Abstract
Background
Enthusiasts suggest that labouring in water and waterbirth increase maternal relaxation, reduce analgesia requirements and promote a midwifery model of care. Critics cite the risk of neonatal water inhalation and maternal/neonatal infection.
Objectives
To assess the evidence from randomised controlled trials about immersion in water during labour and waterbirth on maternal, fetal, neonatal and caregiver outcomes.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register (30 June 2011) and reference lists of retrieved studies.
Selection criteria
Randomised controlled trials comparing immersion in any bath tub/pool with no immersion, or other non-pharmacological forms of pain management during labour and/or birth, in women during labour who were considered to be at low risk of complications, as defined by the researchers.
Data collection and analysis
We assessed trial eligibility and quality and extracted data independently. One review author entered data and the other checked for accuracy.
Main results
This review includes 12 trials (3243 women): eight related to just the first stage of labour: one to early versus late immersion in the first stage of labour; two to the first and second stages; and another to the second stage only. We identified no trials evaluating different baths/pools, or the management of third stage of labour.
Results for the first stage of labour showed there was a significant reduction in the epidural/spinal/paracervical analgesia/anaesthesia rate amongst women allocated to water immersion compared to controls (478/1254 versus 529/1245; risk ratio (RR) 0.90; 95% confidence interval (CI) 0.82 to 0.99, six trials). There was also a reduction in duration of the first stage of labour (mean difference −32.4 minutes; 95% CI −58.7 to −6.13). There was no difference in assisted vaginal deliveries (RR 0.86; 95% CI 0.71 to 1.05, seven trials), caesarean sections (RR 1.21; 95% CI 0.87 to 1.68, eight trials), use of oxytocin infusion (RR 0.64; 95%CI 0.32 to 1.28,five trials), perineal trauma or maternal infection. There were no differences for Apgar score less than seven at five minutes (RR 1.58; 95% CI 0.63 to 3.93, five trials), neonatal unit admissions (RR 1.06; 95% CI 0.71 to 1.57, three trials), or neonatal infection rates (RR 2.00; 95% CI 0.50 to 7.94, five trials).
Of the three trials that compared water immersion during the second stage with no immersion, one trial showed a significantly higher level of satisfaction with the birth experience (RR 0.24; 95% CI 0.07 to 0.80).
A lack of data for some comparisons prevented robust conclusions. Further research is needed.
Authors’ conclusions
Evidence suggests that water immersion during the first stage of labour reduces the use of epidural/spinal analgesia and duration of the first stage of labour. There is limited information for other outcomes related to water use during the first and second stages of labour, due to intervention and outcome variability. There is no evidence of increased adverse effects to the fetus/neonate or woman from labouring in water or waterbirth. However, the studies are very variable and considerable heterogeneity was detected for some outcomes. Further research is needed.
BACKGROUND
This review is one in a series of Cochrane reviews examining pain management in labour. These reviews contribute to an overview of systematic reviews of pain management for women in labour (Jones 2011a), and share a generic protocol (Jones 2011b).
Throughout this review, ‘water immersion’ refers to the immersion in water by a pregnant woman during any stage of labour (first, second, third) where the woman’s abdomen is completely submerged. This implies the use of a receptacle that may be called a pool, tub or bath, and which is larger than a normal domestic bath. The period of immersion by the woman may be for one or more stages of labour, and for any duration. Labour is understood to be as defined by the woman or clinicians at the time, and includes regular painful uterine contractions, leading to full cervical dilation, expulsion of the fetus, and the placenta and membranes.
History
The use of water immersion as a therapeutic medium is not new. Its exact origins are unknown, but there is evidence of immersion in water being used as a treatment for physical and psychological ill health by the Chinese, Egyptians, Japanese and Assyrians, as well as Greeks and Romans (Reid Campion 1990; Reid-Campion 1997). Warm water immersion during labour, including birth, used for relaxation and pain relief, has a long history in lay and clinical care (Garland 2000). Igor Tjarkovsky, a Russian boat builder, stimulated the foundation of a movement to promote waterbirth in Soviet Russia in the 1970s. He became convinced of the benefits of water immersion as a means of maximising physiological potential. Michel Odent subsequently popularised water immersion in other European countries (Odent 1983). Although considered a fad by some, the use of water during labour and birth appeals to both women and their carers, particularly those striving for a woman-centred, intervention free, ‘normal’ experience. In 1995, the first international waterbirth conference was held in London, followed by many subsequent study events and international conferences.
Official acceptance of the use of water immersion as a care option during labour came in the UK in 1993, with the publication of the Changing Childbirth report (Department of Health 1993), which recommended that a pool facility should be an option available to women in all UK maternity units. Professional recognition of the use of water during labour and birth came in 1994 when both the Royal College of Midwives (RCM 1994) and the United Kingdom Central Council for Nursing, Midwifery and Health Visiting (UKCC 1994) published position statements, which incorporated water immersion during labour into the role of the midwife. The use of water during labour/birth is now integrated in the UK Midwifery Rules and standards (Nursing and Midwifery Council 2010), and UK policy for maternity services with section 8.4 of the National Service Framework for Children, Young People and Maternity Service (Department of Health 2004).
Most of the evidence on the use of water immersion is based on observational studies (Garland 1997; Garland 2002; Geissbuehler 2004; Ohlsson 2001; Thoeni 2005). A tension has arisen with regard to the perceived acceptability of randomised controlled trial (RCT) design, as some midwives and women perceive this as obviating maternal choice to what is now a widely available option, while women with strong preferences may decline to participate (Garland 1994). Factors such as depth of water, size of the pool and whether the water is still or aerated/whirlpool water have not been compared, as pool design and practice have tended to be based on local availability and customs.
Water immersion during first stage of labour - what it offers women
The positive physiological effects of hydrotherapy such as buoyancy, hydrostatic pressure, and associated thermal changes, are relevant to women labouring in water, where labour is defined as including the first, second (birth) and third stages. The buoyancy of water enables a woman to move more easily than on land (Edlich 1987). This can facilitate the neuro-hormonal interactions of labour, alleviating pain, and potentially optimising the progress of labour (Ginesi 1998a; Ginesi 1998b). Water immersion may be associated with improved uterine perfusion, less painful contractions, a shorter labour with fewer interventions (Aird 1997; Garland 2000; Moneta 2001; Otigbah 2000; Geissbuehler 2004; Thoeni 2005; Zanetti-Daellenbach 2007). In addition, the ease of mobility that water immersion offers women may optimise fetal position by encouraging flexion (Ohlsson 2001).
Hydrotherapy has marked physiological effects on the cardiovascular system (Cefalo 1978). Shoulder-deep warm water immersion reduces blood pressure due to vasodilatation of the peripheral vessels and redistribution of blood flow. It is suggested that water immersion during labour increases maternal satisfaction and sense of control (Hall 1998; Richmond 2003). A woman who feels in control during childbirth experiences greater emotional wellbeing postnatally (Green 1998).
The UK is promoting water immersion during labour and waterbirth as a means of empowering women and is consistent with the current agenda of normalising birth (Royal College of Midwives 2011), the consensus statement from the maternity Care Working Party (Maternity Care Working Party 2007); both of whom use a waterbirth image to illustrate normal birth, as does the Vision for Midwifery expressed by the Midwifery 2020 programme (Midwifery 2020 Program 2010).
Although the use of additives such as essential oils to the water appears to be gaining popularity (Calvert 2000), to date no trial has generated reliable evidence to support or refute the use of any additive.
Waterbirth (second stage of labour) - what it offers women
It has been suggested that waterbirth may reduce the uptake of pharmacological pain relief and likelihood of perineal trauma (Burke 1995; Burns 2001; Garland 2000; Geissbuehler 2000; Otigbah 2000). There may also be increased maternal satisfaction with the birth experience.
Water immersion and the fetus/neonate (first and/or second stage)
It could be argued that the fetus benefits from a relaxed mother, as this maximises placental oxygen perfusion. ‘Nature’s opiates’, endogenous endorphins, predominate. When the mother is not fearful, oxytocin is released to stimulate effective contractions. Labouring in water, compared to land, has been found to reduce stress hormones, catecholamines, which inhibit oxytocin and labour progress. The fetus may be more likely to adopt a flexed position, because the mother can easily explore different positions to maximise her pelvic diameters if the pool is sufficiently large (Ohlsson 2001).
Concerns raised in a survey and case reports about birth in water for the baby include 1) thermoregulation during labour, 2) infection, 3) respiratory difficulties and 4) snapped cord (Gilbert 1999; Kassim 2005; Mammas 2009; Nguyen 2002; Pinette 2004).
1. Thermo regulation
As with any labouring woman, it is important to avoid her becoming pyrexial. Therefore, the water temperature of a pool should not exceed the maternal body temperature, as immersing a woman in water above her natural core temperature will result in fetal hyperthermia and associated cardiovascular and metabolic disturbances (Johnson 1996). High temperatures have been identified as a safety issue by several authors as being associated with fetal mortality and morbidity, based on individual case studies and/or theory (Deans 1995; Johnson 1996; Rosevear 1993). The theory underpinning this was originally based on a study on pregnant ewes (Cefalo 1978). The fetus responded to an increase in maternal temperature by becoming tachycardiac, reducing resistance in the placenta bed and thus heat dissipation. As the temperature increased, there was a tendency to exceed the heat that could be dissipated by the placenta, leading to an increased risk of fetal mortality (Cefalo 1978). A review of the literature on temperature control in mammalian fetuses, mainly sheep, primates and a limited number of human participants, identified that the fetal metabolic processes produce heat (Power 1989). This heat is transferred to the mother primarily via the circulatory system, the umbilical cord and placenta where the large surface area and constant blood flow facilitate heat transfer. A second pathway for heat transfer is via fetal skin, to amniotic fluid, the uterus and maternal system. To enable this heat transfer, the fetus is 0.5°C warmer than the mother. This difference is apparently constant across species, although the basal temperatures differ (Power 1989). When maternal temperature increases, heat transfer is inhibited and the fetal temperature rises, until transfer is again possible. However, there is a concomitant rise in metabolic activity and oxygen demands as temperature increase, the effect of which may be seen in fetal heart rate changes, and which may contribute to fetal compromise during labour. Temperature regulation, and its assessment, are therefore important during labour/birth irrespective of the use of water immersion.
2. Respiration
The diving reflex prevents a healthy baby born in water from drowning. This is an apnoea on expiration (the opposite of an adult who dives having taken a breath), with a closed larynx. The fetal larynx has a myriad of airway chemoreceptors which prevent fluid aspiration. The diving reflex is stimulated via facial skin receptors conveying stimuli along the trigeminal nerve, triggered as these receptors make contact with the water (Johnson 1996).
Fetal breathing is inhibited at the hypo-pharynx. This mechanism is associated with hormonal factors such as prostaglandin and adenosine; sensors in the oral pharynx, including free nerve endings/taste buds, prevent aspiration, and indeed the normal mechanism is that any lung fluid rising into the oro-pharynx is swallowed. Mild hypoxia further inhibits breathing until chronic sub lethal override point, leading to the belief that an uncompromised human neonate will not breathe under water (Johnson 1996). A compromised neonate born underwater has the potential to gasp before the nose and mouth are above the surface, thus inhaling bath water into the lungs. Inhalation of even a small quantity of fresh water can be absorbed quickly into the circulation causing appreciable haemodilution and fluid overload - as seen in fresh water drowning.
There have been two reports of neonatal death following waterbirth attended by a midwife (Burns 2001; Rosser 1994). These adverse outcomes are very rare, and causality cannot be inferred on the evidence to directly link the reported case studies of rare adverse outcomes with waterbirth.
3. Infection
It has been suggested that fetal/neonatal infection may occur due to cross-contamination from the water and pool, and from the woman (Hawkins 1995; Rawal 1994). However, several comparative studies, cohort studies, and audits report no increase risk of infection for the fetus/neonate (Alderdice 1995; Anderson 1996; Eriksson 1997; Otigbah 2000; Rush 1996; Robertson 1998; Zanetti-Daellenbach 2007). As with all maternity provision, it is incumbent upon practitioners to ensure they have appropriate cleaning protocols for labour and birthing pools, and employ universal precautions.
To date, there is no evidence of increased maternal, fetal or neonatal risk associated with water immersion, compared with labouring and giving birth on land. Two UK national surveys were undertaken during the 1990s: Alderdice 1995 included 2885 women and their neonates, while Gilbert 1999 evaluated the neonatal outcomes for 4032 infants. Both surveys indicated that there was no reliable evidence to justify denying the choice of water immersion for labour and/or birth to women at low risk of complications. In addition, multiple cohort studies/audits have suggested the safety of water immersion during labour and birth for women at low risk of complication (Garland 2006; Geissbuehler 2004; Otigbah 2000; Thoeni 2005). RCTs have been conducted which are the focus of this review.
Maternal adverse effects of water immersion during labour have been theorised. These include the possibility that it may promote unrealistic expectations about labour, restrict choice of analgesia, restrict mobility, reduce contraction effectiveness, and increase perineal trauma (McCandlish 1993). Increased risk to the mother of infection caused by water entering the uterus has been proposed (Rosevear 1993). If warmth has a relaxing effect on the uterine muscles, the uterus may contract less efficiently postpartum (Church 1989; Deans 1995). A theoretical risk of water embolism has been hypothesised (Odent 1983). The logic of this hypothesis has been challenged (Wickham 2005). To date no studies have reported an association between water immersion during labour/birth with this adverse event.
4. Snapped umbilical cord
Concerns have been raised about the dangers of umbilical cords at water births (Gilbert 1999). Cords also snap in land births; there are however, no data for this. Cord snaps associated with waterbirth may be related to undue traction exerted on the cord as the baby is lifted out of the water.
Third stage of labour
We are not aware of any studies which have compared third stage of labour management or whether the placenta was delivered in or out of the water.
Water immersion during labour and birth: what it offers caregivers
Labour and birth is a complex, multifaceted and major life event encompassing physiological, emotional, psychological and social elements. It is therefore highly individualised and its features and outcome cannot be predicted with certainty. Although much of health care is based on understanding pathology and ill health, and while that may be appropriate for some, in maternity care, women are experiencing a normal physiological process. Midwives Downe and McCourt (Downe 2004) advocate that midwifery care should be set in the context of salutogenesis.
The salutogenic theory originated from interviews conducted with Israeli women who had survived the Holocaust about their time in concentration camps during the Second World War. It was noted that some stayed healthy despite horrendous experiences. This epidemiological study stimulated sociologist Antonovsky to develop the salutogenic paradigm as a way of focusing on health rather than disease (Antonovsky 1979; Antonovsky 1987). Central to salutogenesis is a person’s sense of coherence, which Antonovsky defined as a global orientation that denotes the degree of self-esteem and confidence an individual possesses to enable them to deal with life’s vicissitudes. In essence salutogenesis involves fostering a positive outlook and sense of self-worth to empower the individual to realize their potential. Empowerment is a key element of woman-centred care and the drive to normalise birth - an international initiative, led in the UK by the Royal College of Midwives (RCM 2008). The development of normal birth care pathways is consistent with these aims (NHS Wales 2004). Another stimulus to normalise birth is the international concern over the rise in caesarean sections in particular, but the medicalisation of labour and birth in general (RCOG 2001). The medicalisation of childbirth has many women believing that childbirth is inherently dangerous (Green 2007). It can be argued that water as an environment changes the context in which care is provided; it facilitates the paradigm shift, from professional-centred to woman-centred, from pathology dominated to normality expected. The woman is in her own ‘world’ and access to her is mediated by the water.
Water implies relaxation and warmth, and many would suggest it also conveys femininity and sexuality (Odent 1999). Clarke 2007 report an increase in the use of water immersion during labour and birth as one result of increasing the focus of one maternity unit to normality through the use of a care pathway. A birthing pool therefore offers midwives an opportunity to develop the skills required to provide woman-centred care, form a therapeutic rapport with women, facilitate their freedom and participation in decision making, and support them in having choice and control over their care (Nursing and Midwifery Council 2010).
OBJECTIVES
To assess the effects of water immersion during labour and/or birth (labour stages one, two and three) on maternal, fetal, neonatal and caregiver wellbeing. For the purpose of this review wellbeing is defined as outcomes measuring physical and psychological health. This review addresses the benefits and risks of immersion in water versus no immersion during each stage of labour. In addition, the review compares early (cervical dilation less than 5 cm) with late (cervical dilation more than 5 cm) immersion, different pool designs, still versus moving water, and water with or without additives.
METHODS
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) only. (We will not include results from quasi-RCTs in the analyses but we may be discuss them in the text if little other evidence is available.)
We have reported trials that included randomised and non-randomised subjects if the randomised data are presented separately. We have included published, unpublished and ongoing studies with reported data.
Types of participants
Nulliparous or multiparous women in labour with a singleton pregnancy, irrespective of gestation or labour characteristics.
Types of interventions
This review is one in a series of Cochrane reviews examining pain management in labour. These reviews contribute to an overview of systematic reviews of interventions for pain management in labour, and share a generic protocol. To avoid duplication the different methods of pain management have been listed in a specific order, from one to 15. Individual reviews focusing on particular interventions include comparisons with only the intervention above it on the list. Methods of pain management identified in the future will be added to the end of the list. The current list is as follows.
