Abstract
Microvascular injury early after hypoxic ischemia (HI) may contribute to neonatal brain damage. N-methyl-D-aspartate receptor overstimulation activates neuronal nitric oxide synthases (nNOS). We hypothesized that microvascular damage occurs early post-HI via nNOS activation and contributes to brain injury. Postpartum day-7 rat pups were treated with 7-nitroindazole (7-NI) or aminoguanidine (AG) before or after HI. Electron microscopy was performed to measure neuronal and endothelial cell damage. There were vascular lumen narrowing at 1 hour, pyknotic neurons at 3 hours, and extensive neuronal damage and loss of vessels at 24 hours post HI. Early after reoxygenation, there were neurons with heterochromatic chromatin and endothelial cells with enlarged nuclei occluding the lumen. There was also increased 3-nitrotyrosin in the microvessels and decreased cerebral blood perfusion. 7-NI and AG treatment before hypoxia provided complete and partial neuroprotection, respectively. Early post-reoxygenation, the AG group showed significantly increased microvascular nitrosative stress, microvascular interruptions, swollen nuclei that narrowed the vascular lumen, and decreased cerebral perfusion. The 7-NI group showed significantly decreased microvascular nitrosative stress, patent vascular lumen, and increased cerebral perfusion. Our results indicate that microvascular damage occurs early and progressively post HI. Neuronal nitric oxide synthases activation contributes to microvascular damage and decreased cerebral perfusion early after reoxygenation and worsens brain damage.
Keywords: cerebral blood flow, hypoxic ischemia, microvessel, neonatal brain, nNOS
Introduction
Hypoxic ischemia (HI) in neonates triggers sequential cascades of neurotoxic events within hours that last for days to weeks after the injury, resulting in significant neuronal injury.1, 2 Neurons and vascular cells are closely related developmentally and functionally.3 Communication between the nervous and vascular systems is required for maintaining the integrity of the blood–brain barrier (BBB) and promoting neural function.4 Dysfunction of the neurovascular unit that occurs early after injury may disrupt microcirculation and promote the development of neurologic diseases.4, 5 The neurovascular unit, therefore, is the major target of neuroprotection in the neonatal brain.6, 7, 8, 9, 10
Studies in the mature brain have shown that the microvascular responses after ischemia are rapid and temporally linked to neuronal injury.5, 11, 12, 13 Nitric oxide (NO) biosynthesis is a key factor in the pathophysiologic response to ischemia.14 Ischemia causes neuronal membrane depolarization that leads to massive glutamate releases and overstimulation of N-methyl-D-aspartate receptors (NMDAR). Calcium over-influxes through NMDAR-activated neuronal nitric oxide synthases (nNOS) lead to high levels of free radical NO that may react with superoxide to form toxic peroxynitrite, a compound that can add nitrate to tyrosine groups on proteins, lipids, and DNA, and promotes cytotoxic and proinflammatory responses.2 Cerebrovascular nitrosative stress may mediate neurovascular dysfunction.15, 16
Experimental evidence suggests that NMDAR activation occurs very early during HI.1, 2 Although neurons are the cellular target of neonatal HI, injury on microvessels in the early phase post HI may contribute greatly to neuronal damage, as BBB disruption has been linked to the severity of HI injury in neonatal brains.17 Whether microvascular damage occurs early after HI via nNOS activation and contributes to neonatal brain injury remains unknown.
In the model of HI in postnatal (P) day-7 rat pups, permanent ligation of unilateral carotid artery followed by systemic hypoxia progressively damages the hemisphere ipsilateral to the artery occlusion.9 The damage area resembles the hypoxic ischemic brain damage in the asphyxiated full-term newborn.6, 7, 8, 9 The present study used this neonatal model of HI brain injury to test the following hypotheses: (1) microvascular nitrosative stress and damage, and decreased cerebral blood flow (CBF) and cerebral perfusion occur early post-HI reoxygenation; and (2) nNOS inhibition during HI decreases nitrosative stress, normalizes microvasculature, improves CBF and cerebral perfusion early after reoxygenation, and provides significant neuroprotection.
Materials and Methods
Hypoxic Ischemia Injury
This study was approved by the Animal Care Committee of National Cheng Kung University and was performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All efforts were made to minimize animal suffering and the number of animals sacrificed. Rat pups were housed with their dam in a temperature- and humidity-controlled manner with a 12-hour light–dark cycle. Food and water were available ad libitum. The right common carotid artery of postpartum day-7 (P7) Sprague–Dawley male pups was permanently ligated. After 1 hour, the pups were placed in airtight 500-mL containers with 8% oxygen flow rate of 3 L per minute for 2 hours.6, 7, 8, 10 The pups were randomly assigned into different groups: intraperitoneally injected with 7-nitroindazole (7-NI; 75 mg/kg), a nNOS inhibitor; aminoguanidine (AG; 300 mg/kg, in saline), an inducible NOS (iNOS) inhibitor; or vehicle at 30 minutes before or 3 hours after HI. Technicians performed the experiments, while investigators masked to the groupings did the quantitative measurements.
