Abstract
Contrast-enhanced radiotherapy is an innovative treatment that combines the selective accumulation of heavy elements in tumors with stereotactic irradiations using medium energy X-rays. The radiation dose enhancement depends on the absolute amount of iodine reached in the tumor and its time course. Quantitative, postinfusion iodine biodistribution and associated brain perfusion parameters were studied in human brain metastasis as key parameters for treatment feasibility and quality. Twelve patients received an intravenous bolus of iodinated contrast agent (CA) (40 mL, 4 mL/s), followed by a steady-state infusion (160 mL, 0.5 mL/s) to ensure stable intratumoral amounts of iodine during the treatment. Absolute iodine concentrations and quantitative perfusion maps were derived from 40 multislice dynamic computed tomography (CT) images of the brain. The postinfusion mean intratumoral iodine concentration (over 30 minutes) reached 1.94±0.12 mg/mL. Reasonable correlations were obtained between these concentrations and the permeability surface area product and the cerebral blood volume. To our knowledge, this is the first quantitative study of CA biodistribution versus time in brain metastasis. The study shows that suitable and stable amounts of iodine can be reached for contrast-enhanced radiotherapy. Moreover, the associated perfusion measurements provide useful information for the patient recruitment and management processes.
Keywords: brain metastasis, brain perfusion, contrast agents, computed tomography, stereotactic radiation therapy
Introduction
Tumor iodine uptake is routinely handled in clinics guiding the neurosurgical biopsy or the primary brain tumor histopathology diagnosis. It also contributes to the extension work-up of an already known cancer by assessing brain metastasis onset. Little is known about the absolute concentration time course since most of its clinical use only requires qualitative contrast imaging analysis (except for brain perfusion studies).
In this paper, we investigated the quantitative, iodine biodistribution time course in human brain metastasis to develop a new radiation therapy method based on the enhanced dose delivery when a tumor accumulates iodinated contrast agent (CA). We also assessed how the heavy element tumor uptake (mean concentration) correlates with tumor perfusion parameters.
The tumor iodine concentration time course determines the effectiveness of the contrast-enhanced radiotherapy. This is a treatment that combines the selective accumulation of heavy elements in tumors with stereotactic irradiations using medium energy X-rays. Synchrotron Stereotactic Radiation Therapy (SSRT) is a state-of-the-art radiotherapy technique, whereby irradiations are performed using monochromatic medium energy X-rays.1, 2, 3, 4, 5, 6, 7 Contrast-enhanced radiotherapy is of particular interest for brain tumors where a selective CA accumulation occurs after a systemic infusion due to the locally impaired blood–brain barrier (BBB).8 An increased differential effect is obtained when irradiating tumors previously loaded with high atomic number CAs, as a consequence from locally increased energy deposition due to the rise of the photoelectric effect cross section on heavy atoms irradiated with low-to-medium energy X-rays.9, 10 This differential effect is the basis of the tighter dose distribution gradients compared with conventional radiotherapy treatments.5, 7
Synchrotron radiation X-rays produced at the ESRF (European Synchrotron Radiation Facility) medical beamline are used to perform this treatment,11, 12 since the X-ray beam is nearly parallel with high flux, which allows selecting intense tunable monochromatic beams (25 to 100 keV energy range) with a dose rate of around 1 Gy/min at 2-cm depth in water. Solberg et al11 suggested that the use of a monoenergetic beam would result in improved dose distributions. Mesa et al12 performed Monte Carlo simulations and have shown that, besides the iodine concentration, the dose distributions are heavily dependent on the external beam spectrum and could be significantly improved with optimization of this parameter. First, the optimum beam energy can be selected for radiation therapy with a compromise between maximum dose enhancement effects on the heavy element and the bone sparing effect. Another advantage of a monochromatic X-ray beam in medium energy radiotherapy is the absence of the beam hardening effect, leading to a more homogeneous dose distribution in the tumor. Synchrotron radiation is ideal for this kind of treatment because the flux of monochromatic beams remains high enough for medical applications. However, the limited availability of these X-ray sources restricts their application to well-defined clinical trials, on a small cohort of patients, as proof of principle, feasibility, and interest.
Iodine-enhanced SSRT clinical trials (phase I/II) using intravenous iodinated CA infusions are currently taking place at the ESRF. The targets in this trial are brain metastasis, regardless of the primary cancer type.
The main issue in SSRT efficiency is the iodine concentration reached in the tumor since the dose enhancement is directly related to the CA biodistribution: 1 mg/mL of iodine results in a 10% radiation dose enhancement.10 The iodine concentration must remain high and stable throughout the irradiation time.13 A detailed study of the quantitative iodine time course in brain tumors is a prerequisite for this type of clinical trial. To our knowledge, only Mello et al14 have estimated iodine concentrations in two patients bearing gliomas (3 to 4 mg/mL after having injected 40 to 70 g of iodine). Therefore, further research needs to be conducted on CA uptake in brain metastasis.
The purpose of this work is to perform quantitative iodine biodistribution studies in human brain metastasis after iodinated CA systemic infusions. Iodine concentrations, homogeneity, and variability over time were evaluated for 12 patients. The correlation of postinfusion mean iodine concentration over 16 minutes with the usual contrast-enhanced brain perfusion parameters was obtained with the dynamic contrast-enhanced computed tomography (CT)-perfusion technique.15, 16, 17
Materials and methods
Patient Recruitment
Twelve patients were recruited over 1 year by two radiation oncologists (with 4 and 24 years experience). The recruitment in this prospective study was based only on the metastasis size and location (diameter inferior to 3 cm, supratentorial region). These two parameters are the most relevant parameters for the associated radiotherapy clinical trial. The primary cancer type (lung, melanoma, breast, colon, kidney, and bladder) is recorded for informative purpose only (Table 1). The procedures followed in the present study were in accordance with the ethical standards of the responsible committee on human experimentation: the regional ethical committee designed by the French ministry of health approved our protocol before any patient recruitment (comité de protection des personnes sud est V, CS10217, Grenoble cedex 9, France). Each recruited patient signed an informed consent form before inclusion in the present study.
