Abstract
The role Hsp60 might play in various inflammatory and autoimmune diseases is under investigation, but little information exists pertaining to Hashimoto’s thyroiditis (HT). With the aim to fill this gap, in the present work, we directed our attention to Hsp60 participation in HT pathogenesis. We found Hsp60 levels increased in the blood of HT patients compared to controls. The chaperonin was immunolocalized in thyroid tissue specimens from patients with HT, both in thyrocytes and oncocytes (Hurthle cells) with higher levels compared to controls (goiter). In oncocytes, we found Hsp60 not only in the cytoplasm but also on the plasma membrane, as shown by double immunofluorescence performed on fine needle aspiration cytology. By bioinformatics, we found regions in the Hsp60 molecule with remarkable structural similarity with the thyroglobulin (TG) and thyroid peroxidase (TPO) molecules, which supports the notion that autoantibodies against TG and TPO are likely to recognize Hsp60 on the plasma membrane of oncocytes. This was also supported by data obtained by ELISA, showing that anti-TG and anti-TPO antibodies cross-react with human recombinant Hsp60. Antibody-antigen (Hsp60) reaction on the cell surface could very well mediate thyroid cell damage and destruction, perpetuating inflammation. Experiments with recombinant Hsp60 did not show stimulation of cytokine production by peripheral blood mononuclear cells from HT patients. All together, these results led us to hypothesize that Hsp60 may be an active player in HT pathogenesis via an antibody-mediated immune mechanism.
Keywords: Hsp60, Hashimoto's thyroiditis (HT), Thyroglobulin (TG), Thyroid peroxidase (TPO), Autoantibodies, Oncocytes, Hurthle cells, Thyrocytes, Chaperonin, Autoimmunity
Introduction
Hashimoto's thyroiditis (HT) is the commonest cause of primary hypothyroidism in humans (Vanderpump and Tunbridge 2002). It is an autoimmune disease characterized by a prolonged autoimmune response against thyroid tissue that alters significantly the morphology of the gland (Ahmed et al. 2012). Classic histological features of HT include small, degenerated follicles, oncocytic (Hurthle cell) metaplasia, and lymphoid infiltrates arranged in follicles (Lorini et al. 2003; Ahmed et al. 2012). Clinical features include increased levels of antibodies to thyroglobulin (TG) and thyroid peroxidase (TPO), two proteins localized within the thyroid gland cells (Lorini et al. 2003). It has been postulated that, as a result of interaction of the antibodies with TG and TPO, inflammation develops in the thyroid gland, the gland is destroyed, and the patient ultimately is rendered hypothyroid (Ahmed et al. 2012). However, since TG and TPO are localized inside the cells, other mechanisms must also be implicated in cell lysis and gland destruction. These latter mechanisms are still largely unknown.
Numerous molecular chaperones have been identified at sites of autoimmune diseases such as rheumatoid arthritis (Yokota et al. 2000; Kotlarz et al. 2013), systemic lupus erythematosus (Yokota et al. 2000), and other autoimmune conditions (Pockley et al. 2008). One of these chaperones, the chaperonin Hsp60, has been found elevated in autoimmune and chronic inflammatory diseases, such as rheumatoid arthritis (Yokota et al. 2000), Crohn's disease (Rodolico et al. 2010), ulcerative colitis (UC) (Rodolico et al. 2010; Tomasello et al. 2011a, b), periodontitis (Rizzo et al. 2012), and chronic obstructive pulmonary disease (COPD) (Cappello et al. 2011). Furthermore, the data suggested that Hsp60 is likely involved in the maintenance of inflammation in UC and COPD by activation of, respectively, macrophages and neutrophils (Tomasello et al. 2011a, b; Cappello et al. 2011). In contrast, very little is known about the involvement of Hsp60 in HT pathogenesis.
Hsp60 is classically described as an intramitochondrial protein involved in assisting the correct folding of other mitochondrial client proteins (Hartl 1991; Pace et al. 2013). However, during the pathogenetic steps of a number of conditions, Hsp60 accumulates in the cytosol (Cappello et al. 2008), reach the plasma membrane (Cechetto et al. 2000; Campanella et al. 2012), and is secreted via the lipid rafts–exosomal pathways (Gupta and Knowlton 2007; Merendino et al. 2010; Campanella et al. 2012). When in the extracellular environment, Hsp60 may interact with receptors present on immune cells (Osterloh et al. 2007; Zanin-Zhorov et al. 2006; Xie et al. 2010) and also reach the bloodstream acting—analogously to other molecular chaperones—as a “chaperokine” at distant sites (Asea 2003; Gazali 2012). Hsp60 may also elicit production of autoantibodies against itself and, thereby, induce an autoimmune response in several tissues in which Hsp60 occurs (Pockley et al. 1999; Rea et al. 2001; Cappello et al. 2009). Therefore, in the last years, Hsp60 is gaining attention as a key player in a number of chronic inflammatory and autoimmune diseases.
In this work, we investigated the levels of Hsp60 in the blood of HT patients and determined the distribution of the chaperonin in the thyroid gland of subjects affected by HT. Finally, we evaluated its structural similarities with TG and TPO, which might lead to immune cross-reactivity and, thus, to thyroid lesions at the sites in which the chaperonin localization in plasma membrane occurs.
Materials and methods
Patients’ recruitment, clinical and laboratory analyses, and fine needle aspiration
Forty-five patients, 28 male and 17 female of ages between 19 and 44 years (mean, 33) at their first diagnosis of HT, were recruited at the Department of Internal Medicine and Medical Specialties of the University of Palermo, Italy. A blood sample was obtained from each subject for routine hematoclinical analyses, including determinations of free triiothyronine (FT3), free thyroxine (FT4), thyroid stimulating hormone (TSH), and anti-thyroid peroxidase (anti-TPO), and anti-thyroglobulin (anti-TG) antibodies. Both hormones and antibodies were measured using a chemiluminescence immunometric assay (Immulite 2000, Siemens Medical Solutions Diagnostics, Los Angeles, CA, USA), with the following reference ranges: FT3 = 1.8–4.2 pg/mL (conversion factor: pg/mL × 1.536 = pmol/L); FT4 = 0.8–1.9 ng/dL (conversion factor: ng/dL × 12.87 = pmol/L); TSH = 0.4–4 μIU/mL (analytical sensitivity = 0.004 μIU/mL); anti-TPO = 0–35 IU/mL; and anti-TG = 0–40 IU/mL. Moreover, the thyroid gland of each subject was submitted to grayscale ultrasonography (GSUS) to determine its volume using an ultrasound mobile system Logiq P5 (General Electric Medical Systems, Milwaukee, WI, USA) equipped with a wide bandwidth (range, 7–12 MHz) transducer. GSUS was performed at 12 MHz. For each patient included in the study, we recorded thyroid volume by ultrasonography (expressed in milliliters) calculating the ellipsoid formula (longitudinal diameter × transverse diameter × antero-posterior diameter × π/6) applied to each lobe. In addition, ten subjects (five male and five female) selected randomly from the entire population studied (45 subjects) were submitted to fine needle aspiration (FNA) of thyroid for immunocytologic studies (see below). From the latter subjects, a supplementary blood sample was also collected for flow cytometry experiments (see below).
