Abstract
Although the innate immune response to induce postischemic inflammation is considered as an essential step in the progression of cerebral ischemia injury, the role of innate immunity mediator NLRP3 in the pathogenesis of ischemic stroke is unknown. In this study, focal ischemia was induced by middle cerebral artery occlusion in NLRP3−/−, NOX2−/−, or wild-type (WT) mice. By magnetic resonance imaging (MRI), Evans blue permeability, and electron microscopic analyses, we found that NLRP3 deficiency ameliorated cerebral injury in mice after ischemic stroke by reducing infarcts and blood–brain barrier (BBB) damage. We further showed that the contribution of NLRP3 to neurovascular damage was associated with an autocrine/paracrine pattern of NLRP3-mediated interleukin-1β (IL-1β) release as evidenced by increased brain microvessel endothelial cell permeability and microglia-mediated neurotoxicity. Finally, we found that NOX2 deficiency improved outcomes after ischemic stroke by mediating NLRP3 signaling. This study for the first time shows the contribution of NLRP3 to neurovascular damage and provides direct evidence that NLRP3 as an important target molecule links NOX2-mediated oxidative stress to neurovascular damage in ischemic stroke. Pharmacological targeting of NLRP3-mediated inflammatory response at multiple levels may help design a new approach to develop therapeutic strategies for prevention of deterioration of cerebral function and for the treatment of stroke.
Keywords: blood–brain barrier, interleukin-1β, ischemic cerebral injury, NADPH oxidase, Nod-like receptors
Introduction
Ischemic stroke is one of the most common vascular diseases in the center nervous system. Although numerous studies have implicated that several mechanisms are involved in the pathogenesis of ischemic stroke, the innate immune response to induce postischemic inflammation is considered as an essential step in the progression of cerebral ischemia injury.1 Acute cerebral ischemia elicits an innate immune response, leading to a cascade of events that culminates in neuron necrosis and injuries to their supportive structures in the brain.2 The innate immune system is responsible for initiating immune responses to resolve infections and repair damaged tissues, which is triggered by recognition of pathogen-associated molecular patterns or danger-associated molecular patterns by pathogen recognition receptors. Among pathogen recognition receptors, the intracellular Nod-like receptors have recently been identified as key mediators of inflammatory and immune responses.3 Recent studies have highlighted the role of NLRP3, one member of Nod-like receptor family, forming an intracellular multiprotein complex with active caspase-1, known as an inflammasome, to create a platform for regulating secretion of interleukin-1β (IL-1β) and IL-18.4 Except for the critical role of NLRP3 in common autoimmune diseases, studies have indicated that NLRP3 inflammasome is also associated with therosclerosis,5 myocardial ischemia-reperfusion injury,6 and Alzheimer's disease.7 However, so far it is unknown the role of NLRP3 in the pathogenesis of ischemic stroke.
Recent evidence has emerged that microvessels and neurons respond rapidly and simultaneously in focal regions of ischemic injury and are coordinated as a unitary manner rather than as individual components.8 The observation that neurovascular communication is disrupted under ischemic conditions can be described by a theoretical ‘neurovascular unit'.9 The neurovascular unit is composed of neuronal, glial, and vascular cells along with extracellular matrix. Many central nervous system diseases including stroke and Alzheimer's disease, which lead to neurovascular unit dysfunction,10 have common features such as glial activation/transformation and vascular/blood–brain barrier (BBB) alteration.11 However, up to date, it is unknown whether NLRP3 is associated with neurovascular damage in ischemic stroke. Therefore, in the present study, we used an in vivo middle cerebral artery (MCA) occlusion (MCAO) model and in vitro cell cultures by oxygen–glucose deprivation (OGD) for the first time to investigate the expression of NLRP3 in ischemic brains and further provide direct evidence that NADPH oxidase-mediated NLRP3 signaling contributes to cerebral ischemia injury via exacerbation of inflammation and neurovascular damage.
Materials and methods
An extended Materials and methods section can be found in Supplementary Materials.
Animals
NLRP3−/− and NOX2−/− mice on a C57BL/6 background were obtained from Mutant Mouse Regional Resource Centers (Davis, CA, USA) and the Jackson Laboratory (Bar Harbor, ME, USA), respectively. Both genotypes were backcrossed with a C57BL/6 background for at least six generations before use in the experiments. The WT mice in the present study were C57BL/6 obtained from the Jackson Laboratory. All mice were housed under specific pathogen free conditions, and maintained on a 12-hour light/dark cycle with free access to food and water.
In Vivo Stroke Model
In animal studies, all procedures were approved by Institutional Animal Care and Use Committee of Shandong University. The investigation conforms to the US National Institutes of Health Guide for the Care and Use of Laboratory Animals and was performed in accordance with the ARRIVE guidelines (http://www.nc3rs.org/ARRIVE). A total of 55 C57BL/6 mice, 29 NLRP3−/− mice, and 50 NOX2−/− mice were used in this study. Different groups were allocated in a randomized manner and investigators were blinded to the allocation of different groups when doing surgeries and doing outcome evaluations. Sample size was determined by power analysis based on pilot experiments (significance level 0.05, power 80%). Transient MCAO was induced in 12-week-old male NLRP3−/−, NOX2−/−, or C57BL/6 mice (20 to 24 g) as described.12 A successful occlusion was indicated by a decrease in the regional cerebral blood flow to <20% of the baseline by transcranial laser-Doppler (Perimed, Jarfalla, Sweden) measurement in the area of cerebral cortex supplied by the MCA. After 2 hours of MCAO, the suture was carefully removed to restore blood flow. Reperfusion was confirmed by an immediate increase in regional cerebral blood flow. During and after the surgery, rectal temperature was controlled with a homeothermic blanket and kept at 37°C until the complete recovery of the animal from the anesthesia. The sham-operated groups were subjected to the same procedure except for the occlusion of the MCA.13 Overall, ∼15% of animals died during surgery or after recovery from surgical anesthesia and 16% of all animals used in this study were excluded because of insufficient cerebral blood flow reduction or hemorrhage during surgery. The percentage of excluded animals was similar across different genotypes.