Table 1. Metastasis data and measured iodine concentrations.
| Patient # | Tumor primary type | Tumor volume (cm3) | Tumor mean radius (cm) | Mean iodine concentration in tumor volume (mg/mL) | Maximum iodine concentration in tumor (mg/mL) |
|---|---|---|---|---|---|
| 1 | Lung well-differentiated adenocarcinoma | 14.1 | 1.5 | 2.14±0.45 | 3.04±1.12 |
| 2 | Dorsal skin malignant melanoma | 11.17 | 1.39 | 1.76±0.36 | 2.36±0.85 |
| 3 | Lung right superior lobe undifferentiated large cells carcinoma | 1.58 | 0.72 | 2.17±0.37 | 3.28±0.87 |
| 4 | Lung right superior lobe undifferentiated large cells carcinoma | 2.43 | 0.84 | 0.74±0.30 | 0.93±0.67 |
| 5 | Lung right superior lobe squamous cells carcinoma | 13.63 | 1.48 | 1.32±0.34 | 1.25±0.69 |
| 5′ | Lung right superior lobe squamous cells carcinoma | 8.84 | 1.28 | 1.10±0.43 | 1.19±1.07 |
| 6 | Bladder urothelial carcinoma with large cells and giant sarcomatoid cells | 6.74 | 1.17 | 1.14±0.33 | 1.59±0.59 |
| 6′ | Bladder urothelial carcinoma with large cells and giant sarcomatoid cells | 3.71 | 0.96 | 1.64±0.35 | 2.07±0.49 |
| 7 | Lung superior right lobe adenocarcinoma, TTF-1 | 8.66 | 1.27 | 2.43±0.45 | 2.89±1.12 |
| 7′ | Lung superior right lobe adenocarcinoma, TTF-1 | 0.58 | 0.52 | 1.14±0.53 | 2.73±0.64 |
| 8 | Breast invasive duct differentiated carcinoma | 43.51 | 2.18 | 2.82±0.58 | 3.67±0.94 |
| 9 | Lung superior right lobe poorly differentiated with large cells carcinoma | 10.46 | 1.36 | 3.57±0.50 | 4.15±1.31 |
| 10 | Lung middle right lobe adenocarcinoma | 4.17 | 1 | 1.34±0.54 | 2.10±1.21 |
| 11 | Cervical skin malignant melanoma | 1.45 | 0.7 | 2.84±0.56 | 3.42±1.33 |
| 11′ | Cervical skin malignant melanoma | 0.56 | 0.51 | 1.99±0.52 | 2.45±1.12 |
| 12 | Renal | 4.37 | 1.01 | 2.82±0.70 | 4.28±1.11 |
| Mean±s.d. | 8.5 | 1.12 | 1.94±0.12 | 2.59±0.25 |
TTF-1, thyroid transcription factor 1. Summary of primary tumor characteristics and iodine concentrations (average and maximum) reached in the whole volume of metastasis for all recruited patients. The prime symbol is used when a patient has two metastasis in the studied field of view.
Imaging and Infusion Protocols
The imaging protocol was performed on a 16-slice CT scanner (LightSpeed, RT;16 GE Healthcare Technologies, Waukesha, WI, USA). A patient-specific thermo-formed mask was manufactured for each patient and attached to a stereotactic radiotherapy frame (Brainlab, Feldkirchen, Germany) on the CT scanner table. Two remote controlled injectors (CT Exprés 3D; Swiss Medical Care, Lausanne, Switzerland and Volumat Agilia; Fresenius Kabi, Brezins, France) were used for intravenous (antecubital area) iodinated CA injections (Iomeron 400; Bracco, Milano, Italy).
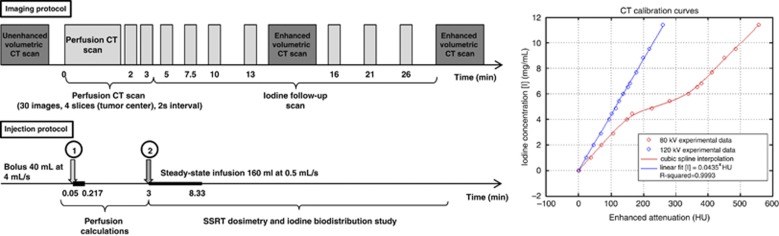
The following imaging and associated infusion protocols were performed (Figure 1):
Unenhanced volumetric CT scan (120 kVp, 376 mA s): helical mode (pitch=0.562), around 80 slices (whole brain), 2.5-mm slice thickness, 50-cm scan field of view (large) and 512 × 512 pixel matrix size.
Computed tomography-perfusion scan (80 kVp, 125 mA s): dynamic axial mode, 4 contiguous 5-mm-thick slices per tube rotation (1 second), 25-cm scan field of view (head) and 512 × 512 pixel matrix size. The acquisitions were performed over 3 minutes (to compute perfusion measurements), according to the following time scale (Figure 1): 30 scans, every 2 seconds; followed by 2 scans at 2 and 3 minutes from the beginning. The first unenhanced images serve as an initial baseline. A bolus of CA (40 mL at 4 mL/s) was injected 3 seconds after the beginning of the first scan.
