Abstract
C-type natriuretic peptide (CNP) is abundant in brain and is reported to exert autocrine function in vascular cells, but its effect on blood–brain barrier (BBB) permeability has not been clarified yet. Here, we examined this effect. Transendothelial electrical resistance (TEER) of in vitro BBB model, composed of bovine brain microvascular endothelial cells and astrocytes, was significantly dose dependently decreased by CNP (1, 10, and 100 nmol/L). C-type natriuretic peptide treatment reduced both the messenger RNA (mRNA) and protein expressions of tight junction (TJ) protein zonula occludens-1 (ZO-1). The effects on TEER, mRNA, and protein expressions of ZO-1 were mimicked by cyclic GMP (cGMP) analog 8-bromo-cGMP (1 μmol/L) and reversed by protein kinase G (PKG) inhibitor Rp-8-CPT-cGMPS (100 μmol/L), thus implying the role of PKG and cGMP signaling in BBB function. Transcription factor JunD knockdown by small interfering RNA resulted in no change of permeability by CNP. In vivo study of mouse brain by fluorimetric analysis with intravenous administration of sodium fluorescein (40 mg/kg) also showed a significant increase in BBB permeability by CNP (10 nmol/kg, intravenously). These findings suggest that CNP modulates the BBB permeability by altering ZO-1 expression.
Keywords: adhesion molecules, blood–brain barrier, endothelium, gene regulation, vascular biology
Introduction
Blood–brain barrier (BBB), comprising the capillary endothelial cells, astrocyte foot process ensheathing vessels, and pericytes embedded in the basement membrane, has a vital role in the normal function of central nervous system (CNS).1 Blood–brain barrier regulates the amount of trans- and paracellular flux and thus contributes to the maintenance of a specific neural tissue environment.1 The absence of fenestrations, the low number of pinocytotic vesicles, and the presence of tight intercellular junctions between the endothelial cells contribute to the barrier properties of the BBB.2 Tight junctions (TJs) are created by several integral membrane proteins, including occludins, claudins, and associated cytoplasmic proteins, e.g., zonula occludens (ZO)-1 or ZO-2. Claudin and occludin interact with plasma membranes of adjacent cells forming the TJ barrier3 whereas ZO proteins connect the integral membrane proteins to the cytoskeleton (i.e. actin).4 Zonula occludens-1 (ZO-1) and ZO-2 are critical in forming and stabilizing TJs by binding occludin to the cytoarchitecture.4, 5 Although BBB restricts the entry of potentially toxic substances to CNS, it also hinders the delivery of therapeutic drugs into the brain.6 Effective modulation of BBB to facilitate the entry of therapeutic drugs could be of great value in a wide range of neurologic diseases.
C-type natriuretic peptide (CNP) belongs to the natriuretic peptide family, together with atrial natriuretic peptide (ANP) and brain natriuretic peptide.7 C-type natriuretic peptide potently stimulates guanylate cyclase activity by binding to guanylate cyclase-B (GC-B) receptor, which enhances the production of cyclic GMP (cGMP). Although CNP is predominantly localized in CNS and has been thought to act principally as a neuropeptide, there are only few reports regarding the functions of CNP in CNS.8, 9, 10, 11 Meanwhile, numerous studies have demonstrated a modulatory role of ANP on vascular permeability both in vitro and in vivo,12, 13, 14 although there have been discrepancies in the results depending upon the doses used, the animal species studied, and the experimental conditions. It has been reported that ANP has no significant effect on BBB permeability.13 Although CNP has been reported to exert autocrine function in the vascular cells, there have been contradictions on its effects, whether it enhances or attenuates the endothelial permeability.14, 15, 16 Despite the abundance of CNP in brain and regulatory function of CNP on vascular cells, the effect of CNP on BBB permeability has not been clarified yet, which led us to undertake this study. Indeed, our results demonstrate that CNP can effectively modulate and enhance the permeability of BBB.
Materials and Methods
Primary Cell Cultures and Drug Administration
Primary bovine brain microvascular endothelial cells (BBMECs) were obtained from DS Pharma Biomedical, Osaka, Japan. BBMECs were seeded (1 × 105 cells per cm2) on collagen-coated culture dishes (Iwaki, Tokyo, Japan) with the media composed of RPMI 1640 (Sigma-Aldrich, St Louis, MO, USA) supplemented with L-glutamine (2 mmol/L), 10% fetal bovine serum, and β-fibroblast growth factor (10 ng/mL). Cells were cultivated in a humidified 37°C incubator with 95% room air, 5% CO2, and culture medium was replaced every 2 days until the cells reached confluence.
Rat primary cultured astrocytes were prepared as previously reported with some modifications.17 Briefly, cerebral cortices were collected from 17- to18-day-old Sprague–Dawley (Japan SLC, Shizuoka, Japan) rat embryos. After carefully and completely removing the meninges, the cortices were finely chopped in cold 50% Dulbecco's modified Eagle medium (Sigma) and 50% phosphate-buffered saline (Dainippon Pharmaceutical, Osaka, Japan). Then, the tissues were rinsed twice with cold Hank's balanced salt solution (Invitrogen, Carlsbad, CA, USA) containing 2% sucrose, and were incubated in warm Hank's balanced salt solution containing 0.25% trypsin (Invitrogen), 1.4 mmol/L EDTA (Nacalai Tesque, Kyoto, Japan), and DNase (40 ng/mL, Nacalai Tesque) for 20 minutes at 37°C with gentle shaking every 5 minutes. After addition of fetal calf serum (ICN, Aurora, Ohio, USA) to a final concentration of 13%, the cells were centrifuged at 45 × g for 1 minute. Then, the supernatant was collected and residual tissues were filtered out with a 40-μm cell strainer (Becton Dickinson, Franklin Lakes, NJ, USA). This procedure was repeated two more times. After centrifugation of the resulting suspension at 180 × g for 5 minutes, the supernatant was removed and the cell pellet was dispersed in RPMI 1640 supplemented with L-glutamine (2 mmol/L), 10% fetal bovine serum, and β-fibroblast growth factor (10 ng/mL). This procedure was repeated two more times. The cells were then seeded on poly-L-lysine-coated dishes (Iwaki, Tokyo, Japan) and incubated using the same medium and conditions as described above for BBMEC.
