Abstract
Human DNAJC12 is a J domain-containing protein whose regulation, subcellular localization, and function are currently unknown. We show here that the abundance of DNAJC12 in human LNCaP prostate cancer cells is upregulated by the stress-inducing drug A23187 and by the stress-regulated transcription factor AIbZIP/CREB3L4. The DNAJC12 gene encodes two isoforms, only one of which (isoform a) is expressed in these cells. Immunofluorescence studies showed that a recombinant DNAJC12 protein is diffusely distributed in the cytoplasm. To identify substrates of DNAJC12, we used an immunoaffinity-mass spectrometry approach in cells that express epitope-tagged DNAJC12. The list of potential DNAJC12-binding proteins that were identified in this screen includes several nucleotide-binding proteins. The most frequently identified partner of DNAJC12 in unstressed cells was Hsc70, a cognate Hsp70 chaperone, whereas in stressed cells, the ER chaperone BiP was frequently associated with DNAJC12. Immunoprecipitation experiments confirmed that the endogenous DNAJC12 and Hsc70 proteins interact in LNCaP cells. These results clarify the role of DNAJC12 in the regulation of Hsp70 function.
Keywords: DNAJC12, Hsc70, CREB3L4, Endoplasmic reticulum stress, LNCaP
Introduction
Heat shock 70 kDa proteins (Hsp70s) are molecular chaperones that play an essential role in ensuring that cellular proteins adopt and preserve the conformation required for their optimal function. These chaperones intervene at various stages of protein synthesis and function, including protein folding, protein transport, the assembly and disassembly of protein complexes, the control of protein activity, and ultimately, protein degradation (Daugaard et al. 2007). Hsp70 proteins are often referred to as “heat shock proteins” by virtue of the fact that the abundance of some of these proteins is upregulated by heat. In fact, in addition to the housekeeping functions mentioned previously, an important aspect of chaperone function includes protecting cellular proteins from the deleterious effects of stresses such as heat or endoplasmic reticulum (ER) stress (discussed below). However, some Hsp70 proteins are constitutively expressed.
The human genome contains 11 genes that encode Hsp70 proteins. Each of these chaperones is composed of two domains, an amino-terminal adenine nucleotide-binding domain with ATPase activity and a carboxy-terminal domain that interacts with the exposed hydrophobic segments of improperly folded “client” proteins (Daugaard et al. 2007, Kampinga and Craig 2010). To enable their client proteins to fold properly, Hsp70 proteins alternately bind and release the misfolded proteins. Hsp70 binding to a client protein prevents the latter from aggregating whereas dissociation from Hsp70 allows the client protein time to adopt its proper conformation. Stable binding of Hsp70 to its client proteins depends on a conformational change in Hsp70 which results from hydrolysis of ATP to ADP by the ATPase domain of Hsp70. However, because the intrinsic ATPase activity of Hsp70 proteins is low, Hsp70 proteins depend on cofactors known as DnaJ proteins to stimulate their ATPase activity and thereby stabilize their interaction with client proteins.
There are 41 genes that encode DnaJ proteins in humans; all of which contain the signature “J domain” through which DnaJ proteins interact with the ATPase domain of Hsp70 proteins to stimulate ATP hydrolysis (Kampinga and Craig 2010, Qiu et al. 2006). DnaJ proteins are named after the founding member of this protein family, the Escherichia coli DnaJ protein, but they are also referred to as heat shock 40 kDa proteins (Hsp40). DnaJ proteins constitute a structurally and functionally diverse group of proteins. In addition to the J domain, most DnaJ proteins contain motifs that confer upon them the ability to interact with other cellular proteins with varying degrees of specificity. In addition to these structural differences, DnaJ proteins also display specificity in their subcellular localization. In fact, some DnaJ proteins are present in several cellular compartments whereas others show a more restricted distribution.
One of the lesser characterized DnaJ proteins is DNAJC12, also named J domain-containing protein 1 (Lee et al. 2000). DNAJC12 contains a single recognizable functional motif, the J domain, and its binding partners, subcellular localization, and function are unknown (Kampinga and Craig 2010). While the specific function of DNAJC12 is unknown, the available data indicate that its abundance is regulated by physiological stimuli. De Bessa et al. (2006) previously reported that female sex steroids (estrogens) upregulate DNAJC12 mRNA levels in estrogen-sensitive MCF7 human breast cancer cells. In addition, we recently made the interesting observation that DNAJC12 mRNA is upregulated by the transcription factor androgen-induced bZIP/CREB3L4 (AIbZIP) in human prostate cells (Ben Aicha et al. 2007). This observation is particularly significant because AIbZIP localizes to the ER, and it is activated by regulated intramembrane proteolysis (RIP) in cells that are exposed to agents that induce ER stress (Ben Aicha et al. 2007).
AIbZIP is a member of the CREB3 family of transcription factors whose other members are CREB3, OASIS, BBF2H7, and CREBH (Asada et al. 2011, Chan et al. 2011). The mechanism whereby AIbZIP (like other CREB3 proteins) is activated by proteolysis is analogous to that of activating transcription factor 6 (ATF6), a transcription factor whose central role in the cellular response to ER stress is well established (Hetz 2012). The ER stress response (also referred to as the unfolded protein response) is a complex adaptive response that is triggered by the accumulation of misfolded proteins in the ER. ATF6 activation in response to ER stress results in the production of the transcriptionally active form of ATF6 which in turn induces the expression of genes that code for chaperones, such as BiP/GRP78, and other proteins that function to restore ER homeostasis. The observation that DNAJC12 is upregulated by AIbZIP suggested that DNAJC12 might function in the ER stress response.