Iodine infusion follow-up procedure (80 kVp, 125 mA s): dynamic axial mode, 4 contiguous 5-mm-thick slices per tube rotation (1 second), 25-cm scan field of view (head) and 512 × 512 pixel matrix size. The acquisitions were performed over 30 minutes (Figure 1), which corresponds to the maximum time required to perform the SSRT treatment. This infusion (referred to as second infusion) is intended to maintain high and stable iodine concentrations in the metastasis during the whole irradiation time. The infusion (160 mL of CA at 0.5 mL/s) started 3 minutes after the bolus injection (see 2). Images were acquired 2, 4.5, 7, 10, 13, 18, and 23 minutes after the beginning of the second infusion.
Two enhanced volumetric CT scans were performed for dosimetry purposes, 15 and 30 minutes after the beginning of the imaging protocol. The acquisition parameters were identical as the ones described in paragraph (1).
Figure 1.
Imaging and infusion protocols and associated computed tomography (CT) calibration curves. Two infusions were performed: one bolus for perfusion measurements (40 mL at 4 mL/s) and one steady state for iodine biodistribution studies (160 mL at 0.5 mL/s). Volumetric scan (120 kVp, 375 mA, pitch=0.562, 2.5 mm thickness). Axial dynamic scan: slices centered on the tumor center for iodine time curves follow-up (80 kVp, 125 mA, 5 mm thickness). The enhanced attenuations (HU) versus iodine concentrations curves are represented on the right of the figure. The iodine concentrations rank from 0 to 12 mg/mL measured in a phantom at 80 and 120 kVp tube voltages. A cubic spline interpolation (at 80 kVp) and a linear fit (120 kVp) were used to retrieve intermediate concentrations. HU, Hounsfield units.
None of the 12 patients encountered an allergic reaction to iodine or nephrotoxicity with no observable effects on the serum creatinine and a renal clearance to creatinine always greater than 60 mL/min.
Radiation Dose to Patients
The total radiation dose received by each patient was evaluated by measuring the Computed Tomography Dose Index (CTDI).18 A 290-mGy volume-weighted CTDIvol was measured for the three helical scans. The weighted CTDIw reached 230 mGy for the 39 axial acquisitions (iodine follow-up). The CTDIvol differs from the CTDIw by taking into account the helical scan's pitch. The total dose length product was equal to 5,000 mGy cm. The radiation dose to patient from CT scan is high when compared with single volumetric CT scans usually performed for dosimetry purposes. However, the total dose (0.55 Gy) related to the imaging procedure is small when compared with the full radiotherapy treatment, which is anyway performed on the patients (3 times 11 Gy for the stereotactic treatment and 10 times 3 Gy for the whole brain radiotherapy).
Computed Tomography Calibration
To retrieve quantitative iodine biodistribution from the images, Hounsfield units (HU) had to be converted into absolute iodine concentrations. Two calibration data sets (HU versus iodine concentration; Figure 1) were derived at 120 kVp (volumetric scans) and at 80 kVp (axial scans) using a 16.5-cm diameter Lucite phantom19 filled with 15 different iodine concentrations ranging from 0.5 to 12 mg/mL (Figure 1). The CT scanner must be calibrated in terms of iodine concentrations for the two voltages used in this study: at 120 kVp for the low noise 3D dosimetric scans and at 80 kVp for the low dose follow-up scans. Although a linear dependence between iodine concentrations and HU has been reported,20 an unexpected but reproducible nonlinearity (three independent measurement campaigns) was obtained on the 80-kVp calibration curve for the range of (150 to 350) HU. A linear fit was used to convert HU into iodine concentrations (mg/mL) at 120 kVp, whereas a smoothing spline curve was used at 80 kVp for the same purpose (Figure 1).
The noise (CT number standard deviation measured in an 8-cm diameter homogenous region of interest (ROI)18) reached 5 HU at 120 kVp and 25 HU at 80 kVp. This leads to an uncertainty on the iodine concentration measurement lower than 0.5 mg/mL.
Perfusion Measurements
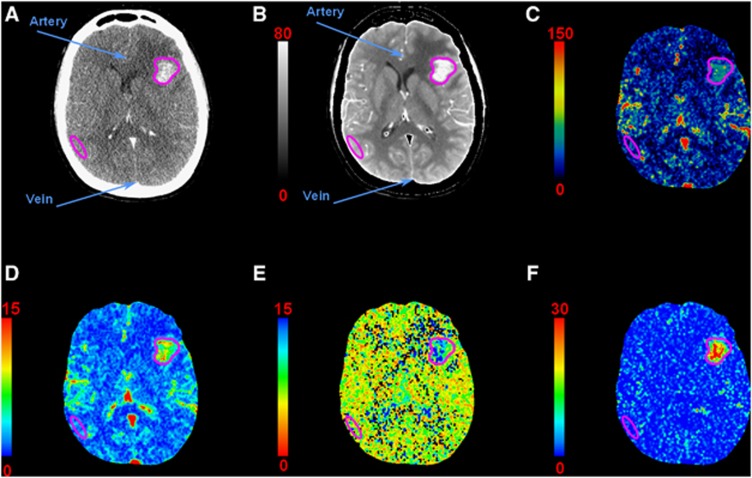
Perfusion maps were generated using the clinical CT-perfusion software (CTperf4; GE Healthcare Technologies, Buc, France). The calculation is performed on the contrast-enhanced images (Figure 2A) and is based on the adiabatic approximation of the Johnson and Wilson model,15, 16, 17, 20 which is particularly relevant when the BBB is disrupted.20
Figure 2.