The cells were treated with CNP (Peptide Institute, Osaka, Japan) at the doses of 1, 10, and 100 nmol/L for the intended duration. Cyclic GMP analog 8-bromo-cGMP (1 μmol/L) (Enzo Life Sciences, Tokyo, Japan) and cGMP-dependent protein kinase inhibitor Rp-8-CPT-cGMPS (100 μmol/L) (Enzo Life Sciences) were used. Rp-8-CPT-cGMPS, when used with CNP, was added 30 minutes before CNP administration.
Construction of In Vitro Blood–Brain Barrier System
The in vitro BBB model was prepared using polycarbonate Greiner transwell inserts (surface area 0.3 cm2; pore size 0.4 μm) coated with poly-L-lysine on the bottom or abluminal surface and type IV collagen and fibronectin on the well of insert or luminal surface. Astrocytes were first seeded on the bottom at a density of 45,000 cells per insert, allowed to adhere to the bottom for 10 minutes and then cultured with inserts placed in 24-well dish. After 3 days, BBMEC were seeded on the luminal surface at a density of 30,000 cells per insert and cultured for additional 3 days.18 The in vitro BBB model was then used for desired experiment. All the treatments were performed on the luminal surface of the inserts.
BBB Kit
The commercially available BBB kit (PharmaCo-Cell Company, Nagasaki, Japan) composed of rat brain capillary endothelial cells, pericytes, and astrocytes was used to further support and confirm the findings of the BBMEC–astrocytes co-culture system. BBB kit also incorporates pericytes in the triple co-culture setting and has been shown to have close resemblance to in vivo permeability.19
Transendothelial Electrical Resistance Measurement
Transendothelial electrical resistance (TEER) of both BBB models were assessed with EVOM2 (epithelial voltohmmeter) and an Endohm-6 chamber attachment (World Precision Instruments, Sarasota, FL, USA). The values were calculated using the formula; TEER (Ω*cm2)=(resistance of insert with cells−resistance of blank insert) * surface area of the insert. For the experiments in which chemical agents were applied separately, measurements of TEER on the BBB models in response to treatments were performed after 24 hour incubation with CNP, 8-bromo-cGMP, and Rp-8-CPT-cGMPS. For time-dependent experiment, measurements were made at 3, 6, 12, 24, and 48 hours.
RNA Isolation and Reverse Transcription Polymerase Chain Reaction
Total RNA was extracted from cells using Trizol LS (Invitrogen) according to the manufacturer's protocols. After reverse transcription using a High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Carlsbad, CA, USA), the resulting cDNA was subjected to PCR using AmpliTaq DNA Polymerase (Applied Biosystems) according to the manufacturer's protocols. Polymerase chain reaction conditions were as follows: 28 to 36 cycles of denaturation at 98°C for 10 seconds, annealing at the appropriate temperature for 1 minute (62°C for CNP, 64°C for GC-B, 55°C for ZO-1, Claudin-5, and Occludin-1, 61°C for glyceraldehyde-3-phosphate dehydrogenase), and extension at 72°C for 1 minute. The primer sequences are shown in Table 1. Glyceraldehyde-3-phosphate dehydrogenase mRNA was used to normalize the results from different samples.
Table 1. Primer sequences.
| mRNA | Sequence (5′–3′) |
|---|---|
| Bovine ZO-1 | |
| Forward | AAC CAg ggg CTg TCT CgA CTC C |
| Reverse | CgA ATg gCA AgC CAg gAC CCC |
| Bovine Claudin-5 | |
| Forward | gCg gAC CAC gAT gTT ggC gA |
| Reverse | Tgg gCT ggg Tgg gCC TgA TT |
| Bovine Occludin-1 | |
| Forward | gAA CgC CgA gTA gCC CTC gC |
| Reverse | gCA gCC Atg gCC AgC Agg AAT |
| Rat ZO-1 | |
| Forward | ggA gCC TgT CCC CTC gCT CA |
| Reverse | Cgg ggA ggC CTg TCA Tgg gA |
| Rat Claudin-5 | |
| Forward | gTg CAg AgC ACC ggg CAC AT |
| Reverse | CCA CAC gCg gCT TCC CAC AT |
| Rat Occludin-1 | |
| Forward | TCA ACC CgA CTg CCC Agg CT |
| Reverse | TCg CAg gAC TCg CCA CCT gT |
| Universal GAPDH | |
| Forward | ACC ACA gTC CAT gCC ATC AC |
| Reverse | TCC ACC CTg TTg CTg TTg CTg TA |
GAPDH, glyceraldehyde-3-phosphate dehydrogenase; mRNA, messenger RNA; ZO-1, zonula occludens-1.
Western Blotting
According to previously reported protocols,20 cells were homogenized in radioimmunoprecipitation assay buffer containing 50 mmol/L Tris–HCl (pH 8.0), 5 mmol/L EDTA, 50 mmol/L NaF, 10 mmol/L sodium pyrophosphate, 1 mmol/L sodium orthovanadate(V), 1% NP-40, 0.5% deoxycholate, 0.1% sodium dodecyl sulfate, and 150 mmol/L NaCl. 5 μg of each protein was run on a polyacrylamide gel, blotted on a polyvinylidene difluoride membrane, and probed with antibodies specific for ZO-1, Claudin-5, Occludin-1 (Invitrogen Corporation, Camarillo, CA, USA), JunD (Cell Signaling Technology, Danvers, MA, USA), β-actin (Santa Cruz Biotechnology, Santa Cruz, CA, USA); horseradish peroxidase-conjugated anti-mouse or anti-rabbit antibodies (Thermo Scientific, Bremen, Germany) were used as secondary antibodies. Bands were visualized using ECL prime (GE Healthcare, Waukesha, WI, USA) and a LAS-1000 mini image analyzer (Fuji Film, Tokyo, Japan).
Immunocytochemistry
Bovine brain microvascular endothelial cells were plated on a cover slip (Matsunami Glass, Tokyo, Japan) coated with type IV collagen. According to the reported protocols for immunocytochemistry,20 cells were fixed for 5 minutes with methanol:acetone (1:1) at −30°C, washed with phosphate-buffered saline, and incubated in phosphate-buffered saline containing 5% bovine serum albumin and 0.3% Triton X-100 for 60 minutes. Cells were then incubated with antibodies specific for ZO-1 and Claudin-5 (Invitrogen Corporation), followed by anti-mouse secondary antibodies coupled to CF488 (Biotium, Hayward, CA, USA). Samples were mounted on cover slips with Dako mounting medium (DakoCytomation, Glostrup, Denmark) containing 4 μmol/L propidium iodide (Sigma-Aldrich). Fluorescent images were obtained using an LSM700 confocal laser scanning microscope (Carl Zeiss, Jena, Germany).