Placebo/no treatment
Hypnosis
Biofeedback (Barragán 2011)
Intracutaneous or subcutaneous sterile water injection (Derry 2011)
Immersion in water (this review)
Aromatherapy (Smith 2011a)
Relaxation techniques (yoga, music, audio)*
Acupuncture or acupressure (Smith 2011b)
Manual methods (massage, reflexology)*
Transcutaneous electrical nerve stimulation (TENS) (Dowswell 2009)
Inhaled analgesia
Opioids (Ullman 2010)
Non-opioid drugs (Othman 2011)
Local anaesthetic nerve blocks (Novikova 2011)
Epidural (including combined spinal epidural) (Anim-Somuah 2005; Simmons 2007)
Accordingly, this review includes comparisons of any kind of bath/tub/pool that enabled immersion during any stage of labour, regardless of care setting, compared with: 1. no treatment (no immersion); 2. hypnosis; 3. biofeedback; 4. intracutaneous or subcutaneous sterile water injection; and 5. immersion during a different stage of labour.
Types of outcome measures
We chose primary outcomes that we thought would be the most clinically valuable in assessing safety and effectiveness for the woman, fetus/neonate and caregivers. We identified all outcomes that were considered to be of interest from the perspective of the woman and her baby, primary caregivers and related service providers. These (list below) are analysed within the comparison groups:
immersion in water versus no immersion during the first stage of labour;
immersion in water versus no immersion during the second stage of labour;
comparison of different types of bath/pool;
additives versus no additives to water used for immersion during labour and/or birth;
early (cervical dilation less than 5 cm) with late (cervical dilation more than 5 cm) immersion.
Primary outcomes
Maternal outcomes
Morbidity - side effects
- ○ Blood loss during labour (first, second, third stage, and immediate postnatal period)
- ○ Infection during labour/postnatal period
- ○ Perineal trauma
- ○ Postpartum depression
- ○ Post-traumatic stress disorder
Labour
- ○ Pain intensity (first and second stage, as defined by trialists)
- ○ Mode of delivery (spontaneous birth, assisted vaginal births and caesarean sections)
- Wellbeing
- ○ Satisfaction with childbirth experience (as defined by trialists)
- ○ Satisfaction with pain relief (as defined by trialists)
- ○ Sense of control in labour (as defined by trialists)
- ○ Effect (negative) on mother/baby interaction
Fetal outcomes
Abnormal heart rate pattern
Meconium liquor
Neonatal outcomes
- Morbidity - side effects
- ○ Apgar score less than seven at five minutes
- ○ Cord pH immediately after birth (arterial and or venous cord blood)
- ○ Admission to special care baby unit/neonatal intensive care unit
- ○ Respiratory support (oxygen/ventilation required)
- ○ Lung hypoplasia
- ○ Infection, including markers of infection such as pyrexia and raised white cell count
- ○ Neurological pathology, e.g. seizures, cerebral palsy
- ○ Snapped cord
- ○ Birth injury
- ○ Poor infant outcomes at long term follow-up (as defined by trialists)
- Wellbeing markers
- ○ Breastfeeding (at specified time points)
Other outcomes
Cost (as defined by trialists)
Secondary outcomes
Maternal outcomes
Mortality
- Labour
- ○ Augmentation of labour (artificial rupture of membranes and/or oxytoxic administration)
- ○ Use of non-pharmacological analgesia
- ○ Use of pharmacological analgesia (including regional and general anaesthesia) during any stage of labour
- ○ Duration of labour (first, second and third stage)
- Wellbeing
- ○ Temperature (first and second stage)
- ○ Pulse and blood pressure (first, second and third stage)
- ○ Maternal self-esteem
- ○ Preference for care in subsequent labour
Fetal outcomes
Birthweight
Gestational age at birth
Neonatal outcomes
Mortality
Caregiver outcomes
Satisfaction
Injuries (any reported physical injury attributed to care of women in water)
Search methods for identification of studies
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co-ordinator (30 June 2011).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co-ordinator and contains trials identified from:
quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
weekly searches of EMBASE;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE and EMBASE, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co-ordinator searches the register for each review using the topic list rather than keywords.
Searching other resources
We used personal contacts to identify other potential trials (published and unpublished) and we retrieved and assessed relevant references referred to in the reviewed papers for appropriateness for inclusion in this review.
We did not apply any language restrictions.
Data collection and analysis
Selection of studies
We re-evaluated trials included and excluded within the previous review and confirmed their inclusion or exclusion. As previously, two review authors independently examined abstracts of all potential studies identified by the search to ascertain which met the inclusion criteria. We resolved any disagreement through discussion among all review authors. We have added one new paper since the last update.
Data extraction and management
We used a data extract template provided by the Cochrane Pregnancy and Childbirth Group and modified for the topic for the evaluation and data identification/extraction process. Elizabeth Cluett (EC) entered data into Review Manager software (RevMan 2011), and Ethel Burns (EB) checked for accuracy.
Assessment of risk of bias in included studies
For this current review two review authors, EC and EB, independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement through discussion.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non-random process, e.g. odd or even date of birth; hospital or clinic record number); or;
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment. We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non-opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3) Blinding (checking for possible performance bias)
Due to the nature of the intervention, blinding is not possible and therefore this could not be considered.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information is reported, or can be supplied by the trial authors, we will re-include missing data in the analyses which we undertake.
We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups, less than 20% loss);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated” analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found. We assessed the methods as:
low risk of bias (where it is clear that all of the study’s prespecified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre-specified outcomes have been reported; one or more reported primary outcomes were not pre-specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we had about other possible sources of bias.
We assessed whether each study was free of other problems that could put it at risk of bias:
low risk of other bias;
high risk of other bias;
unclear whether there is risk of other bias.
(7) Overall risk of bias
We will make explicit judgements about whether studies are at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we will assess the likely magnitude and direction of the bias and whether we consider it is likely to impact on the findings. We will explore the impact of the level of bias through undertaking sensitivity analyses - see Sensitivity analysis.
Measures of treatment effect
Dichotomous data
For dichotomous data, we used odds ratio with 95% confidence intervals. We analysed data for this review as presented in original papers, therefore by allocation (intention to treat).
Continuous data
For continuous data, we use the mean difference if outcomes are measured in the same way between trials.
Dealing with missing data
We have analysed data on all participants with available data in the group to which they were allocated, regardless of whether or not they received the allocated intervention, and irrespective of whether they used additional interventions. If, in the original reports, participants were not analysed in the group to which they were randomised, and there was sufficient information in the trial report, we have attempted to restore them to the correct group. For included studies we have noted levels of attrition.
Where data were not reported for some outcomes or groups we attempted to contact the study authors.
Assessment of heterogeneity
As part of the meta-analyses we examined heterogeneity between trials using the I2 statistic.
Assessment of reporting biases
If there were 10 or more studies in the meta-analysis we planned to investigate reporting biases (such as publication bias) using funnel plots. We would assess funnel plot asymmetry visually, and would use formal tests for funnel plot asymmetry. For continuous outcomes we would use the test proposed by Egger 1997, and for dichotomous outcomes we would use the test proposed by Harbord 2006. If asymmetry was detected in any of these tests or was suggested by a visual assessment, we proposed to perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2011).
Subgroup analysis and investigation of heterogeneity
For the primary outcomes, where data were available, we planned the following subgroup analyses.
Spontaneous labour versus induced labour.
Primiparous versus multiparous.
Term versus preterm birth.
Continuous support in labour versus no continuous support.
Sensitivity analysis
We planned to carry out sensitivity analyses to explore the effect of trial quality assessed by concealment of allocation, high attrition rates, or both, with poor quality studies being excluded from the analyses in order to assess whether this made any difference to the overall result.
RESULTS
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification.
One-to-one care in labour is known to affect labour outcomes (Hodnett 2007), and this was clearly documented in four trials (Cammu 1994; Da Silva 2006; Nikodem 1999; Taha 2000). Where it was stated that normal/routine/standard care was provided, this was understood to mean that the practitioners who normally provided intrapartum care to women in labour in the study centre provided care for the study participants (Da Silva 2006; Eckert 2001; Eriksson 1997; Nikodem 1999; Rush 1996; Schorn 1993; Woodward 2004). Cammu 1994 indicated that care was supervised by obstetric staff.
Water temperature is known to be important in the care of women using water immersion during labour. This varied across trials, with some using a temperature up to 37°C (Cammu 1994; Eckert 2001; Kuusela 1998); others up to 38°C (Da Silva 2006; Eriksson 1997; Taha 2000); and still others not stated (Chaichian 2009; Ohlsson 2001; Schorn 1993; Woodward 2004). Rush 1996 refers to a temperature of 38 to 39°C. The higher temperatures may affect outcomes, but there are no studies comparing outcomes for the use of different water temperatures.
The studies collected a wide range of data, but the specific outcome measures collected were very variable, and collected in different formats. For example, some studies did not consider neonatal wellbeing. Use of Apgar scores was also variable; some used them as continuous data, others as dichotomous, making comparison across studies challenging, and resulting in the reporting of many variables based on the results from one study.
Results of the search
We identified a total of 20 studies (some described in more than one report/paper) for consideration for inclusion in the review. Of these, we have now included 12 and excluded six; two await further assessment while we seek additional information from the authors. For more information see Characteristics of excluded studies.
Included studies
Of the 12 trials included in this review, eight related to the first stage of labour only; one related to early versus late immersion in the first stage of labour; two involved immersion during the first and second stages of labour; and one involved women in the second stage of labour only. There were no studies evaluating the use of different types of baths/pools at any stage of labour or the effects of water immersion on the third stage of labour. We identified no trials that evaluated immersion versus no immersion during pregnancy (i.e. not in labour).
For further details, see Characteristics of included studies.
Excluded studies
We excluded the two studies by Cluett (Cluett 2001; Cluett 2004), primarily because all the women were nulliparous who had been diagnosed as having dystocia in the first stage of labour, and the comparison (control) group all received augmentation of labour. Hence comparison and inclusion in any meta-analysis with women at low risk of complications is inappropriate. In addition, Cluett 2001 was a feasibility study and only involved four women in each arm. Cluett 2004 suggested that in nulliparous women who have been classified as having slow progress in the first stage of labour, labouring in water reduced the incidents of epidural analgesia, although this did not reach statistical significance (P = 0.056). The pain scores were significantly lower, but as the comparison group received augmentation of labour, it could be argued that this was to be expected. This further supports the rationale for not including these women in this review, as doing so would not represent the situation when water immersion would be used, namely in a low-risk labour and birth.
One pilot study (Calvert 2000) compared the use of essential oil of ginger with the use of essential oil of lemon grass. The data from this pilot are not in an appropriate form for analysis and so we excluded the study from the review. Use of other additives, such as salt, has not yet been evaluated in an RCT.
We excluded Benfield 2001 because of inadequate allocation concealment and the nature of the intervention.
Risk of bias in included studies
See details under Characteristics of included studies, Figure 1; Figure 2.
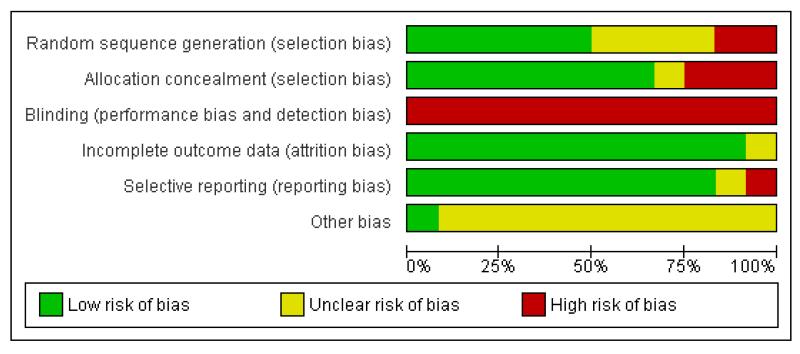
Figure 1.
Risk of bias graph: review authors’ judgements about each risk of bias item presented as percentages across all included studies.
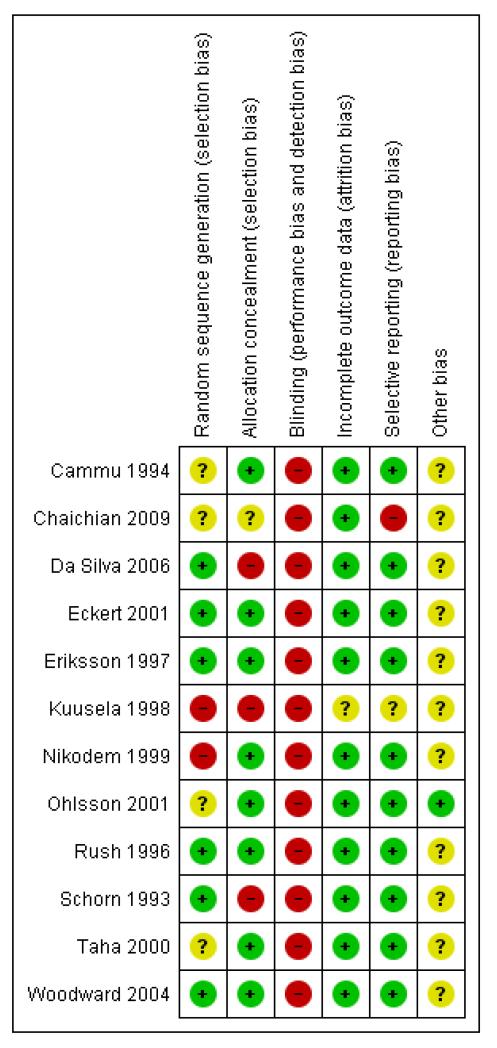
Figure 2.
Risk of bias summary: review authors’ judgements about each risk of bias item for each included study.
Allocation
Randomisation processes varied; those of the best quality used computer-generated, sequentially numbered opaque envelopes containing the group allocation (Eriksson 1997; Rush 1996; Woodward 2004) or a clear description of concealment (Cammu 1994; Eriksson 1997; Nikodem 1999; Ohlsson 2001; Taha 2000). Others were less transparent (Chaichian 2009; Kuusela 1998). Nikodem 1999 and Taha 2000 used allocation in blocks, which is not ideal as this has the potential for breaking concealment at the end of the block.
Blinding
None of the trials cite any blinding of outcome assessment, and this is likely to be difficult to achieve, as use of water during labour is usually clearly documented in case records.
As an intervention, it is not possible to blind participants or carers to water immersion. Not all participants and/or carers will be in a state of equipoise between immersion or non-immersion, that is being equally comfortable and confident about water immersion. This may positively or negatively influence outcomes such as pain perception and hence subsequent analgesia use, maternal satisfaction, self-esteem and postpartum depression. An example of this is Woodward 2004, which reports that some midwives were apparently not supportive of women using water, suggesting a positive bias within the women, and in this case a negative bias within the midwives. Conversely Rush 1996 reports practitioners as maintaining a interest in low-intervention labour practice, suggesting a positive bias towards water immersion. Water immersion, however, is as much a psychological choice as a physical pain management strategy, and as such pragmatic clinical trials are assessing the effect of the whole package.
Incomplete outcome data
Compliance with trial allocation was variable across the trials. Of the trials that involved water immersion in the first stage of labour, Rush 1996 reported that 46% of women allocated to water immersion did not actually enter the water, while Woodward 2004 planned a 2:1 ratio allocation to water anticipating that about 50% of women would not use water, but of the 40 allocated to use water, only 24 used the pool. Four (of 58) women in Da Silva 2006 did not receive the water intervention due to medical/obstetric reasons. Another five trials (Cammu 1994; Eckert 2001; Eriksson 1997; Ohlsson 2001; Woodward 2004) reported some crossover between groups. Kuusela 1998 and Chaichian 2009 did not provide information on this.
Rush 1996 and Da Silva 2006 referred to post-randomisation exclusion. For Rush 1996 this was 41 (of 785) women who were ineligible for the trial but recruited and allocated to a trial arm. They indicate that these 41 women were included in the analysis as it was on an intention-to-treat basis, but they also supply subgroup analysis with these women excluded.
Selective reporting
From the methods indicated, all the outcomes were reported. This was particularly hard to assess in the translated paper (Kuusela 1998), however, the risk of bias from selective reporting could be greater as in all trials there was an absence of a full study protocol.
Other potential sources of bias
The trials adopted a variety of definitions for water immersion, with different size baths/pools containing different volumes of water. To date, there is no evidence as to whether different degrees of immersion, or the amount of mobility possible within the bath/pool, affect outcomes. Schorn 1993 refers to a tub with a moulded seat, which may restrict mobility and the freedom to adopt different positions while immersed. Likewise, Rush 1996 used a pool where the woman could not change position. Schorn 1993 and Rush 1996 used a whirlpool (hot tub with jets) and the effect of moving water during immersion may be different to the effect of still water. Kuusela 1998 refers to a tub that is 70 cm deep and holds 730 litres; Da Silva 2006 indicates tub volume as 194 litres; Eckert 2001 and Eriksson 1997 cite tub depths of 54 cm and 40 cm, respectively. Differences as to what constitutes water immersion makes comparisons of outcomes across trials difficult.
Most of the included trials have small sample sizes and therefore a high risk of bias. These factors limit comparison across trials and the reliability and validity of the trial findings.
Effects of interventions
This section considers the results from the included trials and overall conclusions.
Immersion versus no immersion in the first stage of labour
Eight trials reported on this comparison.
Maternal outcomes
The following maternal outcomes were not reported in the trials: mortality; post-traumatic stress disorder; temperature; satisfaction with childbirth experience; maternal self-esteem; satisfaction with pain relief; sense of control in labour; effect on mother/baby interaction.
Blood loss during labour (first, second, third stage, and immediate postnatal period)
One trial (Eckert 2001) reported on the postpartum haemorrhage rate in each group and there was no difference between groups (risk ratio (RR) 1.58; 95% confidence interval (CI) 0.80 to 3.13), Analysis 1.13. Two trials (Kuusela 1998; Taha 2000) reported on the mean blood loss (ml) in each group and there was no difference between groups (mean difference (MD) −14.33; 95% CI −63.03 to 34.37), Analysis 1.14.