The objectives of the present study were twofold. The first objective was to validate that AIbZIP upregulates DNAJC12 and to determine if DNAJC12 is induced by ER stress. The second objective was to identify the binding partners of DNAJC12 with the expectation that the identification of such partners should considerably increase our understanding of the function of DNAJC12.
Materials and methods
Cell lines and reagents
LNCaP cells were obtained from the American Type Culture Collection and cultured as described (Lessard et al. 2007). The single-vector format of the RheoSwitch conditional expression system (Lessard et al. 2007) and the RheoSwitch cell line (clone 7-11) that produces the nuclear form of AIbZIP (Ben Aicha et al. 2007) have been described previously. The plasmid pZX-DNAJC12 was used to generate stably transfected LNCaP cells (clone 37-3) that support RSL1-dependent conditional expression of recombinant DNAJC12 containing a C-terminal HA tag (hereafter referred to as DNAJC12HA). A pcDNA3 expression plasmid encoding BiP fused to a C-terminal FLAG epitope was used to transiently express BiP. Plasmid details are available upon request. A23187 was dissolved in DMSO and used at a final concentration of 2 μM. RSL1 was dissolved in DMSO and used at a final concentration of 500 nM.
Immunoblotting
Immunoblotting experiments were performed using standard techniques. Equal amounts of protein were separated by SDS-PAGE and transferred to nitrocellulose filters. The primary antibodies were rabbit polyclonal DNAJC12 antibody (12338-1-AP, Proteintech), mouse monoclonal Hsc70 antibody (B-6, Santa Cruz Biotechnology), and rabbit polyclonal B23 antibody (C-19, Santa Cruz Biotechnology). The DNAJC12 antibody was raised against a fusion protein containing the full-length DNAJC12a protein. Antigen–antibody complexes were visualized using an enhanced chemiluminescence detection method.
Promoter activity assays
A fragment of the DNAJC12 gene comprising 1 kb of promoter DNA and the 5′ untranslated region of exon 1 was amplified by PCR using oligonucleotides 5′-GGGACGAGGTACCCAGGAGAAATAGCACAGCCATCTGA-3′ and 5′-GGGACGAC CATGGTGATGACTTAATCAGTCCTTCTTCCTCGGA-3′ and cloned immediately upstream of the luciferase open reading frame in the Kpn I and Nco I sites (underlined in oligonucleotide sequences) of plasmid pGL3-Basic. A pcDNA3 expression plasmid encoding the amino-terminal fragment of AIbZIP (aa 1-290) was constructed by PCR. Transient transfection experiments were performed using standard procedures. LNCaP cells were plated at a density of 4 × 105 cells/well in 12-well plates. In addition to the luciferase and expression plasmids, cells were transfected with a promoter-less renilla luciferase reporter plasmid (pRL-null, Promega) to control for transfection efficiency. Promoter activity was defined as the ratio of firefly luciferase activity to Renilla luciferase activity and expressed as the mean ± SEM of triplicate wells.
Immunofluorescence
LNCaP cells that conditionally express DNAJC12HA (clone 37-3) were grown on 18 mm cover slips and exposed to RSL1 to induce production of DNAJC12HA. To detect BiP, some DNAJC12HA-expressing cells were transfected with a plasmid expressing FLAG-tagged BiP. The cells were fixed in formalin solution, washed in PBS, and then stained with DAPI. Following additional PBS washes, the cells were incubated in TRIS-buffered saline containing 0.2 % triton and then incubated in blocking buffer (2 % milk) for 1 h at room temperature prior to addition of the primary antibodies. A 1:500 dilution of rabbit polyclonal DNAJC12 antibody (12338-1-AP, Proteintech) or a 1:1000 dilution of mouse monoclonal anti-FLAG antibody (F3165, Sigma) was incubated at 4 °C overnight in 2 % milk. Secondary antibodies (1:400 dilutions of either Alexa 594-conjugated goat anti-mouse or Alexa 546-conjugated goat anti-rabbit; Life Technologies) were incubated for 1 h at room temperature, after which, the slides were washed in PBS. After mounting the cover slips to slides, the cells were examined under a microscope (Nikon Eclipse 80i) at ×100 magnification and images were taken using a digital camera (Retiga QICAM FAST 1394 Color 12-bit) and Bioquant Nova Prime software. Color images were converted to black and white. Negative controls (not shown) were performed by incubating control and protein-expressing cells with primary or secondary antibodies alone.
Identification of DNAJC12HA-binding proteins by mass spectrometry
Cells that conditionally express DNAJC12HA (clone 37-3) were treated with vehicle alone, RSL1 (500 nM × 24 h) or RSL1 and A23187 (2 μM × 24 h). Cellular proteins were solubilized in 10 mM Tris–HCl (pH 8.0), 1 mM EDTA, 150 mM NaCl, 1 % NP-40, and protease inhibitors at 4 °C for 1 h. The cell lysates were then incubated with anti-HA antibody resin (Sigma-Aldrich) overnight at 4 °C, after which, the immune complexes were recovered by low speed centrifugation. The resin was washed three times with the same buffer, and bound proteins were then eluted from the resin with urea. The protein samples were subjected to short SDS-PAGE, stained with colloidal blue, excised from the gel, and sent to the proteomics platform of the Québec Genomics Center for digestion with trypsin and analysis by mass spectrometry. The mass spectrometry data were visualized using Scaffold version 4.0 (Proteome Software). Proteins that were detected in all three samples were considered contaminants, and they were not analyzed further.
