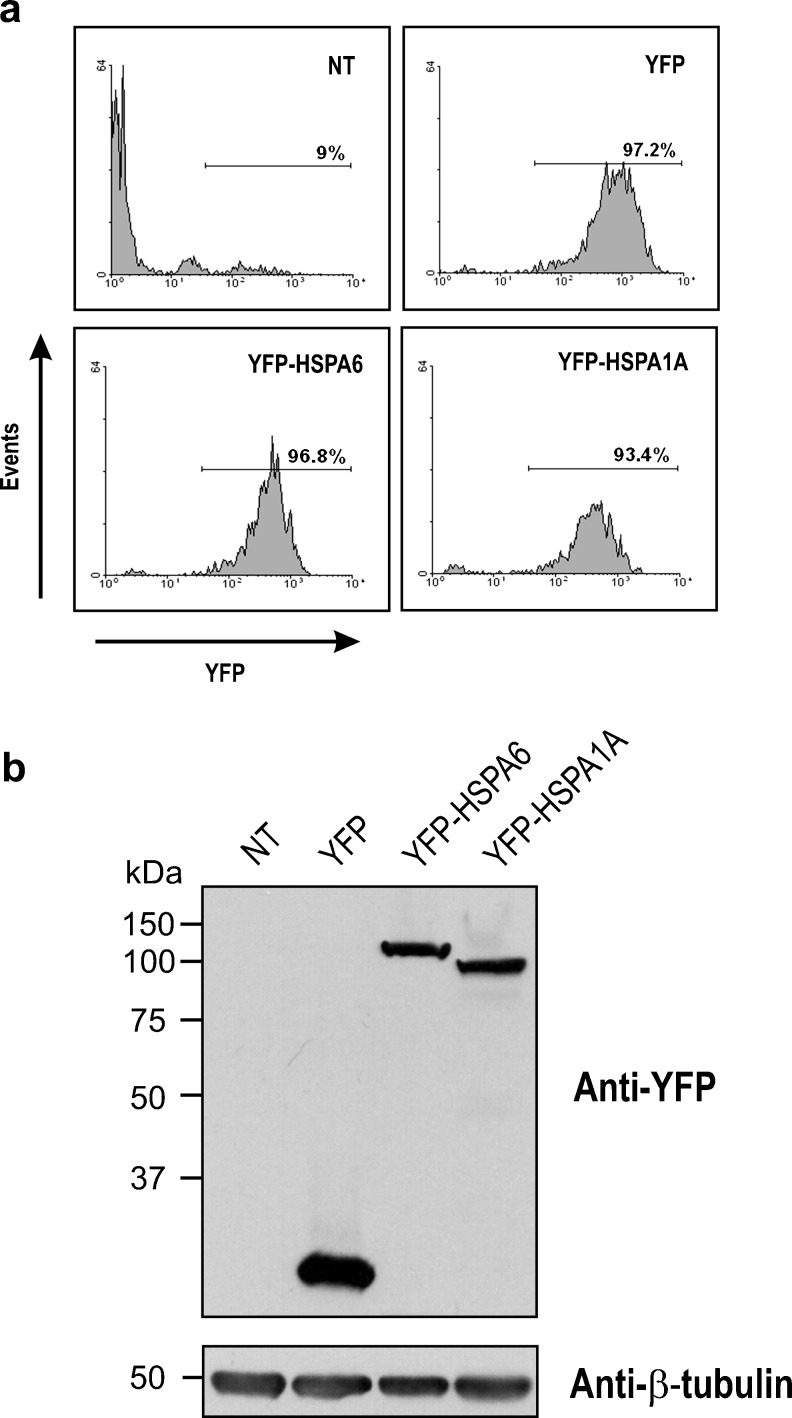
In order to investigate the localization of HSPA6 (HSP70B′) and HSPA1A (HSP70-1) proteins in human neuronal cells following thermal stress, a strong detectable marker, namely, an enhanced YFP was fused to the N terminus. Transfected cells were selected for transgene expression, subjected to fluorescence activated cell sorting, and stable cell lines generated (Fig. 1a). Western blot analysis demonstrated that cell lines expressing YFP-tagged HSPA6, YFP-tagged HSPA1A, and YFP were obtained (Fig. 1b).
Fig. 1.