Infection during labour/postnatal period
There were no significant differences in the incidence of maternal infection (Cammu 1994; Eckert 2001; Kuusela 1998; Rush 1996; Schorn 1993), (15/647 versus 15/648; RR 0.99; 95% CI 0.50 to 1.96), Analysis 1.26.
Perineal trauma
There were no significant differences between the benefits and risks associated with the use of water immersion during labour on outcomes such as perineal trauma: intact perineum (236/678 versus 200/659; RR 1.16; 95% CI 0.99 to 1.35) (Da Silva 2006; Eckert 2001; Rush 1996; Taha 2000; Woodward 2004); episiotomy (207/644 versus 219/628; RR 0.93; 95% CI 0.80 to 1.08), second-degree tears (110/658 versus 112/628; RR 0.94; 95% CI 0.74 to 1.20) and third-/fourth-degree tears (40/1202 versus 29/1199; RR 1.37; 95% CI 0.86 to 2.17) (Eckert 2001; Ohlsson 2001; Rush 1996; Taha 2000; Woodward 2004), Analysis 1.15.
Postpartum depression
Two trials (Eckert 2001; Taha 2000) reported on postpartum depression, which was defined as a score of more than 11 on the Edinburgh Postnatal Depression Scale (EPDS). There was no difference between groups in the incidence of postpartum depression, (RR 1.38; 95% 0.85 to 2.24), Analysis 1.28.
Augmentation of labour (artificial rupture of membranes and/or oxytocic administration)
There has been some concern that water immersion may slow labour, therefore we analysed data on augmentation. There were no differences in the incidence of amniotomy (240/465 versus 233/461; RR 1.02; 95% CI 0.90 to 1.16) (Da Silva 2006; Kuusela 1998; Rush 1996), Analysis 1.19. There were no differences in the use of oxytocin infusion (RR 0.64; 95% CI 0.32 to 1.28), Analysis 1.22. However, considerable heterogeneity was detected within Analysis 1.22, which was not apparent with the exclusion of the Chaichian 2009 study, (heterogeneity: I2= 79%, T2 = 0.41, Chi2 test for heterogeneity P = 0.0008) and so we used a random-effects meta-analysis.
Pain intensity
Four trials (Da Silva 2006; Kuusela 1998; Nikodem 1999; Taha 2000) reported on pain intensity. Nikodem 1999 only reported the results narratively, “More (75% versus 40%) mothers in the water group experienced less pain than they expected” and so these data could not be included in an analysis. Two trials (Da Silva 2006: Kuusela 1998) reported mean visual analogue pain scores (VAS) at the start of assessment and then 30 minutes (Da Silva 2006) and one hour after (Kuusela 1998) the start of assessment and found no difference between groups in pain assessment at the different time points (start of assessment (MD −0.01; 95% CI −0.54 to 0.52), up to one hour after start assessment (MD −0.81; 95% CI −1.34 to −0.28), Analysis 1.17.
One trial (Taha 2000) assessed pain using three ordinal scales: pain reported on a VAS scale, where 1 is no pain and 10 is worst pain imaginable; feelings indicated by means of faces on a scale of 0 to 5; description in words the pain they experience, from no pain at all to unbearable pain. They did not use the McGill Pain Questionnaire. The data were reported at six different time points (before randomisation and then 30 minutes, one hour, two hours, three hours and 24 hours after randomisation) and was dichotomised giving the proportion of patients at different points on the scales. We have included only the data after randomisation in an analysis, Analysis 1.6. Moderate to severe pain according to all three ordinal scales was significantly less in those labouring in water than those not labouring in water when assessed 30 minutes after randomisation (RR 0.75; 95% CI 0.62 to 0.9, Analysis 1.6.1; RR 0.72; 95% CI 0.58 to 0.90, Analysis 1.6.2; RR 0.67; 95% CI 0.51 to 0.90, Analysis 1.6.3), and 24 hours after randomisation (RR 0.64; 95% CI 0.50 to 0.82, Analysis 1.6.13; RR 0.62; 95% CI 0.49 to 0.80, Analysis 1.6.14; RR 0.69; 95% CI 0.54 to 0.87). It was significantly less when assessed at one hour and two hours after randomisation for two out of the three ordinal scales (one hour - RR 0.76; 95% CI 0.63 to 0.91, Analysis 1.6.4; RR 0.68; 95% CI 0.53 to 0.86; Analysis 1.6.6) (two hours - RR 0.76; 95% CI 0.59 to 0.98, Analysis 1.6.7; RR 0.72; 95% CI 0.52 to 0.98, Analysis 1.6.9). However, there was no significant difference between those labouring in water and those not labouring in water when pain was assessed using the VAS 1 to 10 ordinal scale at one or two hours after randomisation (one hour -RR 1.21; 95% CI 0.69 to 2.11, Analysis 1.6.5; two hours - RR 0.83; 95% CI 0.66 to 1.05, Analysis 1.6.8). There was no significant difference between groups at three hours after randomisation on any of the three ordinal scales (Analysis 1.6.10, 1.6.11, 1.6.12), Analysis 1.6.
Use of non-pharmacological analgesia
Two trials (Rush 1996; Woodward 2004) provided data on the use of transcutaneous nerve stimulation (TENS) and there was no significant difference in the use of TENS between groups (RR 1.05; 95% CI 0.37 to 2.94), Analysis 1.3.
Use of pharmacological analgesia (including regional and general anaesthesia) duration of any stage of labour
Six trials (Cammu 1994; Eckert 2001; Kuusela 1998; Ohlsson 2001; Rush 1996; Woodward 2004) provided data on epidural/spinal analgesia/anaesthesia use and there was a significant reduction in the incidence of epidural/spinal/paracervical analgesia/anaesthesia amongst women allocated to immersion in water during the first stage of labour compared to controls (478/1254 versus 529/1245; RR 0.90; 95% CI 0.82 to 0.99), Analysis 1.1. Of these trials, Rush 1996 and Woodward 2004 reported women were allocated to water immersion who did not use water. In Rush 1996, 183 (46%) of the water group did not immerse, but none of the control group immersed. Based on clinical experience, Woodward 2004 anticipated that up to 50% of women allocated to labour in water, would not do so, and this was planned into the recruitment strategy, where the water to control recruitment ratio was 2:1. There was no significant difference in narcotic/pethidine use from the four trials that provide this data (RR 0.85; 95% CI 0.46 to 1.56) (Eckert 2001; Rush 1996; Taha 2000; Woodward 2004), Analysis 1.2. However, substantial heterogeneity was detected (heterogeneity: I2 = 58%, T2 = 0.20, Chi2 test for heterogeneity P = 0.07) and so we used a random-effects meta-analysis. The inclusion of Chaichian 2009, who only documented ‘any analgesia’, also resulted in a non-significant difference for the overall analgesia outcome of ‘any analgesia used’ (RR 0.72; 95% CI 0.46 to 1.12), Analysis 1.4. However, considerable heterogeneity was again detected (heterogeneity: I2 = 93%, T2 = 0.19, Chi2 test for heterogeneity P < 0.00001) and so we used a random-effects meta-analysis.
There was no significant difference for any pharmacological analgesia used from two trials that provide this data (RR 1.05; 95% CI 0.80 to 1.39), Analysis 1.5 (Eckert 2001; Taha 2000).
Mode of delivery (spontaneous birth, assisted vaginal births and caesarean sections)
Seven studies provide data on mode of birth (Cammu 1994; Eckert 2001; Kuusela 1998; Ohlsson 2001; Rush 1996; Taha 2000; Woodward 2004). These showed no significant difference for either the assisted delivery rate (water/land 156/1313 versus 181/1315, (RR 0.86; 95% CI 0.71 to 1.05)) or caesarean section (water/land 72/1358 versus 58/1354, (RR 1.21; 95% CI 0.87 to 1.68)). Chaichian 2009 only indicates the normal birth rate which was significantly higher in the water group (100% compared to 79.2%, (RR 1.26; 95% CI 1.09 to 1.45)), Analysis 1.7.
Duration of labour (first, second and third stage)
Seven trials (Cammu 1994; Chaichian 2009; Eckert2001; Kuusela 1998; Rush 1996; Schorn 1993; Woodward 2004) provided data on duration of the first stages of labour. These showed a significant difference in favour of a shorter labour for the immersion group (MD −32.4 minutes; 95% CI −58.67 minutes to −6.13 minutes), Analysis 1.8. Seven trials (Cammu 1994; Da Silva 2006; Eckert 2001; Kuusela 1998; Rush 1996; Schorn 1993; Woodward 2004) reported on the duration of the second stage of labour; there was no statistical difference (MD 0.47 minutes; 95% CI −3.45 minutes to 4.38 minutes), Analysis 1.9. Three trials (Chaichian 2009; Eckert 2001; Rush 1996) reported on the duration of the third stage of labour; there was no statistical difference (MD −0.52 minutes; 95% CI −1.84 minutes to 0.79 minutes), and moderate heterogeneity was detected (heterogeneity: I2 = 41%, T2 = 0.54, Chi2 test for heterogeneity P = 0.18), Analysis 1.10.
Pulse and blood pressure (first, second and third stage)
One study (Taha 2000) reported the biophysiological effect of immersion in water on the effect of blood pressure changes: systolic (mean 120.3 mmHg versus 127.5 mmHg; MD −7.20; 95% CI −13.12 to −1.28), Analysis 1.23; diastolic (mean 62.8 mmHg versus 73 mmHg; MD −10.20; 95% CI −13.70 to −6.70), Analysis 1.24; and mean arterial pressure (mean 83.7 versus 94.2; MD −10.50, 95% CI −14.68 to −6.32), Analysis 1.25, were statistically significantly reduced in the immersion group.
Preference for care in subsequent labour
One study (Taha 2000) reported the number of women who would not wish to use immersion during labour with a subsequent labour and delivery and there were significantly fewer women in the immersion group who expressed this wish when compared to the control group (RR 0.38; 95% CI 0.14 to 0.98), Analysis 1.18.
Fetal outcomes
Abnormal heart rate pattern
Three trials (Eckert 2001; Schorn 1993; Taha 2000) provided data on abnormal fetal heart rate patterns and there was no significant difference amongst women allocated to immersion in water during the first stage of labour compared to controls (RR 0.75; 95% CI 0.34 to 1.67), Analysis 1.30, and substantial heterogeneity was detected (heterogeneity: I2 = 57%, T2 = 0.22, Chi2 test for heterogeneity P = 0.13) and so we used a random-effects meta-analysis.
Meconium liquor
Five trials (Da Silva 2006; Eckert 2001; Kuusela 1998; Rush 1996; Woodward 2004) provided data on the presence of meconium-stained liquor and there was no significant difference amongst women allocated to immersion in water during the first stage of labour compared to controls (RR 0.95; 95% CI 0.76 to 1.19), Analysis 1.21.
Gestational age at birth/birthweight
There were no differences in gestational age at birth (MD −0.01; 95% CI −0.82 to 0.80), Analysis 1.40 or birthweight (MD −22.74; 95%CI −66.44 to 20.96) (Cammu 1994; Da Silva 2006; Eckert 2001; Kuusela 1998; Ohlsson 2001; Rush 1996; Schorn 1993; Taha 2000; Woodward 2004), Analysis 1.41.
Neonatal outcomes
The following neonatal outcomes were not reported in the trials: mortality; respiratory support (oxygen/ventilation required); lung hypoplasia; neurological pathology, e.g. seizures, cerebral palsy; snapped cord; birth injury; poor infant outcomes at long-term follow-up (as defined by trialists).
Apgar score less than seven at five minutes
Five trials reported when the Apgar score was less than seven at five minutes (Cammu 1994; Eckert 2001; Ohlsson 2001; Schorn 1993; Taha 2000), and there was no significant difference (10/907 versus 6/927; RR 1.58; 95%CI 0.63 to 3.93), Analysis 1.31. Another two studies provided the mean Apgar score at five minutes (Da Silva 2006; Rush 1996) and again there was no difference (MD −0.03 95%CI −0.11 to 0.06), Analysis 1.32.
Cord pH immediately after birth (arterial and or venous cord blood)
One trial reported on umbilical artery pH less than 7.20 (Cammu 1994) and found no difference amongst women allocated to immersion in water during the first stage of labour compared to controls (RR 5.18; 95% CI 0.25 to 105.51), Analysis 1.33.
Admission to special care baby unit/neonatal intensive care unit
There was no significant difference in the three trials that reported admissions to the neonatal intensive care unit (48/789 versus 45/782; RR 1.06; 95% CI 0.71 to 1.57) (Eckert 2001; Ohlsson 2001; Woodward 2004), Analysis 1.35.
Infection, including markers of infection such as pyrexia and raised white cell count
Infection rates were very low (6/647 versus 3/648) and reported in five trials (RR 2.00; 95% CI 0.50 to 7.94) (Cammu 1994; Eckert 2001; Kuusela 1998; Rush 1996; Schorn 1993), Analysis 1.36, although in three trials there were no infections in either group (Cammu 1994; Kuusela 1998; Schorn 1993), as might be hoped, as all three had small sample sizes. Chaichian 2009 indicates there were no statistically significant outcomes. One trial reported temperature greater than 37.8°C as an indicator of infection (Eckert 2001) and found no difference between groups (RR 1.00; 95% CI 0.06 to 15.83), Analysis 1.34
Breastfeeding (at specified time points)
Two trials reported on the number of women not breastfeeding after six weeks post delivery (Eckert 2001; Taha 2000) and found no difference amongst women allocated to immersion in water during the first stage of labour compared to controls (RR 1.17; 95% CI 0.64 to 2.15), Analysis 1.29.
Caregiver outcomes
No trial describes any injuries or satisfaction outcomes for caregivers.
Other outcomes
No trial describes the costs associated with immersion in water in labour and birth.
Immersion versus no immersion in the second stage of labour
Three trials reported on this comparison. One trial evaluated immersion during the second stage of labour (Nikodem 1999) and two trials measured outcomes across the first and second stages (Chaichian 2009; Woodward 2004). We have entered data for these trials in both the first and second stage sections of this review where there are data to compare, although it should be noted that only 10 (25%) of the 40 women allocated to birth in water actually did so in Woodward 2004. All the women birthed in the water in the trial by Chaichian 2009 which is somewhat surprising.
Maternal outcomes
The following maternal outcomes were not reported in the trials: mortality; infection during labour/postnatal period; postpartum depression; post-traumatic stress disorder; augmentation of labour (artificial rupture of membranes and/or oxytoxic administration); use of non-pharmacological analgesia; use of pharmacological analgesia (including regional and general anaesthesia) duration of any stage of labour; pulse and blood pressure (first, second and third stage); maternal self-esteem; satisfaction with pain relief (as defined by trialists); sense of control in labour (as defined by trialists); effect (negative) on mother/baby interaction.
Blood loss during labour (first, second, third stage, and immediate postnatal period)
One trial reported on the postpartum haemorrhage rate in each group (Nikodem 1999) and there was no difference between groups (RR 0.14; 95% CI 0.01 to 2.71), Analysis 2.7.
Perineal trauma
There were no significant differences in incidence of trauma to the perineum; episiotomy (12/100 versus 10/79, RR 0.75; 95% CI 0.35 to 1.60); and second-degree tears (21/100 versus 14/79, RR 1.21; 95% CI 0.65 to 2.24); and third- or fourth-degree tears (RR 1.54; 95% CI 0.07 to 36.11) (Nikodem 1999; Woodward 2004), Analysis 2.4.
Pain intensity (first and second stage, as defined by trialists)
One trial reported on the proportion of women experiencing moderate to severe pain (Nikodem 1999), and found no difference between groups (RR 1.06; 95% CI 0.73 to 1.53), Analysis 2.1.
Mode of delivery (spontaneous birth, assisted vaginal births and caesarean sections)
There were no significant differences in the mode of delivery; assisted vaginal birth (RR 0.73; 95%CI 0.21 to 2.54); caesarean section rate (RR 0.33; 95% CI 0.07 to 1.52) (Nikodem 1999; Woodward 2004), Analysis 2.6.
Duration of labour (first, second and third stage)
Three trials reported on the duration of the second stage of labour (Chaichian 2009; Nikodem 1999; Woodward 2004) and there was no statistical difference between groups (MD −1.24 minutes; 95% CI −8.05 minutes to 5.56 minutes), Analysis 2.5.
Temperature (first and second stage)
One trial reported on maternal temperature (Woodward 2004) and found no difference between groups, (MD 0.20; 95% CI −0.18 to 0.58), Analysis 2.17.
Satisfaction with childbirth experience (as defined by trialists)
Nikodem 1999 demonstrated a significantly higher level of satisfaction with the birth experience (RR 0.24; 95% CI 0.07 to 0.80), Analysis 2.2, with fewer women in the immersion group feeling that they did not cope satisfactorily with their pushing efforts (3/60 versus 12/57). However, another trial (Woodward 2004) which measured satisfaction with labour and birth on a scale of 0-6 where 0 is not at all satisfied, found that both groups were reasonably satisfied, but there were no significant differences between groups (MD 0.03; 95% CI −0.64 to 0.70), Analysis 2.16.
Preference for care in subsequent labour
One trial (Nikodem 1999) reported the number of women who would not wish to use immersion during labour with a subsequent labour and delivery and found there was no difference between groups (RR 0.57; 95% CI 0.18 to 1.55), Analysis 2.3.
Fetal outcomes
The following fetal outcomes were not reported in the trials: abnormal heart rate pattern; birthweight.