Computed tomography (CT)-perfusion maps of a patient with a single metastasis in the left hemisphere: (A) contrast-enhanced CT, (B) temporal averaged image (HU), (C) cerebral blood flow (CBF) map (mL/min per 100 g), (D) cerebral blood volume (CBV) map (mL/100 g), (E) mean transit time (MTT) map (seconds), (F) permeability surface (PS) area product map (mL/min per 100 g). Regions of interest (ROIs) are drawn in the artery, vein, metastasis, and gray matter (GM) for perfusion measurements. Each pixel of a given map is expressed in the corresponding perfusion parameter unit. HU, Hounsfield units.
The adiabatic approximation of the Johnson and Wilson model20 requires that the value in each pixel of the images to be directly proportional to the iodine concentration, and that the scaling factor should be independent from the concentration. The images were thus first converted into absolute iodine concentrations using the calibration curves described above.
Quantitative parametric maps were obtained for cerebral blood flow (CBF) (mL/min per 100 g), cerebral blood volume (CBV) (mL/100 g), mean transit time (MTT) (seconds), permeability surface (PS) area product (mL/min per 100 g), and extraction efficiency (E)20 with
Free-hand ROIs were drawn on an average CA concentration map (Figure 2B) on each of the four slices, the metastasis, the contralateral white and gray matter (GM). Another ROI encompassing the whole tumor seen on the four slices was also drawn. The metastasis necrotic parts were excluded from the ROIs.
These ROIs were reported on each iodine biodistribution map and on each perfusion map to compute the average iodine concentrations, the perfusion values and a recalculated MTT (MTT=CBV/CBF, to be compared with the MTT calculated directly with CTperf4).
Steady-State Data Analysis
The quantitative iodine concentration reached after the second injection was measured at each time point in each ROI described above.
The iodine concentration decay after the second infusion was modeled with a monoexponential function (considering a simple compartmental case) for each patient to deduce the predicted concentration at the end of the protocol and compare it with the measured concentration obtained from the last enhanced CT volumetric scan at 120 kVp.
For a given patient, the overall average iodine concentration refers to the concentration averaged in the entire metastasis (four slices) over all the images acquired after the end of the second infusion (∼500 seconds).
Statistics
Statistical analyses were performed using the R software program.21 An unpaired two-tailed t-test was used to compare perfusion parameters between the tumor and the GM.
A paired two-tailed t-test was used to test the difference between the measured concentration and the predicted concentration using the monoexponential model.
A Pearson–Bravais correlation test was performed between iodine concentrations reached in the metastasis and GM and the hemodynamic parameters measured in the same ROIs.
An asymptotic monoexponential fit (equation similar to the Patlak model22) was used to model the concentrations versus perfusion parameters data. This fit is based on the lowest sum of squared absolute error method.23
where [I] is the iodine concentration in (mg/mL) and y is one of the six perfusion parameters with the same physical units as the perfusion parameter.
Results
Iodine Biodistribution and Time Course
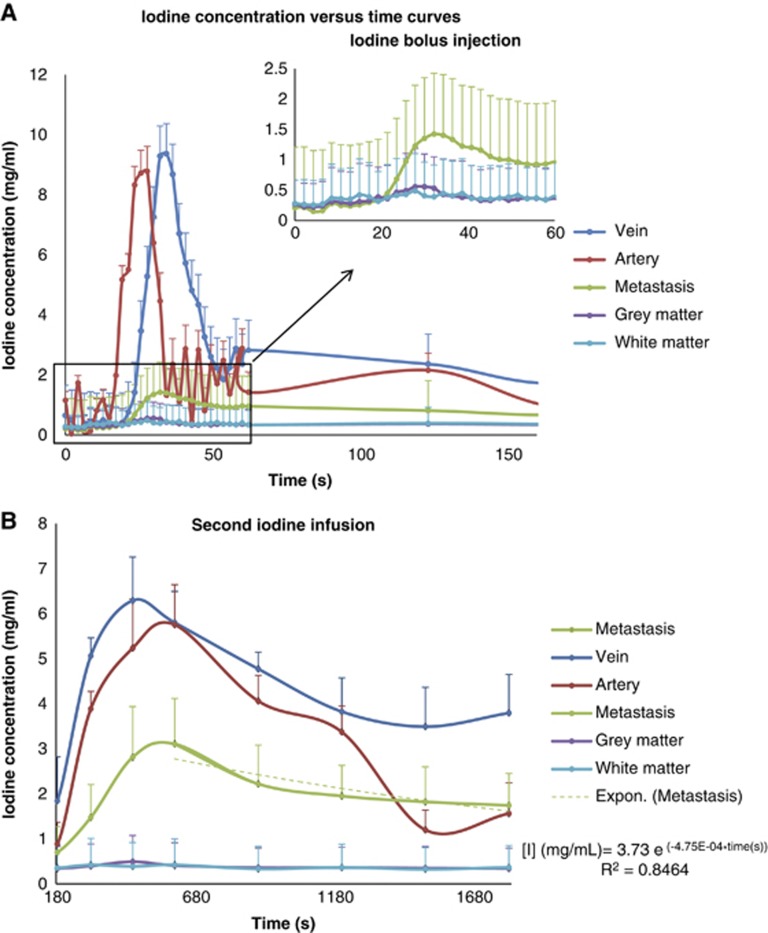
Figure 3 is a plot of iodine concentrations versus time in the various ROIs. The first peak on the bolus curve corresponds to the first pass of the CA and the second peak reflects the recirculation phenomena (Figure 3A). The iodine concentration curves for the second infusion are shown in Figure 3B. This example is representative of the iodine time course observed for each patient, in the major structure of interest for the stereotactic radiotherapy treatments (metastasis, vascular structures, and healthy brain tissue).
Figure 3.