Determination of circulating levels of Hsp60 by ELISA
ELISA was performed as previously described (Rizzo et al. 2012) using a commercial Hsp60 (human) enzyme immunoassay (EIA) kit (ADI EKS 600 ELISA kit from Enzo Life Sciences, Inc., Farmingdale, NY, USA). We performed a quantitative comparison of Hsp60 levels in plasma of the patients with HT with those of 45 age-matched normal controls randomly selected from our files. A whole blood sample from each subject was collected in EDTA-treated tubes. After a centrifugation at 2,000×g for 10 min, plasma was collected, aliquoted, and stored at −20 °C until use. The Hsp60 standard was diluted in sample diluent to generate a standard curve with six points, ranging from 3.125 to 100 ng/mL, and sample diluent alone was used as 0 (zero) standard. First, 100 μL of prepared standards and undiluted plasma was added in duplicate to wells of the immunoassay plate precoated with mouse monoclonal antibody specific for Hsp60 and incubated at 23 °C for 1 h. The primary and the secondary antibodies were diluted according to the manufacturer's instructions. Then, 100 μL of diluted anti-Hsp60 goat polyclonal antibody was added to each well and incubated at 23 °C for 1 h. After washing, 100 μL of diluted horse radish peroxidase-conjugate anti-goat IgG was added to the plate and incubated at 23 °C for 30 min followed by 100 μL of 3,3′,5,5′-tetramethylbenzidine substrate for 15 min in the dark. Finally, 100 μL of Stop Solution was added, and absorbance was measured at 450 nm in a microplate photometric reader (DV990BV4, GDV, Milan, Italy). Sample concentration was calculated by interpolating the sample concentrations in the standard curve. The sensitivity of the human Hsp60, EIA kit was determined to be 3.125 ng/mL. Human Hsp60 EIA kit is specific for Hsp60 and the Hsp60 ELISA has been certified for the detection of human Hsp60.
Collection of histological specimens
Surgical specimens from patients that underwent thyroid gland removal were retrospectively obtained from the Pathologic Anatomy Unit of the Section of Human Pathology of the University of Palermo, Italy. Ten cases of HT and 10 cases of goiter, as controls, were studied. All tissues were formalin-fixed and paraffin embedded. Five-micrometer sections were obtained from the blocks for the immunohistochemical and immunofluorescence studies.
Immunomorphological analyses: immunohistochemistry and immunofluorescence
Immunohistochemistry was performed as previously described (Tomasello et al. 2011a, b) on 5-μm sections of HT and goiter tissues, using Histostain-Pluss IHC detection Kit (Histostain-plus Kit 3rd Gen IHC Detection Kit, Invitrogen Corporation, catalogue no. 85-9073, Camarillo, CA, USA), and a primary antibody against human Hsp60 (mouse anti-Hsp60 monoclonal antibody, Sigma, St. Louis, MO, catalogue no. H4149, dilution 1:400). Appropriate positive controls, and nonimmune serum for negative controls, were run concurrently. Nuclear counterstaining was done using hematoxylin (DAKO, Carpinteria, CA, USA). Three independent observers (FR, FC, and DC) examined the specimens in a blind, code-marked approach and performed a quantitative analysis to determine the percentage of cells positive for Hsp60 in thyrocytes and oncocytes. All the observations were made at a magnification of ×400, and the means of triplicate counts were used for statistical analyses.
Double immunofluorescence tests were performed to assess the colocalization of Hsp60 and TG in 5-μm sections of HT and goiter, as previously described (Serradifalco et al. 2011). Briefly, after dewaxing in xylene, rehydration in ethanol, and washing in phosphate-buffered solution (PBS), sections were incubated with unmasking solution (tri-sodium citrate 10 mM, 0.05% Tween 20) for 10 min at 58 °C and treated with blocking solution (3 % albumin bovine serum in PBS) for 30 min at 23 °C. Then, the sections were incubated with the first primary antibody (rabbit polyclonal antihuman HSP60, Clone H300, Santa Cruz Biotechnology, Dallas, USA, catalogue no. sc-13966) diluted 1:100, overnight at 4 °C. The day after, some sections were incubated with the second primary antibody (Mouse Monoclonal anti-Thyroglobulin, Clone 2H11/6E1, Genemed Biotechnologies, South San Francisco, CA, USA, catalogue no. 61-0064-5) diluted 1:100, overnight at 4 °C. After washing twice in PBS all sections were incubated with fluorescent secondary antibodies such us rabbit IgG antibody conjugated with Texas Red (diluted 1:200; Gene Tex Inc, Irvine, USA) and mouse IgG antibody conjugated with fluorescein isothiocyanate (FITC; diluted 1:200; Sigma-Aldrich, Milan, Italy) respectively.
Double immunofluorescence tests were also performed to assess the colocalization of Hsp60 and integrin (a plasma membrane marker) in cytologic preparations obtained after FNA by thin prep, as described by Tulecke and Wang (2004). The specimens were incubated with unmasking solution (tri-sodium citrate 10 mM, 0.05% Tween 20) for 10 min at 23 °C, treated with blocking solution and incubated with the first primary antibody against Hsp60 diluted 1:100 (rabbit polyclonal anti-Hsp60, Clone H300, Santa Cruz Biotechnology) overnight at 4 °C. Subsequently, the slides were incubated with the second primary antibody against integrin diluted 1:100 (goat polyclonal anti-integrin β3, clone C20, Santa Cruz Biotechnology, catalogue no. sc6626) overnight at 4 °C. After washing twice in PBS, all slides were incubated with fluorescent secondary antibodies (rabbit IgG antibody conjugated with Texas Red, Gene Tex Inc, dilution 1:200; FITC-conjugated donkey antigoat secondary antibody, Gene Tex Inc., dilution 1:200) for 1 h each at 23 °C. The nuclei were counterstained with Hoechst (Sigma-Aldrich, Inc, Milan, Italy) for 15 min at 23 °C. Finally, all slides were mounted with cover slips using a drop of PBS, and readings and imaging were immediately performed with a Leica DM5000 upright fluorescence microscope (Leica Microsystems, Heidelberg, Germany).
Isolation and stimulation of peripheral blood mononuclear cells
Peripheral blood mononuclear cells (PBMC) were isolated from fresh heparinized blood from healthy and HT subjects (the same group studied with FNA, see above) by density gradient centrifugation with Fycoll-Hypaque (Pancoll, Pan Biotech). After washing, the cells were resuspended in RPMI 1640 medium with 10 % heat-inactivated fetal calf serum and supplemented with 2 mM glutamine, 50 U/ml penicillin, and 50 mg/streptomycin. Cell culture reagents were purchased from GIBCO BRL LIFE Technologies (Invitrogen, Italy). Finally, PMBCs (2 × 105 cells) were cultured in six-well tissue-culture plates at 37 °C in a humidified, 5 % CO2, incubator and stimulated with three different doses (1, 5, and 10 μg/ml) of recombinant human Hsp60 (rhHsp60) (Vinci Biochem, Milan, Italy) for various times (8, 24, and 48 h). To verify endotoxin contamination of the commercial rhHsp60, we also determined the amount of endotoxin by the quantitative chromogenic Limulus amebocyte lysate assay (QCL-1000; BioWhittaker, Walkersville, MD, USA) according to the manufacturer's instructions, as described (Bethke et al. 2002). The endotoxin concentration was calculated as EU per milliliter. The endotoxin concentration was <0.5 EU/μg.