Stroke Assessment by Magnetic Resonance Imaging
To analyze infarct dynamics and to scan for possible intracerebral bleedings, serial stroke assessment by magnetic resonance imaging (MRI) was performed as described.14, 15 The MRI was performed on a dedicated 3.0T MR small animal imaging system (Achieva, Philips, Amsterdam, The Netherlands). T2w-TSE sequence parameters were echo time=120 ms, repetition time=5,100 ms, bandwidth=120 Hz/pixel, turbo factor 4, matrix=356 × 256, field of view=50 × 50 mm2, 12 slices, and 1.5 mm slice thickness with 0.2 mm gap. Calculations of edema, corrected stroke volumes, and noninvasive quantification of brain edema were performed as described.16
Evans Blue Extravasation
Blood–brain barrier permeability was estimated by Evan's blue leakage as described.17
Immunofluorescence
Immunofluorescent staining was performed as described using a LSM780 laser scanning confocal microscope (ZEISS, Oberkochen, Germany) equipped with a Plan-Apochromat 63 × /1.4 objective.18
Primary Cell Cultures and Treatments
Primary cortical neuron, microglia, and astrocytes were isolated and cultured as described.19 Primary brain microvessel endothelial cells were prepared as described.20 The purity of neuron, microglia, astrocytes, and endothelial cells was evaluated by immunofluorescence staining using antibodies against NeuN (Cell Signaling Technology, Danvers, MA, USA), CD11b (BD Biosciences, San Diego, CA, USA), glial fibrillary acidic protein (Invitrogen, Carlsbad, CA, USA), and CD34 (BD Biosciences), respectively. Cells were subjected to the model of OGD (1 hour of OGD followed by 24 hours of reoxygenation) as described.12
Transwell Cocultures
The primary microglia cells were cultured on the upper compartment of a two-chamber Transwell system (0.4-μm pore size of polycarbonate membrane coated with poly-L-lysine; Corning, Corning, NY, USA). Neuron/astrocyte cultures (∼70:30%) were grown on a coverslip in the bottom well of the chamber. DNA damage in the target neuron/astrocyte cultures was quantified by counting TUNEL (terminal deoynucleotidyl transferase-mediated 2'-deoxyuridine 5'-triphosphate-biotin nick end labeling)-positive cells as a percentage of DAPI-labeled cells in each well. The damaged cells were neurons by colabeling their nuclei with DAPI and a neuron-specific antibody against NeuN. The detailed procedures of the transwell cultures were followed by previous studies.21, 22
Statistics
Data are expressed as means±SE. The significance of the differences in mean values between and within multiple groups was examined by one-way ANOVA followed by Duncan's multiple range test. P<0.05 was considered as statistically significant.
Results
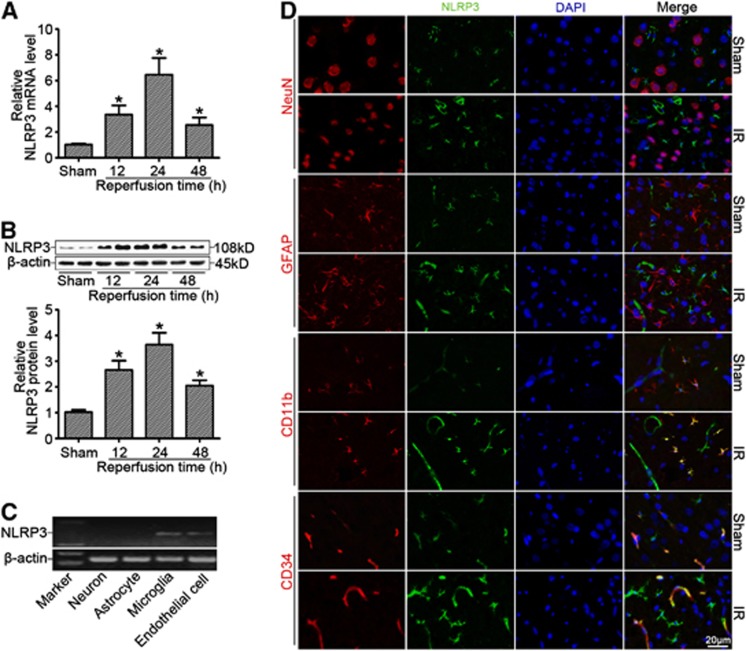
NLRP3 Expression Was Significantly Increased after Cerebral Ischemia Reperfusion
As shown in Supplementary Figure S1A and B, cerebral injury after MCAO was confirmed by 2,3,5-triphenyltetrazolium chloride staining and neurologic deficit score. By real-time RT-PCR (Figure 1A) and western blotting (Figure 1B) analyses, we found that NLRP3 expression was markedly enhanced in the ischemic cerebral hemisphere (ischemic core and penumbra) with a peak expression of NLRP3 at 24 hours after reperfusion. We further found that NLRP3 was mainly expressed in microglia and endothelial cells by confocal immunofluorescent analysis (Figure 1C), which was further confirmed by mRNA detection in primary cultured cortical neurons, astrocytes, microglia, and brain microvessel endothelial cells in vitro (Figure 1D). Moreover, we found that IL-1β and IL-18 levels (Supplementary Figure S1C) were significantly enhanced in the ischemic cerebral hemisphere accompanied by the activation of caspase-1 (Supplementary Figure S1D and E) in a time-dependent manner.
Figure 1.
NLRP3 expression was significantly increased after cerebral ischemia reperfusion (IR). (A) Relative mRNA levels of NLRP3 by real-time RT-PCR analysis in the ischemic cerebral hemisphere from wild-type (WT) mice at different time points after reperfusion. (B) Western blot analysis of NLRP3 protein levels in the ischemic cerebral hemisphere from WT mice at different time points after reperfusion. (C) NLRP3 mRNA detection by RT-PCR from primary cultured cortical neurons, astrocytes, microglia, and brain microvessel endothelial cells in vitro. (D) Cellular localization of NLRP3 at 24 hours of reperfusion after middle cerebral artery occlusion (MCAO) showing that NLRP3 was expressed in microglia and endothelial cells rather than in astrocytes and neurons of ischemic brains by confocal analysis. *P<0.05 vs. sham-operated mice (n=8).
