Abstract
Early poststroke rehabilitation effectively improves recovery of function, likely by engaging multiple plasticity processes through use-dependent activation of neural circuits. The loci of such neuroplastic reorganization have not been examined during the initial phase of behavioral recovery. In the current study, we sought to evaluate sub-components of rehabilitation and to identify brain sites first engaged by early rehabilitation. Rats were subjected to endothelin-1 ischemia and placed in either enriched environment (EE), daily reach training (RT), combination of enriched environment and reach training (ER), or standard housing (ST) starting 7 days post ischemia. Functional and histopathological assessments were made after 2, 5, and 10 days of treatment. Animals exposed to 10 days of ER treatment exhibited significantly more use-dependent neuronal activity (FosB/ΔFosB expression) in perilesional cortex than those exposed to EE, RT, or ST treatments. Similar trends were observed in both perilesional striatum and contralesional forelimb motor cortex. This use-dependent plasticity was not explained by differences in neuronal death, inflammation, or lesion volume. The increased activity likely contributes to the neuroplastic changes and functional recovery observed after extended periods of rehabilitation. Importantly, EE or RT alone did not lead to enhanced activity suggesting that combination therapy is necessary to promote maximum recovery.
Keywords: endothelin-1, enriched environment, motor cortex, neuroplasticity, rehabilitation, stroke
Introduction
Most recovery occurs in the weeks immediately after stroke but many patients reach a plateau where further recovery is limited.1, 2 Similarly, ischemic animals exposed to an enriched environment (EE) and daily reach training (RT) exhibit functional improvement that is most prominent in the early treatment period3, 4 with little evidence of subsequent recovery.5 A better understanding of early recovery processes is needed to optimize rehabilitation and overcome this recovery plateau.
Spontaneous recovery after cortical injury in rodents is associated with neuroplastic changes in perilesional cortex as revealed by Golgi staining6 and two-photon imaging.7 Reach training in monkeys8 and rats9 after motor cortex lesions leads to improved performance and increased motor map representation relating to the impaired limb. Rehabilitation also promotes use-dependent neuroplasticity in the intact hemisphere of ischemic rats.4, 10 These findings indicate that task-specific therapy reorganizes uninjured cortex and promotes recovery.
Early rehabilitation has been found to be more effective than delayed rehabilitation in both rats3 and humans.11 Although events in the early phase after stroke are clearly integral to recovery processes, the mechanisms underlying this critical time window are not fully understood. Ischemia leads to a transient upregulation of growth factors and genes involved in angiogenesis, neurogenesis, and axonal sprouting, potentially ‘priming' the brain for repair.12 For example, blocking brain-derived neurotrophic growth factor inhibits recovery after ischemia,13, 14 whereas effective rehabilitation induces an enhancement of brain-derived neurotrophic growth factor.15 Rehabilitation likely induces use-dependent activation of intact tissue bordering the stroke that mediates neuroplastic changes and subsequent functional improvement. This possibility, although suggested previously,8, 16, 17 has not been directly tested in the earliest stages of poststroke rehabilitation.
This study used a previously reported approach18 to explore the effects of rehabilitation on early neuronal activation after stroke by measuring changes in FosB/ΔFosB expression. Unlike other markers of functional activity, FosB/ΔFosB is activated by chronic or repeated stimulation and can be detected for days,18 making it sensitive to the effects of rehabilitation.19 Potential effects of rehabilitation on delayed cell death and inflammation were also investigated, as enrichment and motor training are known to enhance neurogenesis, as well as growth and survival factors.20, 21 Behavioral assessments were carried out after 2, 5, and 10 days of either EE, daily reach therapy, or a combination of both. Based on previous studies,4 we hypothesized that the combination therapy would be most effective in inducing neuroplastic changes in both the affected and unaffected hemispheres.
Materials and Methods
Subjects
A total of 96 male Sprague–Dawley rats (Charles River Laboratories, Montreal QC, Canada) weighing 325 to 375 g (∼3 months) at the time of surgery were used. Animals were socially housed (two per cage) on a reverse 12 hours light/dark cycle, and all experiments were conducted during the dark phase. Animals were exposed to endothelin-1 (ET-1)-induced focal ischemia and allowed to recover in social housing. Three animals died shortly after surgery while one animal did not exhibit a neurologic deficit and was excluded from the study. Seven days post ischemia, remaining animals were pseudorandomly assigned to either EE alone (EE; n=17), daily reach therapy alone (RT; n=17), a combination of EE and daily reach therapy (ER; n=17) or remained in standard housing (ST; n=17) for the remainder of the experiment. Animals were killed after either 2, 5, or 10 days of treatment. In addition, intact (nonoperated, nonischemic) animals were exposed to the above treatments (n=6 per condition) for 10 days for comparison of histologic parameters. Final group numbers and survival times are summarized in Table 1. All procedures were approved by the Memorial University Animal Care Committee and conformed to the Canadian Council on Animal Care guidelines.
Table 1. Experimental groups.
| Treatment | ST | EE | RT | ER |
|---|---|---|---|---|
| 2 days | 6 | 5 | 6 | 6 |
| 5 days | 5 | 6 | 5 | 6 |
| 10 days | 6 | 6 | 6 | 5 |
| 10 days-intact | 6 | 6 | 6 | 6 |
Abbreviations: EE, enriched environment; ER, enriched rehabilitation; RT, daily reach training; ST, standard housing.
All animals, except those labeled intact, underwent endothelin-1 surgery (i.e., ischemia).
Surgery
Animals were anesthetized with isoflurane (3.5% induction, ∼1.75% maintenance) in 30% oxygen and 70% nitrous oxide and placed in a stereotaxic frame. A midline incision was made in the scalp and three burr holes drilled above the forelimb motor cortex and dorsolateral striatum. Focal ischemia was induced using injections of 400 pmol/μL ET-1 (CalBiochem, Hornby, ON, Canada): 2 μL at each of the forelimb cortical sites and 1 μL at the striatal site:22 (1) forelimb sensorimotor cortex—anteroposterior 0.0 mm/mediolateral+/−2.5 mm/dorsoventral −2.3 mm; (2) forelimb sensorimotor cortex—anteroposterior +2.3 mm/mediolateral +/−2.5 mm/dorsoventral −2.3 mm; and (3) dorsolateral —striatum anteroposterior +0.7 mm/mediolateral +/−3.8 mm/dorsoventral −7.0 mm. The ischemic hemisphere was determined for each animal based on paw preferences observed during preexposure to the reaching apparatus (see below). If the animals showed no obvious paw preference, the hemisphere was selected pseudorandomly.