Detection of cytokines in conditioned medium
After incubation, the supernatants were collected from each plate to measure inflammatory cytokine production after rhHsp60 stimulation. We used the inflammatory human cytokine cytometric bead array (BD™ CBA Human Th1/Th2 Cytokine Kit II, BD Biosciences) to measure interleukin (IL)-2, IL-4, IL-6, IL-10, tumor necrosis factor (TNF), and interferon gamma (IFN-γ) following the manufacturer's directions (Collins et al. 1998). The fluorescence was measured on a FACSCalibur (BD Biosciences) analyzed with FCAP Array software (BD Biosciences).
Bioinformatics evaluation of regional similarity between Hsp60 and, respectively, TG and TPO
The amino-acid sequences of Hsp60, TG, and TPO were obtained from the PubMed website (http://www.ncbi.nlm.nih.gov/genbank/) using, respectively, the following access numbers: Hsp60, Homo sapiens GenBank, accession number NM_002156; thyroglobulin, Homo sapiens GenBank, accession number CAA29104; and thyroid peroxidase, Homo sapiens GenBank, accession number AAA61217. Sequence comparisons were performed, as described (Marino Gammazza et al. 2012), using the open access SIM software (http://expasy.org/tools/sim-prot.html). The three-dimensional model of the Hsp60 monomer was obtained using the 3D-JIGSAW software (http://bmm.cancerresearchuk.org) and visualized by PyMol (http://www.pymol.org). All software used are open access.
Determination of Hsp60 cross-reactivity with TG and TPO by ELISA
Microplates were coated with 1, 5, or 10 μg/mL (50 μL/well) of rhHsp60 (Vinci Biochem, Italy) by overnight incubation at 4 °C. The wells were rinsed with 100 μL of PBS three times and blocked with 200 μL/well of blocking buffer (3 % BSA in PBS) for 1 h at 23 °C. After blocking, the wells were filled with primary antibodies diluted 1:1,000 in PBS for 1 h at 23 °C. We used the primary antibodies we had chosen because they recognize the epitopes of the proteins that bioinformatics analyses showed to have high similarity with Hsp60, as follows: (1) mouse monoclonal antihuman TG (Santa Cruz Biotechnology, Dallas, USA, catalogue no. sc7836) and (2) mouse monoclonal antihuman TPO, (Abcam, Cambridge, UK, catalogue no. ab76935). Morevoer, we used a mouse monoclonal antihuman Hsp60 antibody (Sigma, St. Louis, MO, catalogue no. H4149) as positive control and a goat polyclonal anti-integrin αM antibody (Santa Cruz Biotechnology, Dallas, TX, USA, catalogue no. sc6614) as negative control. Wells without primary antibodies were also prepared and incubated with PBS. After incubation, all the wells were washed extensively with PBS, and the secondary antibodies were added at 1:3000 dilution for 1 h at 23 °C, as follows: antimouse horseradish peroxidase-linked whole antibody (GE Healthcare, Little Chalfont, UK) and antigoat horseradish peroxidase-linked whole antibody (Sigma-Aldrich Italy, Milan, Italy). After washing, 100 μL of o-phenylenediamine dihydrochloride (DAKO) was added to each well and incubated for 30 min at 23 °C in the dark. The reaction was terminated with the addition of 100 μL of 0.5 mol/L sulfuric acid, and absorbance values were read at 492 nm, using an ELISA plate reader (DV990BV4, GDV, Milan, Italy).
Statistical analyses
Statistical analyses were performed using Statview® 5.0 (SAS Institute Inc., Cary, NC, USA). Correlation analyses were performed using the Spearman rank correlation method. Multivariate analysis was performed in order to assess potential independent associations between anti-TPO and anti-TG antibody levels, as well as the thyroid volume and the thyroid hormonal pattern (levels of FT3, FT4, and TSH).
Results
Hsp60 levels and correlation with anatomoclinical parameters in subjects with HT
Hsp60 levels in plasma of HT subjects were undetectable in two of 45 subjects and ranged between 3.125 and 64.218 ng/mL (mean, 16.788; SD, 11.082) in the others, including two very high values; by contrast, in normal subjects, Hsp60 was undetectable in 12 of 45 subjects and ranged between 3.125 and 24.397 ng/mL (mean, 7.586; SD, 4.043) in the others. The difference between Hsp60 levels in HT versus normal subjects was significant (p < 0.02).
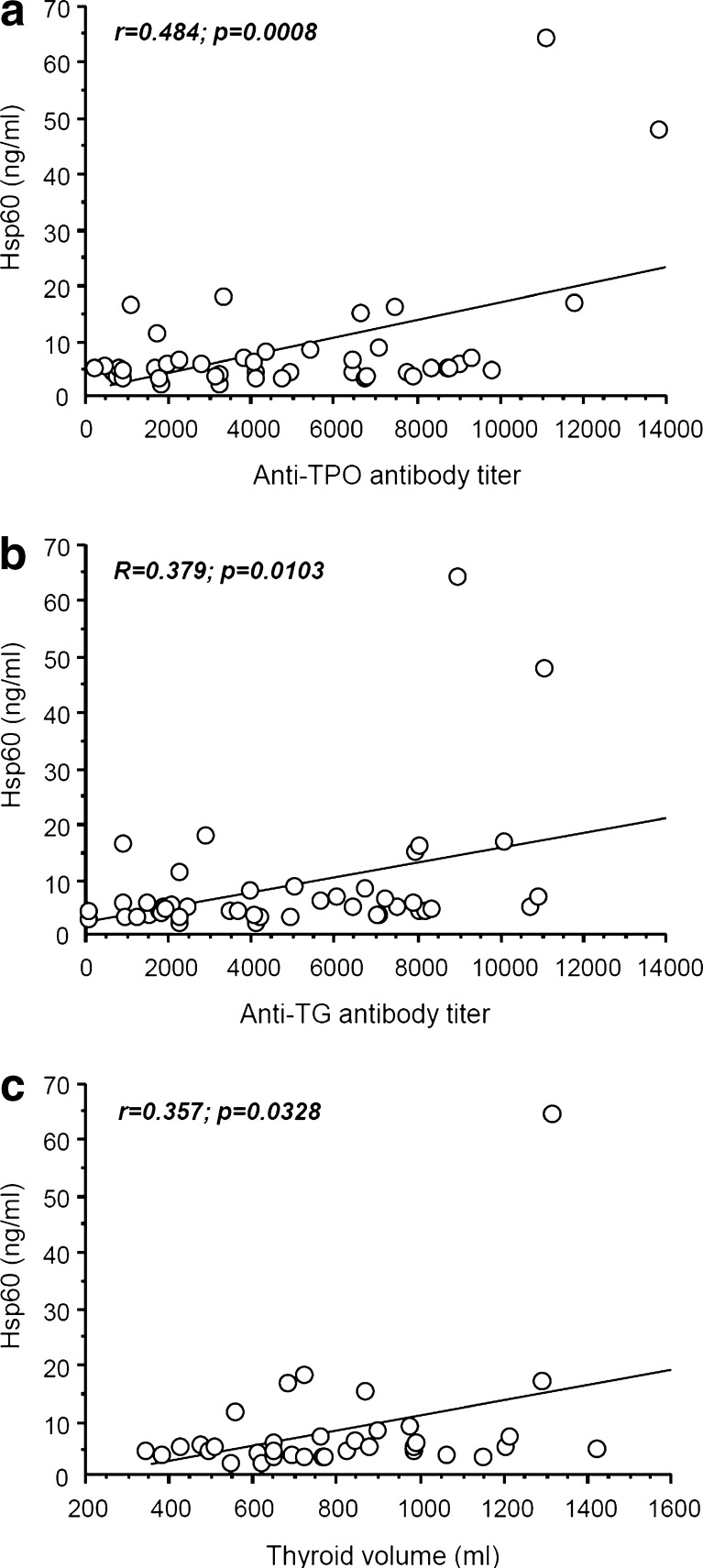
As shown in Fig. 1, Hsp60 plasma levels positively correlated with the levels of antibodies anti-TPO (r = 0.484, p = 0.0008; Fig. 1a) and anti-TG (r = 0.379, p = 0.0103; Fig. 1b), and with the thyroid volume (r = 0.357, p = 0.0328; Fig. 1c). These associations were independent of the thyroid hormonal pattern, as demonstrated by multivariate analysis (data not shown). We adjusted the association between plasma Hsp60 levels and anti-TPO and anti-TG antibody levels and the thyroid volume for FT3, FT4, and TSH, and we found that the Hsp60 plasma levels remained significantly associated with anti-TPO (p = 0.0006) and anti-TG (p = 0.0084) antibody levels and with thyroid volume (p = 0.0323).