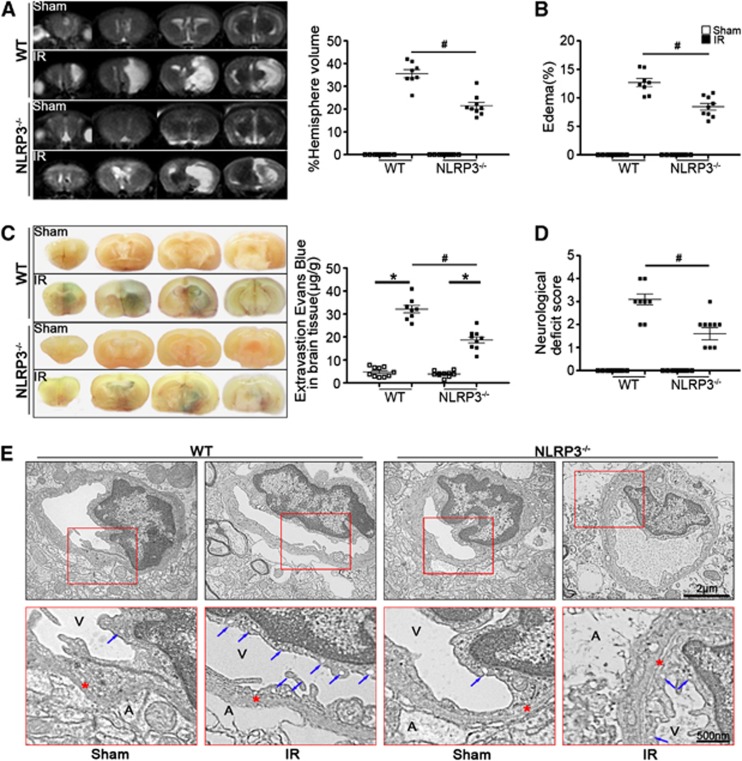
NLRP3 Deficiency Improved Outcomes after Ischemic Stroke
To confirm that the ischemic insult was equivalent among all groups, relevant physiologic parameters were assessed. As shown in Supplementary Table S3, physiologic variables were not significantly different among WT, NLRP3−/−, and NOX2−/− mice before MCAO, during MCAO, or at 24 hours after reperfusion. Using MRI and Evans blue permeability assays, we observed the reduced infarction volume (Figure 2A), edema formation (Figure 2B), and BBB permeability (Figure 2C) in NLRP3−/− ischemic mice, which was consistent with the improved performance in neurologic scoring (Figure 2D). Furthermore, cerebromicrovessel exhibited capillary integrity with normal endothelial cells and basal lamina in sham-operated group by electron microscopy analysis. In ischemic WT mice, the endothelial cells and their nucleus were swollen and deformed, and the integrity of BBB was destroyed, presenting perivascular edema, vacuolation, and membrane damage. However, NLRP3 deficiency significantly blocked ischemia-induced BBB disruption (Figure 2E).
Figure 2.
NLRP3 deficiency ameliorated stroke outcomes. (A) Representative T2 images measured and quantified infarct volume with magnetic resonance imaging (MRI) in wild-type (WT) and NLRP3−/− mice at 24 hours of reperfusion after middle cerebral artery occlusion (MCAO). (B) Calculated cerebral edema in WT and NLRP3−/− mice at 24 hours of reperfusion after MCAO. (C) Representative microscopic images of brain sections after injection of Evans blue and measured Evans blue intensity in WT and NLRP3−/−mice at 24 hours of reperfusion after MCAO. (D) Neurologic deficit scores in WT and NLRP3−/− mice at 24 hours of reperfusion after MCAO. (E) Transmission electronic microscope analysis of the blood–brain barrier (BBB) integrity showing that in ischemic WT mice, the endothelial cells and their nucleus were swollen and deformed, and the integrity of BBB was destroyed, presenting perivascular edema, vacuolation (blue arrows indicated), and membrane (red stars indicated) damage. A represents astrocytes; V represents blood vessel; n=8 to 10 animals per group.*P<0.05 vs. sham-operated mice and #P<0.05 vs. WT ischemic mice. IR, ischemia reperfusion.
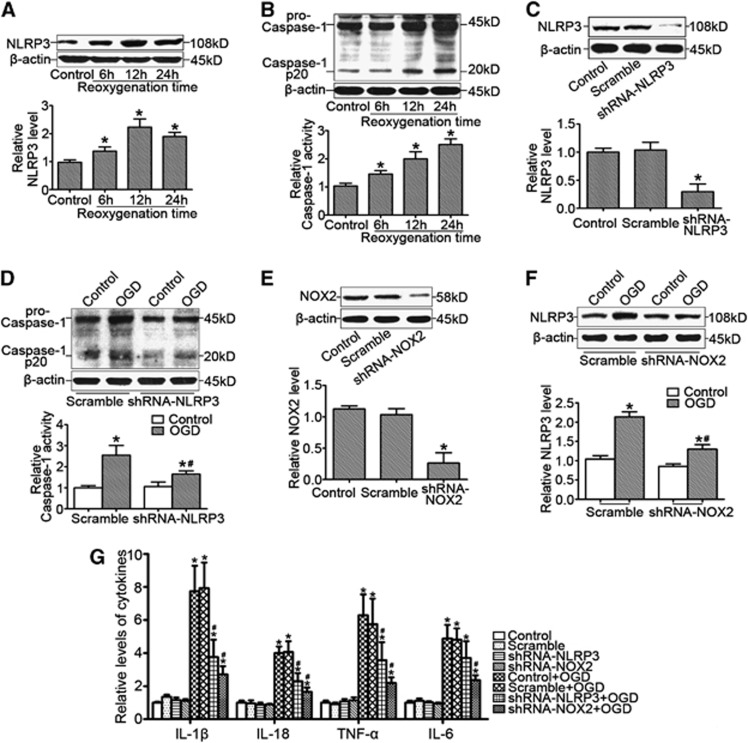
NLRP3 Expression Was Associated with NADPH Oxidase Activity in Primary Microglia Cells Subjected to Oxygen–Glucose Deprivation
In vitro, we found that OGD significantly enhanced microglia NLRP3 expression in a time-dependent manner (Figure 3A) accompanied by enhanced cleavage of caspase-1 and caspase-1 activity (Figure 3B). Further studies showed that gene silencing of NLRP3 by shRNA-NLRP3 transfection (Figure 3C) significantly attenuated OGD-induced caspase-1 cleavage and caspase-1 activity (Figure 3D). Moreover, we found that gene silencing of NOX2 (Figure 3E) inhibited OGD-induced NLRP3 expression (Figure 3F). Finally, we observed that both shRNA-NLRP3 and shRNA-NOX2 blocked OGD-induced increases in cytokine levels (Figure 3G).