Characterization of human neuronal cell lines stably expressing YFP-tagged HSPA6 (HSP70B′) and YFP-tagged HSPA1A (HSP70-1). a Stable cell lines expressing YFP-tagged proteins obtained by fluorescence activated cell sorting. The coding region of human HSPA1A was derived from a previously reported HSPA1A construct [kind gift from Dr. R. L. Anderson, Peter MacCallum Cancer Centre, Melbourne, Australia; (Chow et al. 2009)]. The coding region of HSPA6 was purchased from RZPD (Berlin, Germany). These coding regions were cloned into the pEYFP-C1 plasmid (Clontech, Palo Alto, CA, USA) fused in-frame with enhanced YFP at the N terminus. The human SH-SY5Y cell line (American Type Culture Collection, Manassas, VA, USA) was maintained in Dulbecco’s modified Eagle’s medium supplemented with 10 % fetal bovine serum and cultured at 37 °C in a humidified 5 % CO2 atmosphere. SH-SY5Y cells constitutively expressing YFP-HSPA6 or YFP-HSPA1A were generated by transfection with the respective YFP fusion construct using Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. Transfected cells were selected with 500 μg/ml G418 (Sigma, St Louis, MO, USA) for 6 days. Stable SH-SY5Y cell lines expressing YFP-HSPA6, YFP-HSPA1A, and YFP proteins were then generated by fluorescence activated cell sorting employing a FACSAria cell sorter (Becton Dickinson, Mississauga, ON, Canada) based on comparable YFP fluorescence levels. b Western blot of neuronal cell lines expressing YFP-HSPA6, YFP-HSPA1A, and YFP proteins. NT non-transfected. SH-SY5Y cells were harvested, solubilized in Laemmli buffer, boiled for 20 min, and Lowry assays performed for protein quantification. Equal loadings of 30 μg protein per lane were separated by 12 % SDS-PAGE using the Mini-PROTEAN 3 Electrophoresis Module Assembly (Bio-Rad Laboratories, Hercules, CA, USA) with a stacking gel of 4 % using the standard buffer system of Laemmli before transfer to nitrocellulose membranes. Western blotting was performed with antibodies to YFP (clone JL-8, Clontech) for detection of fusion proteins and β-tubulin (MAB3408, Chemicon, Temecula, CA, USA) as loading control. Horseradish peroxidase conjugated secondary antibodies (Sigma) were detected by enhanced chemiluminescence assay (Amersham, Piscataway, NJ, USA). Western blots representative of three experimental repeats are shown
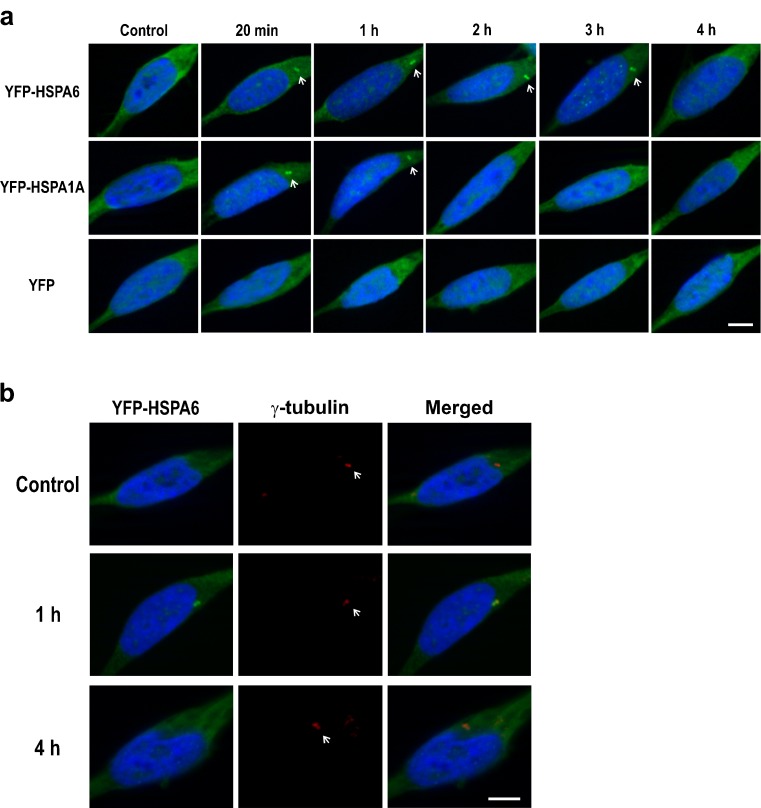
Immediately following thermal stress at 43 °C for 20 min, YFP-tagged HSPA6 and HSPA1A were detected in prominent structures resembling centrioles in the cytoplasm of post-mitotic, differentiating human neurons (Fig. 2a). In controls, the YFP-tagged proteins were diffused throughout the cytoplasm. YFP-HSPA1A was present at putative centrioles at the 1-h time point and not detectable at 3 h. In contrast, YFP-HSPA6 persisted for longer time periods after thermal stress and was still apparent at 3 h. As shown in Fig. 2b, the cytoplasmic structure that was positive for YFP-tagged HSPA6 aligned with the signal of a centriole marker. The appearance of HSPA6 at centrioles was rapid but transient after thermal stress, as signal was not present at 4 h (Fig. 2a, b). The centrioles were still present at 4 h as evidenced by the centriole marker (Fig. 2b).
Fig. 2.