Fig. 1.
Plots of the correlation analyses between the plasma levels of Hsp60 and those of the anti-TPO and anti-TG antibodies, and thyroid volume. The Hsp60 plasma levels positively correlated with those of anti-TPO antibodies (a r = 0.484, p = 0.0008 and anti-TG antibodies (b r = 0.379, p = 0.0103) with thyroid volume (c r = 0.357, p = 0.0328)
Immunohistochemistry for Hsp60 in HT compared with goiter specimens
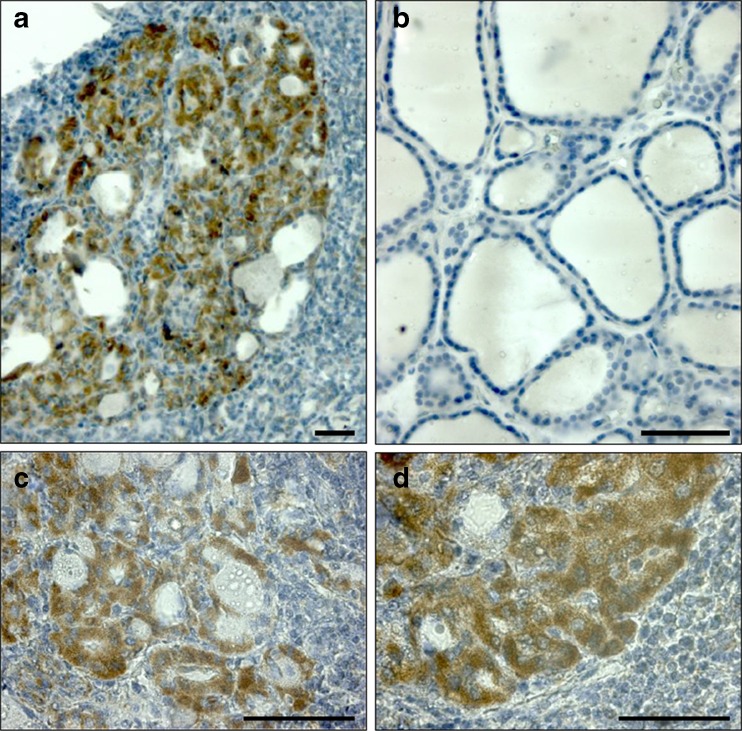
Immunohistochemistry showed that Hsp60 was abundantly present in specimens from HT but not in those from goiter (Fig. 2). Positivity for Hsp60 was localized in thyrocytes of small and degenerated follicles (Fig. 2a, c) and in oncocytic metaplasia areas (Fig. 2b). By contrast, thyrocytes from goiter were negative, or scarcely positive, for Hsp60 (Fig. 2b).
Fig. 2.
Immunohistochemical demonstration of Hsp60 in thyroid tissues. Hsp60 was abundantly present only in thyroid specimens from HT (a) while in goiter it was scarce or absent (b). Positivity for Hsp60 was localized both to small and degenerated follicles (c) and to oncocytic islets (d). Bars: 100 μm
Double immunofluorescence for Hsp60 and TG in HT compared with goiter specimens
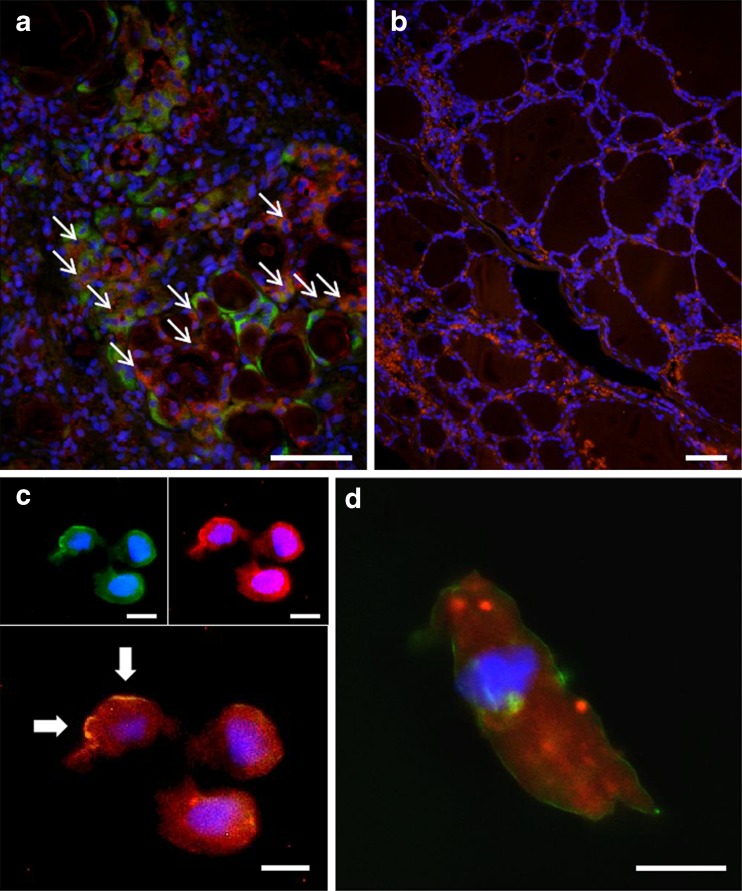
Double immunofluorescence experiments performed on tissue sections revealed that in HT a number of cells showed double positivity for Hsp60 and TG, while others showed only a single staining (Fig. 3a). Double staining positivity indicated that Hsp60 was present in thyrocytes (also positive for TG), although not all thyrocytes were also positive for Hsp60. Single staining positivity for Hsp60 indicated that this molecule was present also in other cells, most of which had the characteristic features of oncocytes. Hence, these experiments suggest that not only thyrocytes but also oncocytes are positive for Hsp60 in HT specimens. In goiter tissues, cells were positive only for TG. (Fig. 3b).
Fig. 3.