Figure 3.
NLRP3 expression was associated with NADPH oxidase activity in primary microglia cells in response to oxygen–glucose deprivation (OGD). (A) Representative western blot gel documents and summarized data showing NLRP3 protein levels in primary microglia cells cultured by the model of OGD at different time points of reoxygenation. (B) Representative western blot gel documents for pro-caspase-1 and caspase-1 p20 and summarized data for caspase-1 activity in primary microglia cells cultured by the model of OGD at different time points of reoxygenation. (C) Representative western blot gel documents and summarized data showing the efficiency of gene silencing of NLRP3 by shRNA-NLRP3 transfection. (D) Representative western blot gel documents for pro-caspase-1 and caspase-1 p20 and summarized data for caspase-1 activity in primary microglia cells transfected with shRNA-NLRP3 cultured by the model of OGD after 12 hours of reoxygenation. (E) Representative western blot gel documents and summarized data showing the efficiency of gene silencing of NOX2 by shRNA-NOX2 transfection. (F) Representative western blot gel documents and summarized data showing NLRP3 protein levels in microglia cells transfected with shRNA-NOX2 cultured by the model of OGD after 12 hours of reoxygenation. (G) The levels of proinflammatory cytokines in microglia cells transfected with shRNA-NLRP3 or shRNA-NOX2. *P<0.05 vs. control and #P<0.05 vs. cells cultured by the model of OGD (n=6).
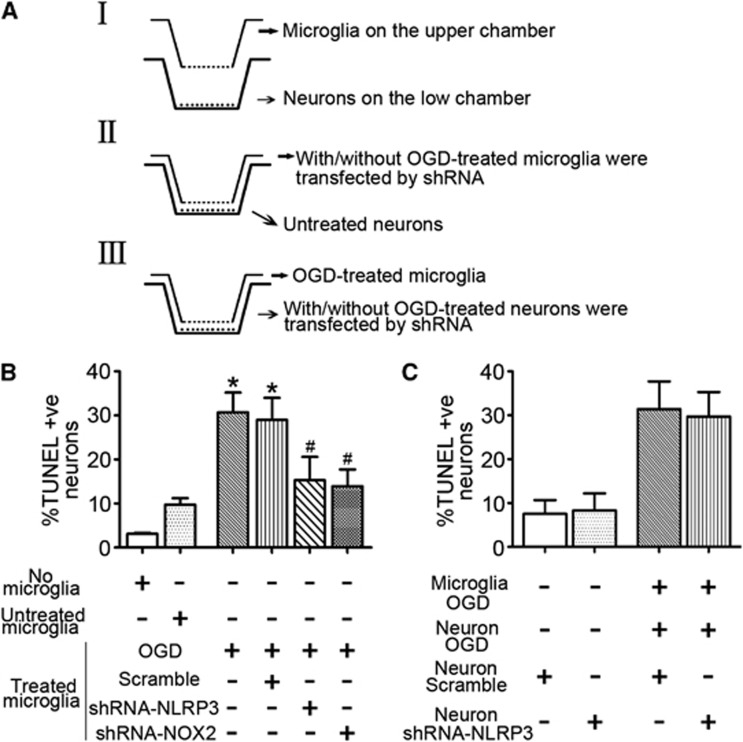
Microglia-Mediated Neuron Damage Was Associated with Oxygen–Glucose Deprivation-Induced Microglial NLRP3 Signaling
The role of microglial NLRP3 was determined by using a two-chamber Transwell system for neuron-microglial cocultures. The experimental paradigm was described in Figure 4A. As shown in Figure 4B, it was ∼3% of the target neurons spontaneously became TUNEL positive, which was increased to 9.76±1.44% when they were exposed to untreated microglia for 24 hours. When neurons were exposed to OGD-stressed microglia, TUNEL-positive neuronal cells were increased to 31.68±4.51%, which was attenuated by NLRP3 or NOX2 knockdown. Expectedly, neuronal cells transfected with shRNA-NLRP3 had no effects on neuron death (Figure 4C). Collectively, our results indicated that microglia-mediated neuron damage was associated with OGD-induced microglial NLRP3 signaling.
Figure 4.
Microglia-mediated neuron damage was associated with oxygen–glucose deprivation (OGD)-induced microglial NLRP3 signaling. (A) The experimental paradigm in this study. (B) Summarized data showing the effects of treating microglia with OGD on the neuronal damage by TUNEL-positive nuclei counting. (C) Summarized data showing the effects of neuronal NLRP3 knockdown on the neuron damage under OGD-stressed microglia coculture condition. *P<0.05 vs. untreated microglia-neuron cocultures and #P<0.05 vs. cells cultured by the model of OGD (n=6). TUNEL, terminal deoynucleotidyl transferase-mediated 2'-deoxyuridine 5'-triphosphate-biotin nick end labeling.