Small Interfering RNA Transfection
Small interfering RNA (siRNA), with sequence 5′-GAGGAGAAAGUGAAGACGCUCAAGA-3′, specific for bovine JunD (NM_001103253, designed by BLOCK-iT RNAi Designer) and control siRNA (Ambion, Austin, TX, USA) were transfected into BBMEC with Lipofectamine RNAiMAX (Life Technologies, Gaithersburg, MD, USA) according to the manufacturer's instructions. Briefly, in vitro BBB kit was prepared as described above in (2); after 2 days of BBMEC seeding, RNAi/Lipofectamine complexes prepared in Opti-MEM (Life Technologies, Gaithersburg, MD, USA) were added and treated with CNP 1 day later for 24 hours. Transendothelial electrical resistance values were measured both before and 24 hours after adding CNP. For western blot, cells were seeded at a density of 1 × 105 cells per cm2 and cultured overnight, then RNAi/Lipofectamine complexes prepared in Opti-MEM were added. After 24 hours of incubation, CNP was added for further 24 hours and then protein isolation was performed.
Animals
Male ICR mice (purchased from Kyudo, Saga, Japan) typically aged 7 to 9 weeks and weighing 30 to 40 g were used for in vivo experiments. Animals were housed under specific pathogen-free environment at Kagoshima University, Kagoshima, Japan with food and water provided ad libitum. All experimental procedures were approved by the Institutional Animal Care and Use Committee of Kagoshima University (ID: MD12123) and were performed in accordance with the guideline for the care and use of animals issued by the Pharmacological Society of Japan and that by the Institutional Animal Care and Use Committee of Kagoshima University.
Drug Administration and Fluorimetric Analysis
C-type natriuretic peptide diluted in saline to the dose of 10 nmol/kg was injected intravenous bolus into mice through the tail vein. The change in BBB permeability was assessed using sodium fluorescein as an indicator for the extravasation of low-weight molecules as previously described.21 Briefly, at indicated times, i.e., 2, 6, 12, and 24 hours after CNP injection, sodium fluorescein (Nacalai Tesque, Kyoto, Japan) was injected at a dose of 40 mg/kg through the tail vein. Thirty minutes later, mice were anesthetized with pentobarbital (50 mg/kg, intraperitoneal) and perfused with ice-cold physiologic saline with 5 U/mL heparin through the left ventricle of the heart. The whole brain was removed and homogenized in a 0.5 mol/L borate buffer pH 10, and centrifuged at 12000 × g for 10 minutes. To 1 mL of the supernatant was added 4 mL of ethanol to precipitate proteins, and then the mixture was centrifuged at 15000 × g for 20 minutes. The aliquot of supernatant (200 μl) was transferred to a Greiner 96-well black assay plate. The fluorescence of the supernatant was measured at an excitation wavelength of 485 μm and emission wavelength of 538 μm using FlexStation 3 Microplate Reader (Molecular Devices, Sunnyvale, CA, USA). The content of sodium fluorescein in each sample was expressed as ng/g of brain tissue using a standardized curve.
The change in BBB permeability was also assessed using 10 kDa FITC-dextran (Sigma-Aldrich) as an indicator for high-weight molecules, as previously described22 with slight modifications. Briefly, 1.0 mg aliquot of FITC-dextran solution in phosphate-buffered saline (10 mg/mL) was injected intravenously through the tail vein 6 hours after intravenous injection of saline, CNP, or positive control adenosine receptor agonist NECA [1-(6-Amino- 9H-purin-9-yl)-1-deoxy-N-ethyl-β-D-ribofuranuronamide] (Abcam, Cambridge Science Park, Cambridge, UK) at 0.08 mg/kg. The brain was removed and homogenized in Tris-Cl, 50 mmol/L, pH 7.6 (100 μL per 100 mg brain), and then centrifuged at 16 × g for 30 minutes. To the supernatant was added the equal volume of absolute methanol and the samples were centrifuged at 16 × g for 30 minutes. The fluorescence of the supernatant was measured and quantified as described above.
Statistical Analysis
Values are shown as the means±s.e. Statistical significance was examined using one-way analysis of variance with post hoc tests, such as Dunnett's or Tukey's test, or Student's t-tests performed with Prism 5 software (GraphPad, San Diego, CA, USA).
Results
C-Type Natriuretic Peptide Enhances Blood–Brain Barrier Permeability In Vitro by Disrupting Tight Junction Protein Zonula Occludens-1
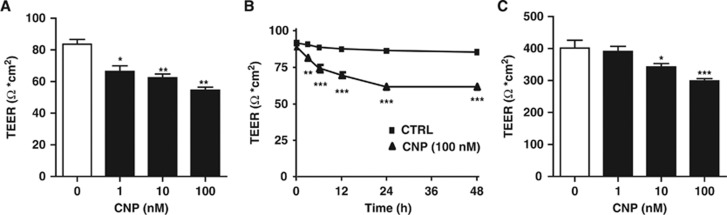
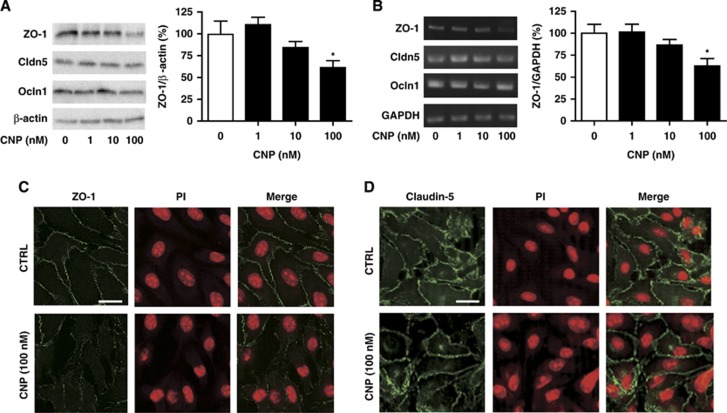
We first examined the mRNA expression of GC-B receptor and CNP on BBMEC; GC-B was expressed whereas CNP was not (data not shown). To assess the functional effect of CNP on BBB, we then evaluated TEER in in vitro BBB model composed of BBMEC and astrocytes. C-type natriuretic peptide decreased the TEER values dose dependently (from 1 to 100 nmol/L) (Figure 1A) and time dependently (from 3 to 48 hours) (Figure 1B), indicating that CNP has a role in enhancing BBB permeability. To investigate the structural changes in TJ proteins, we examined their protein and mRNA expressions. C-type natriuretic peptide treatment resulted in a dose-dependent decrease in both the protein (Figure 2A) and mRNA (Figure 2B) expressions of ZO-1 whereas it had no significant effects on those of Claudin-5 and Occludin-1 (Figures 2A and B). On immunocytochemical analysis, CNP (100 nmol/L) resulted in the diminished expression of ZO-1 (Figure 2C) whereas it did not demonstrate any change on Claudin-5 immunoreactivity (Figure 2D). These findings suggest that CNP brings about an increase in BBB permeability by inducing alteration in TJ protein ZO-1.