YFP-HSPA6 and YFP-HSPA1A at centrioles in human neuronal cells following thermal stress. a Time course of YFP-HSP70 proteins at putative centrioles after heat shock at 43 °C for 20 min and recovery at 37 °C. White arrows show YFP-HSPA6 at putative centrioles for longer time periods compared to YFP-HSPA1A. b YFP-HSPA6 positive cytoplasmic structures aligned with the signal of a centriole marker. Scale bar represents 5 μm. Differentiation of human neuronal SH-SY5Y cells, plated at 3.5 × 104 cells per cm2, was induced by treatment with 10 μM all-trans-retinoic acid in serum free media and incubation at 37 °C for 72 h. Cells were then heat shocked under serum free conditions by immersion in a circulating water bath calibrated at 43 °C ± 0.1 °C for 20 min, returned to incubation at 37 °C, and harvested at the indicated time points, with time zero being the commencement of the heat shock at 43 °C. At the indicated time points, cells were fixed with 4 % paraformaldehyde in phosphate buffered saline (PBS; pH 7.4) at room temperature for 30 min. Cells were then permeabilized with 0.1 % Triton X-100 in PBS containing 100 mM glycine for 30 min, washed, and blocked with 5 % fetal bovine serum (FBS) in PBS for 2 h. Incubation with primary antibodies was performed in 1 % FBS in PBS overnight. Cells were then washed and incubated with fluorescently labeled secondary antibodies before mounting and imaging by structural illumination using an AxioCam HRm camera with an ApoTome module on an AxioVert 200 M microscope (Carl Zeiss, Toronto, ON, Canada). Primary antibody against γ-tubulin (11–543; Exbio, Prague, Czech Republic) was employed in combination with donkey anti-mouse Alexa-Fluor 647 secondary antibody (Invitrogen). DAPI (300 nM) (Invitrogen) was used as a counter stain for nuclei
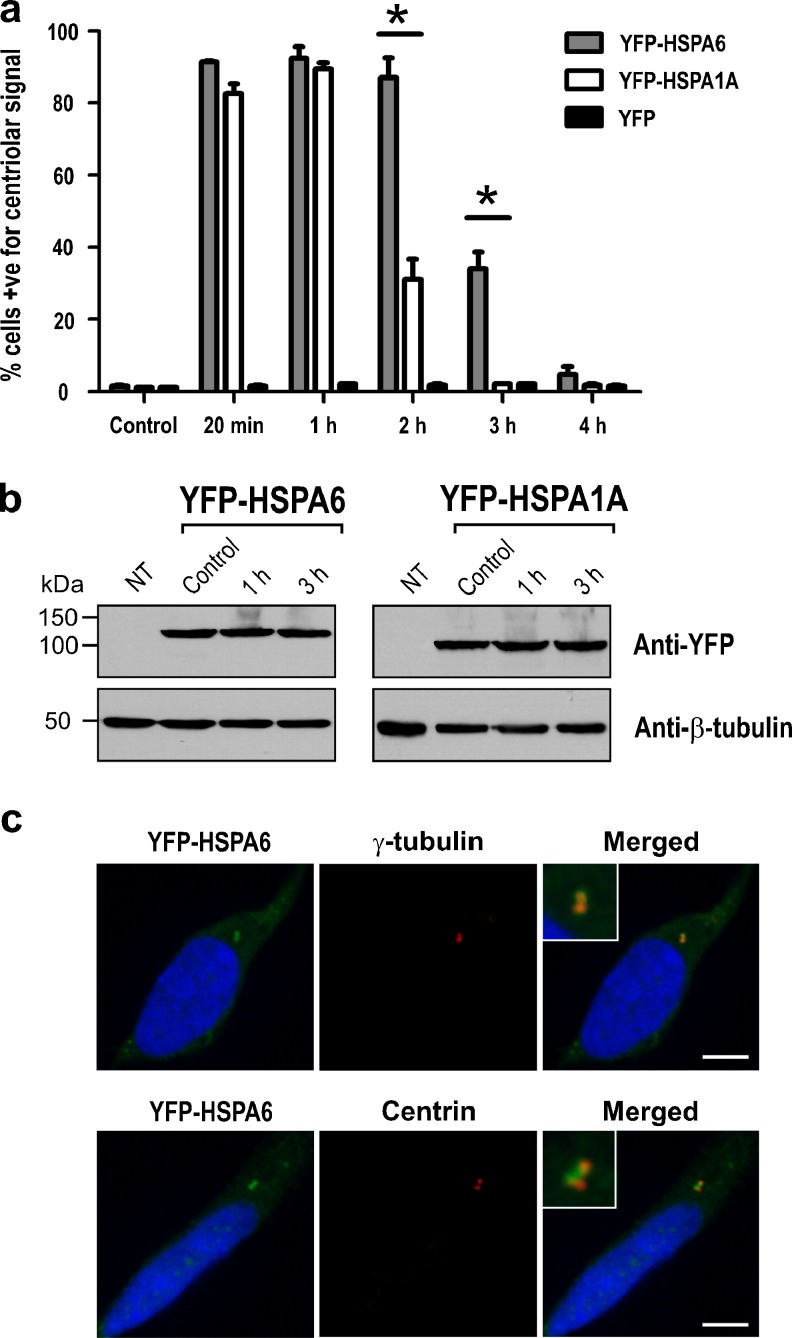
The observation of a prolonged YFP-HSPA6 signal at centrioles was confirmed by quantification as shown in Fig. 3a. At 2 h, 87 % of the neuronal cells in the YFP-HSPA6 line were positive for a signal in centrioles, whereas only 31 % were positive in the YFP-HSPA1A cell line. At 3 h, the percentage of cells positive for a centriole signal was 34 and 2 %, respectively. Analysis of neuronal proteins isolated at time points after thermal stress indicated that levels of YFP-HSPA6 and YFP-HSPA1A did not change, suggesting that the prolonged YFP-HSPA6 signal at centrioles compared to that of YFP-HSPA1A was not due to degradation of YFP-HSPA1A (Fig. 3b).