An example of iodine concentration versus time in artery (before partial volume correction), vein, metastasis, gray (GM) and white matters. (A) Bolus infusion and (B) steady-state follow-up: the decay after the second infusion is fitted with a monoexponential (dashed green curve).
The mean (over time) and maximum (among all times) iodine concentrations reached in the entire metastasis volume (four slices) after the steady-state infusion are summarized in Table 1. No significant correlation was observed between the metastasis volume and the iodine concentrations reached after the steady-state infusion (r=0.4).
The iodine amount reached in the metastasis at the end of the second infusion remained quite stable during 20 minutes with a ±10% maximum deviation from the average. The concentrations reached an average of 1.94±0.12 mg/mL.
The time course study shows that the tumoral iodine concentration decreased following a monoexponential model with a relatively long time constant (Figure 3B). The predicted concentrations using this monoexponential model were compared with the concentrations measured on the last 120 kVp enhanced volumetric CT scan (Table 2). There was no significant difference between the predicted and measured concentrations (P>0.05). These results suggest that the monoexponential model could be a reliable approximation to retrieve concentrations from missing data points. This result also shows that a stable amount of iodine can remain in the target during the treatment time (∼30 minutes). For each patient, the iodine concentration stayed within a ±0.5 mg/mL absolute difference (±20% relative difference) when compared with the concentration measured on the dosimetric scan, 15 minutes after the steady-state injection. This maximum deviation (0.5 mg/mL) leads to a maximum 5% error on the dose in an SSRT treatment, which remains in the radiotherapy standards.
Table 2. Iodine concentrations at the end of the protocol.
| Patient # | Experimental concentration (mg/mL) | Predicted concentration (monoexponential model) (mg/mL) | Error (mg/mL) |
|---|---|---|---|
| 1 | 1.74±0.74 | 1.91±4.59 | 0.17 |
| 2 | 1.35±0.76 | 1.43±5.04 | 0.08 |
| 4 | 0.69±0.61 | 0.75±1.47 | 0.06 |
| 5 | 1.04±0.92 | 1.06±2.67 | 0.02 |
| 5' | 1.37±0.63 | 1.42±1.21 | 0.05 |
| 6 | 1.52±0.64 | 1.00±3.47 | −0.52 |
| 6' | 1.85±0.56 | 1.66±1.85 | −0.19 |
| 7 | 2.39±0.86 | 2.30±3.26 | −0.09 |
| 7' | 1.00±1.05 | 1.01±5.40 | 0.02 |
| 8 | 3.14±1.18 | 3.15±1.80 | 0.01 |
| 9 | 2.95±1.03 | 3.38±3.57 | 0.43 |
| 10 | 1.87±1.03 | 1.59±1.23 | −0.29 |
| 11 | 2.90±1.43 | 2.67±4.21 | −0.28 |
| 11' | 2.17±1.28 | 1.89±3.66 | −0.23 |
| 12 | 2.54±1.05 | 2.59±5.97 | 0.04 |
| Mean±s.d. | 1.90±0.25 | 1.85±0.94 | −0.05±0.23 0.16±0.15a |
A comparison between the calculated values using an exponential model and the experimental values obtained with the volumetric postenhancement computed tomography scan. The error is the difference between the predicted and the experimental concentration. s.d. is the standard deviation from the average. The prime symbol is used when a patient has two metastasis in the studied FOV.
Average error value obtained when the absolute values of the errors are taken.
Perfusion Measurements
An example of perfusion maps is shown in Figure 2. The corresponding average parameters in metastasis and GM (over four slices and all patients) are listed in Table 3. Perfusion parameters were significantly increased in the metastasis when compared with the GM (P<0.05).
Table 3. Correlation between perfusion parameters and iodine concentration.
|
Average value±s.d. |
||||
|---|---|---|---|---|
| Tumor | GM | Pearson's correlation coefficient | 95% Confidence interval | |
| CBF (mL/min per 100 g) | 51.53±4.84 | 25.00±3.12 | r=0.594 | α=0.452, 0.706 |
| CBV (mL/100 g) | 5.01±0.43 | 1.86±0.20 | r=0.627 | α=0.494, 0.732 |
| MTT (seconds) | 7.89±0.65 | 6.08±0.65 | r=0.516 | α=0.359, 0.645 |
| Recalculated MTT (seconds) | 5.68±1.52 | 4.38±1.34 | r=0.593 | α=0.503, 0.739 |
| PS (mL/min per 100 g) | 11.13±1.41 | 2.45±0.51 | r=0.673 | α=0.551, 0.766 |
| E | 0.21±0.03 | 0.09±0.02 | r=0.575 | α=0.430, 0.692 |
CBF, cerebral blood flow; CBV, cerebral blood volume; MTT, mean transit time; PS, permeability surface; E, extraction efficiency; GM, gray matter.
Summary of perfusion parameters (CBF, CBV, MTT, recalculated MTT, PS, and E) computed in metastasis and gray matter averaged over all patients. Correlation results between perfusion parameters and iodine concentration. r: Pearson's linear correlation coefficient and α: 95% confidence interval.
Correlation between Iodine Biodistribution and Perfusion Measurements
The correlation coefficients between iodine concentrations reached at the end of the second infusion (in the metastasis and the GM) and the hemodynamic parameters are summarized in Table 3. Reasonable correlations were observed for the PS product (r=0.67) and the CBV (r=0.63).
The relevance of using an asymptotic monoexponential model (Equation 2) to link the iodine concentration reached after the slow infusion to CBV and PS was also tested (Figure 4). This modeling could help in predicting iodine concentrations for a given patient, based on a single CT-perfusion study. Such a model could lead to easier recruitment for SSRT treatments, by performing only the perfusion CT scan. The benefits would be a shorter imaging procedure (5 minutes versus 40 minutes), reduced radiation doses, and a lower amount of CA injected to the patient.