NLRP3 Contributed to Oxygen–Glucose Deprivation-Induced Brain Microvessel Endothelial Cell Dysfunction
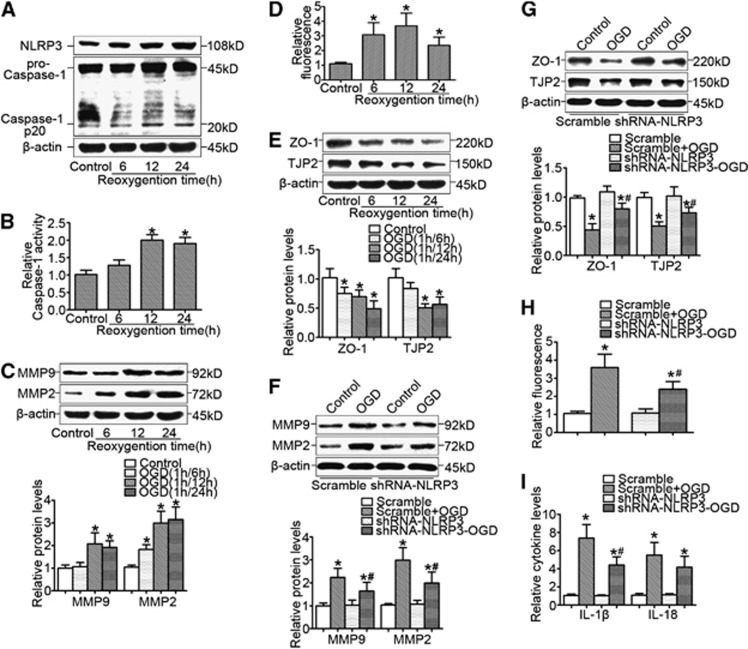
As shown in Figure 5A, we found that in brain microvessel endothelial cells, OGD significantly enhanced NLRP3 expression (Figure 5A), caspase-1 activity (Figure 5B), and matrix metallopeptidase-2 (MMP-2) and MMP-9 expression (Figure 5C) in a time-dependent manner, accompanied by the enhanced endothelial cell permeability (Figure 5D) and reduced expression of tight-junction proteins such as ZO-1 and TJP-2 (Figure 5E). The similar results were also obtained from direct IL-1β treatment (Supplementary Figure S2). Further studies showed that gene silencing of NLRP3 significantly attenuated OGD-induced MMP-2 and MMP-9 expression (Figure 5F) and recovered ZO-1 and TJP-2 expression (Figure 5G). Finally, we found that the increased endothelial cell permeability (Figure 5H) and proinflammatory cytokines levels (Figure 5I) were attenuated by NLRP3 knockdown.
Figure 5.
NLRP3 contributed to oxygen–glucose deprivation (OGD)-induced brain microvessel endothelial cell dysfunction. (A) Representative western blot gel documents for NLRP3, pro-caspase-1, and caspase-1 p20 in brain microvessel endothelial cells cultured by the model of OGD at different time points of reoxygenation. (B) Caspase-1 activity in endothelial cells cultured by the model of OGD at different time points of reoxygenation. (C) Representative western blot gel documents for matrix metallopeptidase-2 (MMP-2) and MMP-9 expression in brain endothelial cells cultured by the model of OGD at different time points of reoxygenation. (D) Endothelial cell permeability by measurement of fluorescence intensity in cells with different treatments. (E) Representative western blot gel documents for ZO-1 and TJP-2 in endothelial cells cultured by the model of OGD at different time points of reoxygenation. (F) Representative western blot gel documents and summarized data showing gene silencing of NLRP3 on the effect of MMP-2 and MMP-9 expression. (G) Representative western blot gel documents and summarized data showing the effects of gene silencing of NLRP3 on MMP-2 and MMP-9 expression. (H) Endothelial cell permeability in brain endothelial cells transfected with shRNA-NLRP3 cultured by the model of OGD after 12 hours of reoxygenation. (I) The levels of proinflammatory cytokines in brain endothelial cells transfected with shRNA-NLRP3 cultured by the model of OGD after 12 hours of reoxygenation. *P<0.05 vs. control and #P<0.05 vs. cells cultured by the model of OGD (n=6).
NLRP3 Expression Was Associated with NOX2-Derived Reactive Oxygen Species in Ischemic Stroke
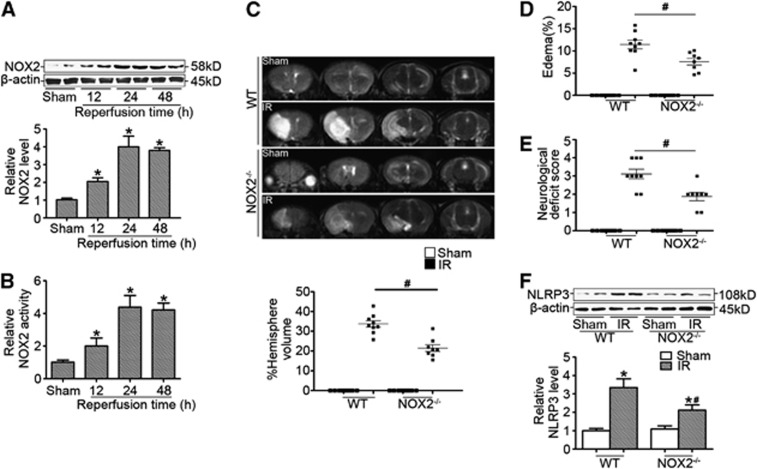
We found that NOX2 expression (Figure 6A) and activity (Figure 6B) was significantly increased in the ischemic brain. Therefore, ischemic NOX2−/− mice were used to further examine whether NOX2 mediates NLRP3 signaling in vivo. Our results showed that the infarction volume (Figure 6C) and edema formation (Figure 6D) at 24 hours after reperfusion was significantly reduced in NOX2−/− mice compared with those of WT ischemic mice, which was consistent with the decreased neurologic deficit score (Figure 6E). Furthermore, we found that NOX2 deficiency reduced NLRP3 expression (Figure 6F), suggesting that NOX2-mediated cerebral injury in ischemic stroke was associated with NLRP3 signaling in vivo.
Figure 6.
NOX2-mediated cerebral injury in ischemic stroke was associated with NLRP3 signaling. (A) Representative western blot gel documents and summarized data showing the relative NOX2 protein levels in the ischemic cerebral hemisphere from wild-type (WT) mice at different time points after reperfusion. (B) Summarized data showing NADPH oxidase activity in the ischemic cerebral hemisphere from WT mice at different time points after reperfusion. (C) Representative T2 images measured and quantified infarct volume with magnetic resonance imaging (MRI) at 24 hours of reperfusion after middle cerebral artery occlusion (MCAO) in WT and NOX2−/− mice (n=8 to 10 animals per group). (D) Calculated cerebral edema at 24 hours of reperfusion after MCAO in WT and NOX2−/− mice. (E) Neurologic deficit scores in WT and NOX2−/− mice at 24 hours of reperfusion after MCAO in WT and NOX2−/− mice. (F) Representative western blot gel documents and summarized data showing the relative NOX2 protein levels in the ischemic cerebral hemisphere at 24 hours of reperfusion after MCAO in WT and NOX2−/− mice. *P<0.05 vs. sham-operated mice and #P<0.05 vs. WT ischemic mice. IR, ischemia reperfusion.