Fig. 3.
Prolonged YFP-HSPA6 at the proximal end of centrioles. a Quantification of cells positive for a centriolar signal in YFP-HSPA6, YFP-HSPA1A, and YFP transfected cells. For each time point, 200 cells were sampled and the average counts of three independent experiments used for statistical analysis. Data were expressed as the mean ± standard error of the means. Two-way analysis of variance followed by Bonferroni test for pair-wise comparison of means was used to assess significant differences (*p < 0.05). Both YFP-HSP70 proteins appear rapidly at centrioles; however, YFP-HSPA6 remained at centrioles for up to 3 h, whereas YFP-HSPA1A did not. b Western blot analysis of YFP-HSPA6 and YFP-HSPA1A levels at time points following thermal stress. Prolonged YFP-HSPA6 at centrioles compared to that of YFP-HSPA1A was not due to degradation of YFP-HSPA1A. NT non-transfected. c Localization of YFP-HSPA6 to the proximal end of centrioles. YFP-HSPA6 colocalized with γ-tubulin, a marker of the proximal end of centrioles and not with centrin (Cat. no. 04-1624; Millipore, Billerica, MA, USA), a marker of the distal end. Scale bar represents 5 μm
Each component of the doublet centriole has a distal and a proximal end (Azimzadeh and Marshall 2010; Bornens 2012). The localization of YFP-tagged proteins was next investigated at higher magnification using marker proteins of either the distal (centrin) or the proximal end (γ-tubulin) of the centriole (Bornens 2002; Brito et al. 2012). YFP-HSPA6 colocalized with γ-tubulin rather than centrin (Fig. 3c), suggesting that YFP-HSPA6 was associated with the proximal but not the distal end of the centriole. Similar results were obtained for YFP-HSPA1A (data not shown).
Centrosomes play key roles in cellular polarity and migration during neuronal differentiation (Tsai and Gleeson 2005; Higginbotham and Gleeson 2007; de Anda et al. 2010; de Anda and Tsai 2011). These structures have also been implicated in neurodegenerative diseases (Bornens 2002; Badano et al. 2005; Diaz-Corrales et al. 2005, 2011; Bradshaw et al. 2008; Kuijpers and Hoogenraad 2011). Centrosomes are composed of two perpendicular barrel-shaped microtubule-based cylinders termed “centrioles” surrounded by pericentriolar material (Bornens 2002, 2012; Azimzadeh and Bornens 2007; Bettencourt-Dias and Glover 2007; Azimzadeh and Marshall 2010; Nigg and Stearns 2011; Brito et al. 2012; Gonczy 2012).
In the present study, rapid localization of YFP-HSPA6 and YFP-HSPA1A to centrioles in the cytoplasm of post-mitotic, differentiating SH-SY5Y human neuronal cells was observed following thermal stress, with YFP-HSPA6 demonstrating a more prolonged association. During in vivo development, young neurons go through a bipolar stage that is critical for their maturation (Nadarajah et al. 2001; LoTurco and Bai 2006; Barnes and Polleux 2009). After treatment with retinoic acid, SH-SY5Y cells appeared as bipolar cells with extended neural cellular processes. In the literature, studies on the localization of HSP70 proteins have focused on dividing cells, regarding the centrosome as an entity rather than individual centrioles with proximal and distal ends (Brown et al. 1996; Hut et al. 2005; Scieglinska et al. 2008). The present study demonstrates that HSP70 proteins localize to the proximal rather than the distal ends of centrioles in post-mitotic, differentiating human neurons following thermal stress.
Centrioles are polar structures that exhibit structural and functional differences at their distal and proximal ends. The distal end is involved in microtubule nucleation, whereas the proximal end has a fibrous network that connects the two centrioles and also a cartwheel structure that forms the assembly and stabilizing base of the barrel-shaped centrioles (Bornens 2002, 2012; Azimzadeh and Bornens 2007; Azimzadeh and Marshall 2010). Interestingly, proteins at the proximal end of the centriole have been implicated in the formation of the primary cilium (Molla-Herman et al. 2008), a stress-sensitive antennae-like structure that plays essential roles in the regulation of sensory and signaling systems during neurogenesis (Breunig et al. 2008; Spassky et al. 2008; Baudoin et al. 2012; Prodromou et al. 2012). Dysfunction of the primary cilium leads to neurological disorders termed “ciliopathies” that have pronounced effects on neural development (Green and Mykytyn 2010; Lee and Gleeson 2011; Louvi and Grove 2011).