Treatment Conditions
Standard housing consisted of a polycarbonate cage (48 cm × 26 cm × 20 cm) with a section of PVC tubing (two rats per cage). Enriched environments consisted of large cages (90 cm × 60 cm × 60 cm) equipped with an array of toys, tubes, ramps, and ropes that provided sensorimotor, cognitive, and social stimulation (five to six rats per cage). Environments were changed regularly to promote exploration. Food and water were available ad libitum. Reaching therapy involved providing access to a reaching apparatus for 6 h per day (0,900 to 1,500).4 Animals were removed from their home cages (ST in the case of RT; EEs in the case of ER and EE) and placed in individual cages with free access to the reaching apparatus baited with 14 g of food pellets (45 mg per each, TestDiet, Richmond, IN, USA) that could only be retrieved using the affected (i.e., contralesional) forepaw. The amount of pellets retrieved was measured and replaced midway through and at the end of each session. Water, but no other food, was available during this period. All animals were exposed to this reaching apparatus with both sides baited for 3 days before surgery to learn the task.
Neurologic Deficit Score
A modified neurologic deficit score (NDS)23 was used to assess sensorimotor impairment in animals before and 3, 6, 12, and 17 days after surgery by an experimenter masked to experimental condition. The test consists of five limb placing tests, which assess both fore- and hindlimb response to tactile and proprioceptive stimulation. In the first task, an assessment of proprioception, rats were placed on the edge of a table and forelimbs gently extended and released to check for replacement to the tabletop. The second task involved a similar assessment of the hindlimbs. Both tests were repeated for five trials in each limb. The third task involved placing the forelimbs on the table surface and gently pushing the animal from behind. Control rats resist the push equally with both forepaws, while ischemic animals fail to do so with the impaired paw. This test was repeated twice, with each forepaw scored separately. The fourth task involved slowly lowering a rat held at the base of the tail toward a table surface. Control rats reach to contact the table surface with both forepaws, while ischemic rats do so only with the less impaired forepaw sometimes twisting their body toward the side of the lesion. This test was repeated twice with each forepaw scored separately. The fifth and final task involved slowly moving the rat laterally toward the table edge until contact with the vibrissae was made. Control rats normally react by raising the ipsilateral forepaw to the table edge, while ischemic rats fail to do so. This evaluation was repeated three times for each side of the body. Tasks 1 to 4 were scored as follows: 2 points for normal response, 1 point for delayed and/or incomplete response, 0 points for no response; while task 5 was scored with 1 point for a response and 0 points for no response. The maximum score (i.e., no impairment) for each side of the body was 31. A subset of tasks (1, 3, and 5) was used to assess forelimb sensorimotor function, with a maximum score of 17.
Histology
On the day after 2, 5, or 10 days of treatment, animals were deeply anaesthetized with isoflurane (4.0%) and transcardially perfused with ice-cold 0.9% heparinized saline followed by 4.0% paraformaldehyde. Brains were removed, immersed in paraformaldehyde for 24 hours at 4°C, and subsequently stored in 20% sucrose in phosphate-buffered saline at 4°C until saturated. Frozen sections (14 μm thick) were taken with a cryostat every 250 μm and slide-mounted for histologic procedures. A series of sections spanning the lesion were stained with cresyl violet, while consecutive series of sections were processed as described below.
For immunohistochemistry, sections were washed with phosphate-buffered saline, treated with 1.0% H2O2, blocked with 5.0% normal goat serum, and incubated overnight at 4°C with either monoclonal mouse anti-rat CD68 (ED-1 for activated microglia; 1:1,000; MCA341R, Serotec, Raleigh, NC, USA) or monoclonal rabbit anti-mouse FosB (102) (FosB/ΔFosB; 1:250; Santa Cruz, CA, USA). The sections were then exposed to either goat anti-rabbit or anti-mouse biotinylated secondary antibodies (1:1,000; Jackson Research Laboratories, West Grove PA, USA), incubated in 10 μg/mL extravadin (Sigma-Aldrich, Oakville, ON, Canada) and reacted for 5 minutes in 3,3′-diaminobenzadine (Sigma-Aldrich). For Fluoro-Jade staining, sections were immersed in 1% NaOH/80% ethanol (5 minutes) followed by 70% ethanol (2 minutes), rinsed in distilled water and incubated in 0.06% KMnO4 (10 minutes). After a rinse in distilled water, sections were transferred to a 0.0001% solution of Fluoro-Jade C (30 minutes; Histo-Chem, Jefferson, AR, USA). Sections were rinsed in distilled water and allowed to dry on a slide warmer overnight before cover slipping in dim light.
An experimenter masked to experimental condition conducted all analyses. Tissue loss (lesion and atrophy) was determined from cresyl violet stained sections using ImageJ software and calculated as follows: volume of tissue loss=volume of tissue in the uninjured hemisphere−volume of tissue remaining in the injured hemisphere. Hemispheric volume was calculated as follows: volume of a hemisphere=area of tissue remaining × distance between sections × number of sections analyzed.
FosB/ΔFosB-positive nuclei and Fluoro-Jade-positive neurons were counted throughout the perilesional area under a microscope using a × 40 objective in combination with StereoInvestigator software (MicroBrightField, Colchester, VT, USA), while ED-1-positive cells were counted throughout the entire lesion area using a × 20 objective. Four sections spanning the lesion were used for cortical analysis, while three sections were used for striatum. Three sections were also used to assess FosB/ΔFosB immunostaining in the contralesional (i.e. uninjured) forelimb motor cortex. The area of interest was traced and the Optical Fractionator24 used to randomly select ∼20 sampling sites (100 μm × 100 μm) throughout the traced region and estimate a total number of positive cells or nuclei in that region. The estimated population of positive cells or nuclei was used to calculate density (per mm2) for each section analyzed and averaged for each animal.