Discussion
In this study, we found that NLRP3 deficiency ameliorated cerebral injury in mice after ischemic stroke and NLRP3 contributed to neurovascular damage by an autocrine/paracrine pattern of NLRP3-mediated proinflammatory mediators. The neurovascular unit was originally proposed as a conceptual framework for an integrative approach to explore how the brain responds to cerebrovascular pathology.23, 24 It has come to the realization that a singular focus on neuronal cell death alone was not enough, and integrated responses in neurons, glia, and brain blood vessels all contribute to the development of acute cerebral injury after stroke, which provides a basis for understanding the multiple pathways to regulate cerebral microvascular permeability under pathologic conditions. In this study, our data clearly showed the upregulation of NLRP3 in ischemic brain and NLRP3 was expressed in microglia and endothelial cells of ischemic brain, indicating that microglial and endothelial cells are major resources of NLRP3 in regions vulnerable to ischemic insult. As the major resident immune cells in the brain, it is not surprising that microglial cells are activated under ischemic condition. Evidence has been cumulated on the role of microglia alterations in their interaction patterns with brain neurons and in the pathway toward neuron injury in stroke. The excessive secretion of proinflammatory mediators from microglia triggers signaling cascades in neurons, ultimately resulting in cerebral injury. Studies have shown that IL-1β can exacerbate stroke pathogenesis and inhibition of IL-1β signaling in vivo has provided improved stroke outcomes.1, 25 Considering that NLRP3 is not expressed in neurons, we wondered whether NLRP3-mediated inflammatory actions contribute to neuronal injury by IL-1β from microglia. By neuron-microglial cocultures, we found that OGD increased NLRP3 expression and activity in microglia, enhanced the production of proinflammatory mediators, and increased their capacity to kill neurons through microglia-mediated neurotoxicity as supported by the fact that gene silencing of microglial NLRP3 attenuated neuron death by OGD-treated microglia, indicating that the activated microglia became neurotoxic, at least in part, through a mechanism mediated by NLRP3 signaling.
The structural basis of the BBB is cerebral endothelial cells, which are characterized by the presence of tight cell–cell junctions and a lack of fenestrations. The BBB serves an important role of restricting the entry of molecules and immune cells from the systemic circulation into the central nervous system.26 Therefore, endothelial dysfunction is a hallmark of cerebral injury leading to BBB permeability and is correlated with the increased risk of acute ischemic stroke. Endothelial cells are one important source of IL-1β and also a target of IL-1β, which causes them to release a range of inflammatory molecules in response to IL-1β stimulation. Thus, endothelial cells are considered as an amplifier of inflammation through the sensing and release of IL-1β. Our results showed that IL-1β is tightly controlled by NLRP3 in brain endothelial cells and NLRP3 contributed to the regulation of MMP-2/-9 and tight-junction protein expressions and endothelial cell permeability, suggesting that NLRP3 may be a potential molecular target for reducing neurovascular damage. It should be noted that although our in vivo data also clearly showed that enhanced caspase-1 activity, MMP-9 expression, and the production of proinflammatory mediators were attenuated in NLRP3−/− ischemic mice (Supplementary Figure S3), we could not exclude the possibility that the attenuation in MMP-9 induction and caspase-1 activity may be due to the smaller infarct volume, as well as the same concerns raised from other studies.27 Therefore, more rigorous and reliable studies need to be further confirmed these findings.
An increasing number of studies have indicated that the activation of the immune system as a result of disturbances in the redox state of cells seems to contribute to the brain damage and cerebrovascular diseases in these conditions. Studies have shown that NADPH oxidase-derived reactive oxygen species (ROS) is central to cerebral ischemia-induced oxidative stress in the brain28 and NOX2 is the most important NADPH oxidase for mediating cerebral injury.29 Mice with genetic NOX2 ablation had smaller infarcts30, 31 and ameliorated BBB damage in experimental stroke.28 The contribution of NOX2 to the inflammatory response accompanying stroke has been confirmed in recent studies showing that mice deficient in NOX2 decreased the levels of proinflammatory mediators.32 Moreover, recent studies have indicated the role of NADPH oxidase-mediated redox signaling in the regulation of NLRP3 inflammasome activation,33, 34 although this conclusion has been challenged by several observations.35, 36 Here, we found that the enhanced NLRP3 expression was attenuated in the ischemic brain of NOX2−/− mice, which was consistent with our in vitro studies showing that NOX2 mediated NLRP3 expression in microglial and endothelial cells, implicating the contribution of NOX2 to the regulation of NLRP3 expression, not only for NLRP3 inflammasome activation per se. Although in this study we did not attempt to explore the mechanisms by which NADPH oxidase mediates NLRP3 expression, HuR may be a potential target. HuR is an RNA-binding protein identified by its ability to bind to an AU-rich element of sequence AUUUA in the 3′ untranslated regions of target genes. HuR has been suggested to be involved in the shuttling of target gene mRNA subsets to the cytosol for translation, as well as controlling the stability of a number of mRNAs.37 Interestingly, 3′ untranslated regions of mouse NLRP3 have six AUUUA domains. In addition, NADPH oxidase-dependent MAPK activation has been shown to be associated with HuR accumulation in the cytoplasm38 and our preliminary results showed that OGD induced the cytoplasmic accumulation of HuR. Therefore, we proposed that the elevated cytoplasmic HuR by OGD-induced NADPH oxidase activity may bind to NLRP3 mRNA 3′ untranslated regions and stabilize mRNA of NLRP3, therefore leading to increased NLRP3 protein levels. Regarding this issue, current studies in our laboratory are designed to test this hypothesis.