Localization of HSP70 proteins to the proximal end of the centriole in differentiating human neurons suggests that this cytoplasmic structure is a stress-sensitive hot spot, and that key proteins in the cellular stress response may play roles in protecting it against stress-induced damage during neuronal differentiation.
Neurogenesis occurs during early development of the brain but also throughout adult life (Lindsey and Tropepe 2006; Ming and Song 2011; Kempermann 2012). Protection of neurogenesis through HSP70-mediated reinforcement of centrioles could be beneficial in the treatment of neurodegenerative diseases, which are characterized by neuronal loss (Mehler and Gokhan 2000; Culmsee and Landshamer 2006). It has been noted that aberrations in centrosomal proteins are linked to brain disorders (Badano et al. 2005; Diaz-Corrales et al. 2005, 2011; Bradshaw et al. 2008; Kuijpers and Hoogenraad 2011).
HSPA6 is present in the human genome; however, it is not found in mouse and rat (Chow and Brown 2007; Noonan et al. 2007a, 2008a). Hence, a component of a centriole defense mechanism in the human brain could be missing in current animal models of human neurodegenerative diseases. Evolution of the very large human brain imposes a high demand on neuronal migration during development (Letinic and Rakic 2001; Rao and Wu 2001). Much greater distances must be traversed in the human brain compared to the rodent brain, as differentiating neurons migrate to their functional sites in the nervous system. In addition, a pathway for neuronal migration has been reported in the human brain that is not present in other mammals (Letinic and Rakic 2001; Rao and Wu 2001; Clowry et al. 2010). This suggests that neuronal migration has played a key role in the evolution of the human brain and its development. The presence in human of HSPA6 that rapidly localizes to the proximal end of centrioles following cellular stress, and resides there longer than HSPA1A, could provide critical benefits to buffering neuronal migration from cellular stress in the human brain. Differences between HSPA6 and HSPA1A have been noted at the N-terminal ATPase domain (Hageman et al. 2011) and the C-terminal alpha helical lid region (Zhu et al. 1996; Noonan et al. 2008a), potentially leading to variation in co-chaperone interaction and regulation of substrate binding kinetics. This difference may relate to the prolonged residence of HSPA6 at the centriole. Furthermore, HSPA6 was found to exhibit specificity for the client protein p53 (Hageman et al. 2011), which has been shown to localize to the centrosome (Tritarelli et al. 2004; Ma et al. 2006).
Acknowledgments
This work was supported by grants from NSERC Canada to I.R.B. who also holds a Canada Research Chair (Tier I) in Neuroscience.
References
- Ali YO, Kitay BM, Zhai RG. Dealing with misfolded proteins: examining the neuroprotective role of molecular chaperones in neurodegeneration. Molecules. 2010;15:6859–6887. doi: 10.3390/molecules15106859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asea AA, Brown IR. Heat shock proteins and the brain: implications for neurodegenerative diseases and neuroprotection. New York: Springer; 2008. [Google Scholar]
- Avila J, Lucas JJ, Hernandez F, editors. Animal models for neurodegenerative disease. Cambridge: RSC; 2011. [Google Scholar]
- Azimzadeh J, Bornens M. Structure and duplication of the centrosome. J Cell Sci. 2007;120:2139–2142. doi: 10.1242/jcs.005231. [DOI] [PubMed] [Google Scholar]
- Azimzadeh J, Marshall WF. Building the centriole. Curr Biol. 2010;20:R816–R825. doi: 10.1016/j.cub.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badano JL, Teslovich TM, Katsanis N. The centrosome in human genetic disease. Nat Rev Genet. 2005;6:194–205. doi: 10.1038/nrg1557. [DOI] [PubMed] [Google Scholar]
- Barnes AP, Polleux F. Establishment of axon–dendrite polarity in developing neurons. Ann Rev Neurosci. 2009;32:347–381. doi: 10.1146/annurev.neuro.31.060407.125536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudoin JP, Viou L, Launay PS, Luccardini C, Espeso Gil S, Kiyasova V, Irinopoulou T, Alvarez C, Rio JP, Boudier T, Lechaire JP, Kessaris N, Spassky N, Metin C. Tangentially migrating neurons assemble a primary cilium that promotes their reorientation to the cortical plate. Neuron. 2012;76:1108–1122. doi: 10.1016/j.neuron.2012.10.027. [DOI] [PubMed] [Google Scholar]
- Bettencourt-Dias M, Glover DM. Centrosome biogenesis and function: centrosomics brings new understanding. Nat Rev Mol Cell Biol. 2007;8:451–463. doi: 10.1038/nrm2180. [DOI] [PubMed] [Google Scholar]