Immunohistochemistry
At different designated time point, post-HI reoxygenation, the brains were perfused and sectioned (14-μm or 50-μm thick) coronally. The sections in the three reference planes, plates 18, 31, and 39 of a rat brain atlas18 were used for immunohistochemistry. The sections were probed with primary antibody, followed by horseradish peroxidase-conjugated anti-immunoglobulin G secondary antibodies (1:200, Chemicon, EMD Millipore, Billerica, MA, USA). Four continuous visual fields (0.145 mm2/field) in the cortex per section (magnification, × 200) (Supplementary Figure 1) and three sections per rat were analyzed.7
Neurons, endothelial cells, and blood–brain barrier damage and microglia activation
The sections (14-μm) were incubated with the following primary antibodies: microtubular-associated protein 2 (1:100, Cell Signaling), rat endothelial cell antigen-1 (1:100, Abcam), biotinylated anti-rat immunoglobulin G antibody (biotinylated 1:400, Chemicon), anti-3 nitrotyrosine (1:100; Chemicon), or anti-ED1 (1:200, Millipore) antibody. The immunohistochemistry staining was quantified using Image-Pro Plus 6.0 at × 200 magnification per visual field (0.145 mm2). The integrated optical density (equals to area × average optical density) of the positive staining of immunoglobulin G signals for BBB damage and 3 nitrotyrosine was calculated, and the number of ED1(+) microglia was analyzed from each section. ED1(+) cells were expressed as the average ED1(+) cell numbers per visual field. The mean integrated optical density values in the ipsilateral hemispheres of each experimental group were compared with those of the control group to obtain the relative integrated optical density ratios.7
Microvascular density measurement
The sections (50 μm) were incubated with anti-rat endothelial cell antigen-1 (1:100). Vascular density was defined as the total length of the microvessel per unit area of the image (mm/mm2). The vascular number in the ipsilateral cortex was counted and compared with that of the contralateral cortex.16
Cerebral Perfusion and Blood Flow
Cerebral blood perfusion
Red blood cells in the vessels were used to measure cerebral perfusion. The brains were obtained without perfusion at 1 hour after reoxygenation and the sections (50 μm) were hydrated and rinsed. 3, 3'-diaminobenzidine was used as a substrate for superoxide peroxidase in red blood cells.19
Cerebral blood flow
Cerebral blood flow was measured using a fiberoptic laser–Doppler device (PERIMED, Stockholm, Sweden) with dual probe tips oriented to the calvarium bilaterally over the parietal cortex. The translucent calvarium of the pups enabled CBF monitoring in real time without opening the skull. The dual probe tips were positioned over the left and right hemisphere 3 mm posterior to the bregma and 3 mm lateral to the midline. Cerebral blood flow was measured continuously during the 2 hours of hypoxia and in the first 3 hours after reoxygenation,8 and expressed as percentage of changes of the baseline for each pup.
Transmission Electron Microscopy
The animals were perfused through the left ventricle with normal saline followed by fixative with 2% paraformaldehyde and 2% glutaldehyde in 0.1 mol/L phosphate buffer (pH=7.2). At 1-, 3-, and 24-hour post-reoxygenation, the perfused cortices (2 × 2 × 2 mm block) were examined for the area between the bregma −3.3 to −5.3 mm and 3.5 mm lateral to the midline. The samples were postfixed overnight, blocked, and fixed in 1% OsO4 aqueous solution for 1 hour, and washed with ddH2O for 10 minutes and then dehydrated in increasingly graded ethanol and pure propylene oxide. The samples were embedded in Epon at room temperature and polymerized in oven at 55°C for 1 day. Sections (80-nm thin) were collected onto the grids, stained with lead citrate and uranyl acetate, and examined using a JOEL 1200 electron microscope (Joel, Tokyo, Japan) at 80 kV.20 The cytologic features and the microvascular lumen area per endothelial cell with identifiable cell nucleus were assessed.
Brain Damage Measurement
Brain damage was determined by Nissl staining at P14. The cross-sectional area of the cortex in the five reference planes, corresponding to plates 15, 18, 27, 31, and 39 in a rat brain atlas18 was calculated. The percentage of cortical area loss in the lesion versus the non-lesion hemisphere was determined.6, 7, 8, 10
NOS Activity
The ipsilateral cortex was excised and the constitutive nitric oxide synthases (NOS) and iNOS activity determined by monitoring the conversion of 3H-arginine into 3H-citrulline (NOS activity kit, Cayman Chemicals, Ann Arbor, MI, USA).21 The reaction was performed to measure the constitutive NOS and iNOS, respectively. The cortices were homogenized, centrifuged, and the supernatant was transferred to microcentrifuge tubes. The reaction buffer containing 1 μL of 3H-arginine monohydrochloride (1 μCi/mL; Perkin-Elmer Life Sciences, Boston, MA, USA), 5 μL of 10 mmol/L NADPH (freshly prepared in 10 mmol/L Tris-HCl, pH 7.4, Sigma, St Louis, MO, USA), 5 μL of 6 mmol/L CaCl2 (or H2O for iNOS test), and 4 μL of deionized H2O was combined with 5 μL of tissue homogenate. The reaction was incubated for 30 or 60 minutes at room temperature for constitutive NOS and iNOS test, respectively. After incubation, the reactions were stopped with stop buffer, 100 μL of equilibrated resin was added to each microtube, and each reaction mix was transferred to a microcentrifuge tube. Samples were centrifuged in a microcentrifuge at full speed for 30 seconds. The resulting elute was transferred to scintillation vials, and 4 mL of liquid scintillation cocktail (Perkin-Elmer Life Sciences) was added. Radioactivity corresponding to 3H-citrulline was measured (cpm) in a liquid scintillation counter (Beckman LS 6500, Brea, CA, USA). The conversion of arginine to citrulline was calculated with the following equation: Percentage conversion=(cpm of the eluate—cpm blank)/total cpm) × 100. Values in each experimental group were compared with those of the control group to obtain ratios.
Statistics
Statistical analysis was performed using the SPSS version 13.0 (SPSS Institute, Chicago). Numerical continuous data were presented as mean±s.e.m. and analyzed using one-way analysis of variance. The Tukey's test was used for post hoc comparisons. The Kruskal–Wallis test and Tukey's test for post hoc comparisons were used to compare brain area between groups. Statistical significance was set at a two-tailed P<0.05.