Figure 4.
Asymptotic monoexponential fit of average iodine concentrations (mg/mL) versus: (A) Permeability surface (PS) (mL/min per 100 g) and (B) cerebral blood volume (CBV) (mL/100 g) in tumor and gray matter (GM). The fit parameters are detailed in a dedicated text box. Error bars are not shown for data clarity.
Discussion
The aim of this clinical study was to evaluate the iodine biodistribution and associated time course in human brain metastasis after dedicated infusion protocols. The study also attempted to determine whether the iodine concentration reached after the infusion protocol could be correlated with brain perfusion parameters. The benefits of dose enhancement in SSRT rely on high and stable iodine concentrations in the metastasis over the irradiation time.10 This step was essential to evaluate SSRT treatments reliability for clinical trials.
Only a few studies mentioned iodine concentrations in human tumors after a systemic injection. Mello et al14 have reported tumoral values (one slice) reached in two patients bearing gliomas after an intravenous CA injection: 2.7 mg/mL (40 g/70 kg) and 4 mg/mL (70 g/70 kg). In our study, the iodine concentrations were evaluated in 12 patients who received 80 g/70 kg of iodine by biphasic injection. The mean concentrations measured in this study are in good agreement with those presented by Mello et al. No correlation was noticed between the metastasis volume and the iodine concentrations reached. Therefore, the metastasis volume parameter does not limit our inclusion criteria, provided that the metastasis is small enough to be treated by stereotactic radiotherapy (diameter<3 cm).
Norman et al13 observed a decrease in glioma enhancements lower than 20% over the first hour after the intravenous CA injection (12 patients). In the present study, a ±10% average deviation from the mean concentration (1.94±0.12 mg/mL) was observed over 20 minutes (12 patients, 16 metastasis). Seven out of twelve patients would have been included in the SSRT trial as an average of 1.5 to 2 mg/mL in the metastasis is required. Since 1 mg/mL of iodine produces a 10% dose enhancement factor for SSRT,10 the averaged dose enhancement factor would be 15% to 20%, which is beneficial for patients when compared with conventional treatments. The associated dose uncertainty due to concentration variations over time would remain in the radiotherapy standards (±5% at maximum if a 0.5 mg/mL absolute deviation from the average value is observed). We have shown in this time course study that a sufficient amount of contrast can be achieved in brain metastasis and may not vary appreciably with time during the irradiation procedure. The dose distribution will not vary by >5% when compared with the calculated dose.
A clinical treatment plan with eight beams was published by Edouard et al.10 However, the CT data were changed to simulate a 10-mg/mL iodine uptake in the target. The current study shows that these concentrations are not realistic. Higher iodine concentrations were expected because the range of the concentrations reported in previous Monte–Carlo studies was between 5 and 20 mg/mL.5, 11, 12 No clinical studies have supported these assumptions and many metastasis are known to provide a lighter contrast uptake when compared with primary brain tumors.24 The same type of treatment plan was realized in this study on patient-specific data corresponding to a representative case. For this treatment, eight conformal beams were simulated. The average dose enhancement at the isocenter due to iodine was 14.6±2.2%, when compared with the treatment without iodine. The average dose enhancement was 17.5±2.3% on average in the whole iodine uptake region (target), when compared with the treatment without iodine. The average tumoral concentration was 1.76±0.37 mg/mL, which is consistent with the 10% dose increase per mg/mL.9 Gliomas could then reach higher concentrations and a better dose enhancement. The concentrations might be further increased in future studies by using BBB opening molecules such as Mannitol25 or by convection enhanced delivery supplying large quantities of iodine directly in the brain.26, 27 The average vascular concentration (arteries and veins) remains below 8 mg/mL, but is two to four times higher than the target concentration. Major vessels are thus to be avoided in the patients treatment planning and X-ray beams are placed accordingly.
Another protocol with a lower injection rate (0.25 mL/s) was initially tested on five patients (seven tumors, data not shown) to study the effect of injection rate on iodine concentrations reached in the tumor. This slower protocol was then disregarded and a faster infusion protocol was chosen for the clinical trials because it has the advantage to significantly shorten the CT procedure and the patient immobilization. Moreover, the concentrations reached in the tumor were smaller with the slower protocol (1.27±0.29 mg/mL instead of 1.94±0.12 mg/mL). Norman et al13 have stated that tumor enhancement is not so much related to the speed of injection but more related to the BBB permeability. Our study confirms this statement for the permeability, however, it is difficult to provide a conclusion for the injection rate since only five patients were recruited for this initial protocol.
The only report on contrast-enhanced medium energy radiotherapy clinical trial was published in the late 90s.24 This trial was performed using a modified CT scanner. It is difficult to conclude positively on the outcome of the treatment since the patients were in the terminal stage of their disease. However, the survival data reported on eight patients were encouraging and ranged between 3 and 41 months after treatment. The main limitations for further investigations were the dose rate and the heating problems associated with the X-ray tube.
A phase I/II clinical trial was designed at the ESRF, according to the one performed by Rose et al. The primary objective of this phase I/II trial was to demonstrate the technical feasibility and the safety of the treatment, not to assess the outcome of the treatment. The study described in the present paper was the preliminary mandatory phase, aimed at studying the iodine time course and justifying the clinical feasibility of iodine-enhanced SSRT. The dynamic CT protocol described in this study is at the core of the recruitment process. The iodine time course was carefully studied in terms of quantity and stability. If enough voxels in the metastasis have an average iodine concentration of >2 mg/mL (i.e., the whole target has an average concentration of higher than 1.5 mg/mL), then the patient will be recruited; provided the iodine concentration remains within ±0.5 mg/mL absolute difference from the average concentration, within a 30-minute follow-up period.