- Bornens M. Centrosome composition and microtubule anchoring mechanisms. Curr Opin Cell Biol. 2002;14:25–34. doi: 10.1016/S0955-0674(01)00290-3. [DOI] [PubMed] [Google Scholar]
- Bornens M. The centrosome in cells and organisms. Science. 2012;335:422–426. doi: 10.1126/science.1209037. [DOI] [PubMed] [Google Scholar]
- Bradshaw NJ, Ogawa F, Antolin-Fontes B, Chubb JE, Carlyle BC, Christie S, Claessens A, Porteous DJ, Millar JK. DISC1, PDE4B, and NDE1 at the centrosome and synapse. Biochem Biophys Res Commun. 2008;377:1091–1096. doi: 10.1016/j.bbrc.2008.10.120. [DOI] [PubMed] [Google Scholar]
- Breunig JJ, Sarkisian MR, Arellano JI, Morozov YM, Ayoub AE, Sojitra S, Wang B, Flavell RA, Rakic P, Town T. Primary cilia regulate hippocampal neurogenesis by mediating sonic hedgehog signaling. Proc Natl Acad Sci U S A. 2008;105:13127–13132. doi: 10.1073/pnas.0804558105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito DA, Gouveia SM, Bettencourt-Dias M. Deconstructing the centriole: structure and number control. Curr Opin Cell Biol. 2012;24:4–13. doi: 10.1016/j.ceb.2012.01.003. [DOI] [PubMed] [Google Scholar]
- Brocchieri L, de Conway Macario E, Macario AJ. Hsp70 genes in the human genome: conservation and differentiation patterns predict a wide array of overlapping and specialized functions. BMC Evol Biol. 2008;8:19. doi: 10.1186/1471-2148-8-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown IR. Heat shock proteins and protection of the nervous system. Ann N Y Acad Sci. 2007;1113:147–158. doi: 10.1196/annals.1391.032. [DOI] [PubMed] [Google Scholar]
- Brown CR, Hong-Brown LQ, Doxsey SJ, Welch WJ. Molecular chaperones and the centrosome. A role for HSP 73 in centrosomal repair following heat shock treatment. J Biol Chem. 1996;271:833–840. doi: 10.1074/jbc.271.2.833. [DOI] [PubMed] [Google Scholar]
- Chow AM, Brown IR. Induction of heat shock proteins in differentiated human and rodent neurons by celastrol. Cell Stress Chaperones. 2007;12:237–244. doi: 10.1379/CSC-269.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow AM, Steel R, Anderson RL. Hsp72 chaperone function is dispensable for protection against stress-induced apoptosis. Cell Stress Chaperones. 2009;14:253–263. doi: 10.1007/s12192-008-0079-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow AM, Mok P, Xiao D, Khalouei S, Brown IR. Heteromeric complexes of heat shock protein 70 (HSP70) family members, including Hsp70B′, in differentiated human neuronal cells. Cell Stress Chaperones. 2010 doi: 10.1007/s12192-009-0167-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow AM, Tang DW, Hanif A, Brown IR. Induction of heat shock proteins in cerebral cortical cultures by celastrol. Cell Stress Chaperones. 2013;18:155–160. doi: 10.1007/s12192-012-0364-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clowry G, Molnar Z, Rakic P. Renewed focus on the developing human neocortex. J Anat. 2010;217:276–288. doi: 10.1111/j.1469-7580.2010.01281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culmsee C, Landshamer S. Molecular insights into mechanisms of the cell death program: role in the progression of neurodegenerative disorders. Curr Alzheimer Res. 2006;3:269–283. doi: 10.2174/156720506778249461. [DOI] [PubMed] [Google Scholar]
- Daugaard M, Rohde M, Jaattela M. The heat shock protein 70 family: highly homologous proteins with overlapping and distinct functions. FEBS Lett. 2007;581:3702–3710. doi: 10.1016/j.febslet.2007.05.039. [DOI] [PubMed] [Google Scholar]
- de Anda FC, Tsai LH. Axon selection: from a polarized cytoplasm to a migrating neuron. Commun Integr Biol. 2011;4:304–307. doi: 10.4161/cib.4.3.14781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Anda FC, Meletis K, Ge X, Rei D, Tsai LH. Centrosome motility is essential for initial axon formation in the neocortex. J Neurosci. 2010;30:10391–10406. doi: 10.1523/JNEUROSCI.0381-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Carlo M, Giacomazza D, San Biagio PL. Alzheimer’s disease: biological aspects, therapeutic perspectives and diagnostic tools. J Phys Condens Matter. 2012;24:244102. doi: 10.1088/0953-8984/24/24/244102. [DOI] [PubMed] [Google Scholar]
- Diaz-Corrales FJ, Asanuma M, Miyazaki I, Miyoshi K, Ogawa N. Rotenone induces aggregation of gamma-tubulin protein and subsequent disorganization of the centrosome: relevance to formation of inclusion bodies and neurodegeneration. Neuroscience. 2005;133:117–135. doi: 10.1016/j.neuroscience.2005.01.044. [DOI] [PubMed] [Google Scholar]