Meconium liquor
Two trials (Nikodem 1999; Woodward 2004) provided data on the presence of meconium-stained liquor and there was no significant difference amongst women allocated to immersion in water during labour compared to controls (RR 1.32; 95%CI 0.63 to 2.80), Analysis 2.8
Gestational age at birth
Two trials (Nikodem 1999; Woodward 2004) provided data on gestational age at birth in days and found no significant difference amongst women allocated to immersion in water during labour compared to controls (MD −1.00; 95% CI −5.13 to 3.13), Analysis 2.21.
Neonatal outcomes
The following neonatal outcomes were not reported in the trials: respiratory support (oxygen/ventilation required); lung hypoplasia; neurological pathology, e.g. seizures, cerebral palsy; snapped cord; birth injury; poor infant outcomes at long-term follow-up (as defined by trialists).
Mortality
One trial provided data on perinatal deaths (Nikodem 1999) and found no difference between groups (RR 3.00; 95% CI 0.12 to 72.20), Analysis 2.15.
Apgar score less than seven at five minutes
Two trials reported on Apgar score; neither Nikodem 1999 nor Woodward 2004 found any significant difference in the incidence of low Apgar score (RR 4.92; 95% CI 0.24 to 100.31, Analysis 2.9), (RR 1.54; 95% CI 0.07 to 36.11, Analysis 2.10), although each used slightly different parameters. Nikodem 1999 reported the number of women in each group with Apgar less than seven at five minutes and Woodward 2004 reported the number of women in each group with Apgar less than eight at five minutes.
Cord pH immediately after birth (arterial and or venous cord blood)
Two trials reported on cord pH; neither Nikodem 1999 nor Woodward 2004 found any significant difference in umbilical artery pH (RR 0.89; 95% CI 0.45 to 1.75, Analysis 2.12) or cord pH immediately after birth (values not estimable) (Analysis 2.13), although each used slightly different parameters.
Admission to special care baby unit/neonatal intensive care unit
There was no significant difference in the two trials that reported admissions to the neonatal intensive care unit (RR 0.79; 95% CI 0.25 to 2.49) (Nikodem 1999; Woodward 2004), Analysis 2.14.
Infection, including markers of infection such as pyrexia and raised white cell count
Nikodem 1999 found no significant difference in the incidence of raised neonatal temperature at birth greater than 37.5° C (8/55 versus 3/54; RR 2.62; 95% CI 0.73 to 9.35), Analysis 2.11. Woodward 2004 found no significant difference in antibiotics given to neonates (RR 1.50; 95% CI 0.17 to 13.52), Analysis 2.19 or in positive neonatal swabs of ear, mouth or umbilicus (RR 1.89; 95% CI 0.90 to 3.96), Analysis 2.20.
Breastfeeding (at specified time points)
Woodward 2004 found no significant difference in the number of women breastfeeding at birth between groups (RR 0.86; 95% CI 0.69 to 1.08), Analysis 2.18.
Caregiver outcomes
No trial describes any injuries or satisfaction outcomes for caregivers.
Other outcomes
No trial describes the costs associated with immersion in water in labour and birth.
Early versus late immersion
One trial compared early versus late immersion during the first stage of labour (Eriksson 1997).
Maternal outcomes
The following maternal outcomes were not reported in the trials: mortality; blood loss during labour (first, second, third stage, and immediate postnatal period); infection during labour/postnatal period; perineal trauma; postpartum depression; post-traumatic stress disorder; pain intensity (first and second stage, as defined by trialists); use of non-pharmacological analgesia; mode of delivery (spontaneous birth, assisted vaginal births and caesarean sections); duration of labour (first, second and third stage); temperature (first and second stage); pulse and blood pressure (first, second and third stage); satisfaction with childbirth experience (as defined by trialists); maternal self-esteem; preference for care in subsequent labour; satisfaction with pain relief (as defined by trialists); sense of control in labour (as defined by trialists); effect (negative) on mother/baby interaction.
Augmentation of labour (artificial rupture of membranes and/or oxytocic administration)
Eriksson 1997 found an increased incidence of augmentation of labour in the early group (57/100 versus 30/100; RR 1.90; 95% CI 1.35 to 2.68), Analysis 5.2.
Use of pharmacological analgesia (including regional and general anaesthesia) duration of any stage of labour
Eriksson 1997 found significantly higher epidural analgesia rates in the early group (42/100 versus 19/100; RR 2.21; 95% CI 1.39 to 3.52), Analysis 5.1.
Fetal outcomes
The following fetal outcomes were not reported in the trials: abnormal heart rate pattern; meconium liquor; gestational age at birth.
Birthweight
Eriksson 1997 found no significant difference in neonatal birthweight in grams between the early and late groups (MD −66.00; 95% CI −189.34 to 57.34), Analysis 5.8.
Neonatal outcomes
The following neonatal outcomes were not reported in the trials: mortality; Apgar score less than seven at five minutes; cord pH immediately after birth (arterial and or venous cord blood); admission to special care baby unit/neonatal intensive care unit; respiratory support (oxygen/ventilation required); lung hypoplasia; neurological pathology, e.g. seizures, cerebral palsy; snapped cord; birth injury; poor infant outcomes at long-term follow-up (as defined by trialists); wellbeing markers; breastfeeding (at specified time points).
Infection, including markers of infection such as pyrexia and raised white cell count
Eckert 2001 found no difference in neonatal infection rate between early and late groups, (RR 3.00; 95% CI 0.12 to 72.77), Analysis 5.6
Caregiver outcomes
No trial describes any injuries or satisfaction outcomes for caregivers.
Other outcomes
No trial describes the costs associated with immersion in water in labour and birth.
DISCUSSION
This review showed that immersion in water during labour significantly reduced the epidural/spinal analgesia rate based on data from six trials. There was also a significant reduction in the duration of the first stage of labour in the immersion group (seven trials, immersion versus no immersion). However, there was a high level of heterogeneity for some of these outcomes and so these results should therefore be examined with caution. The only other statistically significant results were for experience of moderate to severe pain; wish to use water for a subsequent labour, and a reduction in blood pressure, all of which were measured in one trial (Taha 2000), during the first stage of labour.
These results are consistent with observational studies. However, these conclusions need to be considered in the context of small sample sizes (range 33 to 1237); only two trials achieved a total sample size of greater than 300; blinding to the intervention is not possible; and many outcomes were only considered in one or two trials. These factors limit the interpretation of the results. An equivalence study is required to explore whether or not labour and/or birth in water is as safe as labour/birth without immersion in water, in a comparable group of women. It is recognised, however, that as use of water in labour and birth is now widely considered a matter of maternal choice, it is increasingly unlikely that conducting a large, multicentred, randomised controlled trial needed to gain the required evidence will be feasible or acceptable. Large audits and cohort studies should be undertaken in units which provide a pool facility to provide evidence for practice (Geissbuehler 2000; Zanetti-Daellenbach 2007).
The trials reported using different sized pools (only five trials provide information on bath/pool size: Cammu 1994; Da Silva 2006; Eckert 2001; Eriksson 1997; Kuusela 1998); various durations in the water; and still or moving water, each of which had an impact on the outcomes. These factors limit the validity of the findings.
Rush 1996 and Woodward 2004 reported respectively that 46% (n = 183) and 40% (n = 16) of women allocated to water immersion did not actually use water, although in the case of Woodward 2004 this was expected and a recruitment ratio of 2:1 had been adopted. In both studies, analysis was by intention to treat, and they did not report outcomes by actual use. It is possible that subgroup analysis excluding women who did not use the water might have increased the difference between water users and non-users, in favour of less epidural analgesia for those who used water immersion. This would be consistent with the study by Chaichian 2009. This is pertinent, as the authors reported that the main reasons for non-use of the water included early request for epidural, identification of complication precluding water use, as well as non-availability of the pool and change of mind (numbers for each are provided by Woodward 2004 but not by Rush 1996).
Another confounding factor is that the gestational age at which water immersion is permissible varies across the trials, from greater than 34 weeks’ gestation (Eriksson 1997) through 35 weeks (Ohlsson 2001) and 36 weeks (Schorn 1993; Taha 2000; Woodward 2004) to greater than 37 weeks (Cammu 1994; Chaichian 2009; Da Silva 2006; Eckert 2001; Kuusela 1998; Rush 1996). This is due to variations in the definition of ‘preterm’ adopted by different countries. However the baseline characteristics of participants in the included studies showed no difference (see Effects of interventions, Neonatal outcomes for immersion during the first stage of labour).
Although all the trials involved women defined as ‘in labour’, this was interpreted differently, from trials including all women with contractions, or about to have labour induced with a cervical dilatation of as little as 1 cm (Eckert 2001), to trials including only women in active labour with a cervical dilatation of greater than 6 cm (Da Silva 2006). This variability makes comparisons across trials problematic.
Another variation is that the length of the first stage of labour for women in the trial by Cammu 1994 was shorter (mean of 244 minutes) and less variable (small standard deviation of 139 minutes), compared to a first stage length of 846 minutes (standard deviation 432 minutes) in the trial by Schorn 1993. This suggests that the samples may have met different inclusion criteria or experienced a different management protocol during labour, although this was not explicit in the papers. The length of the second stage of labour for the women in the immersion group is much longer than might be expected in the trial by Schorn 1993, which involved nulliparae only, compared to Kuusela 1998 and Chaichian 2009 where the second stage duration was reported as 21 and 20 minutes respectively. This may again relate to different management strategies, in particular definition of the onset of the second stage and the use or not of directed pushing, but again this is not detailed within the papers.
Only one trial investigated early (before a cervical dilatation of 5 cm) versus late (after a cervical dilatation of 5 cm) immersion in water during the first stage of labour, which investigated a higher rate of augmentation and use of pharmacological analgesia in the early immersion group (Eriksson 1997). The main issue arising from this trial is whether or not women in the trial were actually in active labour, and therefore could reasonably be expected to progress spontaneously. Alternatively women may have been in the latent phase, which might have been augmented by mobilisation and other activity within a labour room, compared to relaxation and latent phase contractions ceasing in the water group. It is not possible to preclude that some women may have entered a birthing pool in the latent phase of labour, which could predispose them requiring augmentation. The trial did not consider this possibility.
Although all participants across the included trials were considered at low risk of complications, and trials were excluded where this was not so (Cluett 2001; Cluett 2004), Eckert 2001 reported the inclusion of women whose labour was induced. Rush 1996 indicated that 41 women who did not meet the inclusion criteria had been randomised. When these women were removed from the analysis the P value for epidural analgesia use changes to 0.044 from 0.069, whilst that for instrumental vaginal delivery changes to 0.011 from 0.055. Therefore, when ineligible women are excluded the results indicate that, for women at low risk of complications, labouring in water reduced the likelihood of epidural/narcotic use and of needing an instrumental vaginal delivery (Rush 1996). The definitions adopted for ‘labour’ were varied and may have influenced outcomes. In particular, Cammu 1994 required that the amniotic membranes were ruptured, although there is no indication as to whether this occurred spontaneously or artificially. In contrast the membranes were intact in all participants in the trial by Schorn 1993. Participants in other trials had a mixture of intact and ruptured membranes (Ohlsson 2001; Rush 1996; Taha 2000; Woodward 2004). These differences may affect pain perception, and hence influence analgesia uptake, maternal satisfaction, and possibly labour progress, which makes comparison across trials difficult. There is little or no information about the presence of one-to-one care or not in the trials evaluating first stage of labour outcomes, although Rush 1996 indicated that caregivers tended to be more continuously present with the water immersion participants. As one-to-one care in labour is known to affect outcomes (Hodnett 2007), if this was not balanced across trial arms, this could account for any differences found.
The main conclusion of this review is that labouring in water significantly reduces the incidence of epidural/spinal. It is not possible to conclude whether the differences identified, in particular the reduction in epidural/spinal analgesia, are due to water alone, or the water/pool environment. Water immersion is a care package which includes the actual water and the associated environment, together with the interactions of the woman and her caregivers.
It may be that this last factor, linking midwives/caregivers who support the tranquil, no-obstetric-intervention, salutogenic philosophy espoused by labour and birth in water with like-minded women is the most important component. This would be consistent with the evidence on one-to-one care in labour (Hodnett 2007). It could be argued that, if water immersion facilitates the adoption of a woman-centred approach to care, facilitating normalisation of labour and birth, as many now seek (Maternity Care Working Party 2007; RCM 2008), then immersion in water should be promoted.
AUTHORS’ CONCLUSIONS
Implications for practice
Despite limitations in the validity and reliability of the randomised controlled trial evidence to date due to trial design, the statistically significant reduction in rate of epidural/spinal/paracervical analgesia indicates that water immersion during the first stage of labour reduces the need for this invasive, pharmacological pain mode of analgesia, which disturbs the physiology of labour and is associated with iatrogenic interventions. We found no evidence that this was associated with poorer outcomes for neonates, longer labours or more complex births. The other significant findings come from data from one study only and therefore have to be read with caution. Women can be advised that the use of water immersion in the first stage of labour may reduce the incidence of epidural/spinal/paracervical analgesia, and midwives and other birth attendants can suggest water immersion as part of labour pain management strategy.
There is insufficient evidence about the use of water immersion during second stage of labour and therefore clear implications cannot be stated.
Overall, the evidence indicates that immersion in water during the first stage decreases maternal uptake of epidural/spinal analgesia, and that water immersion during the first stage of labour can be supported for women at low risk of complications.
Immersion during the second stage of labour needs further investigation, but at present there is no clear evidence to support or not to support a woman’s decision to give birth in water.
Implications for research
There is some evidence that immersion in water during the first stage of labour reduces the need for analgesia, but the limited reliability and validity of the studies means that this would benefit from further research, in particular from a study of an appropriate size to assess equivalence. There is a lack of clarity as to what constitutes water immersion, and further evaluation of the relative merits of different shaped/sized pools is required, and of still versus moving water, and the relative merits of water immersion during early labour (latent phase). There is insufficient information to support or not to support the use of immersion during the second stage of labour (birth), or the third stage. The safety regarding infection and neonatal outcomes are not addressed, and large collaborative trials are needed to answer these critical issues. It has been suggested that maternal satisfaction increases with water immersion, although there is a need for a large trial to evaluate this.
There are no data on caregiver outcomes and this warrants investigation.
PLAIN LANGUAGE SUMMARY
Immersion in water in labour and birth
This review includes 12 trials (3243 women). Water immersion during the first stage of labour significantly reduced epidural/spinal analgesia requirements, without adversely affecting labour duration, operative delivery rates, or neonatal wellbeing. One trial showed that immersion in water during the second stage of labour increased women’s reported satisfaction with their birth experience. Further research is needed to assess the effect of immersion in water on neonatal and maternal morbidity. No trials could be located that assessed the immersion of women in water during the third stage of labour, or evaluating different types of pool/bath.
ACKNOWLEDGEMENTS
Thanks are extended to Cheryl Nikodem for establishing the review group and to both Cheryl and Rona McCandlish for their extensive work on previous editions, which underpins the current review.
Thanks to Leanne Jones, Research Associate, Cochrane Pregnancy and Childbirth Group, for restructuring the review and checking data.
SOURCES OF SUPPORT
Internal sources
OCHRAD Oxford Brookes University, UK.
School of Health Sciences, UK.
University Of Southampton
External sources
National Institute for Health Research, UK.