While contributing to oxidative stress exacerbated by reperfusion, the preclinical trials using antioxidant treatments to scavenge ROS failed to show clinical efficacy. Antioxidants may interfere with physiologic functions of ROS or do not reach the crucial target proteins of ROS-induced cerebral injury. Therefore, the key targets in the pathogenic pathways for cerebral injury should be identified to direct toward prevention or treatment of ischemic stroke. Our results indicated that NLRP3 inflammasome is a potential target for drug development. However, inflammasome functions are far more complex than the mere induction of inflammatory cell death or cytokine release; thus efficient inflammasome interference should act on all the different steps which are involved in stimulation of inflammasome assembly, caspase-1 activation, and cytokine release.39 Currently, several compounds targeting inflammasome-activating plasma membrane receptors are available and considered as one of the most promising avenues for drug development. For example, blockers of P2X7 receptor (P2X7R), an ATP-gated ion channel, are promising potential drugs by attenuation of P2X7R-induced inflammasome activation, such as P2X7R antagonist, AFC-5128, an N-indol-3-yl-acetamide and Nazaindol-3-yl-acetamide compound. Collectively, this study for the first time shows the contribution of NLRP3 to neurovascular damage after ischemic stroke and provides direct evidence that NLRP3 as an important target molecule links NADPH oxidase-derived ROS to cerebral injury in ischemic stroke. Pharmacological targeting of NLRP3-mediated inflammatory response at multiple levels may help design a new approach to develop therapeutic strategies for prevention of deterioration of cerebral function and for the treatment of stroke and other cerebrovascular diseases.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
This study was supported by grants from the National 973 Basic Research Program of China (2012CB517700); the National Nature Science Foundation of China (81170772, 81070572, and 81171062); the Shandong Natural Science Fund for Distinguished Young Scholars to Yi F (JQ201121); the Foundation of Program for New Century Excellent Talents in University to Yi F (NCET-11-0311), the Nature Science Foundation of Shandong Province (ZR2010HM112) and the International Science and Technology Cooperation Program of Shandong Province (2011GHZ21801).
Supplementary Material
References
- Lambertsen KL, Biber K, Finsen B. Inflammatory cytokines in experimental and human stroke. J Cereb Blood Flow Metab. 2012;32:1677–1698. doi: 10.1038/jcbfm.2012.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harari OA, Liao JK. NF-κB and innate immunity in ischemic stroke. Ann NY Acad Sci. 2010;1207:32–40. doi: 10.1111/j.1749-6632.2010.05735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh M. The machinery of NOD-like receptors: refining the paths to immunity and cell death. Immunol Rev. 2011;243:235–246. doi: 10.1111/j.1600-065X.2011.01045.x. [DOI] [PubMed] [Google Scholar]
- Eisenbarth SC, Flavell RA. Innate instruction of adaptive immunity revisited: the inflammasome. EMBO Mol Med. 2009;1:92–98. doi: 10.1002/emmm.200900014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundmann S, Bode C, Moser M. Inflammasome activation in reperfusion injury: Friendly fire on myocardial infarction. Circulation. 2011;123:574–576. doi: 10.1161/CIRCULATIONAHA.111.018176. [DOI] [PubMed] [Google Scholar]
- Halle A, Hornung V, Petzold GC, Stewart CR, Monks BG, Reinheckel T, et al. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat Immunol. 2008;9:857–865. doi: 10.1038/ni.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Zoppo GJ. The neurovascular unit, matrix proteases, and innate inflammation. Ann NY Acad Sci. 2010;1207:46–49. doi: 10.1111/j.1749-6632.2010.05760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Zoppo GJ. The neurovascular unit in the setting of stroke. J Intern Med. 2010;267:156–171. doi: 10.1111/j.1365-2796.2009.02199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirnagl U. Pathobiology of injury after stroke: the neurovascular unit and beyond. Ann NY Acad Sci. 2012;1268:21–25. doi: 10.1111/j.1749-6632.2012.06691.x. [DOI] [PubMed] [Google Scholar]
- Maki T, Hayakawa K, Pham LD, Xing C, Lo EH, Arai K. Biphasic mechanisms of neurovascular unit injury and protection in cns diseases. CNS Neurol Disord Drug Targets. 2013;12:302–315. doi: 10.2174/1871527311312030004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Wei X, Liu K, Zhang X, Yang F, Zhang H, et al. NOX2 deficiency ameliorates cerebral injury through reduction of complexin II-mediated glutamate excitotoxicity in experimental stroke. Free Radic Biol Med. 2013;65C:942–951. doi: 10.1016/j.freeradbiomed.2013.08.166. [DOI] [PubMed] [Google Scholar]
- He M, Zhang B, Wei X, Wang Z, Fan B, Du P, et al. HDAC4/5-HMGB1 signalling mediated by NADPH oxidase activity contributes to cerebral ischaemia/reperfusion injury. J Cell Mol Med. 2013;17:531–542. doi: 10.1111/jcmm.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Thompson JF, Taheri S, Salayandia VM, McAvoy TA, Hill JW, et al. Early inhibition of MMP activity in ischemic rat brain promotes expression of tight junction proteins and angiogenesis during recovery. J Cereb Blood Flow Metab. 2013;33:1104–1114. doi: 10.1038/jcbfm.2013.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinschnitz C, Kraft P, Dreykluft A, Hagedorn I, Gobel K, Schuhmann MK, et al. Regulatory T cells are strong promoters of acute ischemic stroke in mice by inducing dysfunction of the cerebral microvasculature. Blood. 2013;121:679–691. doi: 10.1182/blood-2012-04-426734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludewig P, Sedlacik J, Gelderblom M, Bernreuther C, Korkusuz Y, Wagener C, et al. EACAM1 inhibits MMP-9-mediated blood-brain-barrier breakdown in a mouse model for ischemic stroke. Circ Res. 2013;113:1013–1022. doi: 10.1161/CIRCRESAHA.113.301207. [DOI] [PubMed] [Google Scholar]