Results
Microvascular Damage Occurred Early and Progressively after Reoxygenation
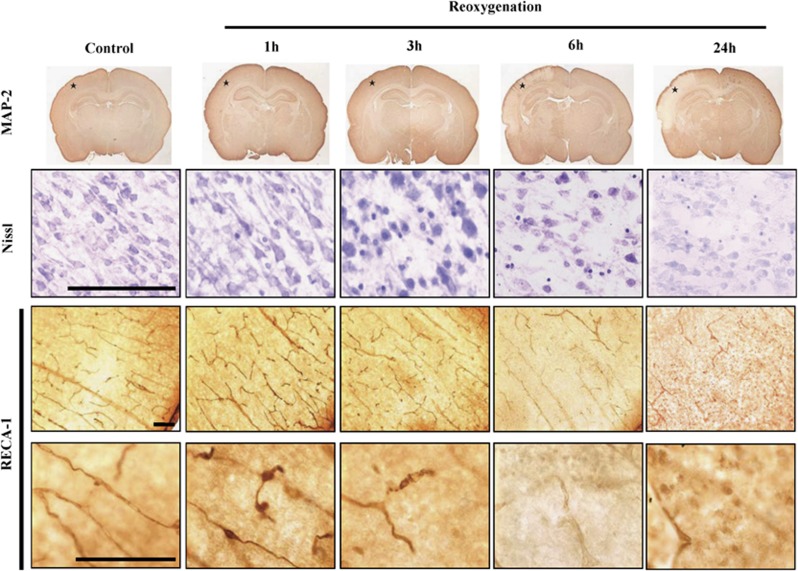
After HI, microtubular-associated protein 2 staining showed neuronal injury at 6 hours and marked neuronal damage at 24 hours after reoxygenation in the ipsilateral cortex (Figure 1). Nissl staining revealed progressive neuronal damage: a small amount of pyknotic neurons at 1 hour, a few neurons with pyknotic nuclei at 3 hours, many pyknotic neurons at 6 hours, and extensively pale and damaged neurons at 24 hours post reoxygenation. In the control pups, rat endothelial cell antigen-1 staining showed a high density of radially penetrating and branching vessels originated from the pial surface of the cortex. There were early and progressive vessel damages: narrowing of the vascular lumen at 1 hour, discontinuation and fragmentation of the microvessels at 3 hours, disappearance of the branching vessels at 6 hours, and extensive loss of microvessels at 24 hours post reoxygenation (Figure 1).
Figure 1.
Neuronal injury progressed from 3 to 24 hours (Nissl staining) and from 6 to 24 hours (microtubular-associated protein 2 (MAP-2) staining) after reoxygenation. Vascular lumen narrowed at 1 hour and progressed to extensive losses of microvessels at 24 hours post reoxygenation (rat endothelial cell antigen-1 (RECA-1)). Nissl and RECA-1 stainings were photographed from the cortex of MAP-2 images marked with asterisks. n=3–4 per time point. Scale bar, 100 μm.
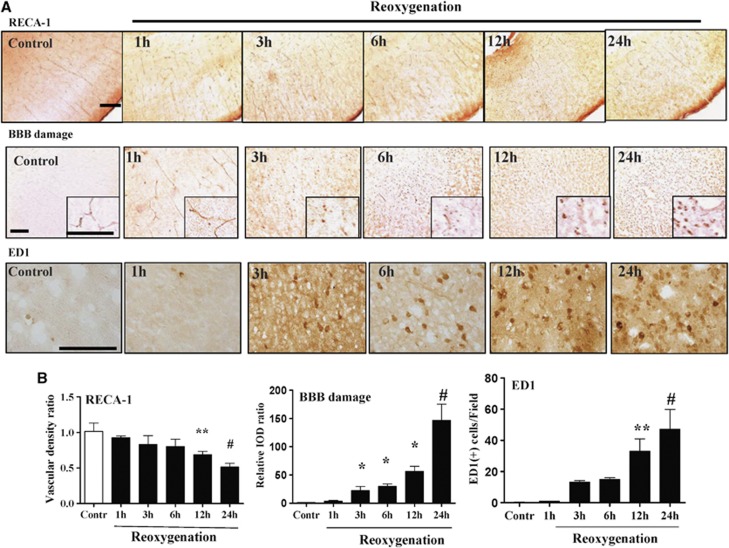
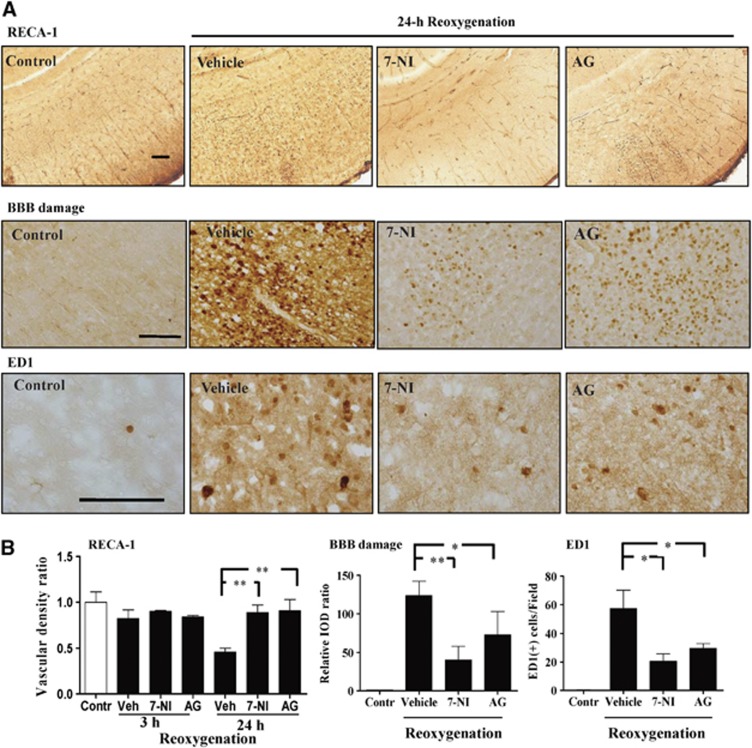
Rat endothelial cell antigen-1 staining showed significantly decreases of vascular number at 12 and 24 hours post reoxygenation (Figure 2). Immunohistochemistry also showed that BBB injury progressed from 3 to 24 hours after reoxygenation, and microglia activation increased from 12 to 24 hours post reoxygenation (Figure 2).
Figure 2.
(A, B) Vascular density (rat endothelial cell antigen-1 (RECA-1) staining) showed significantly decreased vascular number at 12 and 24 hours after reoxygenation. Blood–brain barrier (BBB) injury (immunoglobulin G extravasation) progressed from 3 to 24 hours, and microglia activation (ED1 staining) occurred at 12 to 24 hours after reoxygenation. n=4–5 per time point; values are mean±s.e.m. Scale bar, 100 μm; *P<0.05, **P<0.01, #P<0.001.