Statistics
Neurologic deficit score data were analyzed using the Kruskal–Wallis test for nonparametric data, whereas histologic data were analyzed using a two-way (rehabilitation × survival time) between-subjects analysis of variance (ANOVA). Post hoc analyses were conducted using the Bonferroni correction for multiple corrections. Results were considered significant at P<0.05.
Results
Reach Therapy Performance
A daily measurement of the amount (weight) of pellets retrieved during the last 5 days of treatment revealed no difference in performance between animals exposed to 10 days of RT and ER treatments (RT: 17.1±1.5 g per day, ER: 14.8±1.3 g per day; P=0.30).
Neurologic Deficit Score
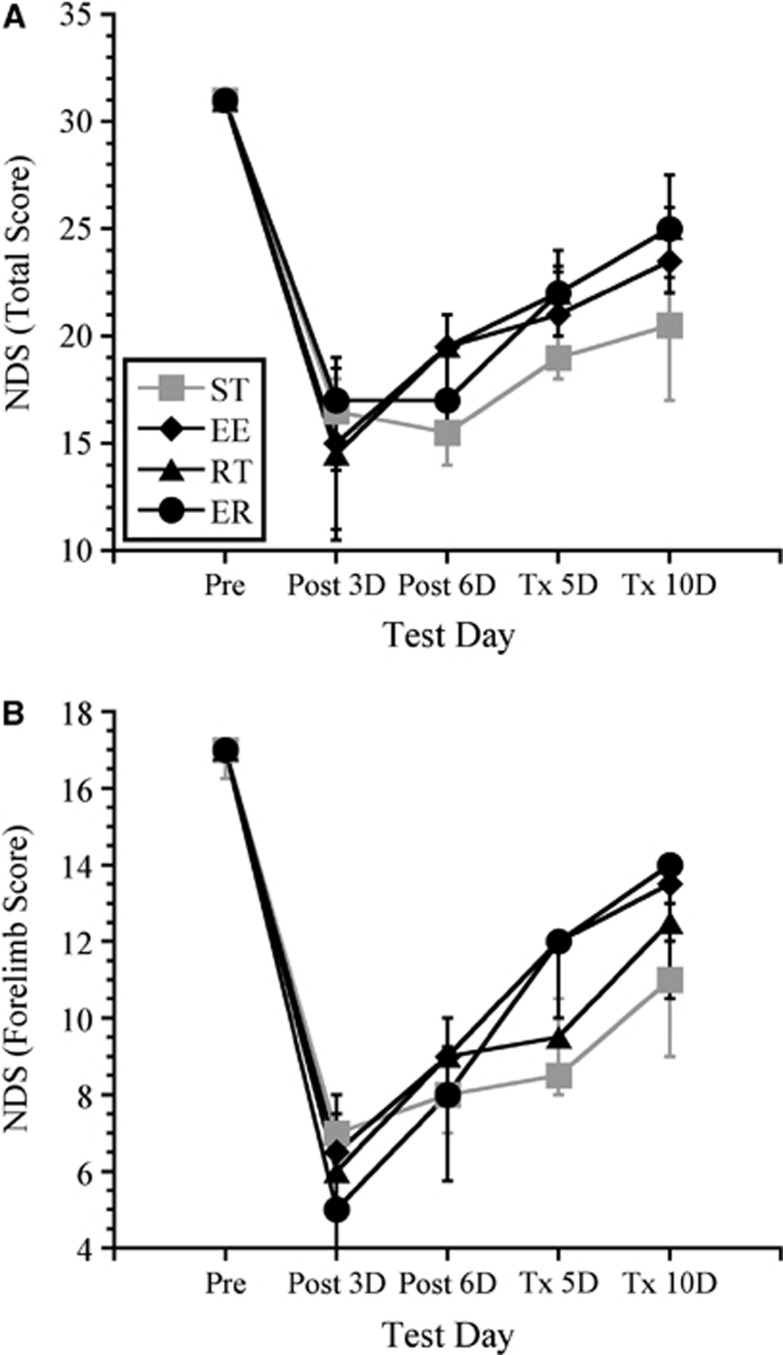
Kruskal–Wallis analyses revealed no significant differences in functional recovery among treatment groups as assessed using either the total NDS score (maximum 31) or the subset of scores for forelimb function (maximum 17) at any survival time. All groups had similar impairments at three days post ischemia, and all groups showed moderate improvements in neurologic function over the course of treatment. Data for animals receiving 10 days of treatment are shown in Figure 1.
Figure 1.
Neurologic deficit score (NDS). All ischemic groups showed profound sensorimotor impairments post surgery on both total NDS (A) and on a subset of scores used to assess forelimb function (B). Although all groups showed some recovery over time, there were no differences among groups. Data shown include only those animals receiving 10 days of treatment. Values are expressed as median scores with interquartile range.
Histopathology of Intact Animals
There were no treatment effects among intact animals for any of the histologic parameters assessed (FosB/ΔFosB Expression, Fluoro-Jade C, or ED-1 expression).