The second part of this study was to find out whether the iodine concentrations in brain metastasis could be correlated with the usual perfusion parameters. It has been shown that iodine accumulation is related to brain perfusion and can be assessed by tracer kinetic models.20 Initially, a correlation of the iodine concentrations with CBF representing the iodinated CA supply or the CBV (representing the blood pool) was expected. This hypothesis was verified for the infusion protocol used in this study.
The Johnson and Wilson model was chosen for perfusion measurements because it is the only model (available on clinical commercial softwares) able to deal with disrupted BBB cases and to provide permeability parametric maps.15, 16, 17 The perfusion parameter values computed in the present study were in good agreement with the ones found in the literature (CBF=20 to 60 mL/min per 100 g, CBV=1 to 3 mL/100 g, and MTT=3 to 5 seconds, approximately in brain metastasis20). Reasonable correlations were obtained (r=0.575 to 0.675) between the iodine concentrations reached in the metastasis and the perfusion values, for each of the perfusion parameters. These correlations could be helpful for future patient recruitment because they provide an initial estimate of the amount of CA that could be reached in the metastasis from a simple and short perfusion exam. The recalculated MTT had a better correlation coefficient with iodine concentration than the MTT processed directly with CTperf4. This difference could rely on the calculation accuracy using CT perfusion: the MTT is detected in every pixel via an impulse residue function, which can possibly be distorted by the high noise level in the CT images. The recalculated MTT is computed by dividing CBV by CBF average values and is less sensitive to noise.
In addition to the simple correlation (linear model), the relationship between the iodine concentration and the perfusion parameters using an asymptotic monoexponential model was also tested (Equation 2). This model is relevant for the CBV since the iodine concentration should increase with the blood volume and reach a plateau when CA homogeneously occupies the whole extracellular space in the voxel (the CA is not metabolized). The monoexponential model is also relevant for PS since the iodine concentration increases with PS as the capillaries permeability allows filling the extravascular space with CA. This permeability limited phase ends when the PS is high enough so that one is in a blood flow limited phase, where PS can increase but the iodine concentration remains stable at its asymptotical value. The asymptotical value (2.6 mg/mL) is on the same scale as the vascular concentration measured at the same time points (around 3.5 mg/mL on average), which is consistent with the model proposed here. More patients would be necessary to refine the relationship between perfusion parameters and the iodine concentration reached in the metastasis.
The current recruitment procedure is uncomfortable for the patient. The procedure lasts almost 2 hours: the patient is lying on the CT couch, in the stereotactic frame. Recruitment based on perfusion measurements could significantly shorten the procedure and improve the patient comfort. If the recruitment process had been based on perfusion measurements, then only 3/12 patients (25%) would have been included as false positives (threshold: 1.5 mg/mL [I]). In that case, they would have followed the whole imaging procedure. None of the patients would have been excluded by mistake. This contrast uptake versus perfusion value study is clearly useful and may help to refine the recruitment protocol by choosing perfusion parameter thresholds above which the patients could safely be included in the SSRT protocol. Therefore, the recruitment process would be significantly reduced to a simple conventional CT perfusion exam.
Acknowledgments
The authors would like to thank Jean Louis Gilet and René Philibert and the radiation oncology department staff of the University Hospital of Grenoble for the help in the patients imaging protocol. We also thank Dr Emmanuel Barbier from Team 5 of the Grenoble Neurosciences Institute for letting us using the in-house Matlab program that was further modified to analyze the CT-scan images. We warmly thank Dr Jeffrey Christopher Crosbie for English editing of the manuscript.
The authors declare no conflict of interest.
Footnotes
This work was funded by the Grenoble University Hospital (clinical investigations department and radiation therapy department). LO and PD were supported by French ministry of research PhD grants.
References
- Adam JF, Elleaume H, Joubert A, Biston MC, Charvet AM, Balosso J, et al. Synchrotron radiation therapy of malignant brain glioma loaded with an iodinated contrast agent: first trial on rats bearing F98 gliomas. Int J Radiat Oncol Biol Phys. 2003;57:1413–1426. doi: 10.1016/j.ijrobp.2003.07.007. [DOI] [PubMed] [Google Scholar]
- Corde S, Joubert A, Adam JF, Charvet AM, Le Bas JF, Esteve F, et al. Synchrotron radiation-based experimental determination of the optimal energy for cell radiotoxicity enhancement following photoelectric effect on stable iodinated compounds. Br J Cancer. 2004;91:544–551. doi: 10.1038/sj.bjc.6601951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biston MC, Joubert A, Adam JF, Elleaume H, Bohic S, Charvet AM, et al. Cure of fisher rats bearing radioresistant F98 glioma treated with cis-platinum and irradiated with monochromatic synchrotron X-rays. Cancer Res. 2004;64:2317–2323. doi: 10.1158/0008-5472.can-03-3600. [DOI] [PubMed] [Google Scholar]