UK NIHR Programme of centrally-managed pregnancy and childbirth systematic reviews of priority to the NHS and users of the NHS: 10/4001/02
CHARACTERISTICS OF STUDIES
Characteristics of included studies [ordered by study ID]
| Methods | Randomisation by sealed opaque envelopes containing method indicator card Methodological qualities:
|
|
| Participants | Study group: n = 54. Control group: n = 56. Inclusion criteria: gestation > 36 weeks; low risk; nulliparous; singleton; cephalic presentation; active labour between 4-5 cm cervical dilatation; ruptured membranes with clear liquor on entry; scalp electrodes used for all participants; ambulation and analgesics were allowed. |
|
| Interventions | The use of an oval-shaped hot tub during labour. Bath temperature not exceeding 37 degrees celsius. No chemicals added. Control group: no water immersion during labour. First stage of labour study, women in both groups received ‘personalised’ care but it is not clear if this is 1-to-1 care or not, although care overseen by obstetricians and all births conducted by house officers (doctors) |
|
| Outcomes | Maternal outcomes: *use of analgesia/anaesthesia; *augmentation of labour; cervical dilatation; *duration of labour and birth; *mode of delivery; *maternal infection. Fetal outcomes: abnormal FHR patterns needing intervention. Neonatal outcomes: *neonatal condition; *admittance to NICU or high dependency care unit; *neonatal infection rates. |
|
| Notes | Academic hospital, Brussels, Belgium. | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No indication of how random sequence was generated. |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes containing method indicator card. |
| Blinding (performance bias and detection bias) All outcomes |
High risk | High risk of bias could have been introduced because women, carers and researcher cannot be blind to group allocation after randomisation due to the nature of the intervention |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | Information available on number of participants asked (water −57/control −56) to number who gave consent (water −53/control −56) to outcome data - no attrition |
| Selective reporting (reporting bias) | Low risk | All outcome detailed in methods are reported on. |
| Other bias | Unclear risk | All women had 1-to-1 care,which is known to affect outcomes, but is common for water immersion care |
| Methods | Randomised control trial; no information on how randomisation was achieved women laboured and delivered in water, or not. | |
| Participants | Water group - n = 53; control group - n = 53. Gestational age 37-42 weeks. No previous CS. Intact membranes. No malpresentations. No placenta abruption or praevia. Well fetus. |
|
| Interventions | Information given to women in pregnancy, then randomised to experimental or control group in labour. Water group labour and birth in warm water pool, but no description of pool size or care protocol given. Control group conventional care at the hospital, but not detailed | |
| Outcomes | Data provided on baseline characteristics or age, gravida, parity, previous abortion, and prolonged rupture of membranes Data provided on outcomes, *normal birth rate, *duration of labour, *use of oxytocin and *analgesia (not stated what type) Data collected on *episiotomy/perineal trauma, *neonatal weight, *Apgar score, gender and breastfeeding initiation but data not given |
|
| Notes | Study undertaken in Iranian hospital affiliated to Iran University of Medical Sciences, between June 2006 and September 2007 Authors contacted twice for further information but no reply |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information given on randomisation processes. |
| Allocation concealment (selection bias) | Unclear risk | No information given on randomisation processes. |
| Blinding (performance bias and detection bias) All outcomes |
High risk | High risk of bias could have been introduced because women, carers and researcher cannot be blind to group allocation after randomisation due to the nature of the intervention |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | No indication that women withdrew from study, or that data was lost/incomplete |
| Selective reporting (reporting bias) | High risk | Outcomes not detailed on perineal trauma, neonatal weight, Apgars, gender and breastfeeding initiation although data collected and described as not significantly different |
| Other bias | Unclear risk | It is surprising that all the women that went to water gave birth in the water. Normally one would expect some who laboured in water to choose to get out for birth, but no evidence of this as number in each group is the same. This calls into question if all who got into the pool are included in study or just those who remained in for birth as well |
| Methods | Randomisation was computer generated, and then recorded on a list (paper copy), where the next allocation was concealed from the research until the next woman had provided consent, was recruited and thus being allocated. Methodological qualities:
|
|
| Participants | Power calculation undertaken. Water n = 58. Control n = 56. Full term, nulliparous, live, cephalic presentation, no complications, cervical dilation of 6 cm or less in established labour |
|
| Interventions | Control group received standard care, including cardiotocography on admission, ambulation, amniotomy and oxytocin augmentation if now cervical progress over 3 hours, intermittent auscultation during labour Intervention group as above with immersion in water when cervix had reached 6-7 cm dilated, for 60 minutes First stage of labour study, all women received 1-to-1 care from the researcher Pool was 194 litres, equipped with a heater. Water temperature ranged from 27 to 38 degrees Celsius |
|
| Outcomes | Pain score on 5-point behavioural scale and numerical pain score from 0 to 10, at 6-7 cm dilated and again 1 hour later In addition the following data were collected: use of augmentation, amniotic liquor conditions, duration of labour, perineal condition, gestational age, Apgar score at 1 and 5 minutes, maternal and water temperature |
|
| Notes | Study done in Sao Paulo, Brazil. | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated random list. |
| Allocation concealment (selection bias) | High risk | Each allocation on the list was covered with a tab, which was removed by the researcher after consent form signed by next participant. This description suggests the process could be open to tampering |
| Blinding (performance bias and detection bias) All outcomes |
High risk | High risk of bias could have been introduced because women, carers and researcher cannot be blind to group allocation after randomisation due to the nature of the intervention |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | Flow chart detailed participants from eligibility to completion; no attrition after instigation of allocated care |
| Selective reporting (reporting bias) | Low risk | All the data mentioned in the methods, and that would reasonably be expected of this study are reported |
| Other bias | Unclear risk | All women had 1-to-1 care,which is known to affect outcomes, but is common for water immersion care. In this study the care was from the researcher, regardless of group |
| Methods | Randomisation by sealed opaque, sequentially numbered envelopes that were kept in the admission ward. Prepared in random blocks of 10, stratified for parity. Methodological qualities:
|
|
| Participants | Study group n = 137. Control group n = 137. Inclusion criteria: gestation > 36 weeks; low risk; singleton; cephalic presentation. Exclusion criteria: planned CS; history of Group B streptococcal infection; epidural anaesthesia; continuous FHR monitoring needed |
|
| Interventions | Women were allocated to a delivery suite with a bath or to a general delivery suite without a bath. The bath group was allowed to use the bath as long as each woman wished, but they had to get out during second stage of labour (first stage only). The bath tub was 120 cm × 160 cm × 54 cm and the maximum water temperature was 37 degrees Celsius. Control group was allowed to use a shower. First stage only study women received care from same midwives but no mention of 1-to-1 second care or not |
|
| Outcomes | Maternal outcomes: *maternal experience and satisfaction of labour; *use of analgesia/anaesthesia; *augmentation of labour; *presence of meconium-stained liquor; *duration of labour and birth; *mode of delivery; *trauma to the birth canal requiring suturing; *blood loss; *postpartum depression; breastfeeding. Fetal outcomes: *abnormal FHR patterns, needing intervention. Neonatal outcomes: *neonatal condition; *admittance to NICU or high dependency care unit; * temperature at birth; *neonatal infection rates. |
|
| Notes | Tertiary referral hospital in Adelaide, Australia. May 1995 - Sept 1998. Some of the results are not in an appropriate format. Further information needed |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random table of numbers, using variable blocks of 10, by a clerk independent of the study. Stratification was by place of birth, hospital or midwifery birth centre |
| Allocation concealment (selection bias) | Low risk | On recruitment midwife telephoned an independent clerk for allocation |
| Blinding (performance bias and detection bias) All outcomes |
High risk | High risk of bias could have been introduced because women, carers and researcher cannot be blind to group allocation after randomisation due to the nature of the intervention. Study says where appropriate the data analyst was blinded to group; however bias is likely to be at point of care |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | Data analysed on intention-to-treat basis. Flow chart reports on participants from eligibility to completion. From randomisation similar numbers (water 58 (42%)/control 53 (39%)) became ineligible or did not use the allocated care option as might be expected in a study of this size which respected women’s right to choice care options; however, this is a high percentage |
| Selective reporting (reporting bias) | Low risk | All the data mentioned in the methods and that would reasonably be expected of this study are reported |
| Other bias | Unclear risk | No mention of 1-to-1 care or not, but no other issue apparent |
| Methods | Randomisation by sealed opaque, sequentially numbered envelopes containing the code. Methodological qualities:
|
|
| Participants | Group 1: n = 100: the “early bath group”. Group 2: n = 100: the “late bath group”. Regional referral hospital in the west of Sweden. Inclusion criteria: gestation > 34 weeks; low risk; singleton; cephalic presentation; spontaneous labour; contractions 3/10 minutes and/or ruptured membranes with cervical dilatation less than 3 cm. Normal FHR pattern. Ambulation and analgesics were allowed. |
|
| Interventions | All women used an oval tub that was 1.5 m long and 40 cm deep. It contained 300 L of waters at a temperature not more than 38 degrees Celsius. Group 1: the “early bath group” had a cervical dilatation of less than 5 cm when immersed in water. Group 2: the “late bath group” had a cervical dilation of 5 cm or more when immersed in water No mention of 1-to-1 care or not. |
|
| Outcomes | Maternal outcomes: *use of analgesia/anaesthesia; *augmentation of labour; duration of labour and birth; *mode of delivery; *maternal infection; *abnormal FHR patterns needing intervention; *neonatal condition; *admittance to NICU or high dependency care unit; *neonatal infection rates (studies that describe additional outcomes that may be of importance will be mentioned in the text); parity; maternal age; birthweight; Bishop score before randomisation. |
|
| Notes | Duration of labour not in acceptable format. Early group 9.80 hours and late group 8. 48 hours P < 0.004. Primipara: 72/100 in early group and 60/100 in late group. Maternal mean age: 26.3 early group; 27.2 late group. Mean birthweight: 3550 g early group; 3616 g late group. Performance bias: caregivers were not blind to group allocation. Not recorded if results were analysed blind. Exclusion bias: *women did not enter bath - groups not mentioned. Thus moderate rate of bias may be present. |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated. |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes containing allocation. |
| Blinding (performance bias and detection bias) All outcomes |
High risk | High risk of bias could have been introduced because women, carers and researcher cannot be blind to group allocation after randomisation due to the nature of the intervention |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | 1 woman in early bath group did not use water, compared to 7 in late bath group; however, this might be expected as a result of different degrees of progression in labour |
| Selective reporting (reporting bias) | Low risk | All outcomes identified in methods are reported. |
| Other bias | Unclear risk | Percentage of primigravida higher in early group, but likely to be due to chance No mention of 1-to-1 care or not. |
| Methods | Randomisation stated but only described as ‘by lots’. Methodological qualities:
|
|
| Participants | 33 women, 18 water, 15 control. In labour (cervix 4 cm dilated). Low risk - term, 1 fetus, no complications in current or any previous pregnancy/birth |
|
| Interventions | Intervention was use of bath for max of 60 minutes. Bath was thermally insulted, oval, size 150 cm by 110 cm, by 70 cm deep. Volume was 730 litres Water temperature 37 degree Celsius. No pharmacological analgesia available to either control or intervention group during study hour After use of bath labour care as normal and could access ‘usual’ pain relief methods, positions No mention of 1-to-1 second care or not. First stage only study. |
|
| Outcomes | Duration of first and second stage of labour. Pain relief used, pain score before and after study period (1 hour), own assessment in postnatal questionnaire on day 2 postpartum Blood loss, perineal trauma, Apgars. Maternal pulse, temperature, blood pressure. |
|
| Notes | Undertaken in Finland - 1 hospital. | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomised ‘by lots’ in translation. |
| Allocation concealment (selection bias) | High risk | Described as randomised but translation does not indicate how concealed |
| Blinding (performance bias and detection bias) All outcomes |
High risk | High risk of bias could have been introduced because women, carers and researcher cannot be blind to group allocation after randomisation due to the nature of the intervention |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | Not reported. |
| Selective reporting (reporting bias) | Unclear risk | Unable to assess this from translation. |
| Other bias | Unclear risk | Full translation not available, just extracts as requested on Cochrane Pregnancy and Childbirth Group translation sheet |
| Methods | Randomisation by sealed opaque, sequentially numbered envelopes containing the code. Prepared in random blocks of 10, stratified for parity. Methodological qualities:
|
|
| Participants | Study group: n = 60. Control group: n = 60. Academic teaching hospital, Johannesburg, South Africa. Inclusion criteria: gestation > 36 weeks; low risk; singleton; cephalic presentation; active phase of labour; normal FHR pattern; ambulation and analgesics were allowed; able to read and understand English. No immersion of water was used during the first stage of labour |
|
| Interventions | Study group: allocated to oval bath tub which contained about 220 L of water. Temperature 34-38 degrees Celsius. Women were allowed to use different postures in the bath. Control group: care the same as study group but they were not allowed to use a bath for birth. All care was the same. Consent obtained early in labour but randomisation took place at full second stage. Same main caregivers for all women. |
|
| Outcomes | Maternal outcomes: *maternal experience and satisfaction of labour; *pain; *use of analgesia/anaesthesia; *augmentation of labour; *blood pressure; *pulse; *duration of labour and birth; *mode of delivery; *trauma to the birth canal requiring suturing; *blood loss; maternal infection; *postpartum depression. Fetal outcomes: *abnormal FHR patterns needing intervention. Neonatal outcomes: *neonatal condition; *admittance to NICU or high dependency care unit; *temperature at birth; *perinatal deaths; delivered in OP position; gravida; age; birthweight; duration in bath. |
|
| Notes | Done in South Africa. 1999. | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not stated only says prepared in random blocks of 10, stratified for parity. Blocks of 10 have potential for breaking concealment for at least participant in each block |
| Allocation concealment (selection bias) | Low risk | Sealed opaque, sequentially numbered envelopes containing the code |
| Blinding (performance bias and detection bias) All outcomes |
High risk | High risk of bias could have been introduced because women, carers and researcher cannot be blind to group allocation after randomisation due to the nature of the intervention |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | Information from approach to women (133) to allocation (60+60); all women completed trial but 3 in control group to not complete follow-up questionnaire |
| Selective reporting (reporting bias) | Low risk | All outcomes identified in methods are reported. Thesis made available with very detailed reporting |
| Other bias | Unclear risk | All women regardless of group had 1-to-1 care from researcher |
| Methods | Randomised when regular contractions and eligible. Sealed opaque envelopes. Methodological qualities:
|
|
| Participants | Study group: KH: n = 364. OH: n = 95; LH: n = 153; total = 612. Control group: KH: n = 376; OH: n = 97; LH: n =152; total = 625. Inclusion criteria: gestation > 35 weeks; previous CS included (VBAC); twins included; active labour > 3 cm cervical dilatation; ruptured membranes on entry also eligible. Ambulation, analgesics and anaesthesia were allowed. 42 were withdrawn (15 from OH, 21 from LH and 6 KH) no indication for study/control group split for withdraws |
|
| Interventions | Study group: warm bath; no information on management of care for either group; no information on water temperature or bath size. Control group: shower allowed. Water use in first stage, no mention of 1-to-1 second care or not |
|
| Outcomes | Maternal outcomes: *use of analgesia/anaesthesia; *mode of delivery; *trauma to the birth canal requiring suturing. Neonatal outcomes: *neonatal condition; *admittance to NICU or high dependency care unit. Additional outcomes: secondary arrest and delivered in OP position. |
|
| Notes | 3 obstetric units in Sweden - 1992-1995. | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not indicated. |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes. |
| Blinding (performance bias and detection bias) All outcomes |
High risk | High risk of bias could have been introduced because women, carers and researcher cannot be blind to group allocation after randomisation due to the nature of the intervention |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | From a total of 1279 women, 42 were excluded across both groups and all centres for obstetric reasons |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned in methods are reported, although detailed results only given for main outcomes, others just stated as not significant in the text |
| Other bias | Low risk | Study was started in 1 unit then after 2 years 2 further obstetric units were involved to achieve the required sample size. Nil else noted |
| Methods | Randomisation by consecutively numbered, computer generated random allocation in sealed opaque envelopes. Methodological qualities:
|
|
| Participants | Academic hospital, Ontario, Canada. Inclusion criteria: gestation > 37 weeks; previous CS included (VBAC); twins included; active labour > 3 cm cervical dilatation; ruptured membranes on entry also eligible. Ambulation, analgesics and anaesthesia were allowed. 800 women were randomised, 15 were withdrawn 8 from study group and 7 from control group. Nearly half (46%) of the women in the study group did NOT use the bath but were still considered experimental subjects with the intent to treat. 41 of the women did not meet eligibility criteria but were still included and results were analysed. Experimental group adds up to 394. |
|
| Interventions | Study group: n = 393. The use of a Parker whirlpool hot tub with jets during labour. Bath temperature between 38-39 degrees celsius. Mean total time in tub was 54 minutes. No births in tub Control group: n = 392. No water immersion during labour. Refer to care being provided by assigned nurse, and all had be trained to care for women using immersion, but not clear if this is 1-to-1 second care First stage only. |
|
| Outcomes | Maternal outcomes: *use of analgesia/anaesthesia; *augmentation of labour; *presence of meconium-stained liquor; *duration of labour and birth; *mode of delivery. Additional outcomes: maternal age; gravida; cervical dilatation; duration in tub; VBAC. |