Figure 1.
C-type natriuretic peptide (CNP) decreases the transendothelial electrical resistance (TEER) of the blood–brain barrier (BBB). (A) C-type natriuretic peptide (1, 10, and 100 nmol/L) treatment for 24 hours resulted in dose-dependent decrease in TEER values of bovine brain microvascular endothelial cells-astrocytes co-culture. Data are mean±s.e. (n=6 per group). (B) Treatment with CNP (100 nmol/L) for 48 hours showed time-dependent decrease in TEER values. Data are mean±s.e. (n=6 per group). (C) C-type natriuretic peptide (CNP) (1, 10, and 100 nmol/L) treatment for 24 hours dose dependently decreased TEER of the commercially available BBB kit. Data are mean±s.e. (n=6 per group). Statistical analyses were performed with analysis of variance with post hoc Dunnett's test and Student's t-test; *P<0.05, **P<0.01, and ***P<0.001.
Figure 2.
C-type natriuretic peptide (CNP) enhances the blood–brain barrier permeability by disrupting the tight junction (TJ) protein zonula occludens-1 (ZO-1). C-type natriuretic peptide (1, 10, and 100 nmol/L) treatment for 24 hours dose dependently decreased both protein (A) and messenger RNA (B) expressions of ZO-1 whereas did not remarkably affect those of Claudin-5 (Cldn-5) and Occludin-1 (Ocln-1) (A, B); with β-actin and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) normalization, respectively. Data are mean±s.e. (n=5 to 8 per group for panel A and n=5 to 6 per group for panel B). (C) C-type natriuretic peptide (CNP) (100 nmol/L) induced disruption in membrane expression of TJ protein ZO-1 after 24-hour treatment but (D) did not affect Claudin-5. Bar, 20 μm. Statistical analyses were performed with analysis of variance with post hoc Dunnett's test; *P<0.05.
C-Type Natriuretic Peptide Increases Blood–Brain Barrier Permeability via Cyclic GMP and Protein Kinase G Signaling
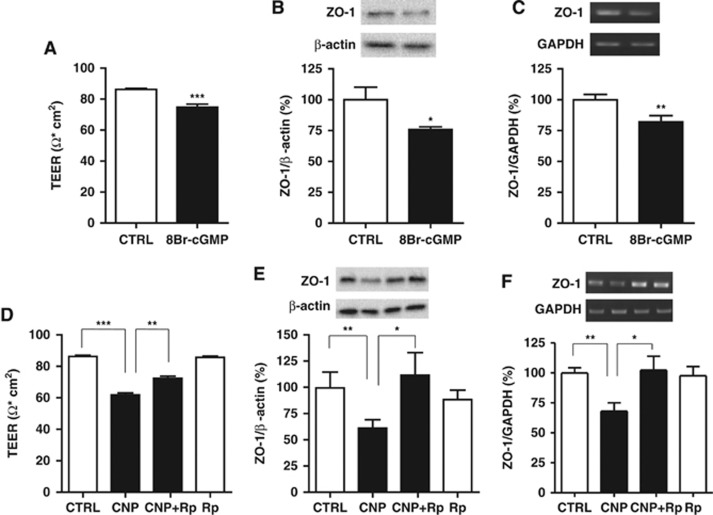
To explore the possible role of cGMP and protein kinase G (PKG) signaling in BBB function, we tested with the cGMP analog 8-bromo-cGMP and the PKG inhibitor Rp-8-CPT-cGMPS in our experimental paradigm. 8-Bromo-cGMP (1 μmol/L) treatment replicated the CNP-induced reductions in TEER (Figure 3A), ZO-1 protein expression, (Figure 3B) and ZO-1 mRNA expression (Figure 3C). Pretreatment with Rp-8-CPT-cGMPS (100 μmol/L) significantly reversed CNP-induced decreases in TEER (Figure 3D), ZO-1 protein expression, (Figure 3E) and ZO-1 mRNA expression (Figure 3F). These data indicate that CNP increases BBB permeability via cGMP and PKG signaling.
Figure 3.
C-type natriuretic peptide (CNP) increases blood–brain barrier (BBB) permeability via cyclic GMP (cGMP) and protein kinase G signaling. 8-Bromo-cGMP (1 μmol/L; abbreviated as 8Br-cGMP) treatment for 24 hours resulted in reduced (A) transendothelial electrical resistance (TEER) of bovine brain microvascular endothelial cells-astrocytes co-culture, (B) zonula occludens-1 (ZO-1) protein expression and (C) ZO-1 messenger RNA (mRNA) expression. Data are mean±s.e. (n=5 to 6 per group). Pretreatment with Rp-8-CPT-cGMPS (100 μmol/L; abbreviated as Rp) reversed CNP-induced decrease in (D) TEER, (E) ZO-1 protein expression and (F) ZO-1 mRNA expression. Data are mean±s.e. (n=5 to 8 per group). Statistical analyses were performed with Student's t-test and analysis of variance with post hoc Tukey's test; *P<0.05, **P<0.01, and ***P<0.001. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
C-Type Natriuretic Peptide Increases the Barrier Permeability of BBB kit (a Commercially Available In Vitro Blood–Brain Barrier Model)
In order to reproduce our findings in a different barrier system, we used commercially available BBB kit, described in ‘materials and methods' section. GC-B receptor mRNA was expressed in the endothelial cells of the kit whereas CNP was not (data not shown). C-type natriuretic peptide dose dependently decreased TEER values of BBB kit at concentrations ranging from 1 to 100 nmol/L (Figure 1C). C-type natriuretic peptide (1 to 100 nmol/L) treatment also resulted in the significantly diminished mRNA expression of ZO-1; however, we could not observe any significant change in mRNA expressions of Claudin-5 and Occludin-1 (Supplementary Figure 1). Thus, these data on BBB kit further support our findings on BBMEC–astrocyte co-culture and strongly suggest that CNP enhances BBB permeability.