FosB/ΔFosB Expression: Perilesional Cortex
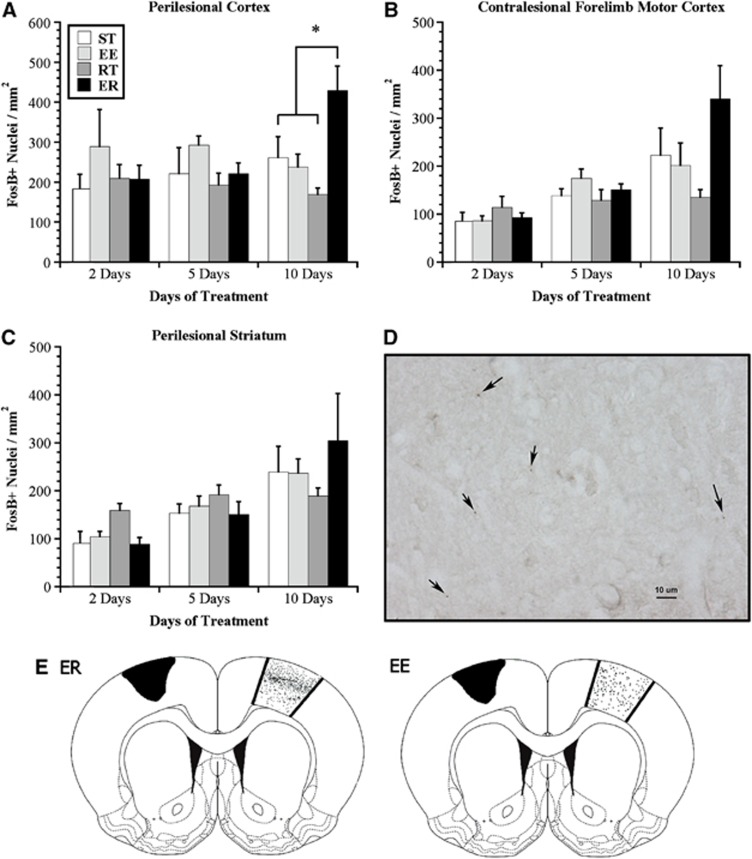
Two-way ANOVA analysis failed to reveal a main effect of rehabilitation or time but a significant interaction in FosB/ΔFosB immunoreactivity in the perilesional cortex (F6,55=2.31, P<0.05). One-way ANOVA at each time point and subsequent analyses with Bonferroni corrections demonstrated that after 10 days of treatment, animals exposed to the ER treatment had significantly more FosB/ΔFosB-positive nuclei in the cortical regions surrounding the lesion than did either ST-, EE-, or RT-treated animals (P<0.05; Figure 2A). Further, these neuroplastic changes were specific to ischemic animals exposed to the ER treatment because intact animals exposed to 10 days of ER treatment had significantly fewer FosB/ΔFosB-positive nuclei than their ischemic counterparts in analogous cortical regions (t9=3.39, P<0.01; data not shown).
Figure 2.
Use-dependent neuronal activation; FosB/ΔFosB expression. (A) Animals exposed to enriched rehabilitation (ER) treatment had significantly higher FosB/ΔFosB expression in perilesional cortex than did those exposed to standard housing (ST), enriched environment (EE), or daily reach training (RT) after 10 days of treatment. There were no differences among groups at earlier time points. (B) A similar trend was observed in contralesional forelimb motor cortex, although not reaching statistical significance. (C) Animals exposed to RT alone had significantly higher FosB/ΔFosB in perilesional striatum after 2 days of treatment, but not at later time points. (D) A representative photomicrograph ( × 40) illustrating FosB/ΔFosB-positive nuclei (black arrows) in perilesional cortex. (E) Representative illustrations (from actual tracings) demonstrating FosB/ΔFosB expression in the contralesional forelimb motor cortex of animals exposed to 10 days of treatment. Note the increased density of FosB/ΔFosB-positive nuclei (represented by black dots) in the region corresponding to cortical layer II/III of ER-treated animals. This increased density was not observed in ST, EE, or RT groups (ST, RT not shown). Values are expressed as mean±s.e.m. (*P<0.05).
Pearson correlation analysis that included all ischemic animals revealed a near-significant correlation (r=0.241, P=0.053) between FosB/ΔFosB immunoreactivity in the perilesional cortex and the total NDS score (as measured on the final day of survival). However, R2=0.06, indicating that only 6% of the variability in NDS was explained by FosB/ΔFosB immunoreactivity.
FosB/ΔFosB Expression: Contralesional Cortex
A two-way ANOVA failed to demonstrate an effect of rehabilitation, but a significant effect of time (F2,55=16.87, P<0.01) and a significant interaction (F6,55=2.66, P<0.05) with respect to the number of positive nuclei in the contralesional motor cortex. Follow-up analysis of this interaction failed to reveal the origin of this interaction; however, at the 10-day time point, one-way ANOVA resulted in a probability value of 0.06 (ER>than other conditions; Figure 2B).
A qualitative analysis of FosB/ΔFosB expression in the contralesional forelimb motor cortex showed that while positive nuclei were present in all cortical layers, they were more densely packed in the region corresponding to layer II/III after 10 days of ER treatment (Figure 2E). This layer-specific density was not evident in ST, EE, or RT groups at any time point. The stratification of cortical layers could not be reliably assessed in the damaged hemisphere because of tissue fragmentation at this early time point.
FosB/ΔFosB Expression: Striatum
Analyses of FosB/ΔFosB expression in the striatum similarly failed to demonstrate an effect of rehabilitation, but a significant effect of time (F2,55=11.32, P<0.01) and a significant interaction (F6,55=2.33, P<0.05). Follow-up analysis demonstrated that at 2 days after rehabilitation initiation, animals in the RR condition had significantly more FosB/ΔFosB expression than all other conditions (P<0.05; Figure 2C). There were no differences among conditions at the other time points; however, analysis approached significance at the 10-day period (P=0.06; Figure 2C).
Fluoro-Jade C
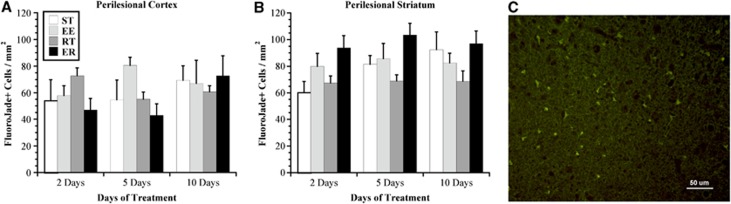
Two-way ANOVA of Fluoro-Jade C-positive cells in the perilesional cortex did not reveal an effect of time or rehabilitation and no interaction. There was, however, a significant effect of rehabilitation (F3,55=9.634, P<0.01) but no effect of time and no interaction between these independent variables with respect to the number of Fluoro-Jade C-stained cells in the perilesional striatum (Figure 3). Post hoc analyses revealed that the RT animals expressed significantly fewer Fluoro-Jade C-positive cells. Interestingly, neuronal death continued after 10 days of treatment (i.e., 17 days post ischemia) and did not show a reduction compared with earlier survival times.
Figure 3.