Microvascular Injury and Nitrative Stress, and Decreased Cerebral Perfusion and Blood Flow Occurred Early after Reoxygenation
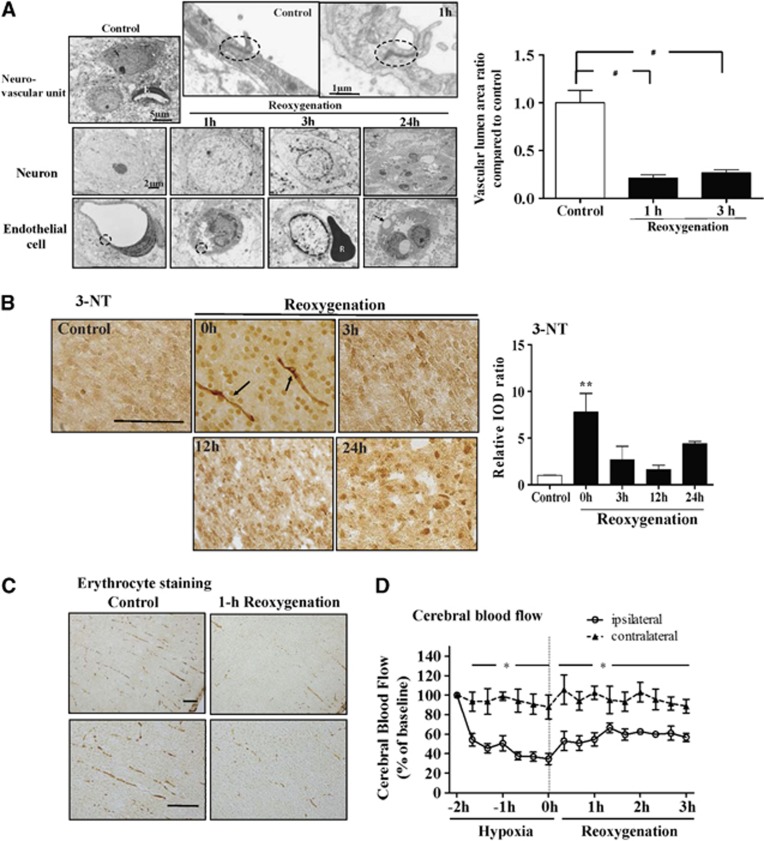
Transmission electron microscopy of the neurovascular unit showed that after HI, neurons had heterochromatic chromatin at 1 hour; condensed nucleus chromatin, swollen mitochondria, cytoplasmic vacuoles, and loss of synapses at 3 hours; and broken cellular nuclear membrane and loss of organelles at 24 hours post reoxygenation (Figure 3A). Endothelial cells showed irregular cell surface and enlarged nuclei that narrowed the vascular lumen at 1 hour; vacuolated cytoplasmic structures containing electron-dense material and loss of tight junction at 3 hours; and large vacuoles in the cytoplasm and nuclei, ballooning of the surface and broken cell membrane at 24 hours post reoxygenation. Compared with the control, the vascular lumen area was significantly decreased at 1 hour and 3 hours after reoxygenation (Figure 3A).
Figure 3.
(A) Transmission electron microscopy of a normal neurovascular unit: neuron (N), microvessel with lumen lined by endothelial (E) cells and visible tight junction (black circle). After hypoxic ischemia, neurons showed heterochromatic chromatin at 1 hour; condensed nucleus chromatin, swollen mitochondria, and cytoplasmic vacuoles at 3 hours; and broken membrane and loss of organelles at 24 hours after reoxygenation. Endothelial cells showed visible tight junction (black circle) but increased enlarged nuclei narrowing the vascular lumen at 1 hour; vacuolated cytoplasm-containing electron-dense material and loss of tight junction at 3 hours, and large vacuoles (arrow) in the cytoplasm and nuclei and broken cell membrane at 24 hours after reoxygenation. R, erythrocyte. The microvascular lumen areas with identifiable endothelial cell nucleus were significantly decreased at 1 hour and 3 hours after reoxygenation. n=30–40 microvessels per group. (B) 3 nitrotyrosine (3-NT) showed markedly increased 3-nitrotyrosin expression in the microvessels (arrows) immediately post reoxygenation compared with the control. (C) Cerebral perfusion evaluated by erythrocyte staining revealed marked interruptions of the erythrocyte column in the microvessels at 1 hour post reoxygenation. (D) Cerebral blood flow showed significantly decreased blood flow during hypoxia and in the first 3 hours after reoxygenation. n=4–5 per group, scale bar, 2 μm (A), 100 μm (B, C). Values are mean±s.e.m. *P<0.05, **P<0.01, #P<0.001. IOD, integrated optical density.
3 nitrotyrosine staining revealed significant increases of 3-nitrotyrosin expression in the microvessels immediately post reoxygenation (Figure 3B). Cerebral perfusion study showed clearly visible and continuous erythrocyte columns filling intact microvessels before hypoxia, but marked interruptions of the erythrocyte columns 1 hour after reoxygenation (Figure 3C). There was significantly decreased CBF (∼40% of baseline) during hypoxia that persisted (∼60% of baseline) within the first 3 hours after reoxygenation (Figure 3D).
Neuronal Nitric Oxide Synthases Inhibition before Hypoxia Afforded Complete Neuroprotection
Constitutive NOS activity increased to 120% of baseline during hypoxia, decreased to 40% to 50% of baseline at 1–12 hours post reoxygenation, and gradually increased to baseline after 24 hours. Inducible NOS activity decreased to 15% of baseline during hypoxia, further decreased to 25% of baseline at 1 hour, returned to baseline at 12 hours, and increased to 300% of baseline at 24 hours post reoxygenation (Supplementary Figure 2A and B).