C-Type Natriuretic Peptide Increases JunD Expression and JunD Knockdown Prevents C-Type Natriuretic Peptide-Induced Permeability Increase
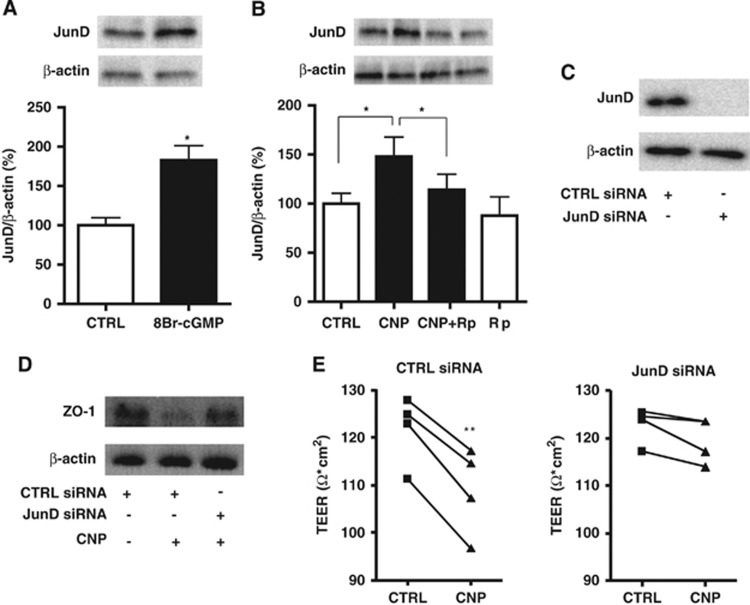
Transcription factor JunD has been reported to suppress ZO-1 expression without altering other TJ molecules.23 To investigate the possible underlying role of JunD in CNP-induced increased permeability, we first performed western blot for JunD expression, where we found that CNP (100 nmol/L) and 8-Bromo-cGMP (1 μmol/L) (Figures 4A and B) resulted in the significant increase of JunD expression whereas Rp-8-CPT-cGMPS (100 μmol/L) significantly reversed the CNP-induced increase (Figure 4B). Next, BBMECs were transfected with siRNA specific for JunD that resulted in marked decrease in JunD levels (Figure 4C). JunD siRNA transfection canceled the suppressive effect of CNP (100 nmol/L) on ZO-1 protein expression (Figure 4D). Furthermore, on treatment with CNP, a significant decrease in TEER values was observed in cells transfected with control siRNA, but not in cells transfected with siRNA specific for JunD (Figure 4E). These results suggest that JunD knockdown prevents the CNP-induced increased permeability and hence is the key transcription factor in the BBB modulatory role of CNP.
Figure 4.
Transcription factor JunD knockdown prevents C-type natriuretic peptide (CNP)-induced increased permeability. (A) 8-Bromo-cGMP (1 μmol/L; abbreviated as 8Br-cGMP) treatment resulted in the significant increase of JunD expression. Data are mean±s.e. (n=5 per group). (B) C-type natriuretic peptide also induced significant increase in JunD expression, which was reversed by pretreatment with Rp-8-CPT-cGMPS (100 μmol/L; abbreviated as Rp). Data are mean±s.e. (n=9 per group). (C) Western blot analysis showed that transfection of bovine brain microvascular endothelial cells with small interfering RNA (siRNA) specific for JunD resulted in marked decrease in JunD levels. (D) C-type natriuretic peptide (100 nmol/L) treatment decreased zonula occludens-1 (ZO-1) protein expression in the cells transfected with control siRNA, whereas JunD siRNA transfection canceled this effect. (E) On treatment with CNP (100 nmol/L), transendothelial electrical resistance (TEER) values decreased significantly in the cells transfected with control siRNA, but not in the cells transfected with siRNA specific for JunD (n=4 per group). Statistical analyses were performed with Student's t-test and analysis of variance with post hoc Tukey's test; *P<0.05 and **P<0.01.
C-type natriuretic peptide Increases Blood-Brain Barrier Permeability in vivo
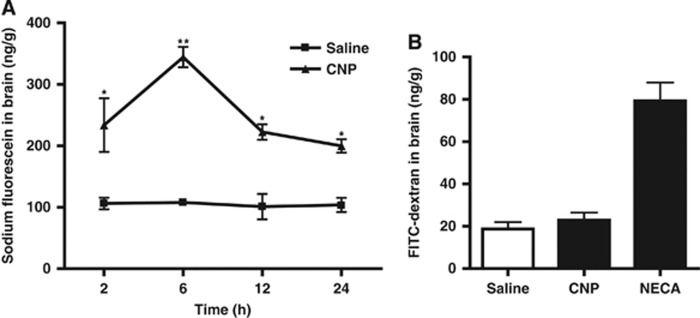
With the purpose of further confirming our in vitro findings, we undertook in vivo studies to assess the extravasation of intravenously administered sodium fluorescein into the CNS of mice upon CNP treatment. We found that CNP (10 nmol/kg, intravenous bolus) significantly increased the brain fluorescein levels at 2, 6, 12, and 24 hours post treatment, with maximum increase at 6 hours post treatment (Figure 5A). To evaluate if CNP facilitates the entry of molecules with relatively higher molecular weight as well, the extravasation of fluorescent-labeled dextran, FITC-dextran (10 kDa) into the brain was assessed at 6 hours of CNP treatment, which did not bring about any significant increase, taken the adenosine receptor agonist NECA as positive control based on a previous report22 (Figure 5B). These results, nevertheless, suggest that CNP increases the BBB permeability in vivo and further verify the in vitro results.
Figure 5.
C-type natriuretic peptide (CNP) increases blood–brain barrier (BBB) permeability in vivo. (A) Extravasation time course of sodium fluorescein (40 mg/kg, intravenous) into mouse brain administered at 2, 6, 12, and 24 hours after intravenous bolus injection of CNP (10 nmol/kg) or vehicle (saline), as measured by fluorimetry (n=3 to 4 animals per group). (B) Extravasation of 10 kDa fluorescein isothiocyanate (FITC)-dextran into mouse brain at 6 hours after treatment with saline (n=3), CNP (10 nmol/kg, intravenous bolus) (n=3), or NECA (0.08 mg/kg, intravenous bolus) as positive control (n=2), measured by fluorimetry. Data are mean±s.e. Statistical analyses were performed with Student's t-test; *P<0.05 and **P<0.01 versus vehicle.