Delayed neuronal death as assessed with Fluoro-Jade C. There was marked neuronal death occurring in both the (A) cortex and (B) striatum throughout the study period, up to 17 days post ischemia (i.e., 10-day treatment). There was no difference in the number of Fluoro-Jade C stained cells in the perilesional cortex (P>0.05), but RT animals exhibited fewer positive cells than the other ischemic conditions in the striatum (P<0.01). (C) A representative photomicrograph ( × 20) illustrating typical Fluoro-Jade C staining in perilesional cortex. Values are expressed as mean±s.e.m. EE, enriched environment; ER, enriched rehabilitation; RT, daily reach training; ST, standard housing.
Endothelin-1 Expression
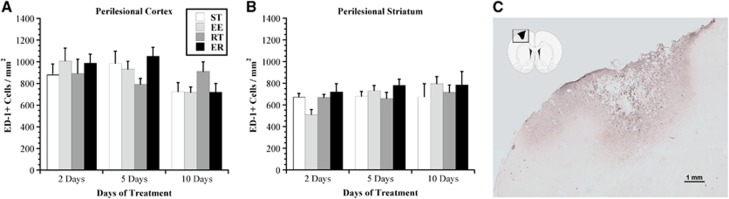
Two-way between-subjects ANOVA of the number of ED-1+ cells in the perilesional cortex did not reveal an effect of rehabilitation, but a significant effect of time (F2,55=4.95, P<0.02) and no interaction between these variables (Figure 4). Follow-up analyses demonstrated a significant decrease in the number of ED-1+ cells over time (subsiding inflammation), where, overall, animals expressed fewer microglia/macrophages at 10 days post rehabilitation than those at either 2 or 5 days (P<0.05), regardless of rehabilitation condition.
Figure 4.
Inflammation as assessed with the anti-CD-68 (ED-1) marker for microglia/macrophages. There was marked ongoing inflammation (activated microglia/macrophages) in and around the injured cortex (A) and striatum (B) throughout the study period. There were no differences among groups at any time point; however, there was a significant decrease in the density of ED-1+ cells over time. (C) A representative photomicrograph ( × 4) illustrating typical ED-1 staining in lesioned cortex. Values are expressed as mean±s.e.m. ED-1, endothelin-1; EE, enriched environment; ER, enriched rehabilitation; RT, daily reach training; ST, standard housing.
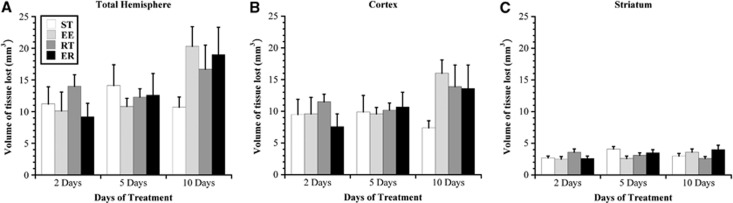
Volume of Tissue Loss
Two-way ANOVA failed to reveal an effect of rehabilitation but a significant effect of time (F2,55=4.23, P<0.02) and no significant interaction between the independent variables with respect to the total volume of hemispheric tissue loss (Figure 5). Post hoc analyses demonstrated that overall, there was a significant progression of ischemic damage with longer survival times (i.e., 10 days significantly larger infarct volumes than 2 days; P<0.05).
Figure 5.
Volume of tissue lost (mm3). Tissue loss (lesion and atrophy), as determined from cresyl violet stained sections, is shown for total hemisphere (A), cortex (B), and striatum (C). There were no differences among groups at any time point. Values are expressed as mean±s.e.m. EE, enriched environment; ER, enriched rehabilitation; RT, daily reach training; ST, standard housing.
There were no effects of either independent variable and no interactions with respect to the volume of tissue loss in the cortex. Similarly, there were no effects of rehabilitation or time on volume of tissue loss in the striatum; however, there was a significant interaction between these variables (F6,55=2.43, P<0.04). Owing to a small effect size, however, subsequent one-way ANOVA at each time point failed to reveal the origin of this interaction.
Discussion
We have shown for the first time that a combination of EE and daily reach therapy (ER) increases neuronal activity in perilesional cortical tissue in rats after focal ischemia. The use of FosB/ΔFosB immunohistochemistry to reveal this enhanced neuronal activity is not commonly employed in preclinical stroke research,19 but provided a means to demonstrate that task-specific training is an instrumental component of early stroke therapy. These results parallel findings in humans,17 and suggest that recruiting intact brain tissue early after stroke may contribute to the neuroplastic changes and functional recovery observed after extended rehabilitation.
In accordance with earlier studies,5, 22 ET-1 applied to the forelimb motor cortex and dorsolateral striatum produced profound impairments in sensorimotor function as demonstrated with the NDS assessments (Figure 1). Although the NDS was used to confirm ischemic injury and persisting sensorimotor deficits over the short time course of this study, these assessments are generally not sensitive to detecting rehabilitative changes in the long term because of the robust spontaneous recovery as observed in the current study. Further, we did not expect to observe profound functional improvements at these early time points (i.e., 10 days of rehabilitation). We previously demonstrated that ER-associated recovery of function requires several weeks of training, and is more effective when started early after ischemia.3, 4, 5, 13, 15 In contrast, the goal of the current study was to identify early neuroplastic changes that may precede and contribute to long-term functional improvements after more extended durations of rehabilitation.
Although previous studies suggest that EE must be combined with reach therapy to achieve functional recovery,4 it is possible that either EE or RT alone is sufficient to improve function. Data from the current study, however, support the combination rehabilitation hypothesis. Importantly, 10 days of ER significantly increased FosB/ΔFosB expression in perilesional cortex, while EE or RT alone did not increase expression above levels observed in ST animals, nor were there any differences among intact animals in any treatment. Similar increases, though not reaching statistical significance, were observed in both perilesional striatum and contralesional forelimb cortex (Figure 2) similar to the architectural changes previously noted.3, 4 These findings indicate that a combination of environmental enrichment and task-specific rehabilitation targeting the primary functional deficit (i.e. skilled reaching as demonstrated in our previous reports)4, 5 most effectively increases neuronal activity around the lesion and in the intact hemisphere. This heightened activity at the cellular level is important in light of previous behavioral evidence from both animals4 and humans25 suggesting that task specificity is an important component of effective rehabilitation. Although the observed increases in FosB/ΔFosB expression may reflect increased motor activity during reach therapy and/or in the EE, it is important to note that the combination of both treatments was required to produce those increases.