Double immunofluorescence for Hsp60, thyroglobulin, and integrin in thyroid tissues and cells. A number of cells in HT specimens (a) showed single staining for Hsp60 (green) or thyroglobulin (red), while others presented a double positivity (orange). Some of the latter are indicated by arrows. In contrast, goiter tissue cells (b) were positive only for thyroglobulin (red). Double immunofluorescence for integrin (green) and Hsp60 (red) in cytological smears obtained by fine needle aspiration from HT thyroids showed Hsp60 positivity in both cytosol and plasma membrane (arrows) in oncocytes (c), but not in thyrocytes (d); in the latter, Hsp60 occurred only inside cells. Bars: 100 μm (a, b) and 10 μm (c, d)
Double immunofluorescence for Hsp60 and integrin in HT cytologic smears
Double immunofluorescence experiments for integrin and Hsp60 in cells carried out in cytologic smears (ThinPreps) obtained by FNA from thyroids of patients with HT showed Hsp60 positivity both in cytosol and in plasma membrane in large size, round cells with large nucleus, all characteristic of oncocytes (Fig. 3c; arrows), but not in cells with a follicular morphology, i.e., with features of thyrocytes (Fig. 3d).
Tests for establishing if Hsp60 would stimulate PBMC from HT patients to produce cytokines
Since we found that Hsp60 levels in HT tend to be higher than in normal subjects and considering that the chaperonin is believed to stimulate cytokine release, we decided to compare levels of cytokines (IL-2, IL-4, IL-6, IL-10, TNF, and IFN-γ) secreted by PBMC obtained from either HT patients or healthy controls. The results would provide insights into whether or not Hsp60 has a proinflammatory role, i.e., acting as chaperokine, in HT. PBMC were either treated with hrHsp60 or left untreated. Cytokine levels did not vary significantly in any of the observed conditions (data not shown). In summary, these data suggest that Hsp60 did not stimulate cytokine production in PBMC from HT patients or normal subjects.
Bioinformatics: primary structure comparison between Hsp60 and TG or TPO
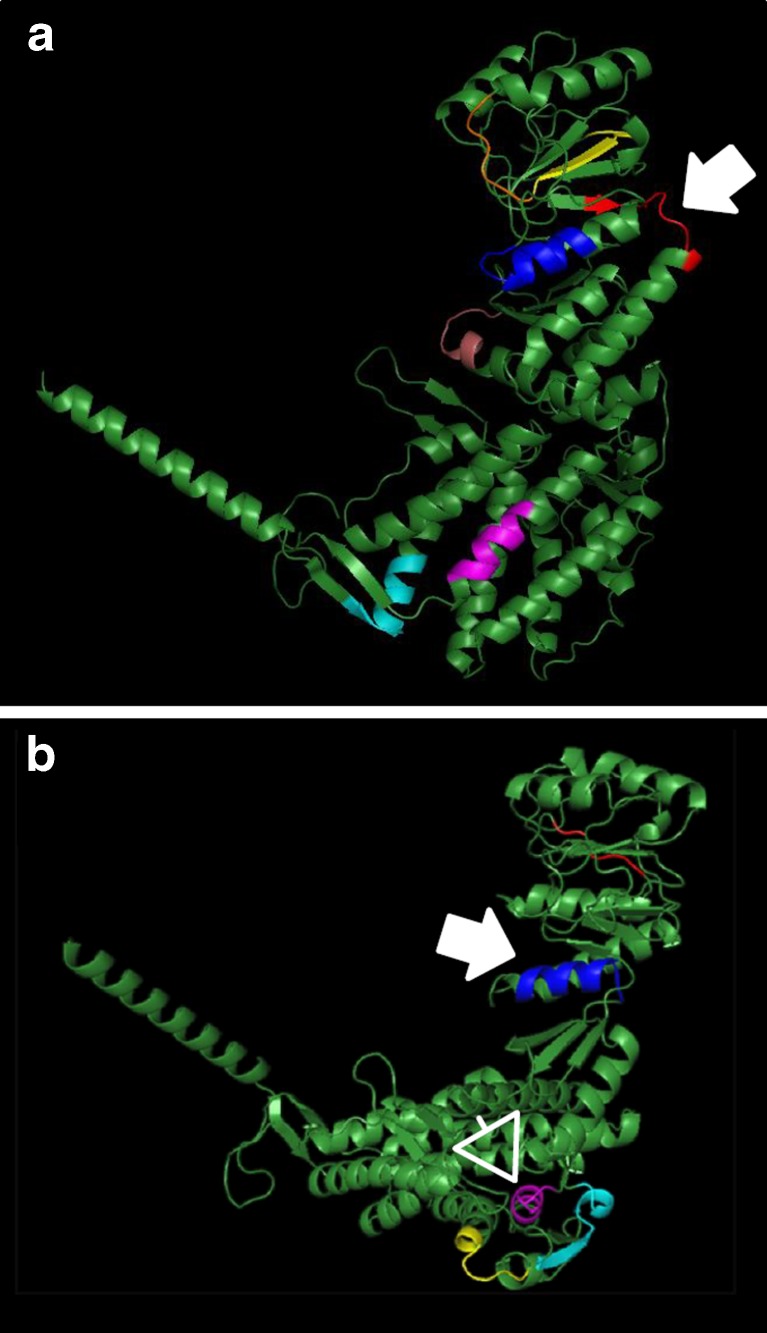
Since it is known that Hsp60 has a high degree of sequence similarity with other human proteins (Jones et al. 1993; Marino Gammazza et al. 2012), we performed bioinformatics comparative analyses of amino-acid sequences between Hsp60 and, either, TG or TPO. These analyses showed a high similarity between Hsp60 and both these thyroid-specific molecules (Table 1). The alignment of the amino-acid sequences of Hsp60 and TPO revealed seven epitopes with a percentage of identity between 50 and 66.7. Likewise, the alignment of the amino-acid sequences of Hsp60 and TPO showed five homologous epitopes with identity percentage between 58.3 and 80. These epitopes were distributed all along the amino-acid sequence (Fig. 4).
Table 1.
Regions of similarity between Hsp60 and either thyroglobulin or thyroid peroxidase amino-acid sequences (all human) revealed by the SIM alignment
| Proteins compared | Amino-acid region | Sequencea | Percentage of identities |
|---|---|---|---|
| Hsp60 vs. thyroglobulin | 357–365 | LLKGKGDKA | 66.7 |
| 171–179 | LLHGVGDKS | ||
| 244–253 | VLLSEKKISS | 60 | |
| 1982–1991 | VPMSEKSISN | ||
| 531–539 | DAAGVASLL | 55.6 | |
| 1014–1022 | DSAGASALL | ||
| 239–247 | FQDAYVLLS | 55.6 | |
| 214–222 | FPDAFVTFS | ||
| 304–314 | GFGDNRKNQLK | 54.5 | |
| 2008–2018 | GFGFLNVSQLK | ||
| 408–415 | GTSDVEVN | 50 | |
| 2049–2056 | GSPDIEVH | ||
| 32–341 | FGADARALML | 50 | |
| 2373–2382 | FGGDPRRVSL | ||
| Hsp60 vs. thyroid peroxidase | 322–326 | GAVFG | 80 |
| 302–307 | GALFG | ||
| 439–447 | GGGCALLRC | 66.7 | |
| 715–723 | GGGTPELRC | ||
| 508–517 | GDFVNMVEKG | 60 | |
| 751–760 | GDFVHCEESG | ||
| 388–399 | EKLNERLAKLSD | 58.3 | |
| 558–569 | EELTERLFVLSN | ||
| 481–487 | KNAGVEG | 57.1 | |
| 917–923 | ESAGMEG |
aSee also Fig. 4
Fig. 4.