Co-immunoprecipitation
Confluent LNCaP cell cultures in 10 cm dishes were lysed in 10 mM Tris–HCl (pH 8.0), 1 mM EDTA, 150 mM NaCl, and 1 % NP-40 for 1 h at 4 °C. The lysates were centrifuged at 13,000 rpm for 15 min at 4 °C. The supernatants were then pre-cleared with protein A-Sepharose for 40 min at 4 °C and subsequently centrifuged. The resulting lysates were incubated with anti-DNAJC12 antibody overnight at 4 °C following which protein A-Sepharose was added and incubation continued for one more hour. Protein complexes were recovered by centrifugation at 3,000 rpm for 3 min, washed three times, and resolved by SDS-PAGE. Cell lysates incubated with protein A beads alone were used as a negative control.
Results and discussion
The human DNAJC12 gene consists of five exons that yield two transcripts by alternative splicing. The longer transcript (variant 1) encodes the full-length 198 amino acid DNAJC12 protein (isoform a) whereas the shorter transcript (variant 2) encodes the 107 amino acid isoform b. Both isoforms contain identical DnaJ domains in their N-terminal portions, but the C-terminal portion of variant b is much shorter than that of variant a. We previously reported that AIbZIP upregulates DNAJC12 mRNA, but the hybridization data from that experiment did not allow us to determine if one or both variants were regulated (Ben Aicha et al. 2007). We therefore conducted experiments to determine (1) which DNAJC12 isoform is expressed in LNCaP cells and (2) if the upregulation of DNAJC12 mRNA by the processed form of AIbZIP results in an appreciable increase in the abundance of the DNAJC12 protein.
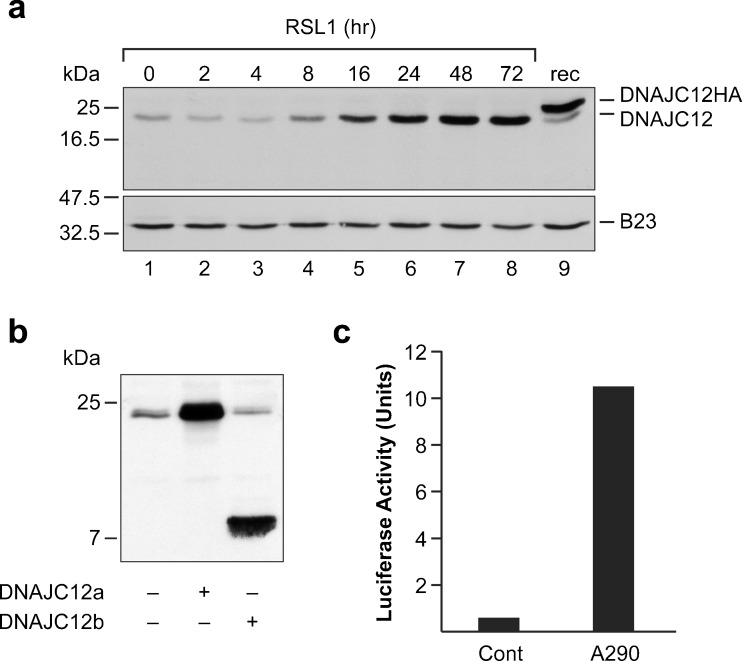
For this, we used LNCaP cells (clone 7-11) that were engineered to conditionally express the transcriptionally active amino-terminal fragment of human AIbZIP (Ben Aicha et al. 2007). The recombinant protein produced in these cells, which we refer to as A290, corresponds to the first 290 amino acids of AIbZIP. The cell line was created using the RheoSwitch expression system that relies on the small molecule RSL1 to activate specially engineered transcription factors which control the expression of the gene of interest (Lessard et al. 2007). The RSL1-dependent production of A290 in these cell lines has been characterized in great detail (Ben Aicha et al. 2007, Lessard et al. 2007). To evaluate the abundance of DNAJC12, we used an antibody that, as confirmed using transiently expressed proteins (Fig. 1b), recognizes both DNAJC12 isoforms. The cells were incubated in the presence of RSL1 for 2 to 72 h to induce A290, and whole-cell extracts were prepared at predetermined intervals. In untreated LNCaP cells, the anti-DNAJC12 antibody detected a polypeptide whose apparent molecular weight was consistent with that of the longer DNAJC12 isoform (Fig.1a). Addition of RSL1 to the culture medium resulted in a time-dependent increase in the abundance of this protein. A polypeptide corresponding to the shorter DNAJC12 isoform was not detected in control or RSL1-treated cells. This result indicates that the processed form of AIbZIP upregulates the abundance of DNAJC12 isoform a.
Fig. 1.
AIbZIP upregulates DNAJC12 in prostate cells. a LNCaP cells that conditionally express A290 were exposed to the inducing agent RSL1 for 2 to 72 h. Whole-cell extracts were incubated with antibodies against DNAJC12 and as a loading control, the nuclear protein B23. Lane 9 contains an extract of cells transfected with a plasmid that expresses HA-tagged DNAJC12 isoform a. b LNCaP cells were transiently transfected with CMV expression plasmids encoding DNAJC12a or DNAJC12b. The recombinant proteins were detected using a polyclonal antibody raised against full-length DNAJC12a. c LNCaP cells were transiently transfected with a DNAJC12 luciferase reporter gene and a plasmid expressing the N-terminal fragment of AIbZIP (A290). Luciferase activity (expressed in arbitrary units) was corrected for transfection efficiency using a cotransfected renilla luciferase construct. The results presented in panels a and b are representative of at least three independent experiments
We then sought to determine if the upregulation of DNAJC12 by AIbZIP results from a stimulatory effect of AIbZIP on DNAJC12 promoter activity (as opposed to an effect on mRNA stability). For these experiments, we constructed a luciferase reporter gene containing approximately 1.2 kb of the human DNAJC12 gene that we cloned immediately upstream of the luciferase ATG codon. The genomic fragment includes 1 kb of 5′ regulatory DNA and the entire untranslated region of the first coding exon (exon 1). To assess the effect of AIbZIP, we transiently expressed the amino-terminal fragment of AIbZIP (A290) and the DNAJC12 reporter construct into LNCaP cells. As shown in Fig.1c, transient expression of A290 produced a robust increase in DNAJC12 promoter activity. These results confirm that the increase in DNAJC12 protein levels observed in cells that express A290 results, at least in part, from a transcriptional effect of AIbZIP on DNAJC12 gene expression.