The recruitment of perilesional cortical circuitry during the acute phase of rehabilitation is likely a cardinal component of early recovery mechanisms. For example, functional imaging in ischemic rats suggests that increased activity in tissue surrounding the infarct is associated with improved behavioral outcome.16 Also, reach training with the ipsilesional (i.e. unimpaired) forelimb reduced FosB/ΔFosB expression in perilesional cortex and worsened behavioral outcome after focal ischemia.19 In the current study, we identified a weak relationship between FosB/ΔFosB expression in perilesional cortex and performance on NDS assessments. More sensitive functional assessments may have identified a stronger relationship consistent with previous research. These data further suggest that maintenance and/or enhancement of activity in surviving cortex is important to facilitate functional recovery.
Functional recovery observed in chronic, long-term experiments appears to be correlated with neuroplastic changes in intact cortex. For example, in primates with small lesions of the primary motor cortex, training on a skilled reaching task led to preservation of intact cortical hand representation, and in some cases expansion into surrounding regions.8 These neuroplastic changes were accompanied by improved reaching performance over the training period. Reorganization of perilesional cortex and enhanced recovery has also been observed in rats exposed to skilled reach training.16, 26 Similar motor map expansions have been detected in stroke patients treated with constraint-induced movement therapy, where use of the impaired arm and hand is encouraged by limiting use of the non-affected limb.27 Notably, these studies assessed cortical reorganization long after rehabilitation had commenced and thus it remains unclear whether behavioral recovery coincides temporally with the formation of new somatosensory maps. Interestingly, we previously showed that ischemic rats exposed to ER begin to exhibit enhanced functional recovery as early as 14 days after onset of ER,3 temporally corresponding with our current findings of increased neuronal activation in periinfarct cortex after 10 days.
Cortical reorganization, increased dendritic complexity, and synaptic plasticity also occur in the contralesional hemisphere of ischemic rats after rehabilitation, coinciding with functional recovery.4, 28 We have demonstrated that improvements in skilled reaching deficits achieved through rehabilitation were reversed by transiently inhibiting neural activity in the contralesional motor cortex.29 These findings suggest that the intact hemisphere is involved in functional recovery of the impaired forepaw despite having a minimal role in normal motor control of that limb. Results from neuroimaging studies in stroke patients support this theory, demonstrating increased recruitment of homotopic motor regions in the contralesional hemisphere by movement of an impaired hand.30 Disruption of the uninjured hemisphere using transcranial magnetic stimulation has also been found to impair movement of the paretic (ipsilateral) hand.31
The trend for increased FosB/ΔFosB expression in the contralesional forelimb motor cortex occurred predominantly in layer II/III, which has been shown to be sensitive to manipulation in forelimb use.32 Previous studies have shown that motor learning can increase synaptic density of both layers II/III33 and V.34 It is possible that more intense motor activity or longer treatment duration is required to increase FosB/ΔFosB expression in layer V, or alternatively that other mechanisms are involved in the dendritic remodeling that has been observed in that layer after prolonged rehabilitation.3, 4 Regardless, the increased neuronal activation in the contralesional cortex after 10 days of ER, although not statistically significant, is consistent with the notion that the intact hemisphere is involved in recovery processes.
It is unclear if similar neuronal activation would occur when rehabilitation is delayed. The effectiveness of ER decreases with time, and therapy started 30 days after stroke produces limited functional recovery and no increase in dendritic complexity as observed with earlier intervention.3 Plasticity mechanisms are likely more active in the acute phase after stroke in keeping with the demonstration that blocking brain-derived neurotrophic growth factor in this time window significantly interferes with rehabilitation efficacy.13 Thus the recruitment of neural networks and facilitation of recovery by rehabilitation clearly appear to be time dependent after ischemia.35
Although young, ‘healthy', animals were used in the current study, it is unknown to what extent the neuroplastic changes translate to an older cohort. Research has shown that age-related changes in an array of neural and genetic responses may act as barriers to neuroplasticity and, potentially, recovery after brain injury and ischemia.35, 36 Recent evidence, however, has shown that middle-aged animals benefit from combination rehabilitation in a model of vascular dementia with resultant neuroplastic architectural changes accompanied by substantial improvements in cognition.37 Further research is required to determine if similar increases in FosB/ΔFosB expression would be observed in aged animals using the current model of stroke.
Numerous Fluoro-Jade C-stained neurons were present at 17 days post ischemia (i.e., 10-day treatment) in all groups. This supports earlier findings that vulnerable cells surrounding the ischemic core continue to die for days after the initial injury,38 and suggests that the lesion may continue to evolve over significantly longer periods than previously thought. Alternatively, it may be that cellular fragments undergoing phagocytosis create a false impression of late cell death.
There were no differences among groups in inflammation as measured by density of activated microglia/macrophages, although inflammation was subsiding by 17 days post ischemia. The inflammatory response to cerebral ischemia begins almost immediately and peaks several days later,39 suggesting that the optimal window for any anti-inflammatory effect might be earlier than the onset of our current treatment. However, inflammation can be detected as long as 9 months after global ischemia,40 and it is likely that inflammatory responses continue to have a role in the sequelae of cerebral ischemia, weeks or months after stroke onset.
In summary, our results demonstrate that regions of intact periinfarct cortex are strongly activated after just a few days of ER, potentially reflecting the earliest phase of poststroke recovery. Notably, the activation of periinfarct cortical neurons was maximal only with a combination of enriched housing and reach training (i.e., ER). In the absence of effects on inflammation, delayed cell death and infarct volumes after ischemia, periinfarct activation may be a harbinger of longer-term neuroplastic changes and cortical reorganization hypothesized to mediate motor relearning and functional improvement. A similar hypothesis was assessed about cognition, where a combination of physical exercise and cognitive activity were more effective than either intervention alone in enhancing cognitive function.41 Taken together, these findings have important implications for current rehabilitation approaches that rely almost exclusively on monotherapies such as repetitive reach training. Combining two or more complementary interventions may be a much more effective means for improving recovery from stroke and related forms of brain injury.