Three-dimensional model of the human Hsp60 showing the amino-acid sequences (potential crossreactive immunogenic/antigenic epitopes; various colors) it shares with thyroid specific molecules. a Shared sequences between Hsp60 and thyroglobulin (TG); the arrow indicates the epitope with the highest similarity (66,7 % identities). b Shared sequences between Hsp60 and thyroid peroxidase (TPO); the epitopes with the highest (80 % identities) and second highest (66.7 % identities) similarities are indicated by an arrow and an arrowhead, respectively. See also Table 1
Determination of cross-reactivity between Hsp60 andTG or TPO by ELISA tests
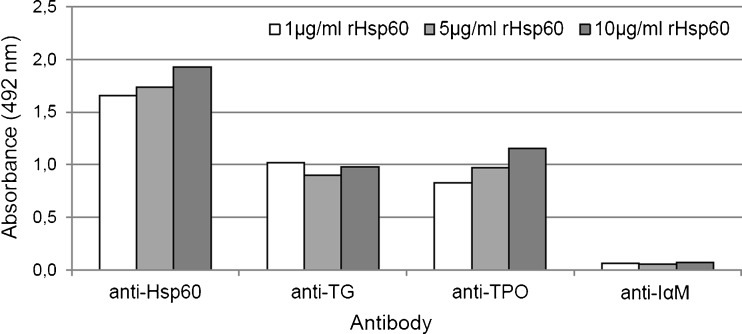
The bioinformatics results described in the preceding section predicted cross-reactivity of Hsp60 with TG and with TPO. This hypothesis was further tested by determining if anti-TG and anti-TPO antibodies crossreact with Hsp60, using ELISA. The results confirmed the prediction (Fig. 5).
Fig. 5.
Hsp60 cross-reactivity with TG and TPO. ELISA tests with various concentrations (1, 5, and 10 μg/ml) of rhHsp60 and antibodies anti-TG, anti-TPO, anti-Hsp60 (positive control), and anti-integrin αM (negative control) show that both anti-TG and anti-TPO antibodies are able to recognise rhHsp60
Discussion
Molecular chaperones are a class of proteins with various fundamental roles in cell and tissue physiology and in response to a variety of stressors (Henderson and Pockley 2010; Macario et al. 2013). Many “chaperones” are also “heat shock proteins” (Hsps), although not all Hsps are chaperones. Since they are important in many physiologic processes, a number of diseases in which chaperone functions are impaired, the chaperonopathies, have been described (Macario et al. 2013). From a pathogenetic point of view, chaperonopathies have been classified in three groups: by defect, for instance due to chaperone gene mutation, e.g., the hereditary spastic paraplegia 13 (Hansen et al. 2002); by excess, due to chaperone gene overexpression, i.e., COPD (Cappello et al. 2011); and by mistake, in which chaperones are apparently normal but are working to the advantage of the disease rather that than to the host organism, e.g., some forms of tumors (Rappa et al. 2012). Among chaperonopathies by mistake may be included some forms of autoimmune diseases in which a supposedly normal chaperone acts as an autoantigen (Macario et al. 2013).
In this study, we found that Hsp60 levels are increased in over one third of HT patients compared to controls in plasma and in all patients studied in thyroid tissue. Moreover, Hsp60 localized to thyroid tissues, both in thyrocytes and oncocytes. In the latter cells, Hsp60 was present not only in the cytoplasm but also in the plasma membrane. Since we found regions of remarkable structural similarity between Hsp60, TG, and TPO, we postulated that autoantibodies against TG and TPO are likely to recognize Hsp60 on the plasma membrane of oncocytes and, thereby, mediate their destruction, perpetuating inflammation. Data from the literature indicate that even a very small sequence segment (e.g., two or three amino acids) (Jorgensen et al. 1992; Singh et al. 2013), and epitopes with <50 % similarity (Frankild et al. 2008), can induce cross-reactivity by, both, humoral and innate immune system activation. It is therefore likely that, as postulated above, plasma membrane Hsp60 may be an antigen for autoantibodies that might be able to recognize also TG and/or TPO considering the high percentage of similarity of their shared epitopes. We confirmed the immunological cross-reactivity of anti-TG and anti-TPO antibodies with human Hsp60 by ELISA tests. All these observations indicate that Hsp60 could have an important role in maintaining autoimmunity in HT subjects, thus contributing to disease progression.
Hsp60 could also have a role in thyroid pathology by inducing the production of inflammatory cytokines in inflammatory cells infiltrating the thyroid. Although our data do not support this mechanism, it cannot be excluded. Our results with PBMC stimulated with preparations of rhHsp60 did not show cytokine production regardless of the origin of the PBMC, healthy individuals or HT patients. Nevertheless, it cannot be ruled out that autologous Hsp60, or even other Hsp60 preparations, recombinant or otherwise, different from the one we used, would have had a different effect. This road remains open for investigation.
We found only a few reports on the involvement of chaperones/Hsps in HT pathogenesis. Bougacha-Elleuch et al. (2004) examined single and haplotypic genetic variations of a number of genes, including Hsp70, but they did not find any such variations. Heufelder et al. (1992) demonstrated the expression of Hsp72 in the thyroid gland from patients with HT. Misaki et al. (1994) suggested that aberrantly expressed Hsp72 may activate part of the thyroid-infiltrating lymphocytes and thereby aggravate autoimmune processes.
A similarity between human Hsp60 and 19 known autoantigens, including TG, has been described in the past (Jones et al. 1993). Hence, Hsp60 has been postulated to be involved in a number of autoimmune diseases, including HT. However, to the best of our knowledge, only one paper dealt with the role of Hsp60 in HT pathogenesis: Kotani et al. (1996) showed increased Hsp60 levels in HT follicular cells compared to controls. Interestingly, no increment was found of anti-Hsp60 antibodies in HT patients, compared to normal subjects. These were the data available in the literature, but they alone seemed us insufficient to postulate a role of Hsp60 in HT pathogenesis. Indeed, Hsp60 may increase in HT tissue as a physiologic response to a number of pericellular stressors, as those present during an inflammation.
Also noteworthy are the facts that one region of sequence similarity between TG and Hsp60 was identified many years ago (Jones et al. 1993) and that modern bioinformatics techniques allowed us now to identify a greater number of sequence similarity regions not only between Hsp60 and TG but also between Hsp60 and TPO, which—to the best of our knowledge—were never described before.
These data, combined with the correlation between Hsp60 and both anti-TG and anti-TPO antibody levels in blood of HT patients, with the presence of Hsp60 in the plasma membrane of oncocytes, and with the cross-reactivity between Hsp60 andTG and TPO, led us to hypothesize that Hsp60 plays a determinant role in HT pathogenesis by being involved in the autoimmune destruction of the gland. However, some questions are still unanswered: Is part of the Hsp60 molecule expressed on the cell surface? If this is so, which epitope(s) are expressed on the surface and are available for antibody binding? Our findings encourage research in this direction.
In conclusion, this work shows for the first time that: (1) Hsp60 levels in thyroid tissue and the blood of HT subjects are increased compared to controls; (2) Hsp60 is expressed both in thyrocytes and oncocytic metaplasia cells; in the latter, Hsp60 is present inside the cell and also at the plasma membrane level; (3) Hsp60 shares structural homology with TG and TPO over various segments of their amino-acid sequences, which constitute potential immunological cross-reactive epitopes; (4) Hsp60 blood levels tend to correlate with those of anti-TG and anti-TPO antibodies in HT patients; (5) rhHsp60 did not induce increment of cytokine (i.e., IL-2, IL-4 , IL-6, IL-10,TNF, and IFN-γ) production by PBMC in HT subjects, under the conditions tested; and (6) antibodies anti-TG and anti-TPO are able to recognize Hsp60. These data considered together lead us to postulate, as a working hypothesis, that Hsp60 is an active player in HT pathogenesis, via an antibody-mediated immune mechanism.