We then conducted experiments to determine if ER stress upregulates DNAJC12 protein levels. For this experiment, we used the calcium ionophore A23187. In LNCaP cells (and in other cell types), A23187 causes ER stress, as evidenced by increased levels of the stress-induced ER chaperone BiP/GRP78, and it induces the processing of AIbZIP by RIP (Ben Aicha et al. 2007). LNCaP cells were incubated with A23187 (2 μM) for 4 to 48 h, and whole-cell extracts were prepared at predetermined intervals and analyzed by immunoblotting using a commercially available anti-DNAJC12 antibody. As shown in Fig.2, A23187 produced an appreciable increase in DNAJC12 levels that was manifest at 8 h and peaked around 16 to 24 h. As observed in A290-expressing cells, the DNAJC12 isoform that was upregulated by ER stress was isoform a. The induction of DNAJC12 by A23187 was only slightly slower than the induction of the ER chaperone BiP (Ben Aicha et al. 2007).
Fig. 2.
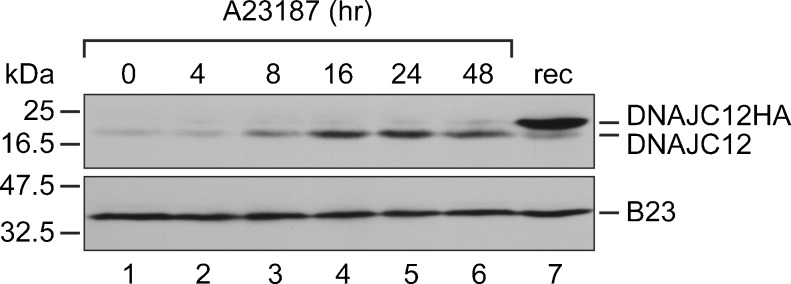
ER stress upregulates DNAJC12 in prostate cells. LNCaP cells were exposed to 2 μM A23187 for 4 to 48 h. Whole-cell extracts were incubated with antibodies against DNAJC12 and as a loading control, the nuclear protein B23. Lane 7 contains an extract of cells transfected with a plasmid that expresses HA-tagged DNAJC12 isoform a. These results are representative of at least three independent experiments
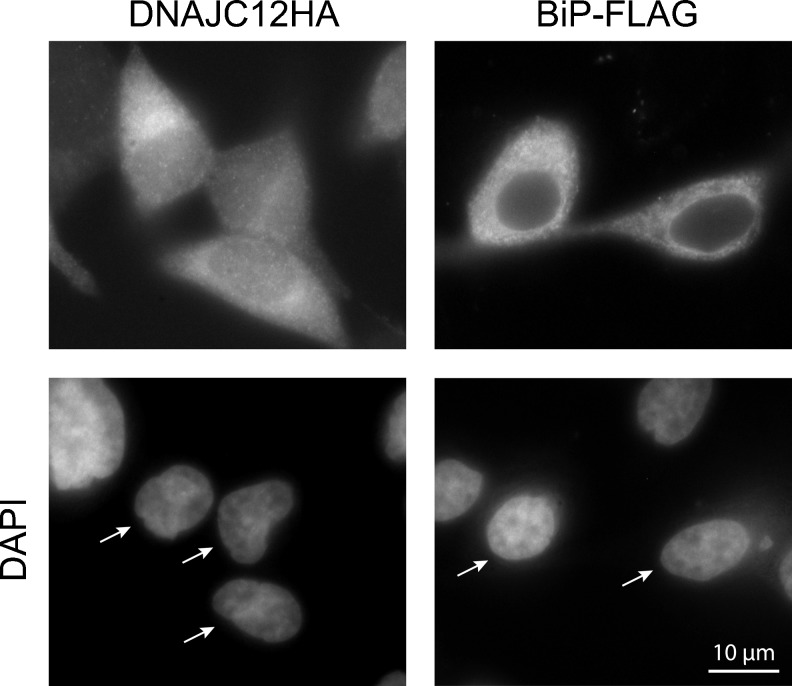
Having established a link between ER stress and DNAJC12, we then sought to clarify the physiological role of DNAJC12 by identifying proteins that associate with DNAJC12. For this, we generated LNCaP cell lines that conditionally express recombinant DNAJC12 isoform a. An HA epitope tag was appended to the C terminus of DNAJC12 to facilitate detection of the recombinant protein as well as to allow the purification of DNAJC12-containing protein complexes. The C-terminal tag is not expected to interfere with the localization of DNAJC12 because DNAJC12 does not contain an ER retention signal at its C terminus. Once we had obtained DNAJC12HA-expressing cells, we proceeded to determine the subcellular localization of DNAJC12 as this information should provide some indication regarding the nature of the potential targets of DNAJC12 and its physiological function. We therefore induced DNAJC12HA production (using RSL1) and detected DNAJC12HA by indirect immunofluorescence using an anti-DNAJC12 antibody and a fluorochrome-conjugated secondary antibody. DNAJC12HA was mainly distributed throughout the cytoplasm and there was slight nuclear staining (Fig.3). For comparison, we assessed the localization of transiently expressed BiP-FLAG in DNAJC12HA-expressing cells. BiP was widely distributed throughout the cytoplasm, a distribution that is consistent with that reported previously by others (Li et al. 2013). These results show that the localization of DNAJC12 partially overlaps that of BiP.