Acknowledgments
The authors thank Garry Chernenko for helpful comments on the manuscript and Shirley Granter-Button and Kayla Churchill for technical support.
The authors declare no conflict of interest.
Footnotes
This work was supported by an operating grant from the Canadian Institutes of Health Research (DC) and a Canada Research Chair in Stroke and Neuroplasticity held by DC. JC was supported by a fellowship from the Natural Sciences and Engineering Research Council of Canada. KD was supported by the Heart and Stroke Foundation of Canada/Canadian Stroke Network/Astra-Zeneca Focus on Stroke Doctoral Fellowship.
References
- Duncan PW, Goldstein LB, Matchar D, Divine GW, Feussner J. Measurement of motor recovery after stroke. Outcome assessment and sample size requirements. Stroke. 1992;23:1084–1089. doi: 10.1161/01.str.23.8.1084. [DOI] [PubMed] [Google Scholar]
- Nakayama H, Jorgensen HS, Raaschou HO, Olsen TS. Recovery of upper extremity function in stroke patients: the Copenhagen Stroke Study. Arch Phys Med Rehabil. 1994;75:394–398. doi: 10.1016/0003-9993(94)90161-9. [DOI] [PubMed] [Google Scholar]
- Biernaskie J, Chernenko G, Corbett D. Efficacy of rehabilitative experience declines with time after focal ischemic brain injury. J Neurosci. 2004;24:1245–1254. doi: 10.1523/JNEUROSCI.3834-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biernaskie J, Corbett D. Enriched rehabilitative training promotes improved forelimb motor function and enhanced dendritic growth after focal ischemic injury. J Neurosci. 2001;21:5272–5280. doi: 10.1523/JNEUROSCI.21-14-05272.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke J, Mala H, Windle V, Chernenko G, Corbett D. The effects of repeated rehabilitation ‘tune-ups' on functional recovery after focal ischemia in rats. Neurorehabil Neural Repair. 2009;23:886–894. doi: 10.1177/1545968309341067. [DOI] [PubMed] [Google Scholar]
- Jones TA, Schallert T. Overgrowth and pruning of dendrites in adult rats recovering from neocortical damage. Brain Res. 1992;581:156–160. doi: 10.1016/0006-8993(92)90356-e. [DOI] [PubMed] [Google Scholar]
- Winship IR, Murphy TH. In vivo calcium imaging reveals functional rewiring of single somatosensory neurons after stroke. J Neurosci. 2008;28:6592–6606. doi: 10.1523/JNEUROSCI.0622-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nudo RJ, Milliken GW, Jenkins WM, Merzenich MM. Use-dependent alterations of movement representations in primary motor cortex of adult squirrel monkeys. J Neurosci. 1996;16:785–807. doi: 10.1523/JNEUROSCI.16-02-00785.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleim JA, Bruneau R, VandenBerg P, MacDonald E, Mulrooney R, Pocock D. Motor cortex stimulation enhances motor recovery and reduces peri-infarct dysfunction following ischemic insult. Neurol Res. 2003;25:789–793. doi: 10.1179/016164103771953862. [DOI] [PubMed] [Google Scholar]
- Jones TA, Chu CJ, Grande LA, Gregory AD. Motor skills training enhances lesion-induced structural plasticity in the motor cortex of adult rats. J Neurosci. 1999;19:10153–10163. doi: 10.1523/JNEUROSCI.19-22-10153.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter K, Jutai J, Hartley M, Foley N, Bhogal S, Bayona N, et al. Impact of early vs delayed admission to rehabilitation on functional outcomes in persons with stroke. J Rehabil Med. 2006;38:113–117. doi: 10.1080/16501970500314350. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Archibeque I, Luke L, Nolan T, Momiy J, Li S. Growth-associated gene expression after stroke: evidence for a growth-promoting region in peri-infarct cortex. Exp Neurol. 2005;193:291–311. doi: 10.1016/j.expneurol.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Ploughman M, Windle V, MacLellan CL, White N, Dore JJ, Corbett D. Brain-derived neurotrophic factor contributes to recovery of skilled reaching after focal ischemia in rats. Stroke. 2009;40:1490–1495. doi: 10.1161/STROKEAHA.108.531806. [DOI] [PubMed] [Google Scholar]
- Clarkson AN, Overman JJ, Zhong S, Mueller R, Lynch G, Carmichael ST. AMPA receptor-induced local brain-derived neurotrophic factor signaling mediates motor recovery after stroke. J Neurosci. 2011;31:3766–3775. doi: 10.1523/JNEUROSCI.5780-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLellan CL, Keough MB, Granter-Button S, Chernenko GA, Butt S, Corbett D. A critical threshold of rehabilitation involving brain-derived neurotrophic factor is required for poststroke recovery. Neurorehabil Neural Repair. 2011;25:740–748. doi: 10.1177/1545968311407517. [DOI] [PubMed] [Google Scholar]
- Dijkhuizen RM, Ren J, Mandeville JB, Wu O, Ozdag FM, Moskowitz MA, et al. Functional magnetic resonance imaging of reorganization in rat brain after stroke. Proc Natl Acad Sci USA. 2001;98:12766–12771. doi: 10.1073/pnas.231235598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binkofski F, Seitz RJ. Modulation of the BOLD-response in early recovery from sensorimotor stroke. Neurology. 2004;63:1223–1229. doi: 10.1212/01.wnl.0000140468.92212.be. [DOI] [PubMed] [Google Scholar]
- McClung CA, Ulery PG, Perrotti LI, Zachariou V, Berton O, Nestler EJ. DeltaFosB: a molecular switch for long-term adaptation in the brain. Brain Res Mol Brain Res. 2004;132:146–154. doi: 10.1016/j.molbrainres.2004.05.014. [DOI] [PubMed] [Google Scholar]