|
| Notes | Data table 1 incorrect. No response from authors. | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated. |
| Allocation concealment (selection bias) | Low risk | Consecutively numbered sealed opaque envelopes. |
| Blinding (performance bias and detection bias) All outcomes |
High risk | High risk of bias could have been introduced because women, carers and researcher cannot be blind to group allocation after randomisation due to the nature of the intervention |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | All participants are accounted for, and 15 withdraws were detailed, as were 41 who did not meet criteria but were recruited. These are small numbers from 800 recruited |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned in methods are reported, and seem appropriate for the study and topic |
| Other bias | Unclear risk | 46% of women did not use the water, but that was expected as women had choice, and sample size was planned for this |
| Methods | Randomisation by packets containing random computer-generated codes. Methodological qualities:
|
|
| Participants | Study group: n = 45. Control group: n = 48. Inclusion criteria: gestation between 36-41 weeks; no major obstetric or medical complication; active labour between 4-7 cm cervical dilatation; intact membranes on entry; normal FHR patterns; ambulation and analgesics were allowed. |
|
| Interventions | Study group: The use of a hot tub with air jets and with a moulded seat during labour. Bath temperature between 32-41 degrees Celsius. Control group: no water immersion during labour. Showers were allowed First stage of labour. |
|
| Outcomes | Maternal age; gestational age; ethnicity; parity; water temperature; duration in bath; *use of analgesia; *augmentation; cervical dilatation; *duration of first stage of labour; *duration of second stage of labour; duration of admission to delivery; duration of ruptured membranes; blood pressure; pulse; maternal temperature; *method of delivery; *FHR patterns; Apgar score at 1 minute; *Apgar score at 5 minutes; neonatal weight; *postnatal maternal infections; re-admissions to hospital. |
|
| Notes | Academic hospital, Houston, Texas, USA. December 1990 to December 1991 | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer-generated code. |
| Allocation concealment (selection bias) | High risk | Midwife know the allocation at the time of recruitment, and risk of bias acknowledged but women apparently would not be recruited if they did not know which allocation they had |
| Blinding (performance bias and detection bias) All outcomes |
High risk | High risk of bias could have been introduced because women, carers and researcher cannot be blind to group allocation after randomisation due to the nature of the intervention |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | All participants are accounted for throughout study with no withdrawals |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned in method are reported, and seem appropriate for the study and topic |
| Other bias | Unclear risk | There were significantly more primigravid women in water group, which could affect outcomes, and is a confounding variable |
| Methods | Randomisation into sequentially numbered sealed opaque envelopes containing the code. Prepared in variable random blocks stratified for parity. Randomised when in active birth labour and met inclusion and exclusion criteria. Methodological qualities:
|
|
| Participants | Study group: n = 59. Control group: n = 61. Inclusion criteria: in active labour; primiparous women with cervical dilatation of 4-7 cm; multiparous women with cervical dilatation of 4-6 cm; low-risk women; read/understand English. Exclusion criteria: poor obstetric history; previous CS; medical disorders; pre-eclampsia; multiple pregnancy; intrauterine growth impairment; < 36 weeks and > 42 weeks; pyrexia; meconium-stained liquor; prolonged ruptured of membranes. |
|
| Interventions | Study group: labour in water; water temperature 34-38 degrees Celsius; analgesia as required; exit for second stage; not out of the water for more than 30 minutes. Control group: encouraged ambulation; if lie down use side analgesia as required. Same midwife for all women (so 1-to-1 second stage care assumed) also same observer/assessor of pain for all First stage study. |
|
| Outcomes | Outcomes reported: maternal outcomes; *pain; *use of analgesia/anaesthesia; *augmentation of labour; *blood pressure; *pulse; *duration of labour and birth; *mode of delivery; *trauma to the birth canal requiring suturing; *blood loss; *postpartum depression; *breastfeeding; fetal outcomes; *abnormal FHR patterns needing intervention. Additional outcomes: studies which describe additional outcomes that may be of importance will bementioned in the text; gestational age; maternal age; gravida; parity; cervical dilatation; duration in tub; meconium-stained liquor. |
|
| Notes | Academic hospital, South Africa. | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random list compiled in different block size of 6 and 8 but not clear how this was achieved or by whom |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered sealed opaque envelopes containing the allocation |
| Blinding (performance bias and detection bias) All outcomes |
High risk | High risk of bias could have been introduced because women, carers and researcher cannot be blind to group allocation after randomisation due to the nature of the intervention |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | All participants are accounted for throughout study with no withdrawals |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned in method are reports, and seem appropriate for the study and topic |
| Other bias | Unclear risk | Researcher recruited and cared for all women and provided 1-to-1 care |
| Methods | Randomisation schedule provided by National Perinatal Epidemiology Unit, Oxford. A person unconnected to study prepared by consecutively numbered, computer-generated random allocation in sealed opaque envelopes. Methodological qualities:
|
|
| Participants | 2 groups in RCT part of study. Water n = 40. Land n = 20 (2:1 ratio as about local experience was 50% of women choose not to use water) Women recruited through community midwife, posters in clinics, and media promotions and interested women contacted researcher or gave permission to own midwife to pass on information Aged 18-50. Low risk. |
|
| Interventions | Women could use pool in first and second stages of labour - results do not distinguish which of the women allocated to pool, did not use pool (16 of 40 women), used pool for first stage only (13 of 40 women), used pool in second stage but not for birth (1 woman), or gave birth in the pool (10 women) (no subgroup analysis) Data entered into both ‘immersion in water versus no immersion during first stage of labour’ AND ‘immersion in water versus no immersion during second stage of labour’ DATA and ANALYSIS section Waterbirth pool - dimensions/volume not described, temperature described as recorded but data not provided No mention of 1-to-1 second care or not. |
|
| Outcomes | Intention-to-treat analysis done. Maternal: age, social history, pulse, temperature, maternal satisfaction on scale of 0-6 immediately post birth and in 6 week postal questionnaire Labour: length of first, second stages; analgesia used; augmentation; mode of birth Fetus/neonate: cord arterial and venous gases, Apgar score at 1, 5 and 10 mins, time to first respiration, rectal temperature at birth, ear swabs, method of feeding, date and time of first feed, admission to neonatal unit (plus any interventions needed) infection, any mortality/morbidity Water; duration in water, water temperature, microbiological analysis at end of labour/use |
|
| Notes | Non-randomised, preference arm data not included although additional 20 participants in this part of study 16 (40%) of water women did not use water. UK study. |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated independent of study. |
| Allocation concealment (selection bias) | Low risk | Consecutively numbered in sealed opaque envelopes. |
| Blinding (performance bias and detection bias) All outcomes |
High risk | High risk of bias could have been introduced because women, carers and researcher cannot be blind to group allocation after randomisation due to the nature of the intervention |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | All participants are accounted for throughout study with no withdrawals |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned in method are reported, and seem appropriate for the study and topic |
| Other bias | Unclear risk | 40% or water group did not use water, which is consistent with choice and other papers on this topic |
prespecified outcomes
CS: caesarean section
FHR: fetal heart rate
KH: Karlskrona Hospital
LH: Lund hospital
NICU: neonatal intensive care unit
OH: Osterund Hospital
OP: occipito posterior
VBAC: vaginal birth after caesarean section
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Bastide 1990 | Unpublished data from 1990 available only. Inadequate data for assessment at this time. We contacted the author for further information, but nothing was provided |
| Benfield 2001 | Only 18 women, 9 in each group. Randomsied by drawing slips of paper from a total of 52 paper slips in a bag which is inadequate allocation concealment. Women were in a limited depth of water; were asked to adopt a semi recumbent positions on a partially inflated air raft with attached head pillow (authors description) for 1 hour, and had cannulation to facilitate repeat blood samples. All of which limits mobility and is not consistent with water immersion in normal labour |
| Calvert 2000 | Results of this pilot study (22 women) are not given in a format that can be used in the review. The aim was to compare the effect of the essential oil of ginger compared to essential oil of lemon grass on the progress of labour. The pilot study showed no differences on frequency of contractions, cervical dilatation or duration of first stage of labour between the 2 groups |
| Cluett 2001 | Feasibility study: only 4 women in each of the 3 trial arms. Women had all been diagnosed as having dystocia in the first stage of labour (less than 1 cm/hr progress after established labour) |
| Cluett 2004 | Women had all been diagnosed as having dystocia in the first stage of labour (less than 1 cm/hr progress after established labour) and the comparison group was women receiving augmentation of labour |
| Labrecque 1999 | The method does not meet the inclusion criteria for this review. It is impossible to disentangle the effects of immersion in water. The aim of the trial was to compare 3 non-pharmacological approaches to relieve back pain. A total of 34 women were randomly allocated to receive 1 of 3 treatments: (1) intracutaneous sterile water injections, (2) transcutaneous electrical nerve stimulation and (3) standard care that included back massage, whirlpool bath and liberal mobilisation. The sample size is small and results should be interpreted within the setting only. Women in the ISW group experienced a decrease in the intensity and unpleasantness of their backache, but they would not like to use this method in a future labour |
ISW: intracutaneous sterile water injection
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Not clear; paper refer to ‘the analysis of 205 deliveries including 100 in control group’ however, this could have been subgroups within a cohort. There is no reference to any randomisation |
| Participants | Primiparous women, full-term uncomplicated pregnancy, in first stage of labour with a cervical dilation of between 2-6 cm, with fetus in a longitudinal lie, vertex presentation, and presented part engaged |
| Interventions | Women in the first stage of labour were immersed in a whirlpool bath for duration of no more than 40 minutes |
| Outcomes | Cervical dilation prior to immersion and at end of immersion period. Duration of first stage of labour. Uterine activity before and after intervention |
| Notes | Abstract in English with limited translation of other parts of paper; however, we were not able to determine with any certainty that this study is a RCT. We have contacted the main author for clarification |
| Methods | Clinical trial of women equally divided into 2 groups of 50 of delivery in water and normal delivery. It is not clear from this if there was any randomisation. The fact that all 50 women in the intervention (water) group all achieved a water birth suggests they were a cohort of women who consecutively achieved a water birth rather than a randomised group allocated to water immersion. Clarification has been sought from the main author |
| Participants | 100 gravida 1 and to women; aged 16-28 years; gestational age of 38-42 weeks |
| Interventions | Immersion in water during the active phase of delivery (not clear if this is first or second stage) |
| Outcomes | Outcomes Amount of analgesia, pain score, oxytocin use, duration of labour fist and second stage, Apgar score, admission to neonatal unit, satisfaction with mode of delivery |
| Notes | We have sought clarification from the main author on whether or not randomisation was used; if so, what measure were taken to conceal the allocation; and for clarity on the nature of the intervention |
RCT: randomised controlled trial
DATA AND ANALYSES
Comparison 1.
Immersion in water versus no immersion during first stage of labour
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Use of epidural/spinal analgesia/paracervical block | 6 | 2499 | Risk Ratio (M-H, Fixed, 95% CI) | 0.90 [0.82, 0.99] |
| 2 Pethidine/narcotic used | 4 | 1240 | Risk Ratio (M-H, Random, 95% CI) | 0.85 [0.46, 1.56] |
| 3 Use of transcutaneous nerve stimulation (TENS) | 2 | 845 | Risk Ratio (M-H, Fixed, 95% CI) | 1.05 [0.37, 2.94] |
| 4 Use of any analgesia | 5 | 653 | Risk Ratio (M-H, Random, 95% CI) | 0.72 [0.46, 1.12] |
| 5 Any pharmacological analgesia | 2 | 394 | Risk Ratio (M-H, Fixed, 95% CI) | 1.05 [0.80, 1.39] |
| 6 Experience of moderate to severe pain | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Ordinal description as moderate to severe, 30 mins after randomisation | 1 | 120 | Risk Ratio (M-H, Fixed, 95% CI) | 0.75 [0.62, 0.91] |
| 6.2 VAS scale 8 to 10, 30 mins after randomisation | 1 | 120 | Risk Ratio (M-H, Fixed, 95% CI) | 0.72 [0.58, 0.90] |
| 6.3 Ordinal scale pain faces 4 to 5, 30 mins after randomisation | 1 | 120 | Risk Ratio (M-H, Fixed, 95% CI) | 0.67 [0.51, 0.90] |
| 6.4 Ordinal description as moderate to severe, 1 hr after randomisation | 1 | 117 | Risk Ratio (M-H, Fixed, 95% CI) | 0.76 [0.63, 0.91] |
| 6.5 VAS scale 8 to 10, 1 hr after randomisation | 1 | 117 | Risk Ratio (M-H, Fixed, 95% CI) | 1.21 [0.69, 2.11] |
| 6.6 Ordinal scale pain faces 4 to 5, 1 hr after randomisation | 1 | 117 | Risk Ratio (M-H, Fixed, 95% CI) | 0.68 [0.53, 0.86] |
| 6.7 Ordinal description as moderate to severe, 2 hrs after randomisation | 1 | 57 | Risk Ratio (M-H, Fixed, 95% CI) | 0.76 [0.59, 0.98] |
| 6.8 VAS scale 8 to 10, 2 hrs after randomisation | 1 | 57 | Risk Ratio (M-H, Fixed, 95% CI) | 0.83 [0.66, 1.05] |
| 6.9 Ordinal scale pain faces 4 to 5, 2 hrs after randomisation | 1 | 57 | Risk Ratio (M-H, Fixed, 95% CI) | 0.72 [0.52, 0.98] |
| 6.10 Ordinal description as moderate to severe, 3 hrs after randomisation | 1 | 32 | Risk Ratio (M-H, Fixed, 95% CI) | 0.52 [0.23, 1.16] |
| 6.11 VAS scale 8 to 10, 3 hrs after randomisation | 1 | 32 | Risk Ratio (M-H, Fixed, 95% CI) | 0.69 [0.39, 1.23] |
| 6.12 Ordinal scale pain faces 4 to 5, 3 hrs after randomisation | 1 | 32 | Risk Ratio (M-H, Fixed, 95% CI) | 0.57 [0.25, 1.27] |
| 6.13 Ordinal description as moderate to severe, 24 hrs after randomisation | 1 | 119 | Risk Ratio (M-H, Fixed, 95% CI) | 0.64 [0.50, 0.82] |
| 6.14 VAS scale 8 to 10, 24 hrs after randomisation | 1 | 119 | Risk Ratio (M-H, Fixed, 95% CI) | 0.62 [0.49, 0.80] |
| 6.15 Ordinal scale pain faces 4 to 5, 24 hrs after randomisation | 1 | 119 | Risk Ratio (M-H, Fixed, 95% CI) | 0.69 [0.54, 0.87] |
| 7 Instrumental/surgical delivery | 9 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Assisted vaginal deliveries | 7 | 2628 | Risk Ratio (M-H, Fixed, 95% CI) | 0.86 [0.71, 1.05] |
| 7.2 Caesarean section | 8 | 2712 | Risk Ratio (M-H, Fixed, 95% CI) | 1.21 [0.87, 1.68] |
| 7.3 Normal versus operative birth | 1 | 106 | Risk Ratio (M-H, Fixed, 95% CI) | 1.26 [1.09, 1.45] |
| 8 Duration of first stage (minutes) | 7 | 1461 | Mean Difference (IV, Fixed, 95% CI) | −32.40 [−58.67, −6.13] |
| 9 Duration of second stage (minutes) | 8 | 1569 | Mean Difference (IV, Fixed, 95% CI) | 0.47 [−3.45, 4.38] |
| 10 Duration of third stage (minutes) | 3 | 1165 | Mean Difference (IV, Random, 95% CI) | −0.52 [−1.84, 0.79] |
| 11 Duration of labour from randomisation till delivery | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Duration of total labour (all three stages) | 1 | 120 | Mean Difference (IV, Fixed, 95% CI) | −27.5 [−133.05, 78.05] |
| 13 Postpartum haemorrhage | 1 | 274 | Risk Ratio (M-H, Fixed, 95% CI) | 1.58 [0.80, 3.13] |
| 14 Blood loss | 2 | 153 | Mean Difference (IV, Fixed, 95% CI) | −14.33 [−63.03, 34.37] |
| 15 Perineal trauma after vaginal birth | 6 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 15.1 Intact | 5 | 1337 | Risk Ratio (M-H, Fixed, 95% CI) | 1.16 [0.99, 1.35] |
| 15.2 Episiotomy | 5 | 1272 | Risk Ratio (M-H, Fixed, 95% CI) | 0.93 [0.80, 1.08] |
| 15.3 Second-degree tear | 5 | 1286 | Risk Ratio (M-H, Fixed, 95% CI) | 0.94 [0.74, 1.20] |
| 15.4 Third- or fourth-degree tears | 5 | 2401 | Risk Ratio (M-H, Fixed, 95% CI) | 1.37 [0.86, 2.17] |
| 16 Satisfication with labour | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.1 Labour and delivery satisfaction index | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.2 Dissatisfied measured using ordinal scale | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Self reports pain score on visual analogue scale of 0-10 | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 17.1 Pain score at start of assessment period (time zero) | 2 | 141 | Mean Difference (IV, Fixed, 95% CI) | −0.01 [−0.54, 0.52] |
| 17.2 Pain score up to 60 minutes later | 2 | 141 | Mean Difference (IV, Fixed, 95% CI) | −0.81 [−1.34, −0.28] |
| 18 Does not wish to use bath with next labour/delivery | 1 | 119 | Risk Ratio (M-H, Fixed, 95% CI) | 0.38 [0.14, 0.98] |
| 19 Artificial rupture of membranes | 3 | 926 | Risk Ratio (M-H, Fixed, 95% CI) | 1.02 [0.90, 1.16] |
| 20 Amniotic fluid index | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21 Presence of meconium stained liquor | 5 | 1260 | Risk Ratio (M-H, Fixed, 95% CI) | 0.95 [0.76, 1.19] |
| 22 Use of oxytocin for augmentation of labour | 5 | 1125 | Risk Ratio (M-H, Random, 95% CI) | 0.64 [0.32, 1.28] |
| 23 Systolic blood pressure | 1 | 120 | Mean Difference (IV, Fixed, 95% CI) | −7.20 [−13.12, −21.28] |
| 24 Diastolic blood pressure | 1 | 120 | Mean Difference (IV, Fixed, 95% CI) | −10.20 [−13.70, −6.70] |
| 25 Mean arterial blood pressure | 1 | 120 | Mean Difference (IV, Fixed, 95% CI) | −10.5 [−14.68, −6.32] |
| 26 Maternal infection (perineal, systemic, uterine or increase in temperature) | 5 | 1295 | Risk Ratio (M-H, Fixed, 95% CI) | 0.99 [0.50, 1.96] |
| 27 Low self-esteem | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 28 Postpartum depression EPDS more than 11 | 2 | 370 | Risk Ratio (M-H, Fixed, 95% CI) | 1.38 [0.85, 2.24] |
| 29 Not breastfeeding after six weeks postdelivery | 2 | 363 | Risk Ratio (M-H, Fixed, 95% CI) | 1.17 [0.64, 2.15] |
| 30 Abnormal fetal heart rate patterns | 3 | 487 | Risk Ratio (M-H, Random, 95% CI) | 0.75 [0.34, 1.67] |
| 31 Apgar score less than seven (five minutes) | 5 | 1834 | Risk Ratio (M-H, Fixed, 95% CI) | 1.58 [0.63, 3.93] |
| 32 Apgar score at five minutes | 2 | 893 | Mean Difference (IV, Fixed, 95% CI) | −0.03 [−0.11, 0.06] |
| 33 Umbilical artery pH less than 7.20 | 1 | 110 | Risk Ratio (M-H, Fixed, 95% CI) | 5.18 [0.25, 105.51] |
| 34 Neonate temperature | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 34.1 Temperature less than 36.2 degrees C at birth |
0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 34.2 Temperature greater than 37.5 degrees C at birth |
0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 34.3 Temperature greater than 37.8 degrees C as an indicator for infection |
1 | 274 | Risk Ratio (M-H, Fixed, 95% CI) | 1.0 [0.06, 15.83] |
| 35 Admission to neonatal intensive care unit | 3 | 1571 | Risk Ratio (M-H, Fixed, 95% CI) | 1.06 [0.71, 1.57] |
| 36 Neonatal infection | 5 | 1295 | Risk Ratio (M-H, Fixed, 95% CI) | 2.00 [0.50, 7.94] |
| 37 Lung hypoplasia present | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 38 Perinatal deaths | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 39 Caregiver injuries | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 40 Neonatal gestational age at birth | 9 | 2820 | Mean Difference (IV, Fixed, 95% CI) | −0.01 [−0.82, 0.80] |
| 41 Birthweight in grams | 9 | 2820 | Mean Difference (IV, Fixed, 95% CI) | −22.74 [−66.44, 20.96] |
Comparison 2.