Fig. 3.
Subcellular localization of DNAJC12. LNCaP cells that conditionally express DNAJC12HA (clone 37-3) were exposed to RSL1 and the localization of DNAJC12HA was assessed by indirect immunofluorescence using an anti-DNAJC12 antibody and Alexa Fluor 546-conjugated antibody. For comparison, DNAJC12HA-expressing cells were transfected with FLAG-tagged BiP and the localization of BiP-FLAG was assessed using an anti-FLAG antibody. The color images were converted to black and white such that DNAJC12, BiP, and cell nuclei appear in white. This figure is representative of at least three independent experiments
Next, we performed large-scale immunopurification experiments to isolate DNAJC12-containing protein complexes. Three experimental conditions were compared in parallel: control cells that were treated with vehicle alone, cells that were treated with RSL1 alone (to induce DNAJC12HA), and cells that were treated with both RSL1 and A23187 (to induce DNAJC12HA and ER stress). Cell lysates from each culture were incubated with an anti-HA antibody resin after which the bound proteins were eluted, digested with trypsin, and the resulting peptides were identified by mass spectrometry. As expected, peptides corresponding to DNAJC12 were not identified in the control cells. In contrast, 17 unique peptides covering 67 % of the DNAJC12 protein sequence were identified in cells treated with RSL1 alone, as well as in cells treated with both RSL1 and A23187. The following discussion pertains to proteins that were identified with a high degree of certitude (≥98 % probability) in cells that expressed DNAJC12HA and that were not detected in the control sample.
In addition to DNAJC12, 44 proteins were immunopurified from cells treated with RSL1 alone (Table 1). These proteins include chaperones of different families, enzymes (e.g., a subunit of ATP synthase), as well as proteins involved in protein biosynthesis (e.g., translation elongation factors, tRNA synthetases). Remarkably, 21 of the 44 proteins (48 %) are nucleotide-binding proteins, which suggest that DNAJC12 may have a particular affinity for nucleotide-binding proteins. In addition, 21 of the 44 proteins localize to mitochondria, which suggest that DNAJC12 may play an important role in mitochondrial processes. However, the most frequently identified protein, both in terms of unique peptides and as a percentage of the total spectra, was the cognate chaperone Hsc70 (also known as Hsp73 or Hsp70-8), that is encoded by the HSPA8 gene, a ubiquitously and constitutively expressed essential housekeeping gene (Daugaard et al. 2007). Hsc70-derived spectra represented 0.29 % of all the spectra (by comparison, DNAJC12-derived peptides represented 1.03 % of all spectra), and 21 unique peptides corresponding to Hsc70 were identified. Interestingly, the second most frequently identified DNAJC12-associated protein was pyruvate carboxylase (PC), a mitochondrial enzyme responsible for the synthesis of oxaloacetate, an intermediate of the tricarboxylic acid cycle (Jitrapakdee et al. 2008). Ten unique peptides corresponding to PC were identified, and PC-derived spectra represented 0.08 % of all the spectra. The other proteins that immunopurified with DNAJC12HA yielded one to seven unique peptides each, and each of these proteins corresponded to 0.05 % or less of the total spectra.
Table 1.
Proteins identified with ≥98 % probability in cells expressing DNAJC12HA (ordered by decreasing number of unique peptides)
| Gene | UniProt | Official name or alternate designation (NCBI) | Function | NBa | Mit Locb |
Uniq Pepsc |
Spec (%)d |
|---|---|---|---|---|---|---|---|
| Symbol | Entry | ||||||
| HSPA8 | P11142 | Heat shock 70 kDa protein 8 | Chaperone | ● | 21 | 0.29 | |
| DNAJC12 | Q9UKB3 | DnaJ homolog, subfamily C, member 12 | Chaperone cofactor | 17 | 1.03 | ||
| PC | P11498 | Pyruvate carboxylase | Glucose and lipid synthesis | ● | ● | 10 | 0.08 |
| HSP90AB1 | P08238 | Heat shock protein 90 kDa alpha, class B member 1 | Chaperone | ● | 7 | 0.05 | |
| IDH2 | P48735 | Isocitrate dehydrogenase 2 (NADP+), mitochondrial | Intermediary metabolism | ● | 6 | 0.04 | |
| ATP5B | P06576 | ATP synthase subunit beta, mitochondrial | ATP synthesis | ● | ● | 5 | 0.03 |
| HSPA4 | O14992 | Heat shock 70 kDa protein 4 | Chaperone | ● | 5 | 0.03 | |
| HSPD1 | P10809 | Heat shock 60 kDa protein 1 | Chaperone | ● | ● | 5 | 0.03 |
| GAPDH | P04406 | Glyceraldehyde-3-phosphate dehydrogenase | Glycolysis | 4 | 0.03 | ||
| UQCRC2 | P22695 | Ubiquinol-cytochrome c reductase core protein II | Respiration | ● | 4 | 0.03 | |
| ALDH1B1 | P30837 | Aldehyde dehydrogenase 1 family, member B1 | Alcohol metabolism | ● | 3 | 0.02 | |
| CLPX | O76031 | ClpX caseinolytic peptidase X homolog | Chaperone | ● | ● | 3 | 0.02 |