- Diaz-Corrales FJ, Miyazaki I, Asanuma M, Ruano D, Rios RM. Centrosomal aggregates and Golgi fragmentation disrupt vesicular trafficking of DAT. Neurobiol Aging. 2011;33:2462–2477. doi: 10.1016/j.neurobiolaging.2011.11.014. [DOI] [PubMed] [Google Scholar]
- Evans CG, Chang L, Gestwicki JE. Heat shock protein 70 (hsp70) as an emerging drug target. J Med Chem. 2010;53:4585–4602. doi: 10.1021/jm100054f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gestwicki JE, Garza D. Protein quality control in neurodegenerative disease. Prog Mol Biol Transl Sci. 2012;107:327–353. doi: 10.1016/B978-0-12-385883-2.00003-5. [DOI] [PubMed] [Google Scholar]
- Gonczy P. Towards a molecular architecture of centriole assembly. Nat Rev Mol Cell Biol. 2012;13:425–435. doi: 10.1038/nrm3373. [DOI] [PubMed] [Google Scholar]
- Green JA, Mykytyn K. Neuronal ciliary signaling in homeostasis and disease. Cell Mol Life Sci. 2010;67:3287–3297. doi: 10.1007/s00018-010-0425-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hageman J, van Waarde MA, Zylicz A, Walerych D, Kampinga HH. The diverse members of the mammalian HSP70 machine show distinct chaperone-like activities. Biochem J. 2011;435:127–142. doi: 10.1042/BJ20101247. [DOI] [PubMed] [Google Scholar]
- Higginbotham HR, Gleeson JG. The centrosome in neuronal development. Trends Neurosci. 2007;30:276–283. doi: 10.1016/j.tins.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Hirsch EC (2007) Animal models in neurodegenerative diseases. J Neural Transm Suppl:87–90. doi:10.1007/978-3-211-73574-9_11 [DOI] [PubMed]
- Hut HM, Kampinga HH, Sibon OC. Hsp70 protects mitotic cells against heat-induced centrosome damage and division abnormalities. Mol Biol Cell. 2005;16:3776–3785. doi: 10.1091/mbc.E05-01-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampinga HH, Hageman J, Vos MJ, Kubota H, Tanguay RM, Bruford EA, Cheetham ME, Chen B, Hightower LE. Guidelines for the nomenclature of the human heat shock proteins. Cell Stress Chaperones. 2009;14:105–111. doi: 10.1007/s12192-008-0068-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G. New neurons for ‘survival of the fittest’. Nat Rev Neurosci. 2012;13:727–736. doi: 10.1038/nrn3319. [DOI] [PubMed] [Google Scholar]
- Kiang JG, Tsokos GC. Heat shock protein 70 kDa: molecular biology, biochemistry, and physiology. Pharmacol Ther. 1998;80:183–201. doi: 10.1016/S0163-7258(98)00028-X. [DOI] [PubMed] [Google Scholar]
- Kim YE, Hipp MS, Bracher A, Hayer-Hartl M, Ulrich Hartl F. Molecular chaperone functions in protein folding and proteostasis. Annu Rev Biochem. 2013;82:323–355. doi: 10.1146/annurev-biochem-060208-092442. [DOI] [PubMed] [Google Scholar]
- Kuijpers M, Hoogenraad CC. Centrosomes, microtubules and neuronal development. Mol Cell Neurosci. 2011;48:349–358. doi: 10.1016/j.mcn.2011.05.004. [DOI] [PubMed] [Google Scholar]
- Lee JE, Gleeson JG. Cilia in the nervous system: linking cilia function and neurodevelopmental disorders. Curr Opin Neurol. 2011;24:98–105. doi: 10.1097/WCO.0b013e3283444d05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letinic K, Rakic P. Telencephalic origin of human thalamic GABAergic neurons. Nat Neurosci. 2001;4:931–936. doi: 10.1038/nn0901-931. [DOI] [PubMed] [Google Scholar]
- Lindsey BW, Tropepe V. A comparative framework for understanding the biological principles of adult neurogenesis. Prog Neurobiol. 2006;80:281–307. doi: 10.1016/j.pneurobio.2006.11.007. [DOI] [PubMed] [Google Scholar]
- LoTurco JJ, Bai J. The multipolar stage and disruptions in neuronal migration. Trends Neurosci. 2006;29:407–413. doi: 10.1016/j.tins.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Louvi A, Grove EA. Cilia in the CNS: the quiet organelle claims center stage. Neuron. 2011;69:1046–1060. doi: 10.1016/j.neuron.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z, Izumi H, Kanai M, Kabuyama Y, Ahn NG, Fukasawa K. Mortalin controls centrosome duplication via modulating centrosomal localization of p53. Oncogene. 2006;25:5377–5390. doi: 10.1038/sj.onc.1209543. [DOI] [PubMed] [Google Scholar]
- Mehler MF, Gokhan S. Mechanisms underlying neural cell death in neurodegenerative diseases: alterations of a developmentally-mediated cellular rheostat. Trends Neurosci. 2000;23:599–605. doi: 10.1016/S0166-2236(00)01705-7. [DOI] [PubMed] [Google Scholar]