Compared with vehicle, 7-NI treatment before hypoxia significantly decreased constitutive NOS activity during hypoxia (vehicle 120% versus 7-NI 30% of baseline), but had no significant difference at 24 hours post reoxygenation (100% versus 110% of baseline). The results showed that 7-NI treatment before hypoxia was sufficient to decrease constitutive NOS activity during HI but not at 24 hours post-HI. In contrast, AG treatment before hypoxia showed no effect on constitutive NOS activity during hypoxia (vehicle 120% versus AG 110% of baseline) and 24 hours after reoxygenation (100% versus 110% of baseline). Compared with vehicle, 7-NI and AG treatment before hypoxia had similar effects on iNOS activity during hypoxia (vehicle 15% versus 7-NI 20% versus AG 25% of baseline), but both 7-NI and AG significantly decreased iNOS activity 24 hours after reoxygenation (285% versus 100% versus 115% of baseline) (Supplementary Figure 2C and D). These data showed that 7-NI and AG treatment before hypoxia significantly decreased iNOS activity 24 hours post-HI reoxygenation.
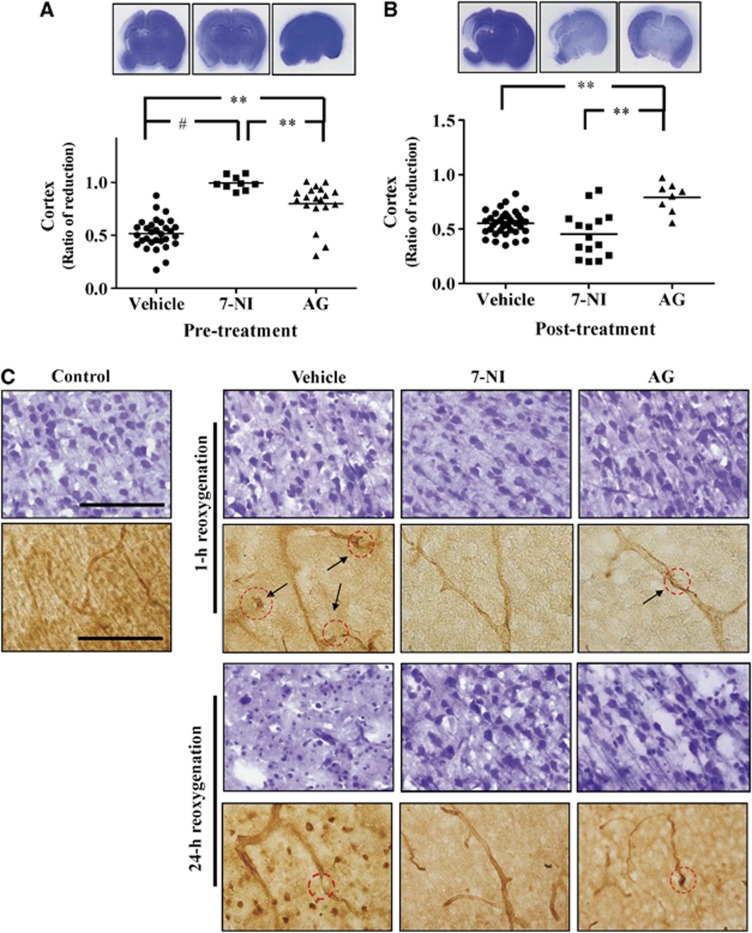
Pathology examination showed that treatment before hypoxia, 7-NI provided complete neuroprotection and AG showed significant but partial neuroprotection compared with vehicle (Figure 4A). 7-nitroindazole had significantly better neuroprotection than AG. In contrast, treatment at 3 hours post reoxygenation, AG, but not 7-NI, still provided significant neuroprotection (Figure 4B).
Figure 4.
(A) 7-nitroindazole (7-NI) treatment before hypoxia provided complete neuroprotection compared with vehicle, while aminoguanidine (AG) treatment showed partial neuroprotection. (B) Treatment at 3 hours post hypoxic ischemia showed that AG but not 7-NI still provided significant neuroprotection. (C) Treatment before hypoxia showed a small amount of pyknotic neurons in the vehicle group but no significant changes in the 7-NI or AG groups at 1 hour after reoxygenation. At 24 hours post-reoxygenation, the vehicle group had extensive neuronal damage, the AG-treated group showed many pyknotic neurons, and the 7-NI group had no neuronal damage. There were vascular interruptions in the vehicle and AG groups (arrows) at 1 hour after reoxygenation, and extensive microvessel loss in the vehicle group and some vascular narrowing in the AG group at 24 hours post reoxygenation. The 7-NI group had patent vascular lumen at 1 hour and 24 hours post reoxygenation. Scale bar, 100 μm; **P<0.01, #P<0.001.
Neuronal Nitric Oxide Synthases Inhibition Attenuated Early and Progressive Microvascular Injury
Examining whether 7-NI or AG treatment before hypoxia provided neurovascular protection, Nissl staining showed the vehicle group had a small amount of pyknotic neurons at 1 hour, and markedly pale and damaged neurons at 24 hours post reoxygenation. The AG group showed no significant neuronal changes at 1 hour but many pyknotic neurons at 24 hours post reoxygenation, while the 7-NI group had no obvious neuronal damage (Figure 4C).
By rat endothelial cell antigen-1 staining, the vehicle group revealed many interruptions in the microvessels at 1 hour and extensive loss or fragmentation at 24 hours post reoxygenation. In the AG group, there were interruptions in some microvessels at 1 hour and 24 hours post reoxygenation. In contrast, the 7-NI group showed patent vascular lumen without interruption or fragmentation in the same time points. Compared with the vehicle, the 7-NI and AG groups had significant increases in vascular density, and decreases of BBB damage and microglia activation (Figure 5) at 24 hours post reoxygenation.
Figure 5.
(A, B) Compared with the vehicle group, the 7-nitroindazole (7-NI) and aminoguanidine (AG) groups had significant increases of vessel number, decreases of blood–brain barrier (BBB) damage and microglia activation at 24 hours post reoxygenation. n=4–5 per group; values are mean±s.e.m. Scale bar, 100 μm; *P<0.05, **P<0.01. Contr, control; IOD, integrated optical density; RECA-1, rat endothelial cell antigen-1; Veh, vehicle.