- Joubert A, Biston MC, Boudou C, Ravanat JL, Brochard T, Charvet AM, et al. Irradiation in presence of iodinated contrast agent results in radiosensitization of endothelial cells: consequences for computed tomography therapy. Int J Radiat Oncol Biol Phys. 2005;62:1486–1496. doi: 10.1016/j.ijrobp.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Boudou C, Balosso J, Esteve F, Elleaume H. Monte Carlo dosimetry for synchrotron stereotactic radiotherapy of brain tumours. Phys Med Biol. 2005;50:4841–4851. doi: 10.1088/0031-9155/50/20/007. [DOI] [PubMed] [Google Scholar]
- Adam JF, Biston MC, Rousseau J, Boudou C, Charvet AM, Balosso J, et al. Heavy element enhanced synchrotron stereotactic radiotherapy as a promising brain tumour treatment. Phys Med. 2008;24:92–97. doi: 10.1016/j.ejmp.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Prezado Y, Fois G, Edouard M, Nemoz C, Renier M, Requardt H, et al. Biological equivalent dose studies for dose escalation in the stereotactic synchrotron radiation therapy clinical trials. Med Phys. 2009;36:725–733. doi: 10.1118/1.3070538. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- Dawson P, Penhaligon M, Smith E, Saunders J. Synergetic cyto-toxicity of iodinated contrast agents and X-radiation. Invest Radiol. 1988;23:S110–S113. doi: 10.1097/00004424-198809001-00012. [DOI] [PubMed] [Google Scholar]
- Edouard M, Broggio D, Prezado Y, Esteve F, Elleaume H, Adam JF. Treatment plans optimization for contrast-enhanced synchrotron stereotactic radiotherapy. Med Phys. 2010;37:2445–2456. doi: 10.1118/1.3327455. [DOI] [PubMed] [Google Scholar]
- Solberg TD, Iwamoto KS, Norman A. Calculation of radiation-dose enhancement factors for dose enhancement therapy of brain-tumors. Phys Med Biol. 1992;37:439–443. doi: 10.1088/0031-9155/37/2/010. [DOI] [PubMed] [Google Scholar]
- Mesa AV, Norman A, Solberg TD, Demarco JJ, Smathers JB. Dose distributions using kilovoltage x-rays and dose enhancement from iodine contrast agents. Phys Med Biol. 1999;44:1955–1968. doi: 10.1088/0031-9155/44/8/308. [DOI] [PubMed] [Google Scholar]
- Norman D, Stevens EA, Wing SD, Levin V, Newton TH. Quantitative aspects of contrast enhancement in cranial computed tomography. Radiology. 1978;129:683–688. doi: 10.1148/129.3.683. [DOI] [PubMed] [Google Scholar]
- Mello RS, Callisen H, Winter J, Kagan AR, Norman A. Radiation-dose enhancement in tumors with iodine. Med Phys. 1983;10:75–78. doi: 10.1118/1.595378. [DOI] [PubMed] [Google Scholar]
- Johnson JA, Wilson TA. A model for capillary exchange. Am J Physiol. 1966;210:1299–1303. doi: 10.1152/ajplegacy.1966.210.6.1299. [DOI] [PubMed] [Google Scholar]
- St Lawrence KS, Lee TY. An adiabatic approximation to the tissue homogeneity model for water exchange in the brain: II. Experimental validation. J Cereb Blood Flow Metab. 1998;18:1378–1385. doi: 10.1097/00004647-199812000-00012. [DOI] [PubMed] [Google Scholar]
- St Lawrence KS, Lee TY. An adiabatic approximation to the tissue homogeneity model for water exchange in the brain: I. Theoretical derivation. J Cereb Blood Flow Metab. 1998;18:1365–1377. doi: 10.1097/00004647-199812000-00011. [DOI] [PubMed] [Google Scholar]
- Association HP, Diagnostic Radiology Topic Group CTWPS, Staff HPA . Measurement of the Performance Characteristics of Diagnostic X-ray Systems Used in Medicine: Measurement and Use of the Associated Performance Parameters: a Guide. The physical specification of computed tomography X-ray scanners. Hospital Physicists' Association: London, UK; 1981. [Google Scholar]
- Elleaume H, Charvet AM, Corde S, Esteve F, Le Bas JF. Performance of computed tomography for contrast agent concentration measurements with monochromatic x-ray beams: comparison of K-edge versus temporal subtraction. Phys Med Biol. 2002;47:3369–3385. doi: 10.1088/0031-9155/47/18/307. [DOI] [PubMed] [Google Scholar]
- Miles K, Cuenod CA. Multidetector Computed Tomography in Oncology: CT Perfusion Imaging. Informa Healthcare: London, UK; 2007. [Google Scholar]
- R Developement Core Team R: A Language and Environment for Statistical Computing2012 . http://www.r-project.org/ .
- Patlak CS, Blasberg RG, Fenstermacher JD. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. J Cereb Blood Flow Metab. 1983;3:1–7. doi: 10.1038/jcbfm.1983.1. [DOI] [PubMed] [Google Scholar]
- Phillips JR.Zunzun.com Online Curve Fitting and Surface Fitting http://www.zunzun.com .
- Drevelegas A. Imaging of Brain Tumors with Histological Correlations. Springer: Berlin, Germany; 2002. [Google Scholar]
- Adam JF, Biston MC, Joubert A, Charvet AA, Le Bas JF, Esteve F, et al. Enhanced delivery of iodine for synchrotron stereotactic radiotherapy by means of intracarotid injection and bloodbrain barrier disruption: quantitative iodine biodistribution studies and associated dosimetry. Int J Radiat Oncol Biol Phys. 2005;61:1173–1182. doi: 10.1016/j.ijrobp.2004.12.026. [DOI] [PubMed] [Google Scholar]
- Morrison PF, Laske DW, Bobo H, Oldfield EH, Dedrick RL. High-flow microinfusion—tissue penetration and pharmacodynamics. Am J Physiol. 1994;266:R292–R305. doi: 10.1152/ajpregu.1994.266.1.R292. [DOI] [PubMed] [Google Scholar]
- Bobo RH, Laske DW, Akbasak A, Morrison PF, Dedrick RL, Oldfield EH. Convection-enhanced delivery of macromolecules in the brain. Proc Natl Acad Sci USA. 1994;91:2076–2080. doi: 10.1073/pnas.91.6.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]