Acknowledgements
This work was partly supported by IEMEST (FC, AJLM) and was carried out under the umbrella of the agreement between IEMEST and IMET signed March 26, 2012.
Footnotes
A. Marino Gammazza and M. Rizzo contributed equally to the present work.
References
- Ahmed R, Al-Shaikh S, Akhtar M. Hashimoto thyroiditis: a century later. Adv Anat Pathol. 2012;19:181–186. doi: 10.1097/PAP.0b013e3182534868. [DOI] [PubMed] [Google Scholar]
- Asea A. Chaperokine-induced signal transduction pathways. Exerc Immunol Rev. 2003;9:25–33. [PMC free article] [PubMed] [Google Scholar]
- Bethke K, Staib F, Distler M, Schmitt U, Jonuleit H, Enk AH, Galle PR, Heike M. Different efficiency of heat shock proteins (HSP) to activate human monocytes and dendritic cells: superiority of HSP60. J Immunol. 2002;169:6141–6148. doi: 10.4049/jimmunol.169.11.6141. [DOI] [PubMed] [Google Scholar]
- Bougacha-Elleuch N, Rebai A, Mnif M, Makni H, Bellassouad M, Jouida J, Abid M, Hammadi A. Analysis of MHC genes in a Tunisian isolate with autoimmune thyroid diseases: implication of TNF −308 gene polymorphism. J Autoimmun. 2004;23:75–80. doi: 10.1016/j.jaut.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Campanella C, Bucchieri F, Merendino AM, Fucarino A, Burgio G, Corona DF, Barbieri G, David S, Farina F, Zummo G, Conway de Macario E, Macario AJL, Cappello F. The odyssey of Hsp60 from tumor cells to other destinations includes plasma membrane-associated stages and Golgi and exosomal protein-trafficking modalities. PLoS One. 2012;7:e42008. doi: 10.1371/journal.pone.0042008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappello F, Conway de Macario E, Marasà L, Zummo G, Macario AJL. Hsp60 expression, new locations, functions and perspectives for cancer diagnosis and therapy. Cancer Biol Ther. 2008;7:801–809. doi: 10.4161/cbt.7.6.6281. [DOI] [PubMed] [Google Scholar]
- Cappello F, Conway de Macario E, Di Felice V, Zummo G, Macario AJL. Chlamydia trachomatis infection and anti-Hsp60 immunity: the two sides of the coin. PLoS Pathog. 2009;5(8):e1000552. doi: 10.1371/journal.ppat.1000552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappello F, Caramori G, Campanella C, Vicari C, Gnemmi I, Zanini A, Spanevello A, Capelli A, La Rocca G, Anzalone R, Bucchieri F, D'Anna SE, Ricciardolo FL, Brun P, Balbi B, Carone M, Zummo G, Conway de Macario E, Macario AJL, Di Stefano A. Convergent sets of data from in vivo and in vitro methods point to an active role of Hsp60 in chronic obstructive pulmonary disease pathogenesis. PLoS One. 2011;6:e28200. doi: 10.1371/journal.pone.0028200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cechetto JD, Soltys BJ, Gupta RS. Localization of mitochondrial 60-kD heat shock chaperonin protein (Hsp60) in pituitary growth hormone secretory granules and pancreatic zymogen granules. J Histochem Cytochem. 2000;48:45–56. doi: 10.1177/002215540004800105. [DOI] [PubMed] [Google Scholar]
- Collins DP, Luebering BJ, Shaut DM. T-lymphocyte functionality assessed by analysis of cytokine receptor expression, intracellular cytokine expression, and femtomolar detection of cytokine secretion by quantitative flow cytometry. Cytometry. 1998;33:249–255. doi: 10.1002/(SICI)1097-0320(19981001)33:2<249::AID-CYTO21>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Frankild S, de Boer RJ, Lund O, Nielsen M, Kesmir C. Amino acid similarity accounts for T cell cross-reactivity and for "holes" in the T cell repertoire. PLoS One. 2008;3:e1831. doi: 10.1371/journal.pone.0001831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazali A. Conference scene: taking the heat out of chaperokine function. Immunotherapy. 2012;4:773–775. doi: 10.2217/imt.12.78. [DOI] [PubMed] [Google Scholar]
- Gupta S, Knowlton AA. HSP60 trafficking in adult cardiac myocytes: role of the exosomal pathway. Am J Physiol Heart Circ Physiol. 2007;292:H3052–H3056. doi: 10.1152/ajpheart.01355.2006. [DOI] [PubMed] [Google Scholar]
- Hansen JJ, Dürr A, Cournu-Rebeix I, Georgopoulos C, Ang D, Nielsen MN, Davoine CS, Brice A, Fontaine B, Gregersen N, Bross P. Hereditary spastic paraplegia SPG13 is associated with a mutation in the gene encoding the mitochondrial chaperonin Hsp60. Am J Hum Genet. 2002;70:1328–1332. doi: 10.1086/339935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartl FU. Heat shock proteins in protein folding and membrane translocation. Semin Immunol. 1991;3:5–16. [PubMed] [Google Scholar]
- Henderson B, Pockley AG. Molecular chaperones and protein-folding catalysts as intercellular signaling regulators in immunity and inflammation. J Leukoc Biol. 2010;88:445–462. doi: 10.1189/jlb.1209779. [DOI] [PubMed] [Google Scholar]
- Heufelder AE, Goellner JR, Wenzel BE, Bahn RS. Immunohistochemical detection and localization of a72-kilodalton heat shock protein in autoimmune thyroid disease. J Clin Endocrinol Metab. 1992;74:724–731. doi: 10.1210/jc.74.4.724. [DOI] [PubMed] [Google Scholar]
- Jones DB, Coulson AF, Duff GW. Sequence homologies between Hsp60 and autoantigens. Immunol Today. 1993;14:115–118. doi: 10.1016/0167-5699(93)90210-C. [DOI] [PubMed] [Google Scholar]
- Jorgensen JL, Reay PA, Ehrich EW, Davis MM. Molecular components of T-cell recognition. Annu Rev Immunol. 1992;10:835–873. doi: 10.1146/annurev.iy.10.040192.004155. [DOI] [PubMed] [Google Scholar]
- Kotani T, Aratake Y, Hirai K, Hirai I, Ohtaki S. High expression of heat shock protein 60 in follicular cells of Hashimoto’s thyroiditis. Autoimmunity. 1996;25:1–8. doi: 10.3109/08916939608994721. [DOI] [PubMed] [Google Scholar]
- Kotlarz A, Tukaj S, Krzewski K, Brycka E, Lipinska B. Human Hsp40 proteins, DNAJA1 and DNAJA2, as potential targets of the immune response triggered by bacterial DnaJ in rheumatoid arthritis. Cell Stress Chaperones. 2013 doi: 10.1007/s12192-013-0407-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorini R, Gastaldi R, Traggiai C, Perucchin PP. Hashimoto's thyroiditis. Pediatr Endocrinol Rev Suppl. 2003;2:205–211. [PubMed] [Google Scholar]
- Macario AJL, Conway de Macario E, Cappello F (2013) The chaperonopathies. Diseases with defective molecular chaperones. Springer, London–New York. http://www.springer.com/biomed/book/978-94-007-4666-4