Neuronal Nitric Oxide Synthases Inhibition Decreased Microvascular Nitrative Stress and Restored Cerebral Blood Flow and Perfusion Early Post Reoxygenation
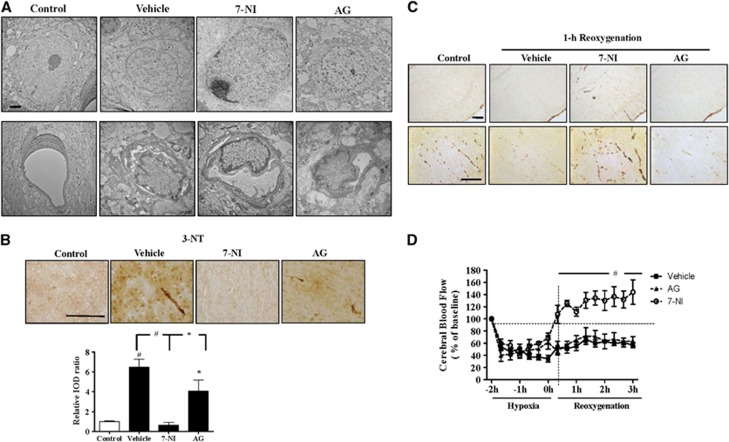
Transmission electron microscopy ultra-structure study at 1 hour post reoxygenation showed neurons with heterochromatin foci and swollen organelles in the vehicle and AG groups, but intact neuron with spherical nucleoli and deeply stained euchromatin in the 7-NI group. The vehicle and AG groups had perivascular edema and swollen nuclei that occluded the vascular lumen, whereas the 7-NI group showed more preserved vascular lumen (Figure 6A). By 3 nitrotyrosine staining, the vehicle and AG groups had markedly increased microvascular nitrotyrosination than the control. The 7-NI group had significantly decreased microvascular nitrotyrosination than the vehicle and AG groups (Figure 6B). Erythrocyte staining at 1 hour post reoxygenation revealed interruptions of the erythrocyte columns in the microvessels of the vehicle and AG groups, but not in the 7-NI group (Figure 6C). Both the vehicle and AG groups had significantly decreased CBF during hypoxia and in the first 3 hours post reoxygenation. In contrast, the 7-NI group showed increased CBF and had significantly higher CBF than the vehicle and AG groups in the first 3 hours after reoxygenation (Figure 6D).
Figure 6.
(A) Transmission electron microscopy examination at 1 hour post reoxygenation showed heterochromatic neurons in the vehicle and aminoguanidine (AG) groups, but intact neuron in the 7-nitroindazole (7-NI) group. The vehicle and AG groups had swollen nuclei that occluded the vascular lumen whereas the 7-NI group had more preserved vascular lumen. (B) The 7-NI group had significantly decreased nitrotyrosination in the microvessels than the vehicle and AG groups immediately after reoxygenation. (C) At 1 hour post reoxygenation, the vehicle and AG groups had interrupted erythrocyte columns in the microvessels, whereas the 7-NI group showed continuous erythrocyte columns. (D) The vehicle and AG groups had significantly decreased cerebral blood flow (CBF) during hypoxia and in the early hours post reoxygenation. The 7-NI group had significantly higher CBF than these two groups in the first 3 hours post reoxygenation. n=4–5 per group; values are mean±s.e.m. Scale bar, 2 μm (A), 100 μm (B, C); *P<0.05, #P<0.001. 3-NT, 3 nitrotyrosine; IOD, integrated optical density.
Discussion
Hypoxic ischemia affects not only neurons but also vessels in the neonatal brain. Impaired CBF and reperfusion through injured microvessels, if early after HI, are likely to aggravate tissue damage. As such, normalization of microvasculature after HI is critical for improving neuroprotective therapies in the neonatal brain. The present study shows microvascular damage occurring early and progressively post-HI reoxygenation. There is increased microvascular nitrative stress and decreased CBF and perfusion early after reoxygenation. Neuronal nitric oxide synthases, but not iNOS, inhibition decreases microvascular nitrative stress, protects against microvascular damage, restores CBF and perfusion early after reoxygenation, and provides complete neuroprotection. These suggest that eliminating NO production via nNOS inhibition normalizes the microvasculature and leads to improved CBF and perfusion early after reoxygenation, and protects the neonatal brain.
The neurovascular unit has been investigated as a framework for the development of treatment that targets neuron and vessels in neurologic diseases.4 Disruption of cerebral circulation impairs the delivery of adequate substrates and interrupts the homeostasis of the microenvironment in which neurons function. Vascular dysfunction may be present early in neurologic diseases, even before the onset of neuronal death, implying that vascular abnormality may actively contribute to neural injury.5, 11, 22 Early hemodynamic alteration may contribute significantly to HI brain lesions, and studies have shown that significant neurovascular protection post HI is related with long-term neuroprotection in neonatal brain.6, 7, 8 The present study provides evidence of early and progressive damage to the cortical microvessels that markedly reduces CBF and perfusion early post HI. The microvessels are irregular, swollen, and discontinuous, and show marked nitrative stress. Vascular normalization or restoration of the normal structure and function in blood vessels early after HI may emerge as a strategy for treating high-risk neonates with asphyxia.
Nitric oxide is produced by nNOS, an enzyme tethered to the NMDAR complex, by the postsynaptic density protein-95. Under physiologic conditions, NO is involved in critical NMDAR-mediated neuroplasticity and contributes to the increase in CBF evoked by neural activity.23 Under pathologic condition, excess NO, formed in response to NMDAR overactivation early during HI, is crucial in neurotoxicity.1, 2 A study using a rat pup model of pure arterial occlusion shows that nNOS inhibition increases CBF in male pups but does not significantly change infarct volumes.24 The present study, using the HI model in male pups, shows that inhibiting nNOS markedly decreases microvascular nitrative stress and significantly increases CBF and perfusion in the early phase post HI. Inhibiting nNOS also protects against microvascular damage at microscopic and ultrastructural levels, and provides complete neuroprotection. These findings suggest that increased microvascular nitrative stress via nNOS overactivation initiated during HI is involved in microvascular damage early post-reoxygenation.