Discussion
Experiments performed in this study reveal that CNP can modulate and enhance BBB permeability both in vitro and in vivo to facilitate the entry of molecules into the brain. To the best of our knowledge, this is the first report demonstrating the modulatory effect of CNP on BBB and the underlying mechanism. We here propose that CNP can increase BBB permeability via cGMP- and PKG-dependent mechanism.
Natriuretic peptides ANP and BNP are secreted from the heart as cardiac hormones and regulate fluid homeostasis and blood pressure, whereas CNP is mainly localized in the CNS.8 Two CNP molecules, 22 and 53 amino acids in length have been identified within the circulation. The 22 amino acid form predominates in plasma and is more potent than the 53 amino acid form.7, 24 Unlike ANP and BNP, CNP lacks a significant natriuretic function, and serves as a vascular regulator in a paracrine or autocrine fashion. There have been contradictory reports regarding the effect of CNP on the vascular and smooth muscle cells. It has been shown that the endothelium-derived vasodilators (natriuretic peptides and nitric oxide) induce apoptosis in rat endothelial cells, which is mediated by cGMP-dependent pathway.15 However, anti-apoptotic effects of CNP have also been reported.16 C-type natriuretic peptide, unlike ANP and BNP, has been shown to have no significant effect on the thrombin-induced pulmonary barrier dysfunction.14 Thus, there have been contradictions over the effects of CNP in the endothelial function and permeability. As CNP is the major natriuretic peptide in CNS, its effects on BBB could be different from those on peripheral endothelial barriers. These observations led us to assess if CNP possesses the properties to modulate BBB permeability.
In the present study, we used three systems characterizing BBB; in vitro BBMEC–astrocyte co-culture system, the commercially available BBB kit and in vivo system studying BBB permeability in mice. Data obtained from experiments in all these systems complemented and supported each other, strongly suggesting that CNP has a vital role in increasing BBB permeability. C-type natriuretic peptide exhibited significant changes in both function and structure, indicated by TEER and expression of TJ protein, respectively. C-type natriuretic peptide decreased TEER of both the in vitro co-culture model and BBB kit on dose-dependent basis. Tight junction proteins contribute to the barrier function but do not actively regulate the passage of molecules. In our study, we demonstrate that CNP (100 nmol/L) significantly alters the TJ protein ZO-1, revealed by its decreased mRNA and protein expressions as well as disruption of connectivity on immunocytochemistry. Zonula occludens-1 is known to have an important role in the integrity of BBB and is well correlated with TEER values.25 Thus, the decreased expression of ZO-1 caused by CNP is consistent with the reduction in TEER values. The BBB kit, comprising pericytes also as a part of triple co-culture, which we employed as the second system for experiments, has been reported to exhibit close resemblance to in vivo permeability and possess relatively higher TEER values.19 The functional and structural effects of CNP were also remarkably replicated in this system.
We propose the possible involvement of cGMP and PKG signaling to bring about the BBB modulatory effect of CNP. C-type natriuretic peptide via GC-B receptor stimulates guanylyl cyclase activity to enhance the production of cGMP, which, through its downstream effectors cGMP-dependent protein kinases (PKG), has been implicated in regulating a variety of physiologic processes.26 It has been demonstrated that CNP causes an increase in cGMP in rat brain microvessels.27 Here, we demonstrate that treatment with cGMP analog replicates the effects of CNP whereas inhibiting PKG reverses those, thereby implying the involvement of cGMP and PKG signaling in the regulation of BBB modulatory effect of CNP. Thus, cGMP activation has an important role in increasing barrier permeability by suppressing ZO-1. It has also been previously reported that cGMP increases the permeability in pial venular capillaries in rats, consequent to the stimulation of histamine H2 and bradykinin B2 receptors.28 Few other reports have demonstrated that nitric oxide,29 vascular endothelial growth factor,30 histamine,31 and bradykinin32 increase the BBB permeability, possibly via stimulation of guanylyl cyclase activity. Transcription factor JunD has been reported to suppress ZO-1 without altering other TJ molecules.23 We demonstrate that CNP and cGMP analog augment the expression of JunD whereas PKG inhibition reverses it. Furthermore, JunD knockdown by siRNA transfection prevented the CNP-induced increase in barrier permeability, which suggests that JunD is a key transcription factor underlying this function of CNP.
In vivo experiments revealed that intravenous administration of CNP (10 nmol/kg) into mice increased the extravasation of sodium fluorescein into the brain at 2, 6, 12, and 24 hours after treatment, with peak increase at 6 hours post treatment. Sodium fluorescein, with a molecular weight of 376 Da, has widely been used as a tracer in studies of BBB permeability.21, 31 It has been reported that >98% of small-molecule drugs <500 Da in size and all large-molecule drugs do not cross the BBB.6 To explore if CNP also harbors the similar property for substances with high molecular weight, we checked the permeation of FITC-dextran (10 kDa) into the brain 6 hours after CNP treatment where CNP did not bring about significant change; NECA, an adenosine receptor agonist was used as positive control based on the previous report.22 We showed that CNP specifically alters ZO-1 without changing other TJ proteins but NECA has been reported to disrupt other TJ proteins too,22 which can explain the difference in their potentials to increase the BBB permeability to molecules with relatively higher weight. However, the enhancement of sodium fluorescein in brain by CNP can be considered to be of functional significance as it mimics the permeation of most of the small-molecule drugs. The difference in the time course of CNP effects in vivo and in vitro could be attributed to the active metabolism of CNP by mainly two mechanisms in vivo: enzymatic degradation by neutral endopeptidases and internalization by the clearance receptor natriuretic peptide receptor-C.24
In the patients with pathologic conditions such as septic shock, CNP levels have been found to be markedly elevated compared with that in healthy controls.33 During the early septic phase, elevated levels of tumor necrosis factor-α and lipopolysaccharide occur, both of which elevate CNP levels in vitro.34 Thus, it has been established that CNP level is increased in septic conditions. However, a variety of evidence has demonstrated that permeability of BBB is increased in sepsis and this phenomenon significantly contributes to the pathophysiology of septic encephalopathy.35 Therefore, considering these two paradigms of findings, there may be possibility of CNP having a pivotal role as a mediator in increasing BBB permeability in septic conditions too. However, experiments specific to septic models and conditions are required to elucidate this property of CNP.