- Ming GL, Song H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron. 2011;70:687–702. doi: 10.1016/j.neuron.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molla-Herman A, Boularan C, Ghossoub R, Scott MG, Burtey A, Zarka M, Saunier S, Concordet JP, Marullo S, Benmerah A. Targeting of beta-arrestin2 to the centrosome and primary cilium: role in cell proliferation control. PLoS One. 2008;3:e3728. doi: 10.1371/journal.pone.0003728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muchowski PJ, Wacker JL. Modulation of neurodegeneration by molecular chaperones. Nat Rev Neurosci. 2005;6:11–22. doi: 10.1038/nrn1587. [DOI] [PubMed] [Google Scholar]
- Murman DL. Early treatment of Parkinson’s disease: opportunities for managed care. Am J Manag Care. 2012;18:S183–S188. [PubMed] [Google Scholar]
- Nadarajah B, Brunstrom JE, Grutzendler J, Wong RO, Pearlman AL. Two modes of radial migration in early development of the cerebral cortex. Nat Neurosci. 2001;4:143–150. doi: 10.1038/83967. [DOI] [PubMed] [Google Scholar]
- Nigg EA, Stearns T. The centrosome cycle: centriole biogenesis, duplication and inherent asymmetries. Nat Cell Biol. 2011;13:1154–1160. doi: 10.1038/ncb2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan EJ, Place RF, Giardina C, Hightower LE. Hsp70B′ regulation and function. Cell Stress Chaperones. 2007;12:393–402. doi: 10.1379/CSC-278e.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan EJ, Place RF, Rasoulpour RJ, Giardina C, Hightower LE. Cell number-dependent regulation of Hsp70B′ expression: evidence of an extracellular regulator. J Cell Physiol. 2007;210:201–211. doi: 10.1002/jcp.20875. [DOI] [PubMed] [Google Scholar]
- Noonan EJ, Fournier G, Hightower LE. Surface expression of Hsp70B′ in response to proteasome inhibition in human colon cells. Cell Stress Chaperones. 2008;13:105–110. doi: 10.1007/s12192-007-0003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan EJ, Giardina C, Hightower L. Hsp70B′ and Hsp72 form a complex in stressed human colon cells and each contributes to cytoprotection. Exp Cell Res. 2008;314:2468–2476. doi: 10.1016/j.yexcr.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Phillips W, Michell A, Pruess H, Barker RA. Animal models of neurodegenerative diseases. Methods Mol Biol. 2009;549:137–155. doi: 10.1007/978-1-60327-931-4_10. [DOI] [PubMed] [Google Scholar]
- Prodromou NV, Thompson CL, Osborn DP, Cogger KF, Ashworth R, Knight MM, Beales PL, Chapple JP. Heat shock induces rapid resorption of primary cilia. J Cell Sci. 2012;125:4297–4305. doi: 10.1242/jcs.100545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao Y, Wu JY. Neuronal migration and the evolution of the human brain. Nat Neurosci. 2001;4:860–862. doi: 10.1038/nn0901-860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scieglinska D, Piglowski W, Mazurek A, Malusecka E, Zebracka J, Filipczak P, Krawczyk Z. The HspA2 protein localizes in nucleoli and centrosomes of heat shocked cancer cells. J Cell Biochem. 2008;104:2193–2206. doi: 10.1002/jcb.21778. [DOI] [PubMed] [Google Scholar]
- Smith KJ, Twal WO, Soodavar F, Virella G, Lopes-Virella MF, Hammad SM (2010) Heat shock protein 70B’ (HSP70B’) expression and release in response to human oxidized low density lipoprotein immune complexes in macrophages. J Biol Chem 285:15985–15993. doi:10.1074/jbc.M110.113605 [DOI] [PMC free article] [PubMed]
- Spassky N, Han YG, Aguilar A, Strehl L, Besse L, Laclef C, Ros MR, Garcia-Verdugo JM, Alvarez-Buylla A. Primary cilia are required for cerebellar development and Shh-dependent expansion of progenitor pool. Dev Biol. 2008;317:246–259. doi: 10.1016/j.ydbio.2008.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavaria M, Gabriele T, Kola I, Anderson RL. A hitchhiker’s guide to the human Hsp70 family. Cell Stress Chaperones. 1996;1:23–28. doi: 10.1379/1466-1268(1996)001<0023:AHSGTT>2.3.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tritarelli A, Oricchio E, Ciciarello M, Mangiacasale R, Palena A, Lavia P, Soddu S, Cundari E. p53 localization at centrosomes during mitosis and postmitotic checkpoint are ATM-dependent and require serine 15 phosphorylation. Mol Biol Cell. 2004;15:3751–3757. doi: 10.1091/mbc.E03-12-0900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai LH, Gleeson JG. Nucleokinesis in neuronal migration. Neuron. 2005;46:383–388. doi: 10.1016/j.neuron.2005.04.013. [DOI] [PubMed] [Google Scholar]
- Young JC. Mechanisms of the Hsp70 chaperone system. Biochem Cell Biol. 2010;88:291–300. doi: 10.1139/O09-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Zhao X, Burkholder WF, Gragerov A, Ogata CM, Gottesman ME, Hendrickson WA. Structural analysis of substrate binding by the molecular chaperone DnaK. Science. 1996;272:1606–1614. doi: 10.1126/science.272.5268.1606. [DOI] [PMC free article] [PubMed] [Google Scholar]