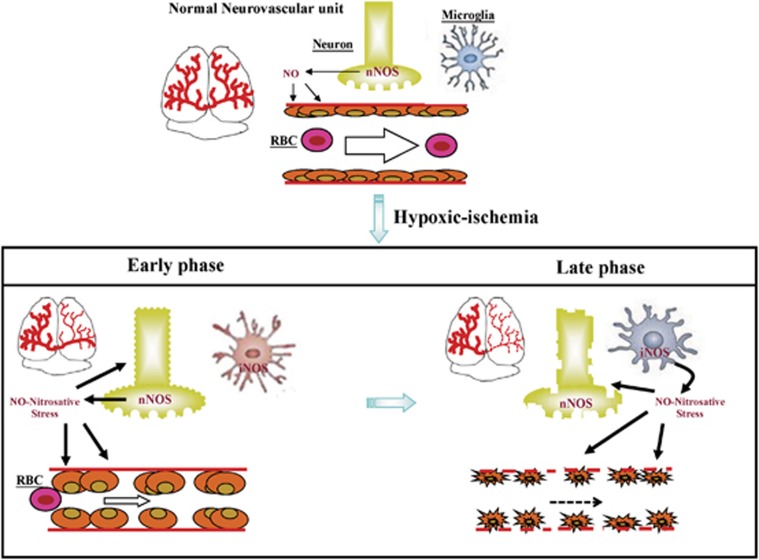
Neuronal nitric oxide synthases has a distinct local role in the physiologic regulation of human microvascular tone in vivo.25 Neuronal nitric oxide synthases-derived NO can influence vascular tone by inducing direct vasodilation in cerebral arteries. Hypoxic ischemia triggers sequential cascades of neurotoxic events that are initiated within hours, last for days, and progressively result in prominent neuronal loss. After HI, there are two peaks of NO metabolites in the injured cortex of neonatal brain: one during hypoxia (involving nNOS) and the other during reoxygenation (involving iNOS).26 Our data showed that during HI, the constitutive NOS activity is upregulated whereas iNOS is downregulated. At 24 hours post-reoxygenation, the constitutive NOS activity returns to baseline levels whereas iNOS is markedly activated that correlated with evident microglial activation at the time point. A diagram is provided to propose that after HI, NO-mediated microvascular damage occurs early and progressively: via nNOS in the early phase and via iNOS in the late phase post reoxygenation (Figure 7).
Figure 7.
Microvascular damage occurs early and progressively post hypoxic ischemia (HI) reoxygenation. There is decreased cerebral blood flow and perfusion early after reoxygenation. Nitric oxide (NO)-mediated microvascular damage occurs in the early phase post reoxygenation via neuronal nitric oxide synthases (nNOS) and in the late phase via inducible NOS (iNOS). RBC, red blood cell.
The present study shows that there is increased microvascular nitrative stress, and decreased CBF and perfusion early after reoxygenation. Neuronal nitric oxide synthases but not iNOS inhibition significantly attenuates microvascular nitrative stress and damage, and markedly increases CBF and perfusion in the early phase post reoxygenation, and subsequently provides complete neuroprotection against HI injury. These data suggest that damage to the microvasculature that occurs early after reoxygenation via nNOS has an important initiation role for the progressive neuronal injury after HI.
Our study also revealed that HI not only causes neuronal and endothelial cell damages but also progressively activates microglia. After reoxygenation, damaged microvessels may recruit activated leukocytes into the injured brain through the disrupted BBB, resulting in sustained microglial activation, which in turn further damages the brain and its vessel through prolonged production of inflammatory cytokines.27 Aminoguanidine has a longer half-life (8 hours) than 7-NI (4 hours). Our study showed that inhibition of iNOS significantly attenuates microglia activation and HI injury suggesting that iNOS and microglia activation that work later after reoxygenation still contribute to HI injury.
The role of eNOS in microvascular damage early post-HI remains unclear. In contrast to the neuroprotective effect observed in nNOS and iNOS knockout mice, eNOS knockout mice are quite susceptible to ischemic injury, suggesting that eNOS activation during hypoxia is protective.28 In conclusion, discovering how neurovascular protection early after HI may salvage neurons can lead to new treatment of neonatal HI brain injury. The microvasculature, acting as a factor contributing to the development and progression of HI, may become a major therapeutic target in the treatment of neonatal HI encephalopathy.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
This study was supported by the Taiwan National Science Council (NSC: NSC100-2314-B006-042-MY3).