- Wang Z, Leng Y, Tsai LK, Leeds P, Chuang DM. Valproic acid attenuates blood-brain barrier disruption in a rat model of transient focal cerebral ischemia: The roles of HDAC and MMP-9 inhibition. J Cereb Blood Flow Metab. 2011;31:52–57. doi: 10.1038/jcbfm.2010.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han H, Wang Y, Li X, Wang PA, Wei X, Liang W, et al. Novel role of NOD2 in mediating Ca2+ signaling: evidence from NOD2-regulated podocyte TRPC6 channels in hyperhomocysteinemia. Hypertension. 2013;62:506–511. doi: 10.1161/HYPERTENSIONAHA.113.01638. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Wei X, Liu L, Liu S, Wang Z, Zhang B, et al. TIPE2, a novel regulator of immunity, protects against experimental stroke. J Biol Chem. 2012;287:32546–32555. doi: 10.1074/jbc.M112.348755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osada T, Gu YH, Kanazawa M, Tsubota Y, Hawkins BT, Spatz M, et al. Interendothelial claudin-5 expression depends on cerebral endothelial cell-matrix adhesion by beta(1)-integrins. J Cereb Blood Flow Metab. 2011;31:1972–1985. doi: 10.1038/jcbfm.2011.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushal V, Schlichter LC. Mechanisms of microglia-mediated neurotoxicity in a new model of the stroke penumbra. J Neurosci. 2008;28:2221–2230. doi: 10.1523/JNEUROSCI.5643-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Li P, Guo Y, Wang H, Leak RK, Chen S, et al. Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke. 2012;43:3063–3070. doi: 10.1161/STROKEAHA.112.659656. [DOI] [PubMed] [Google Scholar]
- Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci. 2003;4:399–415. doi: 10.1038/nrn1106. [DOI] [PubMed] [Google Scholar]
- Hayakawa K, Pham LD, Katusic ZS, Arai K, Lo EH. Astrocytic high-mobility group box 1 promotes endothelial progenitor cell-mediated neurovascular remodeling during stroke recovery. Proc Natl Acad Sci USA. 2012;109:7505–7510. doi: 10.1073/pnas.1121146109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Cadenas I, Del Rio-Espinola A, Giralt D, Domingues-Montanari S, Quiroga A, Mendioroz M, et al. IL1B and VWF variants are associated with fibrinolytic early recanalization in patients with ischemic stroke. Stroke. 2012;43:2659–2665. doi: 10.1161/STROKEAHA.112.657007. [DOI] [PubMed] [Google Scholar]
- Sierra C, Coca A, Schiffrin EL. Vascular mechanisms in the pathogenesis of stroke. Curr Hypertens Rep. 2011;13:200–207. doi: 10.1007/s11906-011-0195-x. [DOI] [PubMed] [Google Scholar]
- Lee KY, Bae ON, Serfozo K, Hejabian S, Moussa A, Reeves M, et al. Asiatic acid attenuates infarct volume, mitochondrial dysfunction, and matrix metalloproteinase-9 induction after focal cerebral ischemia. Stroke. 2012;43:1632–1638. doi: 10.1161/STROKEAHA.111.639427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahles T, Luedike P, Endres M, Galla HJ, Steinmetz H, Busse R, et al. NADPH oxidase plays a central role in blood-brain barrier damage in experimental stroke. Stroke. 2007;38:3000–3006. doi: 10.1161/STROKEAHA.107.489765. [DOI] [PubMed] [Google Scholar]
- Kahles T, Brandes RP. Which NADPH oxidase isoform is relevant for ischemic stroke? The case for nox 2. Antioxid Redox Signal. 2013;18:1400–1417. doi: 10.1089/ars.2012.4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns B, Kim JY, Tang XN, Yenari MA. NOX inhibitors as a therapeutic strategy for stroke and neurodegenerative disease. Curr Drug Targets. 2012;13:199–206. doi: 10.2174/138945012799201676. [DOI] [PubMed] [Google Scholar]
- Chen H, Song YS, Chan PH. Inhibition of NADPH oxidase is neuroprotective after ischemia-reperfusion. J Cereb Blood Flow Metab. 2009;29:1262–1272. doi: 10.1038/jcbfm.2009.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal BH, Grimm MJ, Khan AN, Han W, Blackwell TS. Regulation of innate immunity by NADPH oxidase. Free Radic Biol Med. 2012;53:72–80. doi: 10.1016/j.freeradbiomed.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dostert C, Petrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J. Innate immune activation through NALP3 inflammasome sensing of asbestos and silica. Science. 2008;320:674–677. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abais JM, Zhang C, Xia M, Liu Q, Gehr TW, Boini KM, et al. NADPH oxidase-mediated triggering of inflammasome activation in mouse podocytes and glomeruli during hyperhomocysteinemia. Antioxid Redox Signal. 2013;18:1537–1548. doi: 10.1089/ars.2012.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubartelli A. Redox control of NLRP3 inflammasome activation in health and disease. J Leukoc Biol. 2012;92:951–958. doi: 10.1189/jlb.0512265. [DOI] [PubMed] [Google Scholar]
- Meissner F, Seger RA, Moshous D, Fischer A, Reichenbach J, Zychlinsky A. Inflammasome activation in NADPH oxidase defective mononuclear phagocytes from patients with chronic granulomatous disease. Blood. 2010;116:1570–1573. doi: 10.1182/blood-2010-01-264218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doller A, Pfeilschifter J, Eberhardt W. Signalling pathways regulating nucleo-cytoplasmic shuttling of the mRNA-binding protein HuR. Cell Signal. 2008;20:2165–2173. doi: 10.1016/j.cellsig.2008.05.007. [DOI] [PubMed] [Google Scholar]
- Lin WN, Lin CC, Cheng HY, Yang CM. Regulation of cyclooxygenase-2 and cytosolic phospholipase A2 gene expression by lipopolysaccharide through the RNA-binding protein HuR: Involvement of NADPH oxidase, reactive oxygen species and mitogen-activated protein kinases. Br J Pharmacol. 2011;163:1691–1706. doi: 10.1111/j.1476-5381.2011.01312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Virgilio F. The therapeutic potential of modifying inflammasomes and NOD-like receptors. Pharmacol Rev. 2013;65:872–905. doi: 10.1124/pr.112.006171. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.