| EEF2 | P13639 | Eukaryotic translation elongation factor 2 | Protein biosynthesis | ● | 3 | 0.02 | |
| HSPA1A | P08107 | Heat shock 70 kDa protein 1A | Chaperone | ● | 3 | 0.02 | |
| MTHFD1 | P11586 | Cytoplasmic C-1-tetrahydrofolate synthase | Tetrahydrofolate metabolism | ● | ● | 3 | 0.02 |
| SLC25A3 | Q53HC3 | Solute carrier family 25 member 3, mitochondrial | Phosphate carrier | ● | 3 | 0.02 | |
| B3KUD7 | FLJ39640, Highly similar to MCM7 | DNA replication (presumed) | 3 | 0.02 | |||
| EEF1G | P26641 | Eukaryotic translation elongation factor 1 gamma | Protein biosynthesis | 2 | 0.03 | ||
| ACADSB | P45954 | Acyl-CoA dehydrogenase, short/branched chain | Dehydrogenase | ● | 2 | 0.02 | |
| ETFA | P13804 | Electron-transfer-flavoprotein, alpha polypeptide | Electron transport | ● | 2 | 0.02 | |
| SEC61A1 | P61619 | Sec61 alpha 1 subunit | Protein transport | 2 | 0.02 | ||
| SLC25A5 | P05141 | Solute carrier family 25 member 5 | Adenine nucleotide translocator | ● | 2 | 0.02 | |
| TIMM50 | Q0VAB1 | Translocase of inner mitochondrial membrane 50 homolog | Translocase | ● | 2 | 0.02 | |
| ALDH18A1 | Q3KQU2 | Aldehyde dehydrogenase 18 family, member A1 | Amino acid synthesis | ● | ● | 2 | 0.01 |
| ATL3 | Q6DD88 | Atlastin GTPase 3 | ER/Golgi organization | ● | 2 | 0.01 | |
| HSD17B4 | P51659 | Hydroxysteroid (17-beta) dehydrogenase 4 | Fatty acid metabolism | 2 | 0.01 | ||
| HSPA9 | P38646 | Heat shock 70 kDa protein 9 | Chaperone | ● | ● | 2 | 0.01 |
| NT5DC2 | Q9H857 | 5′-nucleotidase domain-containing 2 | Hydrolase | 2 | 0.01 | ||
| PFKL | P17858 | Phosphofructokinase, liver | Glycolysis | ● | 2 | 0.01 | |
| PYCR2 | Q96C36 | Pyrroline-5-carboxylate reductase family, member 2 | Amino acid synthesis | ● | 2 | 0.01 | |
| PYCRL | Q53H96 | Pyrroline-5-carboxylate reductase-like | Amino acid synthesis | 2 | 0.01 | ||
| SUCLG1 | P53597 | Succinate Co-A ligase, alpha subunit | ATP/GTP synthesis | ● | ● | 2 | 0.01 |
| TRIM28 | Q13263 | Tripartite motif containing 28 | Transcriptional regulation | 2 | 0.01 | ||
| Q68CW5 | DKFZp762A1314 | Unknown | 2 | 0.01 | |||
| ARF3 | P61204 | ADP-ribosylation factor 3 | Protein trafficking | ● | 1 | 0.01 | |
| MVP | Q14764 | Major vault protein | Signal transduction | 1 | 0.01 | ||
| SHMT2 | P34897 | Serime hydroxymethyltransferase 2, mitochondrial | Amino acid synthesis | ● | 1 | 0.01 | |
| CDK1 | P06493 | Cyclin-dependent kinase 1 | Cell cycle regulation | ● | 1 | <0.01 | |
| EIF4A1 | P60842 | Eukaryotic translation initiation factor 4A1 | Protein biosynthesis | ● | 1 | <0.01 | |
| ETFB | P38117 | Electron-transfer-flavoprotein, beta polypeptide | Electron transport | ● | 1 | <0.01 | |
| HIP1R | O75146 | Huntingtin interacting protein 1 related | Endocytosis | 1 | <0.01 | ||
| IARS2 | Q9NSE4 | Isoleucyl-tRNA synthetase 2, mitochondrial | Protein biosynthesis | ● | ● | 1 | <0.01 |
| PKM | P14618 | Pyruvate kinase, muscle | Glycolysis | ● | ● | 1 | <0.01 |
| PSMC6 | P62333 | Proteasome 26S subunit, ATPase, 6 | Protein degradation | ● | 1 | <0.01 | |
| TKT | P29401 | Transketolase | Glycolysis | 1 | <0.01 |
Dots in the column labelled NB indicate that the gene encodes a nucleotide‐binding protein; Dots in the column labelled Mit Loc encode proteins that localize to mitochondria
aNucleotide binding
bMitochondrial localization
cNumber of unique peptides
dPercentage of total spectra
The majority of the 44 proteins that immunopurified with DNAJC12HA in unstressed cells was also identified in A23187-treated cells (data not shown). However, 26 proteins were identified (with ≥98 % probability) only in DNAJC12HA-expressing cells treated with A23187, but not in cells that were not exposed to A23187 (Table 2). Among these proteins, the one that yielded the greatest number of unique peptides and spectra was the Hsp70 chaperone BiP/GRP78. This is an interesting observation in light of the fact that BiP is upregulated by ER stress and plays a central role in the ER stress response (Kozutsumi et al. 1988). On this basis, it is tempting to speculate that BiP might rely on DNAJC12 to stimulate its ATPase activity in stressed cells. The finding that BiP is a major binding partner of DNAJC12 in A23187-treated cells is somewhat surprising considering that BiP is primarily considered an ER protein. While it is possible that the interaction between DNAJC12 and BiP might have occurred in the lysates of stressed cells rather than in whole cells, the immunofluorescence data show marked overlap between the distribution of the two proteins. In addition, there is evidence to suggest that BiP can also localize to other cellular compartments including the plasma membrane, cytoplasm, and mitochondria (Ni et al. 2011). The other proteins that immunopurified with DNAJC12HA in A23187-treated cells yielded one to five unique peptides each, and each protein corresponded to 0.03 % or less of the total spectra.