- Marino Gammazza A, Bucchieri F, Grimaldi LM, Benigno A, Conway de Macario E, Macario AJL, Zummo G, Cappello F. The molecular anatomy of human Hsp60 and its similarity with that of bacterial orthologs and acetylcholine receptor reveal a potential pathogenetic role of anti-chaperonin immunity in myasthenia gravis. Cell Mol Neurobiol. 2012;32:943–947. doi: 10.1007/s10571-011-9789-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merendino AM, Bucchieri F, Campanella C, Marcianò V, Ribbene A, David S, Zummo G, Burgio G, Corona DF, Conway de Macario E, Macario AJL, Cappello F. Hsp60 is actively secreted by human tumor cells. PLoS One. 2010;5:e9247. doi: 10.1371/journal.pone.0009247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misaki T, Takeuchi R, Miyamoto S, Hirano A, Kasagi K, Konishi J. Induction in vitro of 72-kD heat shock protein in a continuous culture of rat thyroid cells, FRTL5. Clin Exp Immunol. 1994;98:234–239. doi: 10.1111/j.1365-2249.1994.tb06131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterloh A, Kalinke U, Weiss S, Fleischer B, Breloer M. Synergistic and differential modulation of immune responses by Hsp60 and lipopolysaccharide. J Biol Chem. 2007;282:4669–4680. doi: 10.1074/jbc.M608666200. [DOI] [PubMed] [Google Scholar]
- Pace A, Barone G, Lauria A, Martorana A, Piccionello AP, Pierro P, Terenzi A, Almerico AM, Buscemi S, Campanella C, Angileri F, Carini F, Zummo G, Conway de Macario E, Cappello F, Macario AJL. Hsp60, a novel target for antitumor therapy: structure–function features and prospective drugs design. Curr Pharm Des. 2013;19:2757–2764. doi: 10.2174/1381612811319150011. [DOI] [PubMed] [Google Scholar]
- Pockley AG, Bulmer J, Hanks BM, Wright BH. Identification of human heat shock protein 60 (Hsp60) and anti-Hsp60 antibodies in the peripheral circulation of normal individuals. Cell Stress Chaperones. 1999;4:29–35. doi: 10.1379/1466-1268(1999)004<0029:IOHHSP>2.3.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pockley AG, Muthana M, Calderwood SK. The dual immunoregulatory roles of stress proteins. Trends Biochem Sci. 2008;33:71–79. doi: 10.1016/j.tibs.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Rappa F, Farina F, Zummo G, David S, Campanella C, Carini F, Tomasello G, Damiani P, Cappello F, Conway de Macario E, Macario AJL (2012) HSP-molecular chaperones in cancer biogenesis and tumor therapy: an overview. Anticancer Res 32:5139–5150 [PubMed]
- Rea IM, McNerlan S, Pockley AG. Serum heat shock protein and anti-heat shock protein antibody levels in aging. Exp Gerontol. 2001;36:341–352. doi: 10.1016/S0531-5565(00)00215-1. [DOI] [PubMed] [Google Scholar]
- Rizzo M, Cappello F, Marfil R, Nibali L, Marino Gammazza A, Rappa F, Bonaventura G, Galindo-Moreno P, O'Valle F, Zummo G, Conway de Macario E, Macario AJL, Mesa F. Heat-shock protein 60 kDa and atherogenic dyslipidemia in patients with untreated mild periodontitis: a pilot study. Cell Stress Chaperones. 2012;17:399–407. doi: 10.1007/s12192-011-0315-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodolico V, Tomasello G, Zerilli M, Martorana A, Pitruzzella A, Gammazza AM, David S, Zummo G, Damiani P, Accomando S, Conway de Macario E, Macario AJL, Cappello F. Hsp60 and Hsp10 increase in colon mucosa of Crohn’s disease and ulcerative colitis. Cell Stress Chaperones. 2010;15:877–884. doi: 10.1007/s12192-010-0196-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serradifalco C, Catanese P, Rizzuto L, Cappello F, Puleio R, Barresi V, Nunnari CM, Zummo G, Di Felice V. Embryonic and foetal Islet-1 positive cells in human hearts are also positive to c-Kit. Eur J Histochem. 2011;55:e41. doi: 10.4081/ejh.2011.e41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh H, Ansari HR, Raghava GP. Improved method for linear B-cell epitope prediction using antigen's primary sequence. PLoS One. 2013;8(5):e62216. doi: 10.1371/journal.pone.0062216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasello G, Rodolico V, Zerilli M, Martorana A, Bucchieri F, Pitruzzella A, Marino Gammazza A, David S, Rappa F, Zummo G, Damiani P, Accomando S, Rizzo M, Conway de Macario E, Macario AJL, Cappello F. Changes in immunohistochemical levels and subcellular localization after therapy and correlation and colocalization with CD68 suggest a pathogenetic role of Hsp60 in ulcerative colitis. Appl Immunohistochem Mol Morphol. 2011;19:552–561. doi: 10.1097/PAI.0b013e3182118e5f. [DOI] [PubMed] [Google Scholar]
- Tomasello G, Sciumé C, Rappa F, Rodolico V, Zerilli M, Martorana A, Cicero G, De Luca R, Damiani P, Accardo FM, Romeo M, Farina F, Bonaventura G, Modica G, Zummo G, Conway de Macario E, Macario AJL, Cappello F. Hsp10, Hsp70, and Hsp90 immunohistochemical levels change in ulcerative colitis after therapy. Eur J Histochem. 2011;55:e38. doi: 10.4081/ejh.2011.e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulecke MA, Wang HH. ThinPrep for cytologic evaluation of follicular thyroid lesions: correlation with histologic findings. Diagn Cytopathol. 2004;30:7–13. doi: 10.1002/dc.10391. [DOI] [PubMed] [Google Scholar]
- Vanderpump MP, Tunbridge WM. Epidemiology and prevention of clinical and subclinical hypothyroidism. Thyroid. 2002;12:839–847. doi: 10.1089/105072502761016458. [DOI] [PubMed] [Google Scholar]
- Xie J, Zhu H, Guo L, Ruan Y, Wang L, Sun L, Zhou L, Wu W, Yun X, Shen A, Gu J. Lectin-like oxidized low-density lipoprotein receptor-1 delivers heat shock protein 60-fused antigen into the MHC class I presentation pathway. J Immunol. 2010;185:2306–2313. doi: 10.4049/jimmunol.0903214. [DOI] [PubMed] [Google Scholar]
- Yokota SI, Hirata D, Minota S, Higashiyama T, Kurimoto M, Yanagi H, Yura T, Kubota H. Autoantibodies against chaperonin CCT in human sera with rheumatic autoimmune diseases: comparison with antibodies against other Hsp60 family proteins. Cell Stress Chaperones. 2000;5:337–346. doi: 10.1379/1466-1268(2000)005<0337:AACCIH>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanin-Zhorov A, Cahalon L, Tal G, Margalit R, Lider O, Cohen IR. Heat shock protein 60 enhances CD4+ CD25+ regulatory T cell function via innate TLR2 signaling. J Clin Invest. 2006;116:2022–2032. doi: 10.1172/JCI28423. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]