- Allred RP, Jones TA. Maladaptive effects of learning with the less-affected forelimb after focal cortical infarcts in rats. Exp Neurol. 2008;210:172–181. doi: 10.1016/j.expneurol.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploughman M, Granter-Button S, Chernenko G, Attwood Z, Tucker BA, Mearow KM, et al. Exercise intensity influences the temporal profile of growth factors involved in neuronal plasticity following focal ischemia. Brain Res. 2007;1150:207–216. doi: 10.1016/j.brainres.2007.02.065. [DOI] [PubMed] [Google Scholar]
- Komitova M, Mattsson B, Johansson BB, Eriksson PS. Enriched environment increases neural stem/progenitor cell proliferation and neurogenesis in the subventricular zone of stroke-lesioned adult rats. Stroke. 2005;36:1278–1282. doi: 10.1161/01.STR.0000166197.94147.59. [DOI] [PubMed] [Google Scholar]
- Windle V, Szymanska A, Granter-Button S, White C, Buist R, Peeling J, et al. An analysis of four different methods of producing focal cerebral ischemia with endothelin-1 in the rat. Exp Neurol. 2006;201:324–334. doi: 10.1016/j.expneurol.2006.04.012. [DOI] [PubMed] [Google Scholar]
- De Ryck M, Van Reempts J, Borgers M, Wauquier A, Janssen PA. Photochemical stroke model: flunarizine prevents sensorimotor deficits after neocortical infarcts in rats. Stroke. 1989;20:1383–1390. doi: 10.1161/01.str.20.10.1383. [DOI] [PubMed] [Google Scholar]
- West MJ. Stereological methods for estimating the total number of neurons and synapses: issues of precision and bias. Trends Neurosci. 1999;22:51–61. doi: 10.1016/s0166-2236(98)01362-9. [DOI] [PubMed] [Google Scholar]
- Dean CM, Shepherd RB. Task-related training improves performance of seated reaching tasks after stroke. A randomized controlled trial. Stroke. 1997;28:722–728. doi: 10.1161/01.str.28.4.722. [DOI] [PubMed] [Google Scholar]
- Ramanathan D, Conner JM, Tuszynski MH. A form of motor cortical plasticity that correlates with recovery of function after brain injury. Proc Natl Acad Sci USA. 2006;103:11370–11375. doi: 10.1073/pnas.0601065103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liepert J, Bauder H, Wolfgang HR, Miltner WH, Taub E, Weiller C. Treatment-induced cortical reorganization after stroke in humans. Stroke. 2000;31:1210–1216. doi: 10.1161/01.str.31.6.1210. [DOI] [PubMed] [Google Scholar]
- Jones TA, Schallert T. Use-dependent growth of pyramidal neurons after neocortical damage. J Neurosci. 1994;14:2140–2152. doi: 10.1523/JNEUROSCI.14-04-02140.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biernaskie J, Szymanska A, Windle V, Corbett D. Bi-hemispheric contribution to functional motor recovery of the affected forelimb following focal ischemic brain injury in rats. Eur J Neurosci. 2005;21:989–999. doi: 10.1111/j.1460-9568.2005.03899.x. [DOI] [PubMed] [Google Scholar]
- Cramer SC, Nelles G, Benson RR, Kaplan JD, Parker RA, Kwong KK, et al. A functional MRI study of subjects recovered from hemiparetic stroke. Stroke. 1997;28:2518–2527. doi: 10.1161/01.str.28.12.2518. [DOI] [PubMed] [Google Scholar]
- Johansen-Berg H, Rushworth MF, Bogdanovic MD, Kischka U, Wimalaratna S, Matthews PM. The role of ipsilateral premotor cortex in hand movement after stroke. Proc Natl Acad Sci USA. 2002;99:14518–14523. doi: 10.1073/pnas.222536799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adkins DL, Bury SD, Jones TA. Laminar-dependent dendritic spine alterations in the motor cortex of adult rats following callosal transection and forced forelimb use. Neurobiol Learn Mem. 2002;78:35–52. doi: 10.1006/nlme.2001.4045. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Lussnig E, Schwarz ER, Comery TA, Greenough WT. Synaptogenesis and Fos expression in the motor cortex of the adult rat after motor skill learning. J Neurosci. 1996;16:4529–4535. doi: 10.1523/JNEUROSCI.16-14-04529.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones TA. Multiple synapse formation in the motor cortex opposite unilateral sensorimotor cortex lesions in adult rats. J Comp Neurol. 1999;414:57–66. [PubMed] [Google Scholar]
- Murphy TH, Corbett D. Plasticity during stroke recovery: from synapse to behaviour. Nat Rev Neurosci. 2009;10:861–872. doi: 10.1038/nrn2735. [DOI] [PubMed] [Google Scholar]
- Burke SN, Barnes CA. Neural plasticity in the ageing brain. Nat Rev Neurosci. 2006;7:30–40. doi: 10.1038/nrn1809. [DOI] [PubMed] [Google Scholar]
- Langdon KD, Granter-Button S, Harley CW, Moody-Corbett F, Peeling J, Corbett D. Cognitive rehabilitation reduces cognitive impairment and normalizes hippocampal CA1 architecture in a rat model of vascular dementia. J Cereb Blood Flow Metab. 2013;33:872–879. doi: 10.1038/jcbfm.2013.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossmann KA. Viability thresholds and the penumbra of focal ischemia. Ann Neurol. 1994;36:557–565. doi: 10.1002/ana.410360404. [DOI] [PubMed] [Google Scholar]
- Morioka T, Kalehua AN, Streit WJ. Characterization of microglial reaction after middle cerebral artery occlusion in rat brain. J Comp Neurol. 1993;327:123–132. doi: 10.1002/cne.903270110. [DOI] [PubMed] [Google Scholar]
- Langdon KD, Granter-Button S, Corbett D. Persistent behavioral impairments and neuroinflammation following global ischemia in the rat. Eur J Neurosci. 2008;28:2310–2318. doi: 10.1111/j.1460-9568.2008.06513.x. [DOI] [PubMed] [Google Scholar]
- Langdon KD, Corbett D. Improved working memory following novel combinations of physical and cognitive activity. Neurorehabil Neural Repair. 2012;26:523–532. doi: 10.1177/1545968311425919. [DOI] [PubMed] [Google Scholar]