Table 2.
Proteins identified with ≥98 % probability only in DNAJC12HA-expressing cells exposed to A23187 (ordered by decreasing number of unique peptides)
| Gene | UniProt | Official name or alternate designation (NCBI) | Function | NBa | Mit Locb |
Uniq Pepsc |
Spec (%)d |
|---|---|---|---|---|---|---|---|
| Symbol | Entry | ||||||
| HSPA5 | P11021 | Heat shock 70 kDa protein 5 | Chaperone | ● | 21 | 0.17 | |
| RUVBL2 | Q9Y230 | RuvB-like 2 | Transcriptional regulation | ● | 5 | 0.03 | |
| TIMM44 | O43615 | Translocase of inner mitochondrial membrane 44 homolog | Translocase | ● | ● | 5 | 0.03 |
| YWHAE | P62258 | 14-3-3 epsilon | Signal transduction | 5 | 0.03 | ||
| DARS | P14868 | Aspartyl-tRNA synthetase | Protein biosynthesis | ● | 4 | 0.02 | |
| EPRS | P07814 | Gultamyl-prolyl-tRNA synthetase | Protein biosynthesis | ● | 4 | 0.02 | |
| PFKP | Q01813 | Phosphofructokinase, platelet | Glycolysis | ● | 3 | 0.02 | |
| RARS | P54136 | Arginyl-tRNA synthetase | Protein biosynthesis | ● | 3 | 0.02 | |
| Q6N0B1 | DKFZ p686D0880 | Unknown | 3 | 0.02 | |||
| COPB1 | P53618 | Coatomer protein complex, subunit beta 1 | Protein transport | 2 | 0.02 | ||
| PSMC2 | P35998 | Proteasome 26S subunit, ATPase, 2 | Protein degradation | ● | 2 | 0.02 | |
| AK4 | P27144 | Adenylate kinase 4 | Nucleotide homeostasis | ● | ● | 2 | 0.01 |
| DNM2 | P50570 | Dynamin 2 | Microtubule binding | ● | 2 | 0.01 | |
| HNRNPD | Q12771 | Heterogeneous nuclear ribonucleoprotein D | mRNA processing | ● | 2 | 0.01 | |
| KPNB1 | Q14974 | Importin beta 1 | Protein transport | 2 | 0.01 | ||
| NIPSNAP1 | Q9BPW8 | Nipsnap homolog 1 | Vesicular transport | ● | 2 | 0.01 | |
| RUVBL1 | Q9Y265 | RuvB-like 1 | Transcriptional regulation | ● | 2 | 0.01 | |
| TCP1 | P17987 | T-complex 1 | Chaperone | ● | 1 | 0.01 | |
| AARS | P49588 | Alanyl-tRNA synthetase | Protein biosynthesis | ● | 1 | 0.01 | |
| CCT6A | P40227 | Chaperonin containing TCP1, subunit 6A (zeta 1) | Chaperone | ● | 1 | 0.01 | |
| ISOC2 | Q6ZN91 | Isochorismatase domain-containing 2 | Protein destabilization | ● | 1 | 0.01 | |
| MARS | P56192 | Methionyl-tRNA synthetase | Protein biosynthesis | ● | 1 | 0.01 | |
| MCM6 | Q14566 | Minichromosome maintenance complex component 6 | DNA replication | ● | 1 | 0.01 | |
| NSF | P46459 | N-ethylmaleimide-sensitive factor | Membrane fusion | ● | 1 | 0.01 | |
| RAN | P62826 | RAN, member RAS oncogene family | Protein transport | ● | 1 | 0.01 | |
| PRKDC | P78527 | Protein kinase, DNA-activated, catalytic subunit | DNA repair | ● | 1 | <0.01 |
Dots in the column labelled NB indicate that the gene encodes a nucleotide‐binding protein; Dots in the column labelled Mit Loc encode proteins that localize to mitochondria
aNucleotide binding
bMitochondrial localization
cNumber of unique peptides
dPercentage of total spectra
Since Hsc70 was immunopurified with DNAJC12HA in both unstressed and stressed conditions, this chaperone appeared as a potentially important physiological binding partner of DNAJC12. We therefore sought to validate the interaction between DNAJC12 and Hsc70 by performing co-immunoprecipitation experiments in LNCaP cells. The cells were exposed to vehicle or A23187 (to upregulate endogenous DNAJC12), and the endogenous DNAJC12 protein was immunoprecipitated using a commercially available antibody. The presence of endogenous DNAJC12 and Hsc70 in immunoprecipitates was assessed by immunoblotting. In agreement with our earlier observations (Fig.1a), A23187 upregulated DNAJC12 protein levels, and accordingly, DNAJC12 was more efficiently immunoprecipitated from A23187-treated cells than from control cells (Fig.4). In addition, Hsc70 co-immunoprecipitated with DNAJC12 in A23187-treated cells, thereby confirming that the endogenous DNAJC12 and Hsc70 proteins interact. The fact that Hsc70 was not recovered from unstressed control cells is most likely due to the low abundance of DNAJC12 in these cells.