Owing to the impediment caused by BBB in the delivery of drugs into CNS, safe and effective methods to facilitate the entry of drugs across BBB for treatment of several neurologic disorders are of paramount importance. Three categories for CNS drug delivery have been described: chemical BBB opening,29, 30, 31, 32, 36 physical BBB disruption,37 and drug modification.38 These procedures have their own associated risks and adverse effects. Further experiments should be required to explore if the CNP-mediated increase in BBB permeability could be of use in drug delivery system. Although the clinical adverse effects of CNP are not well known at present, CNP could be of great therapeutic benefit if it possesses fewer side effects like other members of natriuretic peptide family.39
Taken together, our findings in this study represent that CNP can effectively enhance the permeability of BBB both in vitro and in vivo via cGMP/PKG and JunD-dependent mechanism. The significant enhancement of BBB permeability to sodium fluorescein can be considered to be of functional significance as it mimics the permeation of various small molecular weight drugs across BBB. Indeed, further experiments should be required to refine the system for delivery of therapeutic compounds into the CNS, which needs specific attention to determining the proper timing and control of BBB permeability. Nevertheless, the results we presented here demonstrate the pivotal role of CNP in modulating BBB permeability by altering ZO-1 expression.
Acknowledgments
We thank Dr Takashi Kurihara and Dr Kazuhiko Inoue for their helpful discussion and advice.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
This work was supported by Grant-in-Aid for Scientific Research (23592100) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Supplementary Material
References
- Abbott NJ, Revest PA. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- Crone C, Olesen SP. Electrical resistance of brain microvascular endothelium. Brain Res. 1982;241:49–55. doi: 10.1016/0006-8993(82)91227-6. [DOI] [PubMed] [Google Scholar]
- Tsukamoto T, Nigam SK. Role of tyrosine phosphorylation in the reassembly of occludin and other tight junction proteins. Am J Physiol Renal Physiol. 1999;276:F737–F750. doi: 10.1152/ajprenal.1999.276.5.F737. [DOI] [PubMed] [Google Scholar]
- Itoh M, Furuse M, Morita K, Kubota K, Saitou M, Tsukita S. Direct binding of three tight junction-associated MAGUKs, ZO-1, ZO-2, and ZO-3, with the COOH termini of claudins. J Cell Biol. 1999;147:1351–1363. doi: 10.1083/jcb.147.6.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kniesel U, Wolburg H. Tight junctions of the blood-brain barrier. Cell Mol Neurobiol. 2000;20:57–76. doi: 10.1023/A:1006995910836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardridge WM. Drug delivery to the brain. J Cereb Blood Flow Metab. 1997;17:713–731. doi: 10.1097/00004647-199707000-00001. [DOI] [PubMed] [Google Scholar]
- Sudoh T, Minamino N, Kangawa K, Matsuo H. C-type natriuretic peptide (CNP): a new member of the natriuretic peptide family identified in porcine brain. Biochem Biophys Research Commun. 1990;168:863–870. doi: 10.1016/0006-291x(90)92401-k. [DOI] [PubMed] [Google Scholar]
- Minamino N, Makino Y, Tateyama H, Kangawa K, Matsuo H. Characterization of immunoreactive human C-type natriuretic peptide in brain and heart. Biochem Biophys Res Commun. 1991;179:535–542. doi: 10.1016/0006-291x(91)91404-z. [DOI] [PubMed] [Google Scholar]
- Samson WK, Skala KD, Huang FL. CNP-22 stimulates, rather than inhibits, water drinking in the rat: evidence for a unique biological action of the C-type natriuretic peptides. Brain Res. 1991;568:285–288. doi: 10.1016/0006-8993(91)91410-3. [DOI] [PubMed] [Google Scholar]
- Miyajima M, Arai H, Okuda O, Hishii M, Nakanishi H, Ishii H, et al. Effect of C-type natriuretic peptide (CNP) on water channel aquaporin-4 (AQP4) expression in cultured astrocytes. Brain Res Mol Brain Res. 2004;122:109–115. doi: 10.1016/j.molbrainres.2003.10.026. [DOI] [PubMed] [Google Scholar]
- DiCicco-Bloom E, Lelievre V, Zhou X, Rodriguez W, Tam J, Waschek JA. Embryonic expression and multifunctional actions of the natriuretic peptides and receptors in the developing nervous system. Dev Biol. 2004;271:161–175. doi: 10.1016/j.ydbio.2004.03.028. [DOI] [PubMed] [Google Scholar]
- Hempel A, Noll T, Bach C, Piper HM, Willenbrock R, Hohnel K, et al. Atrial natriuretic peptide clearance receptor participates in modulating endothelial permeability. Am J Physiol. 1998;275:H1818–H1825. doi: 10.1152/ajpheart.1998.275.5.H1818. [DOI] [PubMed] [Google Scholar]
- Nag S, Pang SC. Effect of atrial natriuretic factor on blood–brain barrier permeability. Can J Physiol Pharmacol. 1989;67:637–640. doi: 10.1139/y89-101. [DOI] [PubMed] [Google Scholar]
- Klinger JR, Warburton R, Carino GP, Murray J, Murphy C, Napier M, et al. Natriuretic peptides differentially attenuate thrombin-induced barrier dysfunction in pulmonary microvascular endothelial cells. Exp Cell Res. 2006;312:401–410. doi: 10.1016/j.yexcr.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Suenobu N, Shichiri M, Iwashina M, Marumo F, Hirata Y. Natriuretic peptides and nitric oxide induce endothelial apoptosis via a cGMP-dependent mechanism. Arterioscler Thromb Vasc Biol. 1999;19:140–146. doi: 10.1161/01.atv.19.1.140. [DOI] [PubMed] [Google Scholar]
- Itoh T, Nagaya N, Murakami S, Fujii T, Iwase T, Ishibashi-Ueda H, et al. C-type natriuretic peptide ameliorates monocrotaline-induced pulmonary hypertension in rats. Am J Respir Crit Care Med. 2004;170:1204–1211. doi: 10.1164/rccm.200404-455OC. [DOI] [PubMed] [Google Scholar]