Immersion in water versus no immersion during second stage of labour
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Experience of moderate to severe pain | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Ordinal description as moderate to severe | 1 | 117 | Risk Ratio (M-H, Fixed, 95% CI) | 1.06 [0.73, 1.53] |
| 1.2 Labour Agentry scale | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 Line scale | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Satisfied with labour | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Labour and delivery satisfaction index | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Little or not satisfied with coping experience | 1 | 117 | Risk Ratio (M-H, Fixed, 95% CI) | 0.24 [0.07, 0.80] |
| 3 Does not wish to use bath next delivery | 1 | 117 | Risk Ratio (M-H, Fixed, 95% CI) | 0.57 [0.22, 1.47] |
| 4 Perineal trauma after vaginal birth | 2 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Episiotomy | 2 | 179 | Risk Ratio (M-H, Fixed, 95% CI) | 0.75 [0.35, 1.60] |
| 4.2 Second-degree tear | 2 | 179 | Risk Ratio (M-H, Fixed, 95% CI) | 1.21 [0.65, 2.24] |
| 4.3 Third- or fourth-degree tears | 1 | 60 | Risk Ratio (M-H, Fixed, 95% CI) | 1.54 [0.07, 36.11] |
| 5 Duration of second stage (minutes) | 3 | 286 | Mean Difference (IV, Fixed, 95% CI) | −1.24 [−8.05, 5.56] |
| 6 Instrumental/surgical delivery | 3 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Assisted vaginal deliveries | 2 | 180 | Risk Ratio (M-H, Fixed, 95% CI) | 0.73 [0.21, 2.54] |
| 6.2 Caesarean section | 2 | 180 | Risk Ratio (M-H, Fixed, 95% CI) | 0.33 [0.07, 1.52] |
| 6.3 Any operative versus normal birth | 1 | 106 | Risk Ratio (M-H, Fixed, 95% CI) | 0.04 [0.00, 0.72] |
| 7 Postpartum haemorrhage more than 500 ml | 1 | 120 | Risk Ratio (M-H, Fixed, 95% CI) | 0.14 [0.01, 2.71] |
| 8 Presence of meconium-stained liquor | 2 | 180 | Risk Ratio (M-H, Fixed, 95% CI) | 1.32 [0.63, 2.80] |
| 9 Apgar score less than seven (five minutes) | 1 | 119 | Risk Ratio (M-H, Fixed, 95% CI) | 4.92 [0.24, 100.31] |
| 10 Apgar less than eight at five minutes | 1 | 60 | Risk Ratio (M-H, Fixed, 95% CI) | 1.54 [0.07, 36.11] |
| 11 Neonate temperature | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 11.1 Temperature less than 36.2 degrees C at birth |
1 | 109 | Risk Ratio (M-H, Fixed, 95% CI) | 0.98 [0.30, 3.20] |
| 11.2 Temperature greater than 37.5 degrees C at birth |
1 | 109 | Risk Ratio (M-H, Fixed, 95% CI) | 2.62 [0.73, 9.35] |
| 11.3 Temperature greater 37.8 degrees C as an indicator for infection | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Umbilical artery pH less than 7.20 | 1 | 116 | Risk Ratio (M-H, Fixed, 95% CI) | 0.89 [0.45, 1.75] |
| 13 Cord arterial pH | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Admission to neonatal intensive care unit | 2 | 180 | Risk Ratio (M-H, Fixed, 95% CI) | 0.79 [0.25, 2.49] |
| 15 Perinatal deaths | 1 | 120 | Risk Ratio (M-H, Fixed, 95% CI) | 3.0 [0.12, 72.20] |
| 16 Satisfaction with labour and birth on scale of 0-6 where 0 is not at all satisfied | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.03 [−0.64, 0.70] |
| 17 Maternal temperature | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [−0.18, 0.58] |
| 18 Breastfeeding | 1 | 60 | Risk Ratio (M-H, Fixed, 95% CI) | 0.86 [0.69, 1.08] |
| 19 Antibiotics given to neonate | 1 | 60 | Risk Ratio (M-H, Fixed, 95% CI) | 1.5 [0.17, 13.52] |
| 20 Positive neonatal swab of ear, mouth or umbilicus | 1 | 154 | Risk Ratio (M-H, Fixed, 95% CI) | 1.89 [0.90, 3.96] |
| 21 Neonatal gestational age at birth in days | 2 | 180 | Mean Difference (IV, Fixed, 95% CI) | −1.0 [−5.13, 3.13] |
Comparison 5.
Early versus late immersion in water
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Epidural/spinal analgesia/paracervical block | 1 | 200 | Risk Ratio (M-H, Fixed, 95% CI) | 2.21 [1.39, 3.52] |
| 2 Use of oxytocin | 1 | 200 | Risk Ratio (M-H, Fixed, 95% CI) | 1.9 [1.35, 2.68] |
| 3 Instrumental or surgical delivery | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Abnormal fetal heart rate patterns | 1 | 200 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Apgar score less than seven at one minute | 1 | 200 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Neonatal infection | 1 | 200 | Risk Ratio (M-H, Fixed, 95% CI) | 3.0 [0.12, 72.77] |
| 7 Admission to neonatal special care unit | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Neonatal birthweight in grams | 1 | 200 | Mean Difference (IV, Fixed, 95% CI) | −66.0 [−189.34, 57.34] |
FEEDBACK
Wein, December 2006
Summary
How can the review authors conclude “Overall, the evidence indicates that immersion in water decreases maternal reported pain levels and the uptake of pharmacological analgesia” when their analysis reports the odds ratio for pharmacological analgesia as 1.08 (95% CI 0.71 to 1.65)?
(Summary of comment from Peter Wein, December 2006)
Reply
In the authors’ conclusions section of the previous update of this review (Cluett 2002), the statement “immersion in water decreases maternal reported pain levels” was based on the one trial (Taha 2000) that reported this outcome (OR 0.23, 95% CI 0.08 to 0.63). The limitation of only one study is indicated in the maternal outcome section of the review. The reference to a decrease in maternal ‘uptake of pharmacological analgesia’ was based on the outcome ‘use of epidural/spinal/paracervical block’ (OR 0.84, 95% CI 0.71 to 0.99), which included data from four trials, not the outcome ‘any pharmacological analgesia’ which include data from two trials and is the one cited by Wein above. We accept the wording was ambiguous, and have clarified it in the current update.
Interestingly, in this update data for these outcomes have altered minimally: use of epidural/spinal/paracervical block is now OR 0.82, 95% CI 0.70 to 0.98, with data from six trials; ‘any pharmacological analgesia’, remains unchanged, as do the data for maternal pain experience.
(Response from Elizabeth Cluett, October 2008)
Contributors
Peter Wein
WHAT’S NEW
Last assessed as up-to-date: 23 August 2011.
| Date | Event | Description |
|---|---|---|
| 14 December 2011 | Amended | Corrected error in Abstract and in Analysis 1.17. |
HISTORY
Protocol first published: Issue 3, 1996
Review first published: Issue 3, 1997
| Date | Event | Description |
|---|---|---|
| 30 June 2011 | New search has been performed | Papers from June 2011 search reviewed and data incorporated as appropriate. 1 new study included (Chaichian 2009) and 2 added to Characteristics of studies awaiting classification pending more information from the authors. Risk of bias tables generated. Text updated, although no change in overall conclusions |
| 5 January 2009 | New citation required but conclusions have not changed | Change in authorship. |
| 20 November 2008 | New search has been performed | Search updated. New trials identified, appraised and data are included Title changed to reflect focus on water immersion in labour and birth, so pregnancy removed from title, and outcomes updated accordingly Background information updated. Results and discussion sections updated but no change to overall conclusions |
| 20 November 2008 | Feedback has been incorporated | Response from authors to feedback from Wein incorporated. |
| 29 October 2008 | Amended | Converted to new review format. |
| 25 April 2004 | New search has been performed | Search updated. Five new trials are included (Eckert 2001; Eriksson 1997; Nikodem 1999; Ohlsson 2001; Taha 2000). |
| 25 April 2004 | New citation required and conclusions have changed | The inclusion of the new trials has resulted in a change in the implications for practice, which now indicates that immersion in water during the first stage of labour reduces reported maternal pain and the use of analgesia. The outcome measures have been modified to ensure clarity. Neonatal outcomes have been added to reflect current methods of wellbeing assessment Change in authorship for this update. |
Footnotes
DECLARATIONS OF INTEREST The first review author (E Cluett) is chief investigator of two trials related to the subject of this review (Cluett 2001; Cluett 2004); we have excluded both.
INDEX TERMS
Medical Subject Headings (MeSH)
*Immersion; *Labor Stage, First; *Labor Stage, Second; *Water; Analgesia, Obstetrical [utilization]; Natural Childbirth; Randomized Controlled Trials as Topic
MeSH check words
Female; Humans; Pregnancy
REFERENCES
References to studies included in this review
* Indicates the major publication for the study
- Cammu 1994.Cammu H, Clasen K, Van Wetteren L. Is having a warm bath during labour useful? Journal of Perinatal Medicine. 1992;20(Suppl 1):104. [Google Scholar]; * Cammu H, Clasen K, Van Wetteren L, Derde M. ‘To bathe or not to bathe’ during the first stage of labor. Acta Obstetricia et Gynecologica Scandinavica. 1994;73:468–72. doi: 10.3109/00016349409013433. [DOI] [PubMed] [Google Scholar]
- Chaichian 2009.Chaichian S, Akhlaghi A, Rousta F, Safavi M. Experience of water birth delivery in Iran. Archives of Iranian Medicine. 2009;12(5):468–71. [PubMed] [Google Scholar]
- Da Silva 2006 *.Da Silva FM, De Oliveira SM. The effect of immersion baths on the length of childbirth labor [O efeito do banho de imersao na duracao do trabalho de parto] Revista da Escola de Enfermagem da USP. 2006;40(1):57–63. doi: 10.1590/s0080-62342006000100008. [DOI] [PubMed] [Google Scholar]; Da Silva FMB, De Olivera SMJV, Nobre MRC. A randomised controlled trial evaluating the effect of immersion bath on labour pain. Midwifery. 2009;Vol. 25(issue 3):286–294. doi: 10.1016/j.midw.2007.04.006. [DOI] [PubMed] [Google Scholar]
- Eckert 2001 *.Eckert K, Turnbull D, MacLennan A. Immersion in water in the first stage of labor: a randomised controlled trial. Birth. 2001;28(2):84–93. doi: 10.1046/j.1523-536x.2001.00084.x. [DOI] [PubMed] [Google Scholar]; Eckert KA, MacLennan AH, Turnbull DA. Immersion in water in the first stage of labour: a randomised controlled trial. 4th Annual Congress of the Perinatal Society of Australia & New Zealand; 1998 March 30-April 4; Australia: Alice Springs; 1998. [Google Scholar]
- Eriksson 1997 *.Eriksson M, Mattson L, Ladfors L. Early or late bath during the first stage of labour: a randomised study of 200 women. Midwifery. 1997;13:146–8. doi: 10.1016/s0266-6138(97)90005-x. [DOI] [PubMed] [Google Scholar]; Ladfors L, Mattsson I, Eriksson M. Early or late tub bath during the first stage of labor: a randomized study of 200 women. American Journal of Obstetrics and Gynecology. 1997;176(1 Pt 2):S141. doi: 10.1016/s0266-6138(97)90005-x. [DOI] [PubMed] [Google Scholar]
- Kuusela 1998.Kuusela P, Koivisto A-M, Heinonen PK. Warn tub bath during opening phase of labor [Lammin kylpy synnytyksen avautumisvaiheessa] Suomen Laakarilehti. 1998;11:1217–21. [Google Scholar]
- Nikodem 1999.Nikodem C, Hofmeyr GJ, Nolte AGW, De Jager M. The effects of water on birth: a randomized controlled trial. Proceedings of the 14th Conference on Priorities in Perinatal Care in South Africa; South Africa. 1995 March 7-10.1995. pp. 163–6. [Google Scholar]; * Nikodem VC. Immersion in water during birth: a randomized controlled trial [thesis] University of Witwatersrand; South Africa: 1999. [Google Scholar]; Nikodem VC. Guidelines for underwater deliveries: evidence from randomized controlled trial. Fifteenth Conference on Priorities in Perinatal Care in South Africa; March 5-8; South Africa: Goudini Spa; 1996. [Google Scholar]
- Ohlsson 2001 *.Ohlsson G, Buchhave P, Leandersson U, Nordstrom L, Rydhstrom H, Sjolin I. Warm tub bathing during labor: maternal and neonatal effects. Acta Obstetricia et Gynecologica Scandinavica. 2001;80:311–4. doi: 10.1034/j.1600-0412.2001.080004311.x. [DOI] [PubMed] [Google Scholar]; Rydhstrom H. Trial to test the effect of bathing vs no bathing in labour on transfer to neonatal intensive care. Personal communication. 1994.
- Rush 1996.Rush J, Burlock S, Lambert K, Loosley-Millman M, Hutchison B, Enkin M. The effects of whirlpool baths in labor: a randomized controlled trial. Birth. 1996;23:136–43. doi: 10.1111/j.1523-536x.1996.tb00474.x. [DOI] [PubMed] [Google Scholar]
- Schorn 1993.Schorn MN, McAllister JL, Blanco JD. Water immersion and the effect on labor. Journal of Nurse-Midwifery. 1993;38(6):336–42. doi: 10.1016/0091-2182(93)90014-8. [DOI] [PubMed] [Google Scholar]
- Taha 2000.De Jager M, Nolte AGW, Hofmeyr GJ, Nikodem VC. Immersion in water during first stage of labour. A randomised controlled trial. Personal communication. 2001. ; * Taha M. The effects of water on labour: a randomised controlled trial [thesis] Rand Afrikaans University; Johannesburg: 2000. [Google Scholar]; Taha M, Nolte AGW, Hofmeyr GJ, Dorfling CS. Water as a method of pain relief: a randomised controlled trial. 20th Conference on Priorities in Perinatal Care in Southern Africa; 2001 March 6-9; South Africa: KwaZulu-Natal; 2001. [Google Scholar]
- Woodward 2004.Woodward J, Kelly SM. A pilot study for a randomised controlled trial of waterbirth versus land birth. BJOG: an International Journal of Obstetrics and Gynaecology. 2004;111:537–45. doi: 10.1111/j.1471-0528.2004.00132.x. [DOI] [PubMed] [Google Scholar]
References
References to studies excluded from this review
- Bastide 1990.Bastide A. Personal communication. 1990. A randomised controlled trial of the effects of a whirlpool bath on labour, birth and postpartum. [Google Scholar]
- Benfield 2001.Benfield RD. The effects of hydrotherapy in labor; a psychophysiological study (thesis) University of South Carolina USA; 1993. [Google Scholar]; Benfield RD, Herman J, Katz VL, Wilson SP, Davis JM. Hydrotherapy in labor. Research in Nursing and Health. 2001;24:57–67. doi: 10.1002/1098-240x(200102)24:1<57::aid-nur1007>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Calvert 2000.Calvert I. The evaluation of the use of herbal substances in the bath water of labouring women. Personal communication. 2000.