Fig. 4.
DNAJC12 interacts with Hsc70 in LNCaP cells. The cells were treated with A23187 (+) or vehicle (−) for 24 h. Cell lysates were either immunoprecipitated with an anti-DNAJC12 antibody (lanes 3 and 4) or incubated with protein A sepharose alone (lanes 5 and 6). Hsc70 (upper panel) and DNAJC12 (lower panel) were detected by immunoblotting. These results are representative of three independent experiments
To summarize, the observations reported here provide much new information regarding the localization, regulation, and function of DNAJC12. These experiments are especially enlightening as they identify DNAJC12 as a cofactor of the chaperone Hsc70. This finding assigns a specific function to DNAJC12 and conversely identifies a novel Hsc70 cofactor. In addition, we have identified a number of other potential DNAJC12-binding proteins. Most interestingly, many of these proteins bind nucleotides, especially ATP, and many of these proteins are enzymes. Further work will be required to validate these interactions and characterize their functional significance. Finally, we showed that ER stress upregulates DNAJC12 and provides evidence supporting a role for the ER-bound transcription factor AIbZIP in the regulation of DNAJC12 expression. More elaborate experiments will be required to clarify the regulation of DNAJC12 expression during ER stress.
Acknowledgments
This work was supported by an operating grant to C.L. from the Canadian Institutes of Health Research (MOP-77599). We thank C. Guillemette, N. Vallières, and L. Boudreau for the reagents and advice.
References
- Asada R, Kanemoto S, Kondo S, Saito A, Imaizumi The signalling from endoplasmic reticulum-resident bZIP transcription factors involved in diverse cellular physiology. J Biochem. 2011;149(5):507–518. doi: 10.1093/jb/mvr041. [DOI] [PubMed] [Google Scholar]
- Ben Aicha S, Lessard J, Pelletier M, Fournier A, Calvo E, Labrie C. Transcriptional profiling of genes that are regulated by the endoplasmic reticulum-bound transcription factor AIbZIP/CREB3L4 in prostate cells. Physiol Genomics. 2007;31(2):295–305. doi: 10.1152/physiolgenomics.00097.2007. [DOI] [PubMed] [Google Scholar]
- Chan CP, Kok KH, Jin DY. CREB3 subfamily transcription factors are not created equal: recent insight from global analyses and animal models. Cell Biosci. 2011;1(1):6. doi: 10.1186/2045-3701-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugaard M, Rohde M, Jaattela M. The heat shock protein 70 family: highly homologous proteins with overlapping and distinct functions. FEBS Lett. 2007;581(19):3702–3710. doi: 10.1016/j.febslet.2007.05.039. [DOI] [PubMed] [Google Scholar]
- De Bessa SA, Salaorni S, Patrao DFC, Neto MM, Brentani MM, Nagai MA. JDP1 (DNAJC12/Hsp40) expression in breast cancer and its association with estrogen receptor status. Int J Mol Med. 2006;17(2):363–367. [PubMed] [Google Scholar]
- Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol. 2012;13(2):89–102. doi: 10.1038/nrm3270. [DOI] [PubMed] [Google Scholar]
- Jitrapakdee S, St. Maurice M, Rayment I, Cleland WW, Wallace JC, Attwood PV. Structure, mechanism and regulation of pyruvate carboxylase. Biochem J. 2008;413(3):369–387. doi: 10.1042/BJ20080709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampinga HH, Craig EA. The HSP70 chaperone machinery: J proteins as drivers of functional specificity. Nat Rev Mol Cell Biol. 2010;11(8):579–592. doi: 10.1038/nrm2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozutsumi Y, Segal M, Normington K, Gething MJ, Sambrook J. The presence of malfolded proteins in the endoplasmic reticulum signals the induction of glucose-regulated proteins. Nature. 1988;332(6163):462–464. doi: 10.1038/332462a0. [DOI] [PubMed] [Google Scholar]
- Lessard J, Ben Aicha S, Fournier A, Calvo E, Lavergne E, Pelletier M, Labrie C. Characterization of the RSL1-dependent conditional expression system in LNCaP prostate cancer cells and development of a single vector format. Prostate. 2007;67(8):808–819. doi: 10.1002/pros.20559. [DOI] [PubMed] [Google Scholar]
- Lee J, Hahn Y, Yun JH, Mita K, Chung JH. Characterization of JDP genes, an evolutionarily conserved J domain-only protein family, from human and moths. Biochim Biophys Acta. 2000;1491(1–3):355–363. doi: 10.1016/S0167-4781(00)00047-6. [DOI] [PubMed] [Google Scholar]
- Li N, Zoubeidi A, Beraldi E, Gleave ME. GRP78 regulates clusterin stability, retrotranslocation and mitochondrial localization under ER stress in prostate cancer. Oncogene. 2013;32(15):1933–1942. doi: 10.1038/onc.2012.212. [DOI] [PubMed] [Google Scholar]
- Ni M, Zhang Y, Lee AS. Beyond the endoplasmic reticulum: atypical GRP78 in cell viability, signalling and therapeutic targeting. Biochem J. 2011;434(2):181–188. doi: 10.1042/BJ20101569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu XB, Shao YM, Miao S, Wang L. The diversity of the DnaJ/Hsp40 family, the crucial partners for Hsp70 chaperones. Cell Mol Life Sci. 2006;63(22):2560–2570. doi: 10.1007/s00018-006-6192-6. [DOI] [PMC free article] [PubMed] [Google Scholar]