- Frangakis MV, Kimelberg HK. Dissociation of neonatal rat brain by dispase for preparation of primary astrocyte cultures. Neurochem Res. 1984;12:1689–1698. doi: 10.1007/BF00968079. [DOI] [PubMed] [Google Scholar]
- Gaillard PJ, Voorwinden LH, Nielsen JL, Ivanov A, Atsumi R, Engman H, et al. Establishment and functional characterization of an in vitro model of the blood-brain barrier, comprising a co-culture of brain capillary endothelial cells and astrocytes. Eur J Pharm Sci. 2001;12:215–222. doi: 10.1016/s0928-0987(00)00123-8. [DOI] [PubMed] [Google Scholar]
- Nakagawa S, Deli MA, Kawaguchi H, Shimizudani T, Shimono T, Kittel A, et al. A new blood-brain barrier model using primary rat brain endothelial cells, pericytes and astrocytes. Neurochem Int. 2009;54:253–263. doi: 10.1016/j.neuint.2008.12.002. [DOI] [PubMed] [Google Scholar]
- Kambe Y, Miyata A. Role of mitochondrial activation in PACAP dependent neurite outgrowth. J Mol Neurosci. 2012;48:550–557. doi: 10.1007/s12031-012-9754-0. [DOI] [PubMed] [Google Scholar]
- Baba M, Oishi R, Saeki K. Enhancement of blood-brain barrier permeability to sodium fluorescein by stimulation of μ opioid receptors in mice. Naunyn Schmiedebergs Arch Pharmacol. 1988;337:423–428. doi: 10.1007/BF00169534. [DOI] [PubMed] [Google Scholar]
- Carman AJ, Mills JH, Krenz A, Kim DG, Bynoe MS. Adenosine receptor signaling modulates permeability of the blood-brain barrier. J Neurosci. 2011;31:13272–13280. doi: 10.1523/JNEUROSCI.3337-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Xiao L, Rao JN, Zou T, Liu L, Bellavance E, et al. JunD represses transcription and translation of the tight junction protein zona occludens-1 modulating intestinal epithelial barrier function. Mol Biol Cell. 2008;19:3701–3712. doi: 10.1091/mbc.E08-02-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr CS, Rhodes P, Struthers AD. C-type natriuretic peptide. Peptides. 1996;17:1243–1251. doi: 10.1016/s0196-9781(96)00110-6. [DOI] [PubMed] [Google Scholar]
- Krause D, Mischeck U, Galla HJ, Dermietzel R. Correlation of zonula occludens ZO-1 antigen expression and transendothelial resistance in porcine and rat cultured cerebral endothelial cells. Neurosci Lett. 1991;128:301–304. doi: 10.1016/0304-3940(91)90284-z. [DOI] [PubMed] [Google Scholar]
- Lucas KA, Pitari GM, Kazerounian S, Ruiz-Stewart I, Park J, Schulz S, et al. Guanylyl cyclases and signaling by cyclic GMP. Pharmacol Rev. 2000;52:375–414. [PubMed] [Google Scholar]
- Vigne P, Frelin C. C-type natriuretic peptide is a potent activator of guanylate cyclase in endothelial cells from brain microvessels. Biochem Biophys Res Commun. 1992;183:640–644. doi: 10.1016/0006-291x(92)90530-x. [DOI] [PubMed] [Google Scholar]
- Sarker MH, Fraser PA. The role of guanylyl cyclases in the permeability response to inflammatory mediators in pial venular capillaries in the rat. J Physiol. 2002;540:209–218. doi: 10.1113/jphysiol.2001.012912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayhan WG. Nitric oxide donor-induced increase in permeability of the blood-brain barrier. Brain Res. 2000;866:101–108. doi: 10.1016/s0006-8993(00)02254-x. [DOI] [PubMed] [Google Scholar]
- Mayhan WG. VEGF increases permeability of the blood-brain barrier via a nitric oxide synthase/cGMP-dependent pathway. Am J Physiol. 1999;276:C1148–C1153. doi: 10.1152/ajpcell.1999.276.5.C1148. [DOI] [PubMed] [Google Scholar]
- Schilling L, Wahl M. Opening of the blood-brain barrier during cortical superfusion with histamine. Brain Res. 1994;653:289–296. doi: 10.1016/0006-8993(94)90403-0. [DOI] [PubMed] [Google Scholar]
- Unterberg A, Wahl M, Baethmann A. Effects of bradykinin on permeability and diameter of pial vessels in vivo. J Cereb Blood Flow Metab. 1984;4:574–585. doi: 10.1038/jcbfm.1984.82. [DOI] [PubMed] [Google Scholar]
- Hama N, Itoh H, Shirakami G, Suga S, Komatsu Y, Yoshimasa T, et al. Detection of C-type natriuretic peptide in human circulation and marked increase of plasma CNP level in septic shock patients. Biochem Biophys Res Commun. 1994;198:1177–1182. doi: 10.1006/bbrc.1994.1166. [DOI] [PubMed] [Google Scholar]
- Kalra PR, Anker SD, Struthers AD, Coats AJ. The role of C-type natriuretic peptide in cardiovascular medicine. Eur Heart J. 2001;22:997–1007. doi: 10.1053/euhj.2000.2395. [DOI] [PubMed] [Google Scholar]
- Ballabh P, Braun A, Nedergaard M. The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis. 2004;16:1–13. doi: 10.1016/j.nbd.2003.12.016. [DOI] [PubMed] [Google Scholar]
- Neuwelt EA, Maravilla KR, Frenkel EP, Rapaport SI, Hill SA, Barnett PA. Osmotic blood-brain barrier disruption. Computerized tomographic monitoring of chemotherapeutic agent delivery. J Clin Invest. 1979;64:684–688. doi: 10.1172/JCI109509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho EE, Drazic J, Ganguly M, Stefanovic B, Hynynen K. Two-photon fluorescence microscopy study of cerebrovascular dynamics in ultrasound-induced blood-brain barrier opening. J Cereb Blood Flow Metab. 2011;31:1852–1862. doi: 10.1038/jcbfm.2011.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt KA, Gillespie TJ, Huber JD, Egleton RD, Davis TP. Peptide drug modifications to enhance bioavailability and blood-brain barrier permeability. Peptides. 2001;22:2329–2343. doi: 10.1016/s0196-9781(01)00537-x. [DOI] [PubMed] [Google Scholar]
- Brunner-La Rocca HP, Kiowski W, Ramsay D, Sütsch G. Therapeutic benefits of increasing natriuretic peptide levels. Cardiovasc Res. 2001;51:510–520. doi: 10.1016/s0008-6363(01)00302-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.