Supplementary Material
References
- Ferriero DM. Neonatal brain injury. New Engl J Med. 2004;351:1985–1995. doi: 10.1056/NEJMra041996. [DOI] [PubMed] [Google Scholar]
- Johnston MV, Fatemi A, Wilson MA, Northington F. Treatment advances in neonatal neuroprotection and neurointensive care. Lancet Neurol. 2011;10:372–382. doi: 10.1016/S1474-4422(11)70016-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam SJ, Watts RJ. Connecting vascular and nervous system development: angiogenesis and the blood-brain barrier. Ann Rev Neurosci. 2010;33:379–408. doi: 10.1146/annurev-neuro-060909-152829. [DOI] [PubMed] [Google Scholar]
- Quaegebeur A, Lange C, Carmeliet P. The neurovascular link in health and disease: molecular mechanisms and therapeutic implications. Neuron. 2011;71:406–424. doi: 10.1016/j.neuron.2011.07.013. [DOI] [PubMed] [Google Scholar]
- del Zoppo GJ. Stroke and neurovascular protection. New Engl J Med. 2006;354:553–555. doi: 10.1056/NEJMp058312. [DOI] [PubMed] [Google Scholar]
- Lee HT, Chang YC, Tu YF, Huang CC. VEGF-A/VEGFR-2 signaling leading to cAMP response element-binding protein phosphorylation is a shared pathway underlying the protective effect of pre-conditioning on neurons and endothelial cells. J Neurosci. 2009;29:4356–4368. doi: 10.1523/JNEUROSCI.5497-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu YF, Lu PJ, Huang CC. Moderate dietary restriction reduces p53-mediated neurovascular damage and microglia activation after hypoxic ischemia in neonatal brain. Stroke. 2012;43:491–498. doi: 10.1161/STROKEAHA.111.629931. [DOI] [PubMed] [Google Scholar]
- Lee BF, Wang LW, Lin SH, Jhuo TJ, Chiu NT, Huang CC, et al. Tc-99m-HL91 imaging in early detection of neuronal injury in a neonatal rat model of hypoxic ischemia. Crit Care Med. 2012;40:1930–1938. doi: 10.1097/CCM.0b013e31824e1883. [DOI] [PubMed] [Google Scholar]
- Vannucci RC, Vannucci SJ. Perinatal hypoxic-ischemic brain damage: evolution of an animal model. Dev Neurosci. 2005;27:81–86. doi: 10.1159/000085978. [DOI] [PubMed] [Google Scholar]
- Lin WY, Chang YC, Ho CJ, Huang CC. Ischemic pre-conditioning reduces neurovascular damage after hypoxia-ischemia via the cellular inhibitor of apoptosis 1 in neonatal brain. Stroke. 2013;44:162–169. doi: 10.1161/STROKEAHA.112.677617. [DOI] [PubMed] [Google Scholar]
- del Zoppo GJ, Mabuchi T. Cerebral microvessel responses to focal ischemia. J Cereb Blood Flow Metab. 2003;23:879–894. doi: 10.1097/01.WCB.0000078322.96027.78. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Yanez M, Castellanos M, Blanco M, Mosquera E, Castillo J. Vascular protection in brain ischemia. Cerebrovasc Dis. 2006;21 (Suppl 2:21–29. doi: 10.1159/000091700. [DOI] [PubMed] [Google Scholar]
- Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57:173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- Bolanos JP, Almeida A. Roles of nitric oxide in brain hypoxia-ischemia. Biochimica et Biophysica Acta. 1999;1411:415–436. doi: 10.1016/s0005-2728(99)00030-4. [DOI] [PubMed] [Google Scholar]
- Girouard H, Park L, Anrather J, Zhou P, Pardee C, Iadecola C. Cerebrovascular nitrosative stress mediates neurovascular and endothelial dysfunction induced by angiotensin II. Arterioscler Thromb Vasc Biol. 2007;27:303–309. doi: 10.1161/01.ATV.0000253885.41509.25. [DOI] [PubMed] [Google Scholar]
- Sirinyan M, Sennlaub F, Dorfman A, Sapieha P, Gobeil F, Jr, Hardy P, et al. Hyperoxic exposure leads to nitrative stress and ensuing microvascular degeneration and diminished brain mass and function in the immature subject. Stroke. 2006;37:2807–2815. doi: 10.1161/01.STR.0000245082.19294.ff. [DOI] [PubMed] [Google Scholar]
- Muramatsu K, Fukuda A, Togari H, Wada Y, Nishino H. Vulnerability to cerebral hypoxic-ischemic insult in neonatal but not in adult rats is in parallel with disruption of the blood-brain barrier. Stroke. 1997;28:2281–2288. doi: 10.1161/01.str.28.11.2281. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C.The rat brain in stereotaxic coordinates2nd edn, Academic Press: San Diego, CA; 1986 [Google Scholar]
- Yemisci M, Gursoy-Ozdemir Y, Vural A, Can A, Topalkara K, Dalkara T. Pericyte contraction induced by oxidative-iterative stress impairs capillary reflow despite successful opening of an occluded cerebral artery. Nat Med. 2009;15:1031–1037. doi: 10.1038/nm.2022. [DOI] [PubMed] [Google Scholar]
- Puka-Sundvall M, Gajkowska B, Cholewinski M, Blomgren K, Lazarewicz JW, Hagberg H. Sub-cellular distribution of calcium and ultra-structural changes after cerebral hypoxia-ischemia in immature rats. Brain Res Dev Brain Res. 2000;125:31–41. doi: 10.1016/s0165-3806(00)00110-3. [DOI] [PubMed] [Google Scholar]
- Li J, Wilson A, Kuruba R, Zhang Q, Gao X, He F, et al. FXR-mediated regulation of eNOS expression in vascular endothelial cells. Cardiovasc Res. 2008;77:169–177. doi: 10.1093/cvr/cvm016. [DOI] [PubMed] [Google Scholar]
- Iadecola C. The overlap between neuro-degenerative and vascular factors in the pathogenesis of dementia. Acta Neuropathol. 2010;120:287–296. doi: 10.1007/s00401-010-0718-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garthwaite J. Concepts of neural nitric oxide-mediated transmission. Eur J Neurosci. 2008;27:2783–2802. doi: 10.1111/j.1460-9568.2008.06285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnin P, Leger PL, Villapol S, Deroide N, Gressens P, Pocard M, et al. Dual action of NO synthases on blood flow and infarct volume consecutive to neonatal focal cerebral ischemia. Exp Neurol. 2012;236:50–57. doi: 10.1016/j.expneurol.2012.04.001. [DOI] [PubMed] [Google Scholar]
- Seddon MD, Chowienczyk PJ, Brett SE, Casadei B, Shah AM. Neuronal nitric oxide synthase regulates basal microvascular tone in humans in vivo. Circulation. 2008;117:1991–1996. doi: 10.1161/CIRCULATIONAHA.107.744540. [DOI] [PubMed] [Google Scholar]
- Higuchi Y, Hattori H, Kume T, Tsuji M, Akaike A, Furusho K. Increase in nitric oxide in the hypoxic-ischemic neonatal rat brain and suppression by 7-nitroindazole and aminoguanidine. Eur J Pharmacol. 1998;342:47–49. doi: 10.1016/s0014-2999(97)01524-0. [DOI] [PubMed] [Google Scholar]
- Dammann O, Durums S, Leviton A. Do white cells matter in white matter damage. Trends Neurosci. 2001;24:320–324. doi: 10.1016/s0166-2236(00)01811-7. [DOI] [PubMed] [Google Scholar]
- Huang Z, Huang PL, Ma J, Meng W, Ayata C, Fishman MC, et al. Enlarged infarcts in endothelial nitric oxide synthase knock-out mice are attenuated by nitro-L-arginine. J Cereb Blood Flow Metab. 1996;16:981–987. doi: 10.1097/00004647-199609000-00023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.