- Cluett 2001.Cluett ER, Pickering RM, Brooking JI. An investigation into the feasibility of comparing three management options (augmentation, conservative and water) for nulliparae with dystocia in the first stage of labour. Midwifery. 2001;17(1):35–43. doi: 10.1054/midw.2000.0233. [DOI] [PubMed] [Google Scholar]
- Cluett 2004.Cluett ER, Pickering RM, Getliffe G, Saunders NJSG. Randomised controlled trial of labouring in water compared with standard of augmentation for the management of dystocia in first stage of labour. BMJ. 2004;328(7435):314–20. doi: 10.1136/bmj.37963.606412.EE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrecque 1999.Labrecque M, Nouwen A, Bergeron M, Rancourt JF. A randomized controlled trial of nonpharmacologic approaches for relief of low back pain during labor. Journal of Family Practice. 1999;48(4):259–63. [PubMed] [Google Scholar]
References
References to studies awaiting assessment
- Malarewicz 2005.Malarewicz A, Wydrzynski G, Szymkiewicz J, Adamczyk-Gruszka O. The influence of water immersion on the course of first stage of parturition in primiparous women [Wplyw immersji wodnej na przebieg i okresu porodu u pierwiastek] Med wieku Rozwoj. 2005;9(4):773–80. [PubMed] [Google Scholar]
- Torkamani 2010.Torkamani SA, Kangani F, Janani F. The effects of delivery in water on duration of delivery and pain compared with normal delivery. Pakistan Journal of Medical Sciences. 2010;26(3):551–5. [Google Scholar]
References
Additional references
- Aird 1997.Aird IA, Luckas MJM, Buckett WM, Bousfielf P. Effects of intrapartum hydrotherapy on labour parameters. Australian and New Zealand Journal of Obstetrics and Gynaecology. 1997;37(2):137–42. doi: 10.1111/j.1479-828x.1997.tb02240.x. [DOI] [PubMed] [Google Scholar]
- Alderdice 1995.Alderdice F, Renfrew M, Marchant S, Ashurst H, Hughes PM, Berridge G, et al. Labour and birth in water in England and Wales: survey report. British Journal of Midwifery. 1995;3(7):376–82. doi: 10.1136/bmj.310.6983.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson 1996.Anderson B, Gyhagen M, Neilse NTF. Warm bath during labour: effects on labour duration and maternal and fetal infectious morbidity. Journal of Obstetrics and Gynaecology. 1996;16:326–30. [Google Scholar]
- Anim-Somuah 2005.Anim-Somuah M, Smyth Rebecca MD, Howell Charlotte J. Epidural versus non-epidural or no analgesia in labour. Cochrane Database of Systematic Reviews. 2005;(Issue 4) doi: 10.1002/14651858.CD000331.pub2. [DOI: 10.1002/14651858.CD000331.pub2] [DOI] [PubMed] [Google Scholar]
- Antonovsky 1979.Antonovsky A. Health, Stress and Coping. Jossey-Bass; San Francisco: 1979. [Google Scholar]
- Antonovsky 1987.Antonovsky A. Unravelling the Mystery of Health. How People Manage Stress and Stay Well. Jossey-Bass; San Francisco: 1987. [Google Scholar]
- Barragán 2011.Barragán LIM, Solà I, Juandó PC. Biofeedback for pain management during labour. Cochrane Database of Systematic Reviews. 2011;(Issue 6) doi: 10.1002/14651858.CD006168.pub2. [DOI: 10.1002/14651858.CD006168.pub2; : CD006168] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke 1995.Burke E, Kilfoyle A. A comparative study: waterbirths and bed births. Midwives. 1995;108(1284):3–7. [Google Scholar]
- Burns 2001.Burns E. Waterbirths. MIDIRS Midwifery Digest. 2001;11(3):S10–S13. [Google Scholar]
- Cefalo 1978.Cefalo RC, Andre U, Hellgers E. The effects of maternal hyperthermia on maternal and fetal cardiovascular and respiratory function. American Journal of Obstetrics and Gynecology. 1978;131(6):687–94. doi: 10.1016/0002-9378(78)90833-5. [DOI] [PubMed] [Google Scholar]
- Church 1989.Church LK. Water birth: one birthing center’s observations. Journal of Nurse-Midwifery. 1989;34(4):165–70. doi: 10.1016/0091-2182(89)90076-1. [DOI] [PubMed] [Google Scholar]
- Clarke 2007.Clarke P, Bowcock M, Gales P. Development of an integrated care pathway for natural birth. British Journal of Midwifery. 2007;15(1):12–5. [Google Scholar]
- Deans 1995.Deans AC, Steer PH. Temperature of pool is important. BMJ. 1995;311:390–1. doi: 10.1136/bmj.311.7001.390b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Department of Health 1993.Department of Health . Changing Childbirth. HMSO; 1993. [Google Scholar]
- Department of Health 2004.Department of Health . National Service Framework for Children, Young People and Maternity Service. Department of Health; London: 2004. [DOI] [PubMed] [Google Scholar]
- Derry 2011.Derry S, Straube S, Moore RA, Hancock H, Collins SL. Intracutaneous or subcutaneous sterile water injection for relieving pain in labour. Cochrane Database of Systematic Reviews. 2011;(Issue 5) doi: 10.1002/14651858.CD009107.pub2. [DOI: 10.1002/14651858.CD009107] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downe 2004.Downe S, McCourt C. From being to becoming; reconstructing childbirth knowledge. In: Downe S, editor. Normal Childbirth; Evidence and Debate. Churchill Livingston; Edinburgh: 2004. pp. 3–24. [Google Scholar]
- Dowswell 2009.Dowswell T, Bedwell C, Lavender T, Neilson James P. Transcutaneous electrical nerve stimulation (TENS) for pain relief in labour. Cochrane Database of Systematic Reviews. 2009;(Issue 2) doi: 10.1002/14651858.CD007214.pub2. [DOI: 10.1002/14651858.CD007214.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlich 1987.Edlich RF, Towler MA, Goitz RJ, Wilder RP, Buschbacher LP, Morgan RF, et al. Bioengineering principles of hydrotherapy. Journal of Burn Care and Rehabilitation. 1987;8(6):580–4. [PubMed] [Google Scholar]
- Egger 1997.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clinical research ed.) 1997;315(7109):629–34. doi: 10.1136/bmj.315.7109.629. [PUBMED: 9310563] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland 1994.Garland D, Jones K. Waterbirth, first stage immersion or non immersion? British Journal of Midwifery. 1994;2(3):113–20. [Google Scholar]
- Garland 1997.Garland D, Jones K. Waterbirth; updating the evidence. British Journal of Midwifery. 1997;5(6):368–73. [Google Scholar]
- Garland 2000.Garland D, Jones Waterbirths: supporting practice with clinical audit. MIDIRS Midwifery Digest. 2000;10(3):333–6. [Google Scholar]
- Garland 2002.Garland D. Collaborative waterbirth audit - supporting practice with audit. MIDIRS Midwifery Digest. 2002;12(4):508–11. [Google Scholar]
- Garland 2006.Garland D. On the crest of a wave. Completion of a collaborative audit MIDIRS. Midwifery Digest. 2006;16(1):81–5. [Google Scholar]
- Geissbuehler 2000.Geissbuehler V, Eberhard J. Waterbirths a comparative study. A prospective study on more than 2,000 waterbirths. Fetal Diagnosis and Therapy. 2000;15(5):291–300. doi: 10.1159/000021024. [DOI] [PubMed] [Google Scholar]
- Geissbuehler 2004.Geissbuehler V, Stein S, Eberhard J. Waterbirths compared with landbirths: an observational study of nine years. Journal of Perinatal Medicine. 2004;32(4):308–14. doi: 10.1515/JPM.2004.057. [DOI] [PubMed] [Google Scholar]
- Gilbert 1999.Gilbert R, Tookey P. Perinatal mortality and morbidity among babies delivered in water: surveillance study and postal survey. BMJ. 1999;319:483–7. doi: 10.1136/bmj.319.7208.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginesi 1998a.Ginesi L, Niescierowicz R. Neuroendocrinology and birth 1: stress. British Journal of Midwifery. 1998;6(10):659–63. [Google Scholar]
- Ginesi 1998b.Ginesi L, Niescierowicz R. Neuroendocrinology and birth 2: The role of oxytocin. British Journal of Midwifery. 1998;6(12):791–6. [Google Scholar]
- Green 1998.Green JM, Coupland VA, Kitzinger JV. Great Expectations: a Prospective Study of Women’s Expectations and Experiences of Childbirth. 2nd Edition Books for Midwives; Cheshire: 1998. [Google Scholar]
- Green 2007.Green JM, Baston HA. Have women become more willing to accept obstetric interventions and does this relate to mode of birth? Data from a prospective study. Birth. 2007;34(1):6–13. doi: 10.1111/j.1523-536X.2006.00140.x. [DOI] [PubMed] [Google Scholar]
- Hall 1998.Hall SM, Holloway IM. Staying in control: women’s experiences of labour in water. Midwifery. 1998;14(1):30–6. doi: 10.1016/s0266-6138(98)90112-7. [DOI] [PubMed] [Google Scholar]
- Harbord 2006.Harbord RM, Egger M, Sterne JA. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Statistics in Medicine. 2006;25(20):3443–57. doi: 10.1002/sim.2380. [DOI] [PubMed] [Google Scholar]
- Hawkins 1995.Hawkins S. Water versus conventional birth: infections rates compared. Nursing Times. 1995;91(15):38–40. [PubMed] [Google Scholar]
- Higgins 2011.Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration; [updated March 2011]. 2011. www.cochrane-handbook.org Available from. [Google Scholar]
- Hodnett 2007.Hodnett ED, Gates S, Hofmeyr GJ, Sakala C. Continuous support for women during childbirth. Cochrane Database of Systematic Reviews. 2007;(Issue 3) doi: 10.1002/14651858.CD003766.pub2. [DOI: 10.1002/14651858.CD003766.pub2] [DOI] [PubMed] [Google Scholar]
- Johnson 1996.Johnson P. Birth under water - to breathe or not to breathe. British Journal of Obstetrics and Gynaecology. 1996;103:202–8. doi: 10.1111/j.1471-0528.1996.tb09706.x. [DOI] [PubMed] [Google Scholar]
- Jones 2011a.Jones L, Othman M, Dowswell T, Alfirevic Z, Gates S, Newburn M, et al. Pain management for women in labour: an overview of systematic reviews. Cochrane Database of Systematic Reviews. 2011;(Issue 7) [DOI: 10.1002/14651858.CD009234] [Google Scholar]
- Jones 2011b.Jones L, Dou L, Dowswell T, Alfirevic Z, Neilson James P. Pain management for women in labour: generic protocol. Cochrane Database of Systematic Reviews. 2011;(Issue 6) doi: 10.1002/14651858.CD009234.pub2. [DOI: 10.1002/14651858.CD009167] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassim 2005.Kassim Z, Sellars M, Greenough A. Underwater birth and neonatal respiratory distress. BMJ. 2005;330(7499):1071–2. doi: 10.1136/bmj.330.7499.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mammas 2009.Mammas IN, Thiagarajan P. Water aspiration syndrome at birth - report of two cases. Journal of Maternal-Fetal and Neonatal Medicine. 2009;22(4):365–7. doi: 10.1080/14767050802556067. [DOI] [PubMed] [Google Scholar]
- Maternity Care Working Party 2007.Maternity Care Working Party . Making Normal Birth a Reality. NCT, RCM, RCOG; London: 2007. [Google Scholar]
- McCandlish 1993.McCandlish R, Renfrew M. Immersion in water during labor and birth: the need for evaluation. Birth. 1993;20(2):79–85. doi: 10.1111/j.1523-536x.1993.tb00421.x. [DOI] [PubMed] [Google Scholar]
- Midwifery 2020 Program 2010.Midwifery 2020 Program . Midwifery 2020 Delivering expectation. Midwifery 2020; [accessed 2010]. 2010. http://www.midwifery2020.org/default.asp [Google Scholar]
- Moneta 2001.Moneta J, Okninska A, Wielgos M, Przybos A, Szymusik I, Marianowski L. Patient’s preferences concerning the course of labor. Ginekologia Polska. 2001;72(12):1010–8. [PubMed] [Google Scholar]
- Nguyen 2002.Nguyen S, Kuschel C, Teele R, Spooner C. Water birth--a near-drowning experience. Pediatrics. 2002;110(2 Pt 1):411–3. doi: 10.1542/peds.110.2.411. [DOI] [PubMed] [Google Scholar]
- NHS Wales 2004.NHS Wales . The All Wales clinical pathway for normal labour. Health in Wales; [accessed March 2007]. 2007. http://www.wales.nhs.uk/sites3/home.cfm?orgid=327 [Google Scholar]
- Novikova 2011.Novikova N, Cluver C. Local anaesthetic nerve block for pain management in labour. Cochrane Database of Systematic Reviews. 2011;(Issue 7) doi: 10.1002/14651858.CD009200.pub2. [DOI: 10.1002/14651858.CD009200] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nursing and Midwifery Council 2010.Nursing and Midwifery Council . Midwives Rules and Standards. NMC; London: 2010. [Google Scholar]
- Odent 1983.Odent M. Birth under water. Lancet. 1983;2:1476–7. doi: 10.1016/s0140-6736(83)90816-4. [DOI] [PubMed] [Google Scholar]
- Odent 1999.Odent M. The Scientification of Love. Free Association Books Limited; London: 1999. [Google Scholar]
- Othman 2011.Othman M, Jones L, Neilson JP. Non-opioid drugs for pain management in labour. Cochrane Database of Systematic Reviews. 2011;(Issue 7) doi: 10.1002/14651858.CD009223.pub2. [DOI: 10.1002/14651858.CD009223] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otigbah 2000.Otigbah CM, Dhanjal MK, Harmsworth G. A retrospective comparison of water births and conventional vaginal deliveries. European Journal of Obstetrics & Gynecology and Reproductive Biology. 2000;91(1):15–20. doi: 10.1016/s0301-2115(99)00238-9. [DOI] [PubMed] [Google Scholar]
- Pinette 2004.Pinette MG, Wax J, Wilson E. The risks of underwater birth. American Journal of Obstetrics and Gynecology. 2004;5:1211–5. doi: 10.1016/j.ajog.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Power 1989.Power GG. Biology of temperature: the mammalian fetus. Journal of Developmental Physiology. 1989;12:295–304. [PubMed] [Google Scholar]
- Rawal 1994.Rawal J, Shah A, Stirk F, Mehtar S. Waterbirth and infection in babies. BMJ. 1994;309:511. doi: 10.1136/bmj.309.6953.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RCM 1994.Royal College of Midwives . The Use of Water During Birth. Position Statement 1a. Royal College of Midwives; London: 1994. [Google Scholar]
- RCM 2008.Royal College of Midwives [accessed 2008];The campaign for normal birth. http://www.rcmnormalbirth.org.uk/
- RCOG 2001.Royal College of Obstetricians and Gynaecologists Clinical Effectiveness Support Unit . National Sentinel Caesarean Section Audit Report. Royal College of Obstetricians and Gynaecologists; London: 2001. [Google Scholar]
- Reid Campion 1990.Reid-Campion M. Adult Hydrotherapy. A Practical Approach. 1st Edition Heinemann; Oxford: 1990. [Google Scholar]
- Reid-Campion 1997.Reid-Campion M. Hydrotherapy: Principles and Practice. 2nd Edition Butterworth Heineman; Oxford: 1997. [Google Scholar]
- RevMan 2011.The Nordic Cochrane Centre . The Cochrane Collaboration. Review Manager (RevMan). 5.1. The Nordic Cochrane Centre, The Cochrane Collaboration; Copenhagen: 2011. [Google Scholar]
- Richmond 2003.Richmond H. Women’s experiences of waterbirth. Practising Midwife. 2003;6(3):26–31. [PubMed] [Google Scholar]
- Robertson 1998.Robertson PA, Huang LJ, Croughan-Minihane MS, Kilpatrick SJ. Is there an association between water baths during labour and the development of chorioamnionitis or endometritis? American Journal of Obstetrics and Gynecology. 1998;178(6):1215–21. doi: 10.1016/s0002-9378(98)70325-4. [DOI] [PubMed] [Google Scholar]
- Rosevear 1993.Rosevear SK, Fox R, Marlow N, Stirrat GM. Birthing pools and the fetus. Lancet. 1993;342:1048–9. doi: 10.1016/0140-6736(93)92902-6. [DOI] [PubMed] [Google Scholar]
- Rosser 1994.Rosser J. Is water birth safe? The facts behind the controversy. Midwifery Digest. 1994;4:4–6. [Google Scholar]
- Royal College of Midwives 2011.Royal College of Midwives . Campaign for Normal Birth. Royal College of Midwives; [accessed Feb 2011]. 2011. http://www.rcmnormalbirth.org.uk website. [Google Scholar]
- Simmons 2007.Simmons SW, Cyna AM, Dennis AT, Hughes D. Combined spinal-epidural versus epidural analgesia in labour. Cochrane Database of Systematic Reviews. 2007;(Issue 3) doi: 10.1002/14651858.CD003401.pub2. [DOI: 10.1002/14651858.CD003401.pub2] [DOI] [PubMed] [Google Scholar]
- Smith 2011a.Smith CA, Collins CT, Crowther CA. Aromatherapy for pain management in labour. Cochrane Database of Systematic Reviews. 2011;(Issue 7) doi: 10.1002/14651858.CD009215. [DOI: 10.1002/14651858.CD009215] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith 2011b.Smith CA, Collins CT, Crowther CA, Levett KM. Acupuncture or acupressure for pain management in labour. Cochrane Database of Systematic Reviews. 2011;(Issue 7) doi: 10.1002/14651858.CD009232. [DOI: 10.1002/14651858.CD009232] [DOI] [PubMed] [Google Scholar]
- Thoeni 2005.Thoeni A, Zech N, Moroder L, Ploner F. Review of 600 water births. Does water birth increase the risk of neonatal infection? Journal of Maternal-Fetal and Neonatal Medicine. 2005;17(5):357–61. doi: 10.1080/14767050500140388. [DOI] [PubMed] [Google Scholar]
- UKCC 1994.United Kingdom Central Council for Nursing. Midwifery and Health Visiting . Position Statement on Waterbirths. Annexe 1 to Registrar’s letter 16/1994. UKCC; London: 1994. [Google Scholar]
- Ullman 2010.Ullman R, Smith Lesley A, Burns E, Mori R, Dowswell T. Parenteral opioids for maternal pain relief in labour. Cochrane Database of Systematic Reviews. 2010;(Issue 9) doi: 10.1002/14651858.CD007396.pub2. [DOI: 10.1002/14651858.CD007396.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham 2005.Wickham S. The birth of water embolism. Practising Midwife. 2005;8(11):37. [PubMed] [Google Scholar]
- Zanetti-Daellenbach 2007.Zanetti-Daellenbach RA, Tschudin S, Zhong XZ, Holzgreve W, Lapaire O, Hösli I. Maternal and neonatal infection and obstetrical outcome in water birth. European Journal of Obstetrics & Gynecology and Reproductive Biology. 2007;134(1):37–43. doi: 10.1016/j.ejogrb.2006.09.012. [DOI] [PubMed] [Google Scholar]
References
References to other published versions of this review
- Cluett 2002.Cluett ER, Nikodem VC, McCandlish RE, Burns EE. Immersion in water in pregnancy, labour and birth. Cochrane Database of Systematic Reviews. 2002;(Issue 2) doi: 10.1002/14651858.CD000111.pub2. [DOI: 10.1002/14651858.CD000111.pub2] [DOI] [PubMed] [Google Scholar]
- Cluett 2009.Cluett ER, Burns E. Immersion in water in labour and birth. Cochrane Database of Systematic Reviews. 2009;(Issue 2) doi: 10.1002/14651858.CD000111.pub3. [DOI: 10.1002/14651858.CD000111.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]